An Embedded Sensing and Communication Platform, and a Healthcare Model for Remote Monitoring of Chronic Diseases
Abstract
:1. Introduction
2. Review of the State of the Art and Main Contributions of the Work
- -
- The “1:1” scenario, where each of the patient has a set of biomedical sensors and an embedded acquisition, processing, and communication platform (hereafter called a gateway) to exchange data and healthcare protocols with a remote service center and/or HIS for telemedicine, where a doctor is connected.
- -
- The “1:N” scenario where the embedded acquisition and communication telemedicine platform is manged by a nurse, e.g., assigned by the PHS to a number N of different patients. The nurse is visiting and taking care of data acquisition from a set of N patients. The relevant biomedical data are then transmitted to the remote service center and/or HIS. In the “1:N” scenario it is the nurse that is moving and visiting patients at their homes.
- -
- The “point of care” scenario where a local building, e.g., a pharmacy, or a point of care in a school or a residence for elderly people, hosts the embedded acquisition and communication telemedicine platform and the set of sensors. The patients, instead of being hospitalized, with increased cost and waiting lists, can take the needed measurements at a specific point of care, which is then connected to the remote service center and/or HIS. In this scenario the patients are moving toward the point of care where a nurse supervises the biomedical measurement acquisitions to be collected and transmitted.
- -
- -
- -
- -
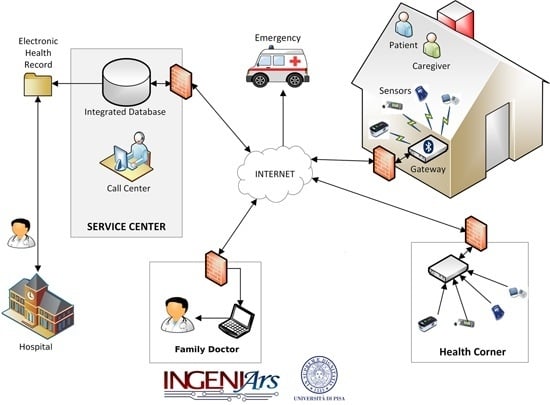
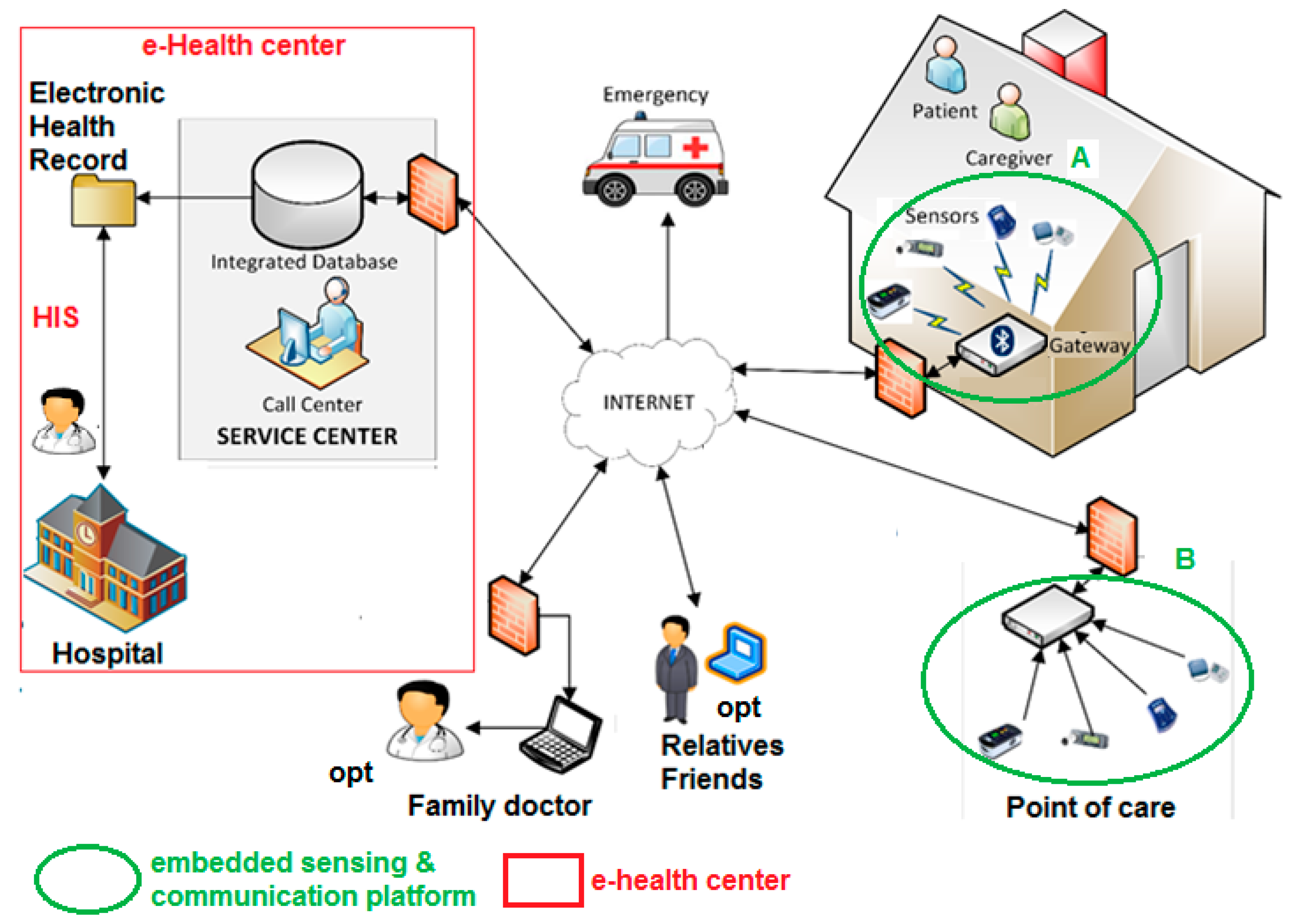
3. Remote Healthcare Model and Embedded Sensing/Communication Platform
- -
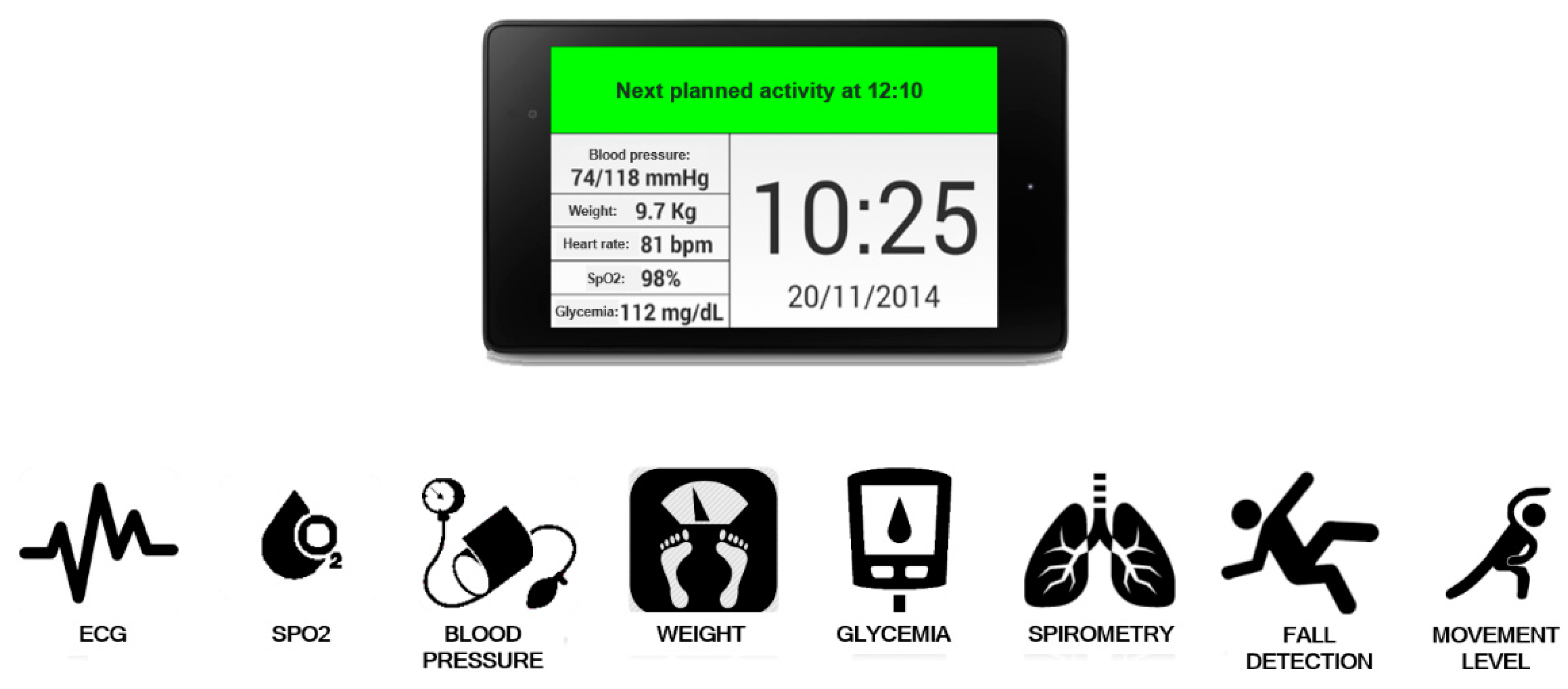
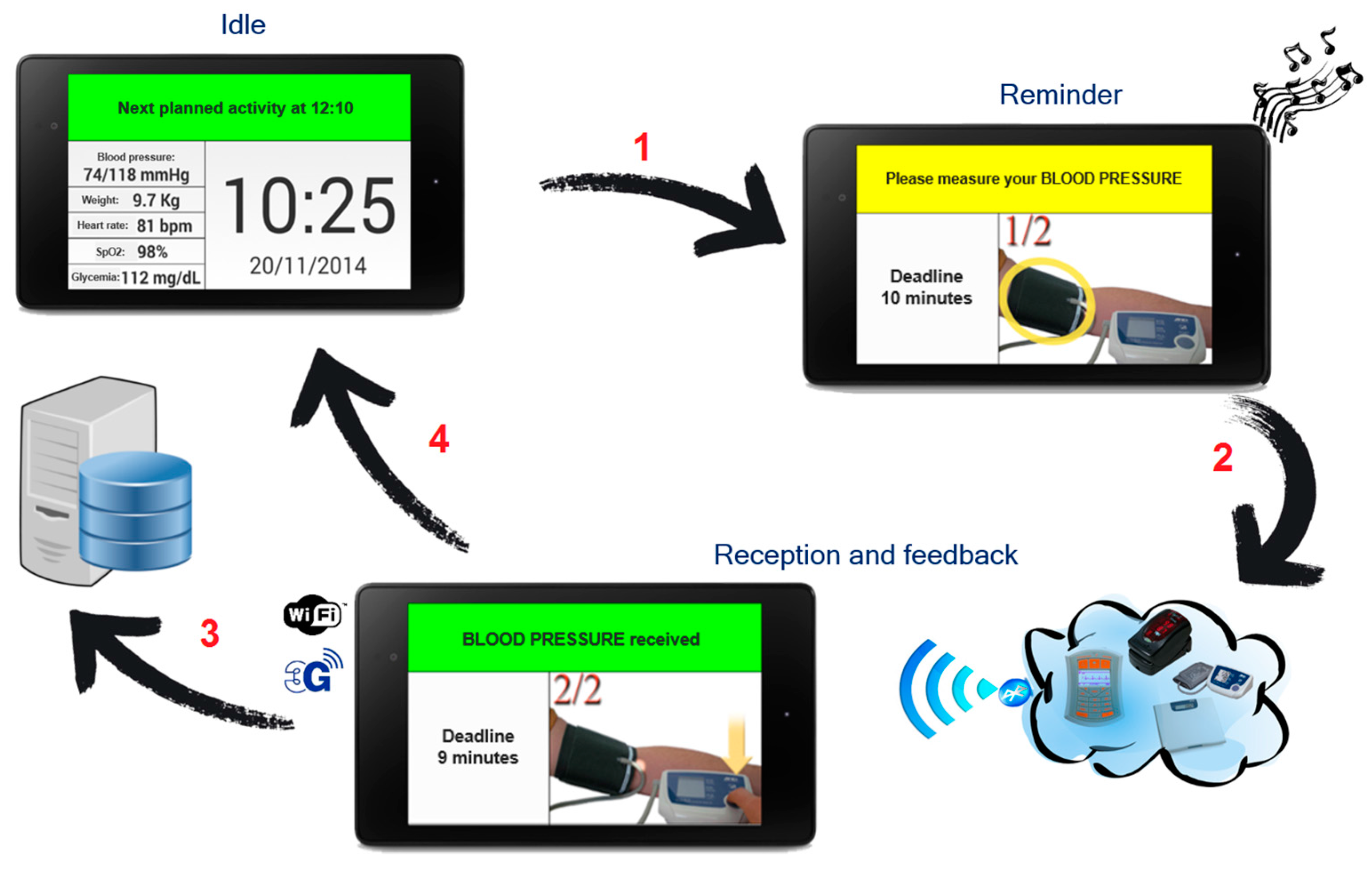
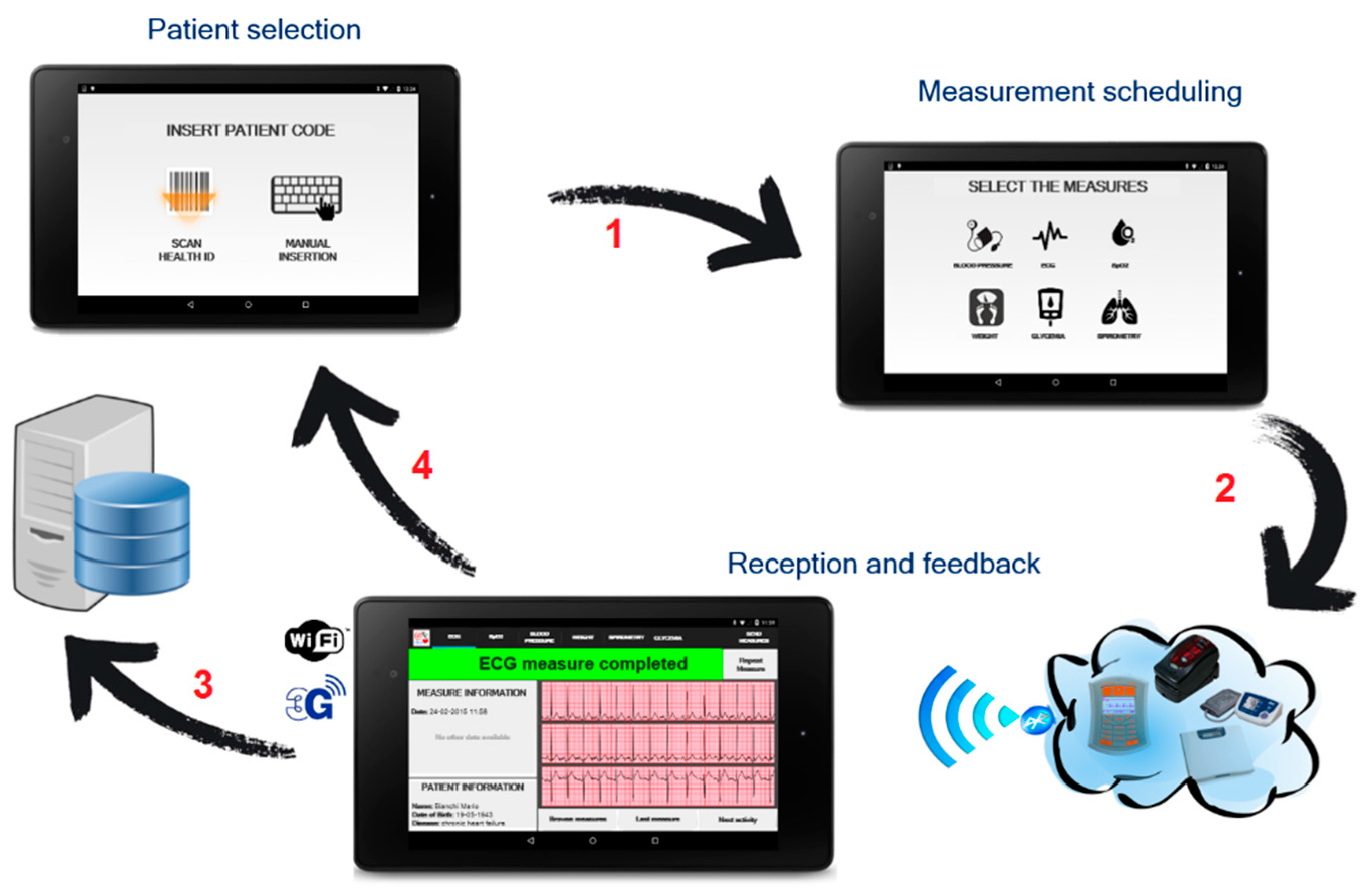
- A monitoring kit (embedded sensing and communication platform, oval A with green borders in Figure 1, whose description is detailed in Section 3.1, Section 3.2 and Section 3.3) which, depending if the “1:1” or “1:N” scenario is implemented, can be used by patients for self-measurement or by caregivers, e.g., nurses, during planned home visits.
- -
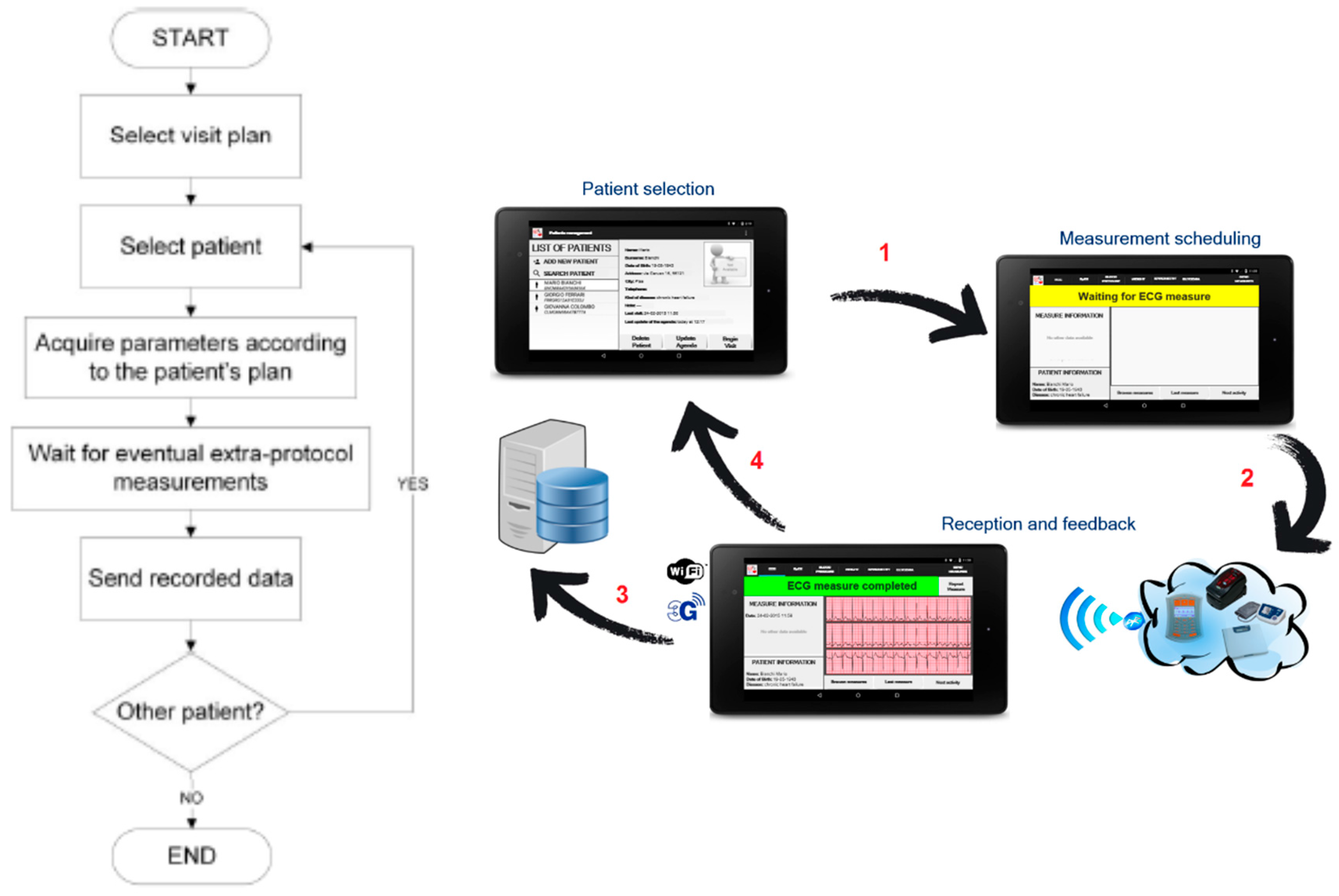
- A totem for the monitoring of the biomedical parameters to be installed at point of cares (e.g., pharmacies, residences for elderly people, or other healthcare points); this is the embedded sensing and communication platform indicated with oval B with green borders in Figure 1, whose description is detailed in Section 3.1 and Section 3.4).
- -
- Management platform of the electronic health record and of the home-care plan (e-Health center detailed in Section 4), integrated with the HIS, and available to specialist doctors directly or through operators of a service center. Optionally, through the service center the data of the electronic health record can also be made available to the family doctor. Alarms can be automatically generated by the embedded sensing/communication units (thanks to local signal processing capability) at home or at the point of care, or by a caregiver analyzing the data. Automatically generated alarms should be validated by a caregiver. An alarm, generated or validated by a caregiver, is communicated to emergency units for a fast re-hospitalization of the patient and, optionally, to a pre-selected list of relatives/friends.
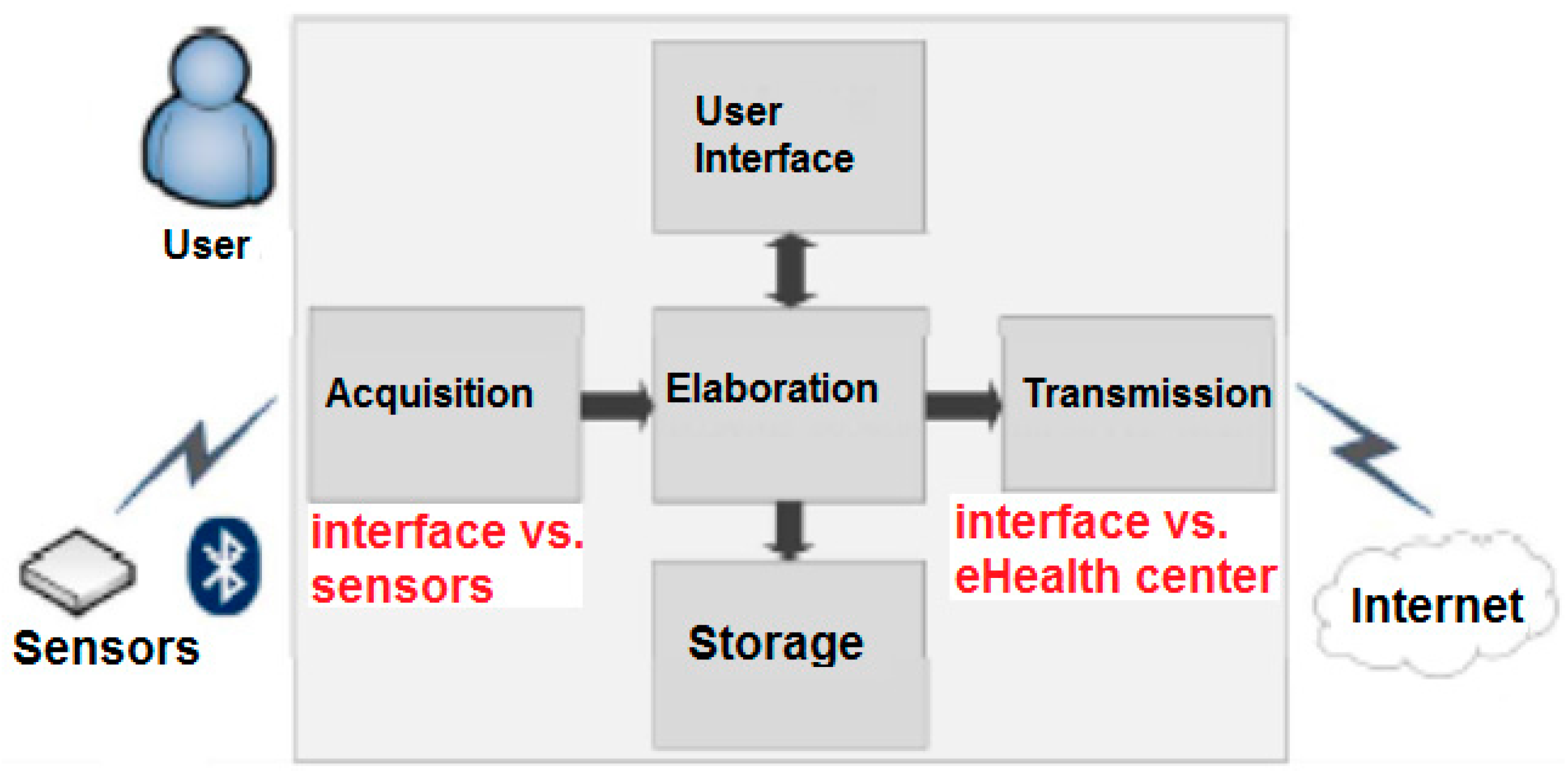
3.1. Biomedical Sensing and Communication Platform
- -
- Collection of the acquired data from the configured BT or BLE sensors to create statistics of the biomedical parameters acquired according to the specific plan.
- -
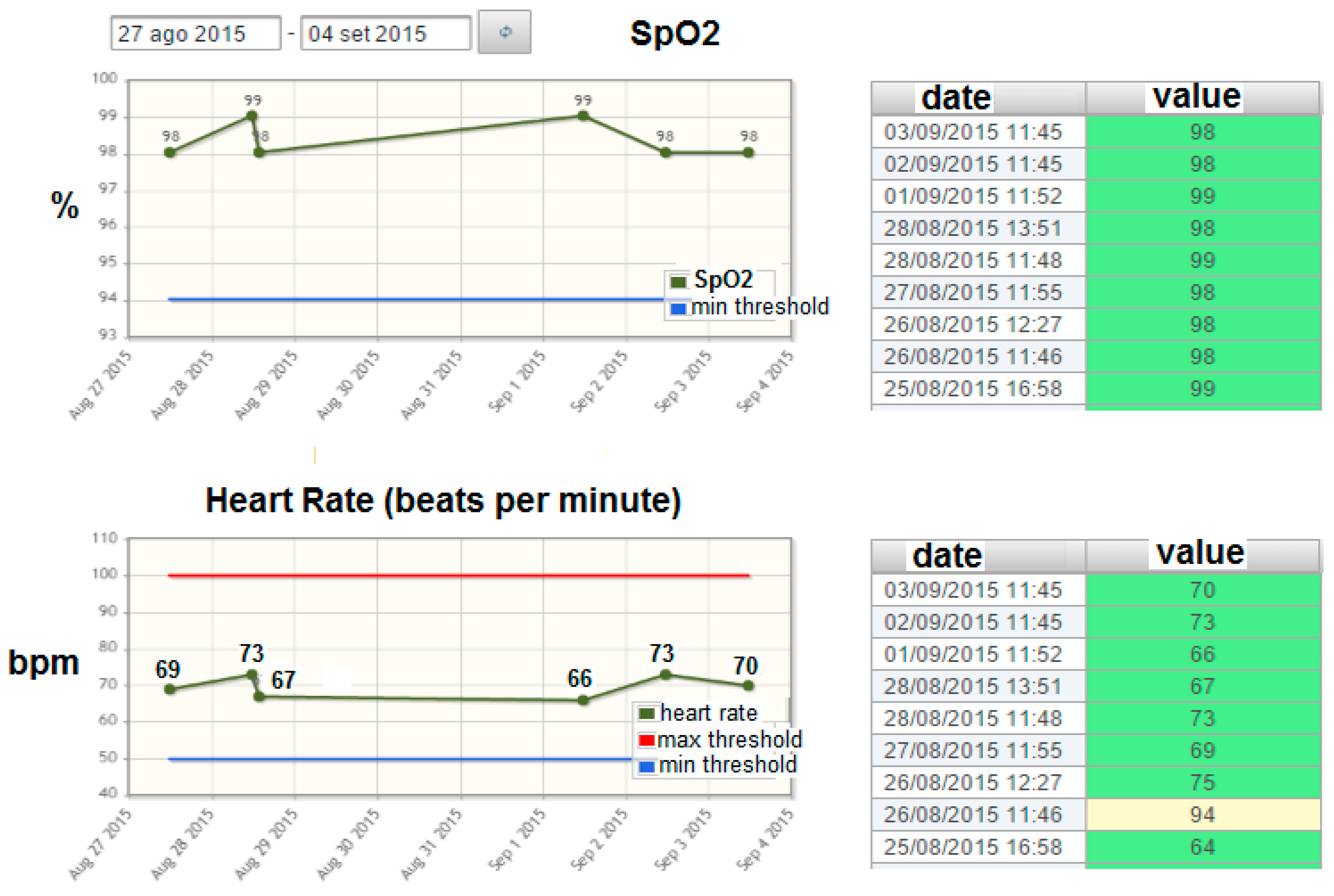
- Graphical rendering for the visualization of the historical evolution of the biomedical parameters acquired according to the specific plan (see an example in Figure 3 related to the evolution of the SpO2 parameter). The statistics and graphical rendering of the historical evolution of biomedical parameters are useful at the gateway side mainly in the “1:N” and in the “point-of-care” scenarios (where the remote acquisition of bio-signals is supervised by a professional caregiver). They are also made available at the remote server side (service center and HIS).
- -
- Threshold-based analysis of the acquired data so that an early warning can be sent when one of the acquired parameters is above or below a specific threshold that can be changed dynamically and remotely by the doctor. The early warning can be used to force an immediate hospitalization in case the chronic illness enters into an acute phase.
3.2. “1:1” Scenario
3.3. “1:N” Scenario
3.4. “Point of Care” Scenario
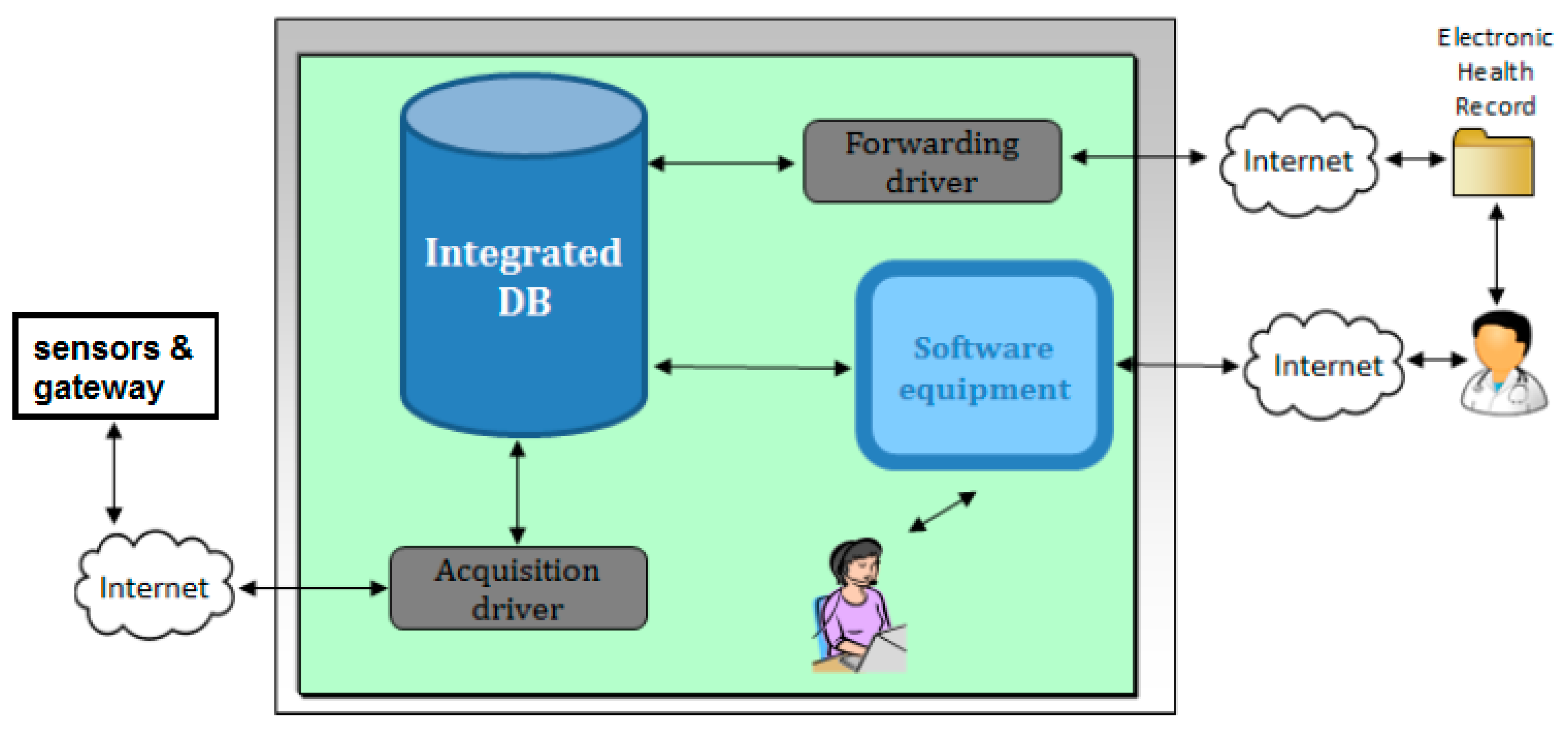
4. Home Monitoring Unit vs. e-Health Center Client–Server Communication
- -
- Relational database: stores all the data and contains most application logic—including object-oriented PL/SQL data models, patient schedules, and alarm triggers. It is in charge of enforcing users’ permissions.
- -
- Java Enterprise Edition (JEE) web application, which implements and publishes the AJAX-based web 2.0 interface.
- -
- Driver: receives the raw data sent by gateways, parses them, and inserts the parsed measurements into the database.
- -
- Audit and Security System: monitoring component that detects and reports any malfunctioning. It also records the system activity.
- -
- Receives raw data embedded into XML tags from the client gateway.
- -
- Transmits to the client gateway the agenda of the configured patient.
- -
- Allows the complete management of patients.
- -
- Transmits data to the server of the service centers with specific adapters.
- -
- Receives agenda by external clinical data management tools.
5. Wireless Biomedical Sensors
5.1. Wireless Sensor Selection
- -
- A single-lead ECG sensing device, patent-filed technology [33], which allows for self-monitoring of the heart in an easy way without the need to connect lots of electrodes in different parts of the body, but simply placing the two hands of the patient on top of a couple of electrodes. This sensor is further discussed in Section 5.2. This sensor, although simple and easy to use, can provide a graphical trace of the ECG and automatic measurement of heart rate and its statistics, thus being useful for arrhythmia monitoring.
- -
- An integrated multi-channel Biomedical ASIC with a configurable sensor front-end [40,41], which allows multiple electrodes for multi-channel ECG or EEG or EMG measurements plus body temperature and blood pressure monitoring. The ASIC also supports automatic detection of pacemaker signals to avoid false alarm generation. This sensor is further discussed in Section 5.3.
- -
- A motion sensor for correct detection of the patient’s posture and possible falls. Indeed, the measurement of most biomedical parameters is influenced by posture. Therefore, the posture of the patient has to be acquired during remote monitoring to reduce the rate of false alarms or missed detection. As an additional service, motion sensors allow for the detection of patient falls and consequent alarm generation. Falls in elderly people are one of the main causes of accidents at home. This sensor is further discussed in Section 5.4.
5.2 Single-Lead ECG Sensor
5.2.1. State of the Art Review and Specifications of Devices for Patient ECG Self-Measurements
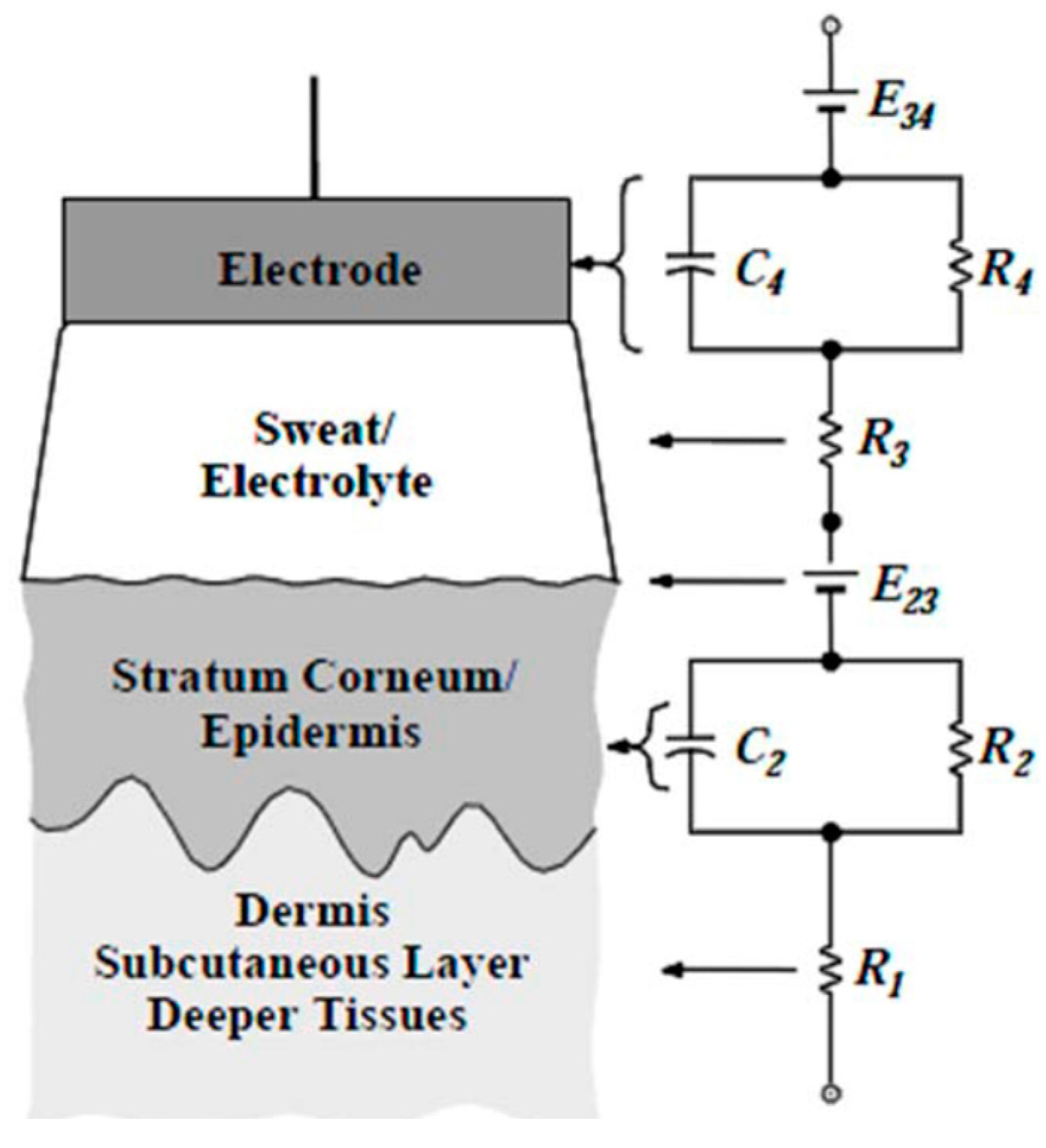
5.2.2. Analysis of the Skin–Electrode Contact and the Shape of a Hand-Based Single-Lead ECG Device
- (1)
- paddles kept singularly in each hand with the palms in contact with the metal electrodes;
- (2)
- paddles attached to each other on the insulated back and kept in contact with the skin through the pressure of the hands;
- (3)
- paddles placed on a plane with the electrodes on the top, in contact with the fingers of each hand;
- (4)
- paddles placed on a plane with the electrodes on the top, in contact with the proximal part of the palm of each hand.
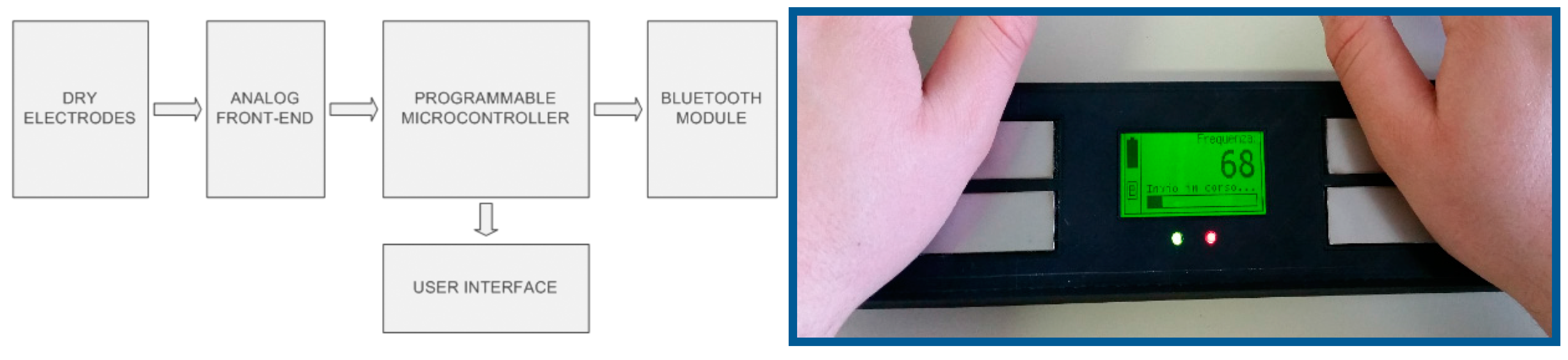
5.2.3. Architecture of a Single-Lead ECG Device
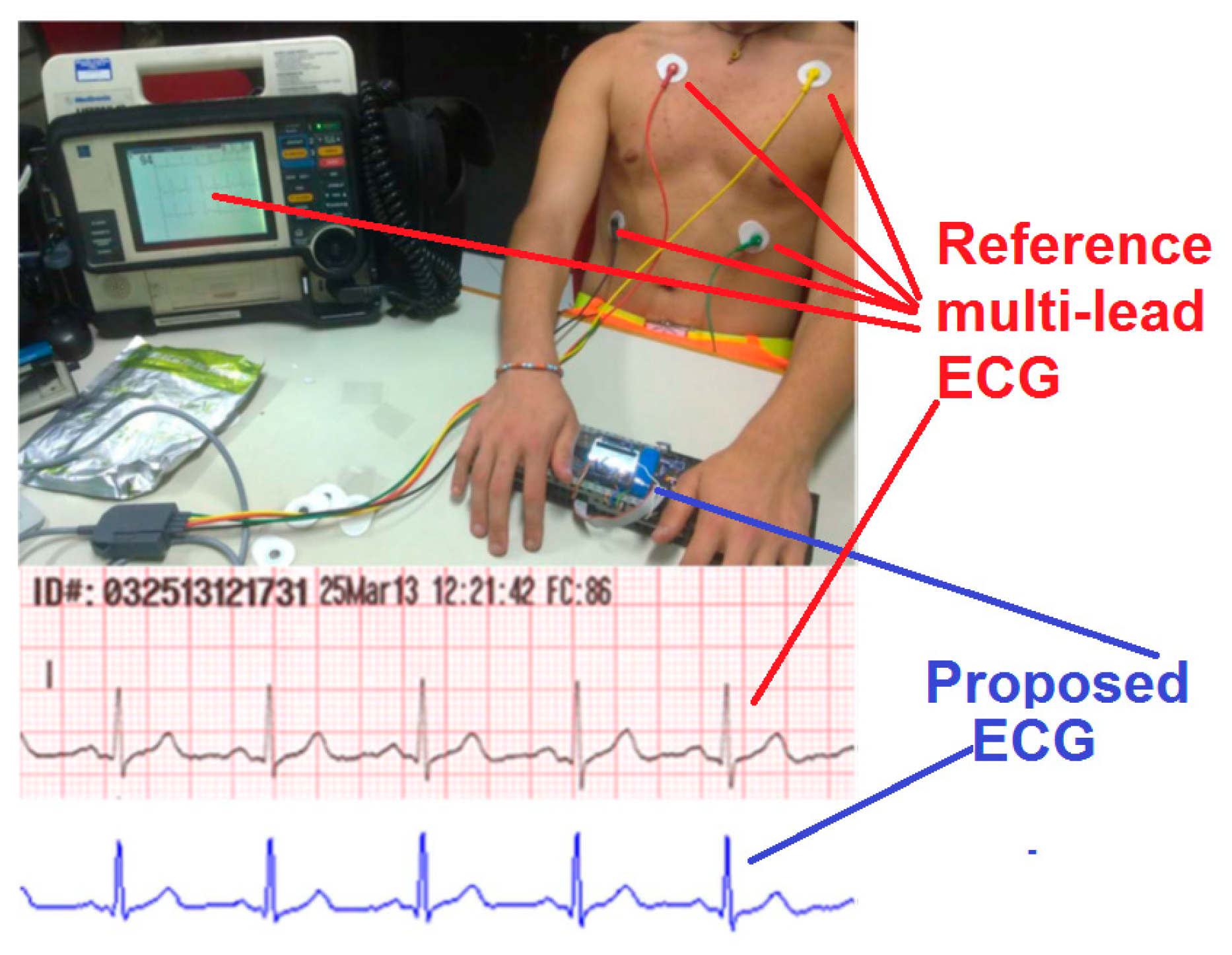
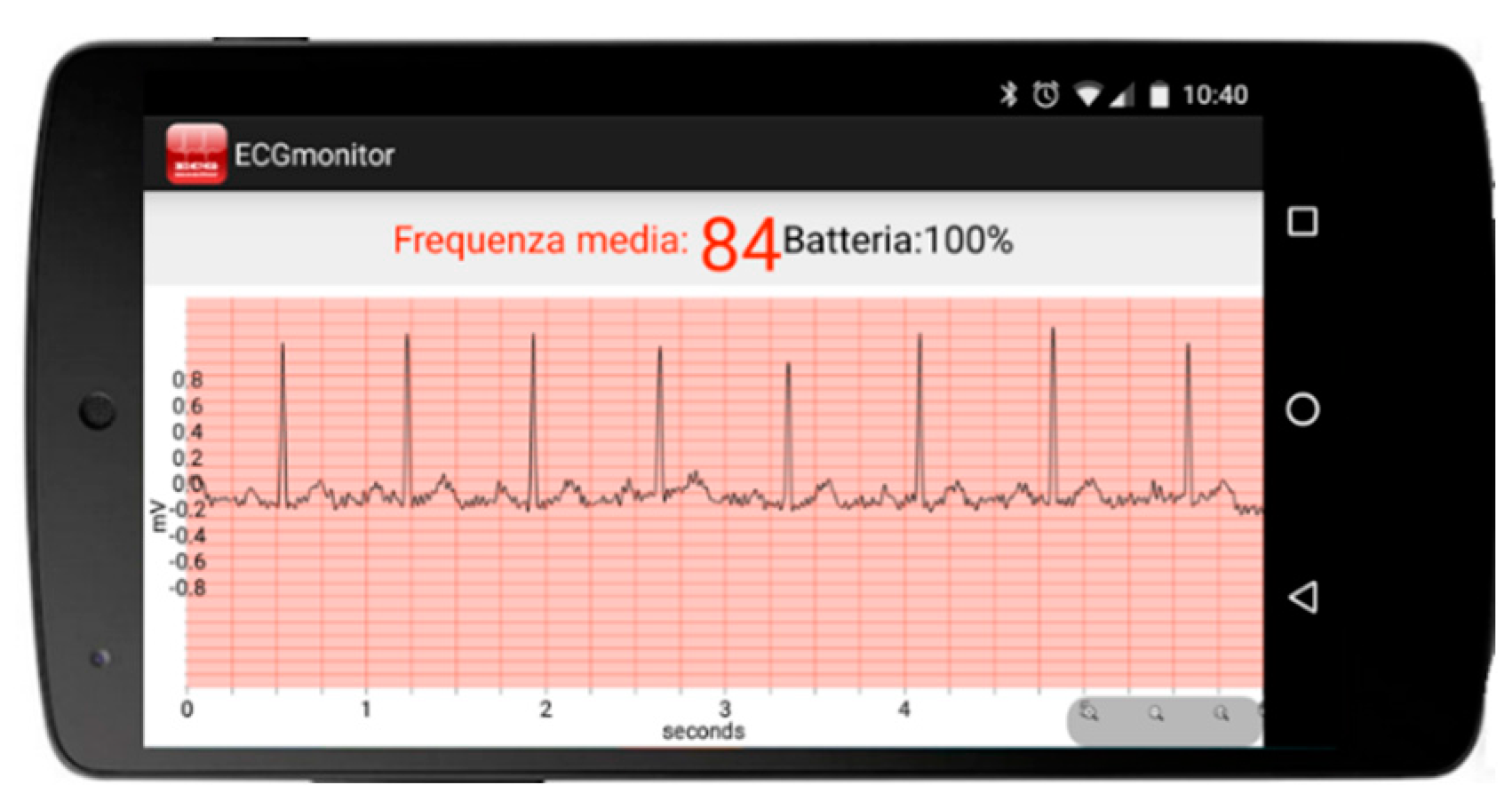
5.2.4. Testing of a Prototype Single-Lead ECG Device
5.3. Multi-Channel Biomedical ASIC Sensor
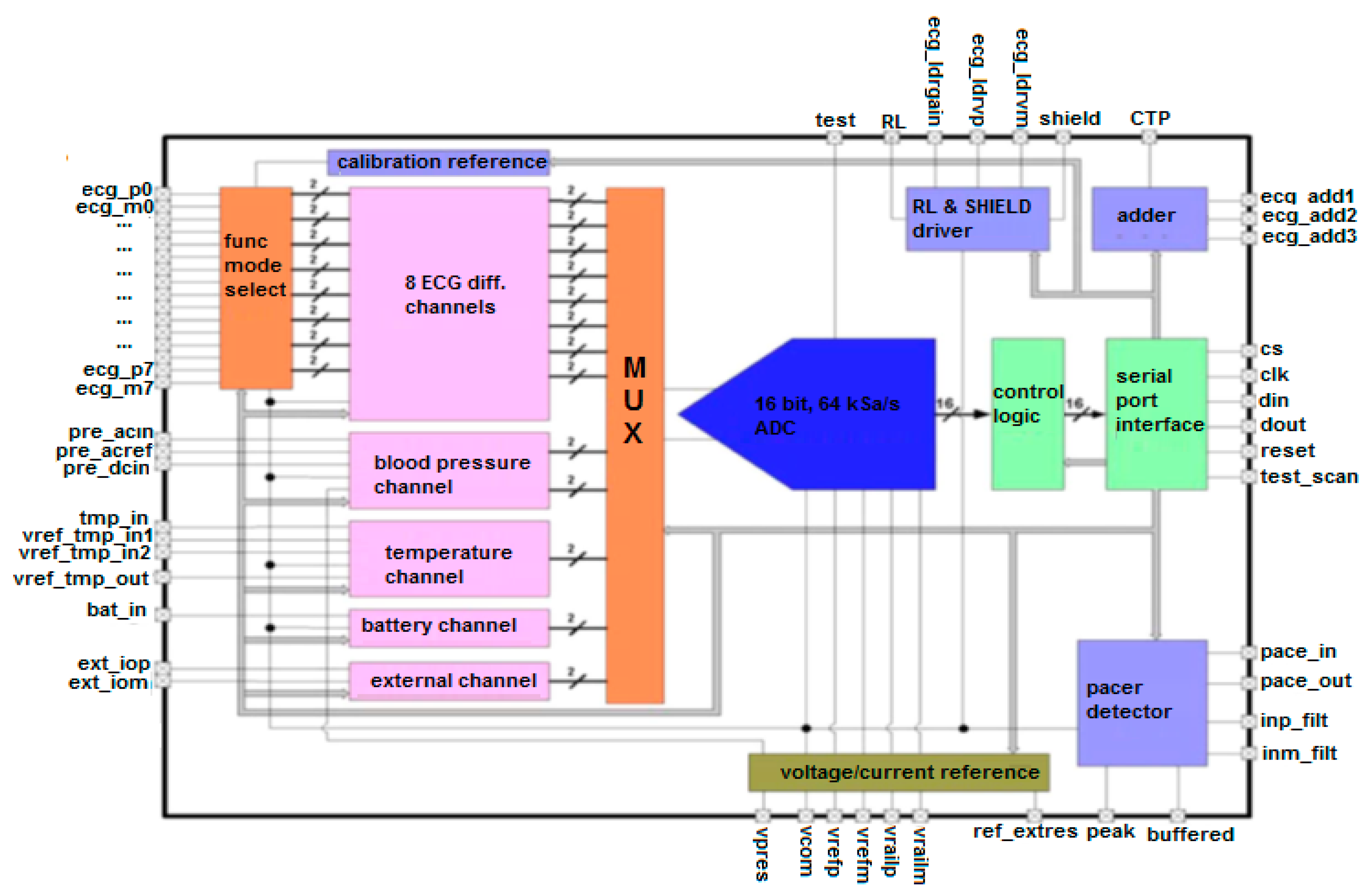
5.3.1. General Architecture of the Biomedical ASIC
5.3.2. Analysis of Specific ASIC Channels
5.4. Motion Sensor for Fall and Posture Detection
6. Experimental Trials
6.1. Experimental Trial Projects and Motivations
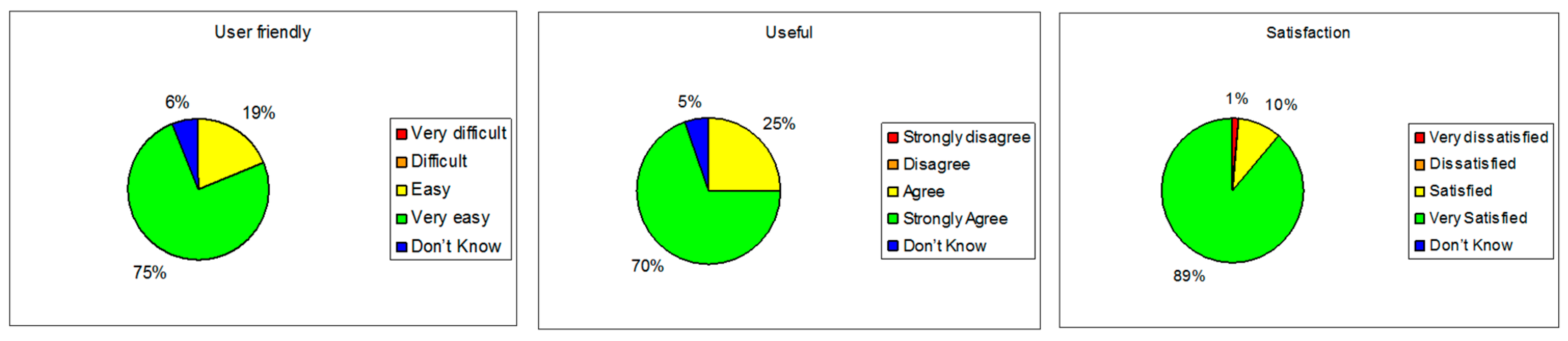
6.2. Health@Home Experimental Trial Results
6.3. Domino Experimental Trial Results
- -
- Synchronization of the vital signs of the patients as soon as they are acquired in the e-Health center, through an automatic procedure that minimizes the possibility of errors due to manual insertion of values.
- -
- Clinical information sharing among the family doctor, the specialists, and the rest of the caregivers, without any time or distance barriers.
- -
- Reduction of the duration of the domiciliary visits, and a better scheduling of the work flows.
6.4. RIS Experimental Trial Results
7. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Go, S.A.; Mozaffarian, D.; Roger, V.L.; Benjamin, E.J.; Berry, J.D.; Borden, W.B.; Bravata, D.M.; Dai, S.; Ford, E.S.; Fox, C.S.; et al. Heart disease and stroke statistics 2013 update: A report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation 2013, 127, 6–245. [Google Scholar] [CrossRef] [PubMed]
- Berry, C.; Murdoch, D.R.; McMurray, J.J. Economics of chronic heart failure. Eur. J. Heart Fail. 2001, 3, 283–291. [Google Scholar] [CrossRef]
- Masella, C.; Zanaboni, P.; Borghi, G.; Castelli, A.; Marzegalli, M.; Tridico, C. Introduction of a telemonitoring service for patients affected by chronic heart failure. In Proceedings of the 11th International Conference on e-Health Networking, Applications and Services, Sydney, Australia, 16–18 December 2009; pp. 138–145.
- Fanucci, L.; Saponara, S.; Bacchillone, T.; Donati, M.; Barba, P.; Sanchex-Tato, I.; Carmona, C. Sensing Devices and Sensor Signal Processing for Remote Monitoring of Vital Signs in CHF Patients. IEEE Trans. Instrum. Meas. 2013, 62, 553–569. [Google Scholar] [CrossRef]
- Parissis, J.; Athanasakis, K.; Farmakis, D.; Boubouchairopoulou, N.; Mareti, C.; Bistola, V.; Ikonomidis, I.; Kyriopoulos, J.; Filippatos, G.; Lekakis, J. Determinants of the direct cost of heart failure hospitalization in a public tertiary hospital. Int. J. Cardiol. 2015, 180, 46–49. [Google Scholar] [CrossRef] [PubMed]
- McMurray, J.J.V.; Adamopoulos, S.; Anker, S.D.; Auricchio, A.; Böhm, M.; Dickstein, K.; Falk, V.; Filippatos, G.; Fonseca, C.; Gomez-Sanchez, M.A.; et al. ESC guidelines for diagnosis and treatment of acute and chronic heart failure 2012. Eur. Heart J. 2012, 14, 803–869. [Google Scholar] [CrossRef]
- Go, A.S.; Mozaffarian, D.; Roger, V.L.; Benjamin, E.J.; Berry, J.D.; Blaha, M.J.; Dai, S.; Ford, E.S.; Fox, C.S.; Franco, S.; et al. Heart disease and stroke statistics, 2014 Update. Circulation 2014, 129, 28–292. [Google Scholar] [CrossRef] [PubMed]
- Zannad, F.; Agrinier, N.; Alla, F. Heart failure burden and therapy. Europace 2009, 11, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Gardner, R.S.; McDonagh, T.A. Chronic heart failure: Epidemiology, investigation and management. Medicine 2014, 42, 562–567. [Google Scholar] [CrossRef]
- Ambrosy, A.P.; Ambrosy, A.P.; Fonarow, G.C.; Butler, J.; Chioncel, O.; Greene, S.J.; Vaduganathan, M.; Nodari, S.; Lam, C.S.; Sato, N.; et al. The global health and economic burden of hospitalization for heart failure. J. Am. Cardiol. 2014, 63, 1123–1133. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.C.; Chavez, Y.E.; Baker, T.; Luce, B.R. Economic burden of heart failure: A summary of recent literature. Heart Lung 2004, 33, 362–371. [Google Scholar] [CrossRef] [PubMed]
- Fergenbaum, J.; Bermingham, S.; Krahn, M.; Alter, D.; Demers, C. Care in the home for the management of chronic heart failure. Cardiovasc. Nurs. J. 2015, 30, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Ekeland, A.G.; Bowes, A.; Flottorp, S. Effectiveness of telemedicine: A systematic review of reviews. Int. J. Med. Inform. 2010, 79, 736–771. [Google Scholar] [CrossRef] [PubMed]
- Finet, P.; Le Bouquin Jeannès, R.; Damerond, O.; Gibaudb, B. Review of current telemedicine applications for chronic diseases. IRBM 2015, 36, 133–157. [Google Scholar] [CrossRef]
- Kligfield, P.; Gettes, L.S.; Bailey, J.J.; Childers, R.; Deal, B.J.; Hancock, E.W.; van Herpen, G.; Kors, J.A.; Macfarlane, P.; Mirvis, D.M.; et al. Recommendations for the standardization and interpretation of electrocardiogram. Circulation 2007, 155, 1306–1324. [Google Scholar]
- Lopez Sendon, J. The heart failure epidemic. Medicographia 2011, 33, 363–368. [Google Scholar]
- Benini, A.; Donati, M.; Iacopetti, F.; Fanucci, L. User-friendly single-lead ECG device for home telemonitoring application. In Proceedings of the 2014 8th International Symposium on Medical Information and Communication Technology (ISMICT), Firenze, Italy, 2–4 April 2014.
- Donati, M.; Bacchillone, T.; Fanucci, L.; Saponara, S.; Cstalli, F. Operating protocol and networking issues of a telemedicine platform integrating from wireless home sensors to the hospital information system. Hindawi J. Comput. Netw. Commun. 2013, 2013, 1–12. [Google Scholar] [CrossRef]
- Li, J.-F.; Wang, Q.-H.; Liu, X.-M.; Cao, S.; Liu, F.-L. Pedestrian Dead Reckoning System Integrating Low-Cost MEMS Inertial Sensors and GPS Receiver. J. Eng. Sci. Technol. Rev. 2014, 7, 197–203. [Google Scholar]
- Weinberg, H. Using the ADXL202 in Pedometer and Personal Navigation Applications; AD AN-602 App. Note; Analog Devices: Norwood, MA, USA, 2002. [Google Scholar]
- Abbate, S.; Avvenuti, M.; Bonatesta, F.; Cola, G.; Corsini, P.; Vecchio, A. A smartphone-based fall detection system. Pervasive Mobile Comp. 2012, 8, 883–899. [Google Scholar] [CrossRef]
- Bourke, A.K.; O’Brein, J.V.; Lyons, G.M. Evaluation of a threshold-based tri-axial accelerometer fall detection algorithm. Gait Posture 2007, 26, 194–199. [Google Scholar] [CrossRef] [PubMed]
- Cleland, I.; Kikhia, B.; Nugent, C.; Boytsov, A.; Hallberg, J.; Synnes, K.; McClean, S.; Finlay, D. Optimal placement of accelerometers for the detection of everyday activities. Sensors 2013, 13, 9183–9200. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. mHealth: New Horizons for Health through Mobile Technologies: Second Global Survey oneHealth; WHO: Geneva, Switzerland, 2011; p. 1. [Google Scholar]
- Bacchillone, T.; Donati, M.; Saponara, S.; Fanucci, L. A flexible home gateway system for telecare of patients affected by chronic heart failure. In Proceedings of the 2011 5th International Symposium on Medical Information and Communication Technology, Montreux, Switzerland, 27–30 March 2011; pp. 1–5.
- Zhou, F.; Yang, H.-I.; Reyes Álamo, J.M.; Wong, J.S.; Chang, C.K. Mobile personal health care system for patients with diabetes. In Aging Friendly Technology for Health and Independence; Lecture Notes in Computer Science; Springer: London, UK, 2011; Volume 6159, pp. 94–101. [Google Scholar]
- Yadav, J.; Rani, A.; Singh, V.; Murari, B.M. Near-infrared LED based non-invasive blood glucose sensor. In Proceedings of the 2014 International Conference on Signal Processing and Integrated Networks (SPIN), Noida India, 20–21 February 2014; pp. 591–594.
- Gan, K.B.; Yahyavi, E.S.; Ismail, M.S. Contactless respiration rate measurement using optical method and empirical mode decomposition. Technol. Health Care 2016, 1–8, preprint. [Google Scholar]
- Daw, W.; Kingshott, R.; Scott, A.; Saatchi, R.; Elphick, H. Development of the ‘BreathEasy’ contactless portable respiratory rate monitor (CPRM). Eur. Respir. J. 2015, 46, PA1583. [Google Scholar] [CrossRef]
- Fang, D.; Hu, J.; Wei, X.; Shao, H.; Luo, Y. A Smart Phone Healthcare Monitoring System for Oxygen Saturation and Heart Rate. In Proceedings of the IEEE International Conference on Cyber-Enabled Distributed Computing and Knowledge Discovery, Shanghai, China, 13–15 October 2014; pp. 245–247.
- Lutze, R.; Waldhor, K.A. Smartwatch Software Architecture for Health Hazard Handling for Elderly People. In Proceedings of the IEEE International Conference on Healthcare Informatics, Dallas, TX, USA, 21–23 October 2015; pp. 356–361.
- Stankevich, E.; Paramonov, I. Using Bluetooth on Android Platform for mHealth Development. In Proceedings of the 10th Conference of FRUCT Association, Tampere, Finland, 7–11 November 2011; pp. 140–145.
- Fanucci, L.; Benini, A.; Donati, M.; Iacopetti, F. Self Electrocardiogram Acquisition Device. Patent N. WO2015083036 A1, 11 June 2015. [Google Scholar]
- V-Patch Medical System. Available online: http://www.vpatchmedical.com (accessed on 18 June 2016).
- Isansys Lifecare. Available online: http://www.isansys.com (accessed on 18 June 2016).
- LifeWatch. Available online: http://www.lifewatch.com/ACT (accessed on 18th June 2016).
- Docobo. Available online: http://www.docobo.co.uk (accessed on 18 June 2016).
- Augustyniak, P.; Tadeusiewicz, R. Telemedical Solutions in Cardiac Diagnostics. In Chapter 2 in Ubiquitous Cardiology: Emerging Wireless Telemedical Applications, Medical Information Science Reference; IRMA book: Hershey, PA, USA, 2009; pp. 72–109. [Google Scholar]
- SolutionMD. Available online: http://www.solutionmd.com (accessed on 18 June 2016).
- Saponara, S.; Petri, E.; Fanucci, L.; Terreni, P. Sensor modeling, low-complexity fusion algorithms, and mixed-signal IC prototyping for gas measures in low-emission vehicles. IEEE Trans. Instrum. Meas. 2011, 60, 372–384. [Google Scholar] [CrossRef]
- Bacciarelli, L.; Lucia, G.; Saponara, S.; Fanucci, L.; Forliti, M. Design, testing and prototyping of a software programmable I2C/SPI IP on AMBA bus. In Proceedings of the IEEE PRIME, Otranto, Italy, 12–15 June 2006; pp. 373–376.
- Baba, A.; Burke, M.J. Electrical characterisation of dry electrodes for ECG recordings. In Proceedings of the 12th WSEAS International Conference on Circuits, Heraklion, Greece, 22–24 July 2008.
- Costantino, N.; Serventi, R.; Tinfena, F.; D’Abramo, P.; Chassard, P.; Tisserand, P.; Saponara, S.; Fanucci, L. Design and test of an HV-CMOS intelligent power switch with integrated protections and self-diagnostic for harsh automotive applications. IEEE Trans. Ind. Electron. 2011, 58, 2715–2727. [Google Scholar] [CrossRef]
- Saponara, S.; Martina, M.; Casula, M.; Fanucci, L.; Masera, G. Motion estimation and CABAC VLSI co-processors for real-time high-quality H.264/AVC video coding. Microprocess. Microsyst. 2010, 34, 316–328. [Google Scholar] [CrossRef]
- Fanucci, L.; Saleti, R.; Saponara, S. Parametrized and reusable VLSI macro cells for the low-power realization of 2-D discrete-cosine-transform. Microelectron. J. 2001, 32, 1035–1045. [Google Scholar] [CrossRef]
- Chimienti, A.; Fanucci, L.; Locatelli, R.; Saponara, S. VLSI architecture for a low-power video codec system. Microelectron. J. 2002, 33, 417–427. [Google Scholar] [CrossRef]

| Sensor | Chronic Illness | Device Characteristics |
|---|---|---|
| Cardioline Microtel Cardiette | CHF | 3/6/12 channels derivation; ECG continuous measurement; 0.05–150 Hz; sampling rate 500 Hz; pacemaker detection; BT 2.0 SPP |
| A&D weighting scale (UC-321PBT) | CHF | Range 0–200 kg, resolution 100 g; BT 2.0 SPP |
| A&D blood pressure (UA-767PBT) | CHF, COPD | Range: pressure 20–280 mmHg, pulse: 40–200 bpm; Accuracy: pressure ± 3 mmHg, pulse: ±5%; BT 2.0 SPP |
| Nonin saturimeter (Onyx II 9560) | CHF, COPD | Range: SpO2 0%–100%, pulse 20–250 bpm; Accuracy: SpO2 ± 1, pulse ± 3 bpm |
| Lifescan Glucometer (onetouch ultraeasy) | diabetes | Range: 20–600 md/dL; Accuracy: ±5%; BT 2.0 SPP |
| MIR spirometer (Spirodoc) | COPD | Range: flux ± 16 L/s, Accuracy: flux ± 5%; BT 2.0 SPP |
| Dimensions (mm) | Sampling | Analog Band (Hz) | Digital Band (Hz) | Notch Filter (Hz) | ECG Trace | Processing | Outputs |
|---|---|---|---|---|---|---|---|
| 300 × 70 × 40 | 12 bit 500 sps | 0.5–50 | 0.6–37 | 50 | First lead | Heart rate detection | LCD display 128 × 64 pixel Green and red LEDs BT 2.0 SPP 115 kbps |
| Tech. | Temp. V Supply | ADC | Channels | ECG Channel | Gateway Connection |
|---|---|---|---|---|---|
| CMOS 0.8 μm | 0 to 70 °C | 16 b 64 kSa/s | 8 ECG, 1 blood press | 100 dB CMRR, 57 dB gain | BT 2.0 SPP |
| TQFP128 pin | 3 V to 5 V | INL/DNL1/0.75 LSB | 1 temp., 1 batt status | 100 dB PSRR, 0.1 to 150 Hz | |
| 14 × 14 × 1.4 mm3 | offset/gain error 0.5/1.5% | Pacer detect, | 10 µVrms/150 Hz input noise |
| Sampling Rare | V Supply | Sensor | Digital Core | Processing | Output |
|---|---|---|---|---|---|
| 50 sps | 3.3 V | 9D IMU (gyroscope, accelerometer, digital compass) | STM32 | Fall event detection Posture detection | BLE 4.0 |
| Macro-Parameter | Score |
|---|---|
| Simply decision and increase effectiveness of diagnosis and treatment of patient based on better evidence | 9,125/10 |
| In general terms, easy to use with clear and understandable interactions | 9,5/10 |
| Flexibility of the system and compatibility with other systems already in use | 9,75/10 |
| Quality of the provided signal | 9,1/10 |
| Sensibility of the alarm detection function | 9,15/10 |
| In favor of the adoption of the H@H system | 9,2/10 |
| “The patient selection process and the measurement acquisition process are quickly and easy to use” |
| “The medical devices provided with the gateway cover acquisition of all the requested vital signs” |
| “The telemedicine kit, composed of a gateway and medical devices, represents an important improvement and optimization of the domiciliary visit with respect to the traditional model” |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saponara, S.; Donati, M.; Fanucci, L.; Celli, A. An Embedded Sensing and Communication Platform, and a Healthcare Model for Remote Monitoring of Chronic Diseases. Electronics 2016, 5, 47. https://doi.org/10.3390/electronics5030047
Saponara S, Donati M, Fanucci L, Celli A. An Embedded Sensing and Communication Platform, and a Healthcare Model for Remote Monitoring of Chronic Diseases. Electronics. 2016; 5(3):47. https://doi.org/10.3390/electronics5030047
Chicago/Turabian StyleSaponara, Sergio, Massimiliano Donati, Luca Fanucci, and Alessio Celli. 2016. "An Embedded Sensing and Communication Platform, and a Healthcare Model for Remote Monitoring of Chronic Diseases" Electronics 5, no. 3: 47. https://doi.org/10.3390/electronics5030047
APA StyleSaponara, S., Donati, M., Fanucci, L., & Celli, A. (2016). An Embedded Sensing and Communication Platform, and a Healthcare Model for Remote Monitoring of Chronic Diseases. Electronics, 5(3), 47. https://doi.org/10.3390/electronics5030047