Recent Developments of Ruthenium Complexes for Dye-Sensitized Solar Cells
Abstract
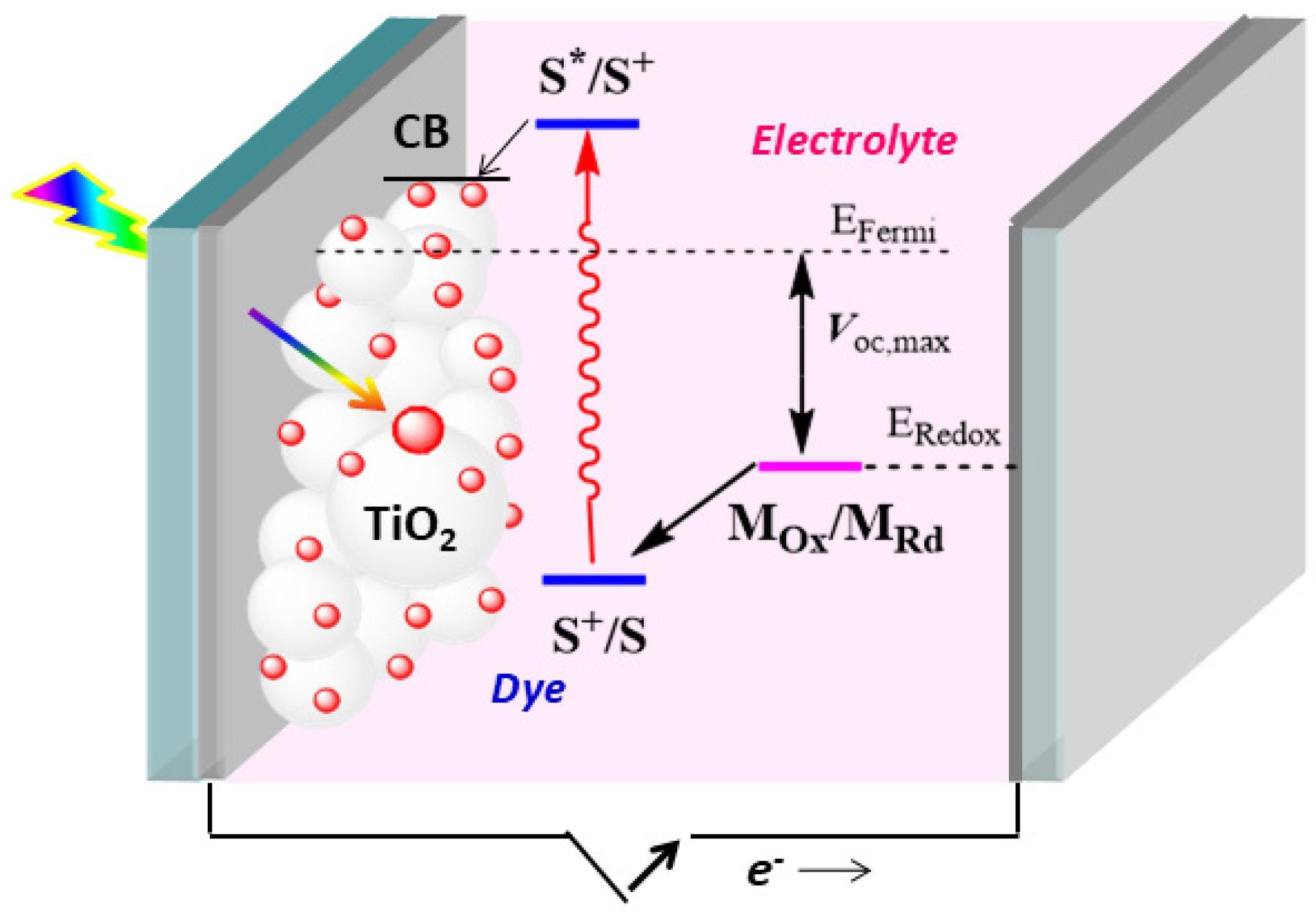
1. Introduction
- (a)
- The short-circuit current density (Jsc, which is the maximum current density in the DSSC when the applied voltage is zero);
- (b)
- The open-circuit potential (Voc, which represents the maximum voltage available from the DSSC when there is no current);
- (c)
- The fill factor (FF, which is the ratio between the maximum power of the DSSC and the product of Voc and Jsc; having values between 0 and 1, it reflects electrical and electrochemical losses during operation of the cell);
- (d)
- The intensity of the incident light (IS, commonly set to 100 mW cm−2, under air mass 1.5 global illumination, AM1.5 G).
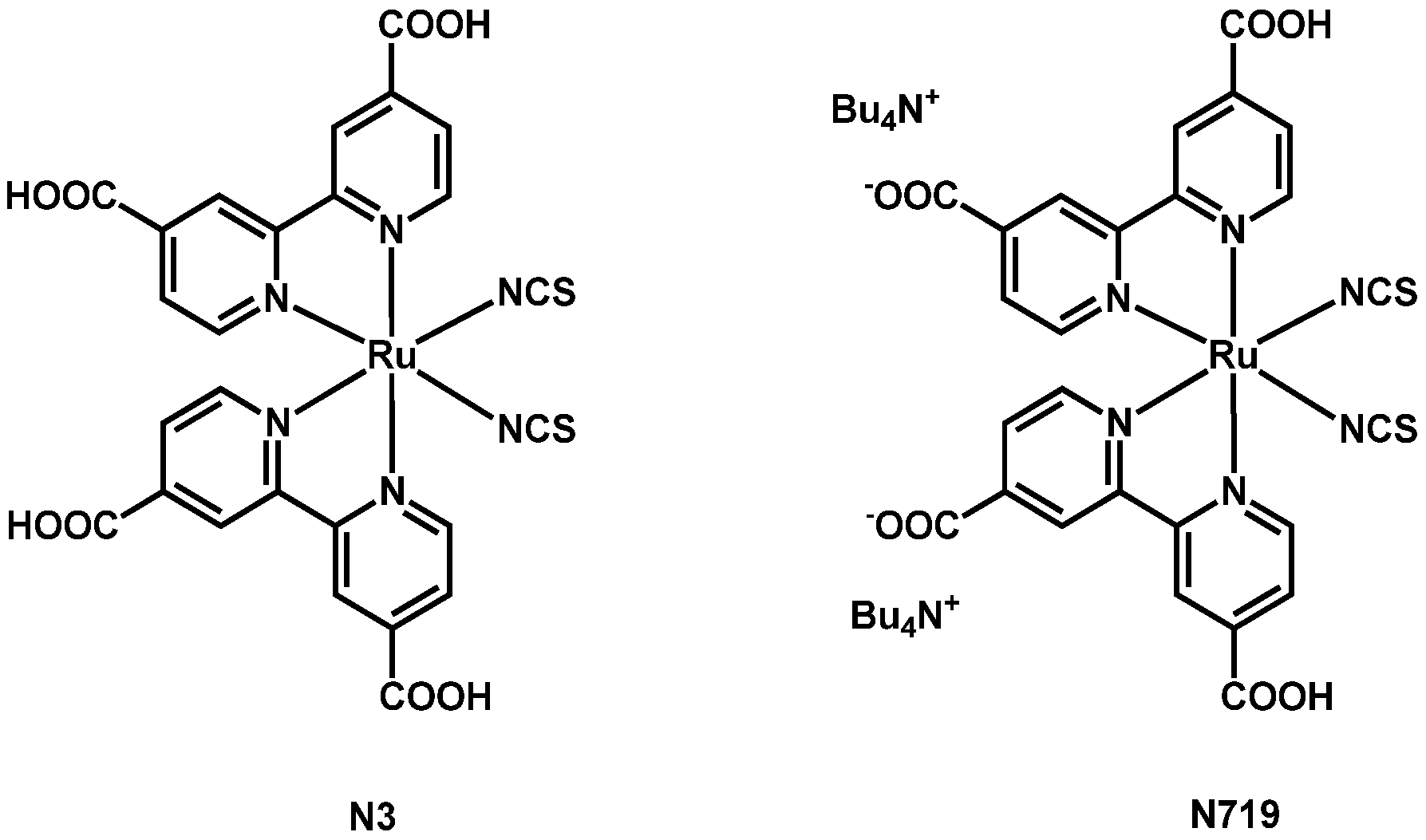
2. Dyes Presenting Thiocyanate Ligands
2.1. Ru–Thiocyanate Dyes with Terpyridines Bearing the Anchoring Group
2.2. Ru–Thiocyanate Dyes with Bipyridines Bearing the Anchoring Groups
2.3. Co-Sensitization Between N719 and Organic Chromophores
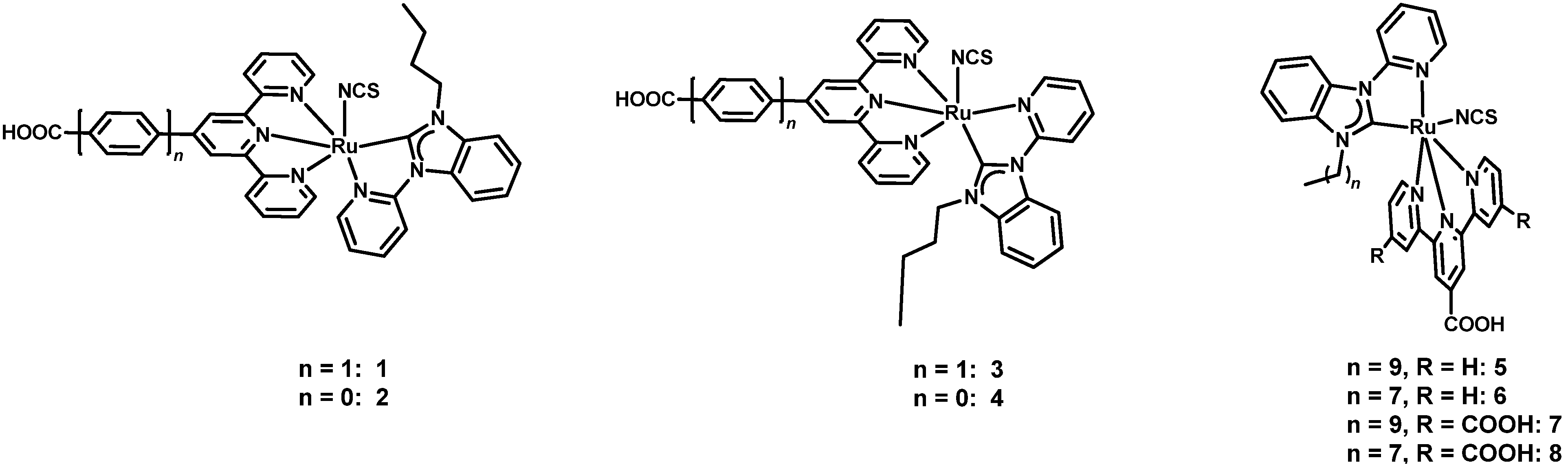
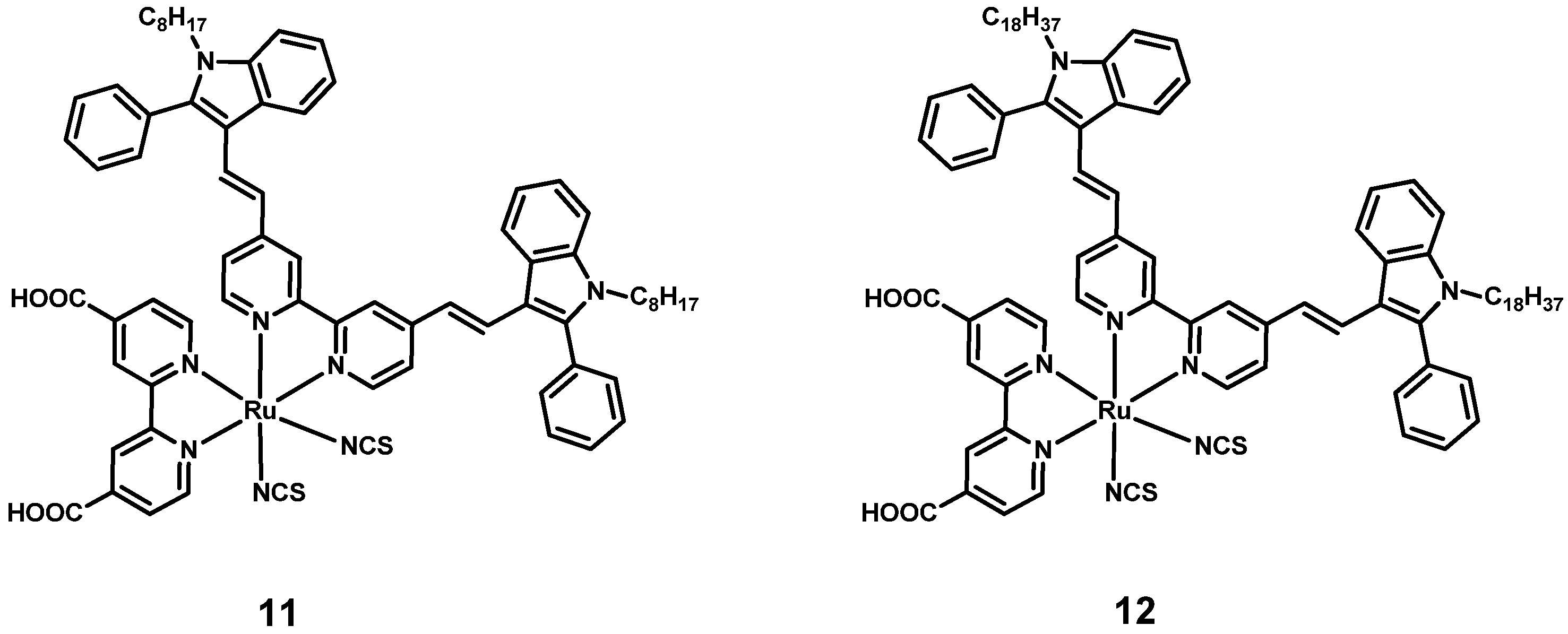
3. Thiocyanate-Free Dyes
3.1. Thiocyanate-Free Ru Dyes with Terpyridines Bearing the Anchoring Group
3.2. Thiocyanate-Free Ru Dyes with Bipyridines Bearing the Anchoring Groups
3.3. Co-Sensitization Between N719 and Thiocyanate-Free Ru Dyes
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACN | Acetonitrile |
| AM1.5 G | Air mass 1.5 global illumination |
| bpy | 2,2′-bipyridine |
| DSSC | Dye-sensitized solar cell |
| FF | Fill factor |
| JSC | Short-circuit current density |
| LUMO | Lowest unoccupied molecular orbital |
| VOC | Open-circuit voltage |
References
- Nozik, A.J.; Miller, J. Introduction to solar photon conversion. Chem. Rev. 2010, 110, 6443–6445. [Google Scholar] [CrossRef] [PubMed]
- O’Regan, B.; Grätzel, M. A low-cost, high-efficiency solar cell based on dye-sensitized colloidal TiO2 films. Nature 1991, 353, 737–740. [Google Scholar] [CrossRef]
- Grätzel, M. Recent Advances in Sensitized Mesoscopic Solar Cells. Acc. Chem. Res. 2009, 42, 1788–1798. [Google Scholar] [CrossRef]
- Vougioukalakis, G.C.; Philippopoulos, A.I.; Stergiopoulos, T.; Falaras, P. Contributions to the development of ruthenium-based sensitizers for dye-sensitized solar cells. Coord. Chem. Rev. 2011, 255, 2602–2621. [Google Scholar] [CrossRef]
- Nazeeruddin, M.K.; Zakeeruddin, S.M.; Humphry-Baker, R.; Jirousek, M.; Liska, P.; Vlachopoulos, N.; Shklover, V.; Fischer, C.-H.; Graetzel, M. Acid−Base Equilibria of (2,2′-Bipyridyl-4,4′-dicarboxylic acid)ruthenium(II) Complexes and the Effect of Protonation on Charge-Transfer Sensitization of Nanocrystalline Titania. Inorg. Chem. 1999, 38, 6298–6305. [Google Scholar] [CrossRef] [PubMed]
- Caramori, S.; Bignozzi, C.A. Recent Developments in the design of dye sensitized solar cell components. In Electrochemistry of Functional Supramolecular Systems; J. Wiley & Sons: Hoboken, NJ, USA, 2010; pp. 523–579. [Google Scholar]
- Nazeeruddin, M.K.; Baranoff, E.; Grätzel, M. Dye-sensitized solar cells: A brief overview. Sol. Energy 2011, 85, 1172–1178. [Google Scholar] [CrossRef]
- Nazeeruddin, M.K.; Péchy, P.; Renouard, T.; Zakeeruddin, S.M.; Humphry-Baker, R.; Comte, P.; Liska, P.; Cevey, L.; Costa, E.; Shklover, V.; et al. Engineering of Efficient Panchromatic Sensitizers for Nanocrystalline TiO2-Based Solar Cells. J. Am. Chem. Soc. 2001, 123, 1613–1624. [Google Scholar] [CrossRef]
- Chen, C.-Y.; Wang, M.; Li, J.-Y.; Pootrakulchote, N.; Alibabaei, L.; Ngoc-le, C.-H.; Decoppet, J.-D.; Tsai, J.-H.; Grätzel, C.; Wu, C.-G.; et al. Highly Efficient Light-Harvesting Ruthenium Sensitizer for Thin-Film Dye-Sensitized Solar Cells. ACS Nano 2009, 3, 3103–3109. [Google Scholar] [CrossRef]
- Chen, W.-C.; Kong, F.-T.; Li, Z.-Q.; Pan, J.-H.; Liu, X.-P.; Guo, F.-L.; Zhou, L.; Huang, Y.; Yu, T.; Dai, S.-Y. Superior Light-Harvesting Heteroleptic Ruthenium(II) Complexes with Electron-Donating Antennas for High Performance Dye- Sensitized Solar Cells. ACS Appl. Mater. Interfaces 2016, 8, 19410–19417. [Google Scholar] [CrossRef]
- Chen, C.-Y.; Pootrakulchote, N.; Wu, S.-J.; Wang, M.; Li, J.-Y.; Tsai, J.-H.; Wu, C.-G.; Zakeeruddin, S.M.; Grätzel, M. New Ruthenium Sensitizer with Carbazole Antennas for Efficient and Stable Thin-Film Dye-Sensitized Solar Cells. J. Phys. Chem. C. 2009, 113, 20752–20757. [Google Scholar] [CrossRef]
- Gao, F.; Cheng, Y.; Yu, Q.; Liu, S.; Shi, D.; Li, Y.; Wang, P. Conjugation of Selenophene with Bipyridine for a High Molar Extinction Coefficient Sensitizer in Dye-Sensitized Solar Cells. Inorg. Chem. 2009, 48, 2664–2669. [Google Scholar] [CrossRef]
- Boschloo, G.; Haggman, L.; Hagfeldt, A. Quantification of the Effect of 4-tert-Butylpyridine Addition to I-/I3- Redox Electrolytes in Dye-Sensitized Nanostructured TiO2 Solar Cells. J. Phys. Chem. B 2006, 110, 13144–13150. [Google Scholar] [CrossRef] [PubMed]
- Kusama, H.; Konishi, Y.; Sugihara, H.; Arakawa, H. Influence of alkylpyridine additives in electrolyte solution on the performance of dye-sensitized solar cell. Sol. Energy Mater. Sol. Cells 2003, 80, 167–179. [Google Scholar] [CrossRef]
- Andersen, A.R.; Halme, J.; Lund, T.; Asghar, M.I.; Nguyen, P.T.; Miettunen, K.; Kemppainen, E.; Albrektsen, O. Charge Transport and Photocurrent Generation Characteristics in Dye Solar Cells Containing Thermally Degraded N719 Dye Molecules. J. Phys. Chem. C 2011, 115, 15598–15606. [Google Scholar] [CrossRef]
- Wadman, S.H.; Kroon, J.M.; Bakker, K.; Lutz, M.; Spek, A.L.; van Klink, G.P.M.; van Koten, G. Cyclometalated ruthenium complexes for sensitizing nanocrystalline TiO2 solar cells. Chem. Commun. 2007, 19, 1907–1909. [Google Scholar] [CrossRef] [PubMed]
- Bomben, P.G.; Robson, K.C.D.; Sedach, P.A.; Berlinguette, C.P. On the Viability of Cyclometalated Ru(II) Complexes for Light-Harvesting Applications. Inorg. Chem. 2009, 48, 9631–9643. [Google Scholar] [CrossRef]
- Bomben, P.G.; Koivisto, B.D.; Berlinguette, C.P. Cyclometalated Ru Complexes of Type [RuII(N∧N)2(C∧N)]z: Physicochemical Response to Substituents Installed on the Anionic Ligand. Inorg. Chem. 2010, 49, 4960–4971. [Google Scholar] [CrossRef]
- Robson, K.C.D.; Bomben, P.G.; Berlinguette, C.P. Cycloruthenated sensitizers: Improving the dye-sensitized solar cell with classical inorganic chemistry principles. Dalton Trans. 2012, 41, 7814–7829. [Google Scholar] [CrossRef]
- Bomben, P.G.; Borau-Garcia, J.; Berlinguette, C.P. Three is not a crowd: Efficient sensitization of TiO2 by a bulkytrichromic trisheteroleptic cycloruthenated dye. Chem. Commun. 2012, 48, 5599–5601. [Google Scholar] [CrossRef]
- Abbotto, A.; Coluccini, C.; Dell’Orto, E.; Manfredi, N.; Trifiletti, V.; Salamone, M.M.; Ruffo, R.; Acciarri, M.; Colombo, A.; Dragonetti, C.; et al. Thiocyanate-free cyclometalated ruthenium sensitizers for solar cells based on heteroaromatic-substituted 2-arylpyridines. Dalton Trans. 2012, 41, 11731–11738. [Google Scholar] [CrossRef]
- Dragonetti, C.; Valore, A.; Colombo, A.; Roberto, D.; Trifiletti, V.; Manfredi, N.; Salamone, M.M.; Ruffo, R.; Abbotto, A. A new thiocyanate-free cyclometallated ruthenium complex for dye-sensitized solar cells: Beneficial effects of substitution on the cyclometallated ligand. J. Organomet. Chem. 2012, 714, 88–93. [Google Scholar] [CrossRef]
- Dragonetti, C.; Colombo, A.; Magni, M.; Mussini, P.; Nisic, F.; Roberto, D.; Ugo, R.; Valore, A.; Valsecchi, A.; Salvatori, P.; et al. Thiocyanate-Free Ruthenium(II) Sensitizer with a Pyrid-2-yltetrazolate Ligand for Dye-Sensitized Solar Cells. Inorg. Chem. 2013, 52, 10723–10725. [Google Scholar] [CrossRef]
- Pogozhev, D.V.; Bezdek, M.J.; Schauer, P.A.; Berlinguette, C.P. Ruthenium(II) Complexes Bearing a Naphthalimide Fragment: A Modular Dye Platform for the Dye-Sensitized Solar Cell. Inorg. Chem. 2013, 52, 3001–3006. [Google Scholar] [CrossRef] [PubMed]
- Hu, F.-C.; Wang, S.-W.; Chi, Y.; Robertson, N.; Hewat, T.; Hu, Y.; Liu, S.-H.; Chou, P.-T.; Yang, P.-F.; Lin, H.-W. Geometrical Isomerism of RuII Dye-Sensitized Solar Cell Sensitizers and Effects on Photophysical Properties and Device Performances. Chem. Phys. Chem. 2014, 15, 1207–1215. [Google Scholar] [CrossRef]
- Siu, C.-H.; Ho, C.-L.; He, J.; Chen, T.; Majumda, P.; Zhao, J.; Li, H.; Wong, W.-Y. Optimizing the photovoltaic performance of thiocyanate-free ruthenium photosensitizers by structural modification of C^N cyclometalating ligand in dye-sensitized solar cells. Polyhedron 2014, 82, 71–79. [Google Scholar] [CrossRef]
- Colombo, A.; Dragonetti, C.; Valore, A.; Coluccini, C.; Manfredi, N.; Abbotto, A. Thiocyanate-free ruthenium(II) 2,2′-bipyridyl complexes for dye-sensitized solar cells. Polyhedron 2014, 82, 50–56. [Google Scholar] [CrossRef]
- Ngo, K.T.; Lee, N.A.; Pinnace, S.D.; Rochford, J. Engineering of Ruthenium(II) Photosensitizers with Non-Innocent Oxyquinolate and Carboxyamidoquinolate Ligands for Dye-Sensitized Solar Cells. Chem. A Eur. J. 2017, 23, 7497–7507. [Google Scholar] [CrossRef]
- Nguyen, T.-D.; Lin, C.-H.; Wu, C.-G. Effect of the CF3 Substituents on the Charge-Transfer Kinetics of High-Efficiency Cyclometalated Ruthenium Sensitizers. Inorg. Chem. 2016, 56, 252–260. [Google Scholar] [CrossRef]
- Nguyen, T.-D.; Lan, Y.-P.; Wu, C.-G. High-Efficiency Cycloruthenated Sensitizers for Dye-Sensitized Solar Cells. Inorg. Chem. 2018, 57, 1527–1534. [Google Scholar] [CrossRef]
- Lavrova, M.A.; Mishurinskiy, S.A.; Smirnov, D.E.; Kalle, P.; Krivogina, E.V.; Kozyukhin, S.A.; Emets, V.V.; Mariasina, S.S.; Dolzhenko, V.D.; Bezzubov, S.I. Cyclometalated Ru(ii) complexes with tunable redox and optical properties for dye-sensitized solar cells. Dalton Trans. 2020, 49, 16935–16945. [Google Scholar] [CrossRef]
- Fiorini, V.; Marchini, E.; Averardi, M.; Giorgini, L.; Muzzioli, S.; Dellai, A.; Argazzi, R.; Sanson, A.; Sangiorgi, N.; Caramori, S.; et al. New examples of Ru(ii)-tetrazolato complexes as thiocyanate-free sensitizers for dye-sensitized solar cells. Dalton Trans. 2020, 49, 14543–14555. [Google Scholar] [CrossRef]
- Pirashanthan, A.; Thanihaichelvan, M.; Mariappan, K.; Velauthapillai, D.; Ravirajan, P.; Shivatharsiny, Y. Synthesis of a carboxylic acid-based ruthenium sensitizer and its applicability towards Dye-Sensitized Solar Cells. Sol. Energy 2021, 225, 399–406. [Google Scholar] [CrossRef]
- Carella, A.; Borbone, F.; Centore, R. Research Progress on Photosensitizers for DSSC. Front. Chem. 2018, 6, 481. [Google Scholar] [CrossRef] [PubMed]
- Tomar, N.; Agrawal, A.; Dhaka, V.S.; Surolia, P.K. Ruthenium complexes based dye sensitized solar cells: Fundamentals and research trends. Sol. Energy 2020, 207, 59–76. [Google Scholar] [CrossRef]
- Mauri, L.; Colombo, A.; Dragonetti, C.; Roberto, D.; Fagnani, F. Recent Investigations on Thiocyanate-Free Ruthenium(II) 2,2′-Bipyridyl Complexes for Dye-Sensitized Solar Cells. Molecules 2021, 26, 7638. [Google Scholar] [CrossRef]
- Mary, A.; Jain, N.; Sakla, R.; Jose, D.A.; Yadav, B.S.; Naziruddin, A.R. Ruthenium (II) complexes bearing N-heterocyclic carbene-based C^N donor sets in dye-sensitized solar cells. Appl. Organomet. Chem. 2023, 37, e6873. [Google Scholar] [CrossRef]
- Jain, N.; Mary, A.; Singh, T.; Gadiyaram, S.; Jose, D.A.; Naziruddin, A.R. Heteroleptic ruthenium(II) complexes featuring N-heterocyclic carbene-based C^N donor sets for solar energy conversion. New J. Chem. 2023, 47, 13476–13485. [Google Scholar] [CrossRef]
- Jain, N.; Mary, A.; Dalal, P.M.; Sakla, R.; Jose, A.; Jain, M.; Naziruddin, A.R. Ruthenium Complexes Bearing Bis-N-heterocyclic Carbene Donors in TiO2 Sensitization for Dye-Sensitized Solar Cells. Eur. J. Inorg. Chem. 2022, 2022, e20220050.2. [Google Scholar] [CrossRef]
- Ashraf, S.; Su, R.; Akhtar, J.; Shuja, A.; Siddiqi, H.M.; El-Shafei, A. Molecular engineering of ruthenium-based photosensitizers with superior photovoltaic performance in DSSCs: Novel N-alkyl 2-phenylindole-based ancillary ligands. New J. Chem. 2022, 46, 2739–2746. [Google Scholar] [CrossRef]
- Chen, C.-Y.; Lin, T.-Y.; Chiu, C.-F.; Lee, M.M.; Li, W.-L.; Chen, M.-Y.; Hung, T.-H.; Zhang, Z.-J.; Tsai, H.-H.G.; Sun, S.-S.; et al. Steric Effects on the Photovoltaic Performance of Panchromatic Ruthenium Sensitizers for Dye-Sensitized Solar Cells. ACS Appl. Mater. Interfaces 2024, 16, 12647–12660. [Google Scholar] [CrossRef]
- Zafar, S.; Ashraf, S.; Iqbal, U.; Yousuf, S.; Siddiqi, H.M.; Liaqat, F.; Rehman, H.M.; Akhtar, Z.; El-Shafei, A.; Shahzad, N. Synthesis of new benzimidazole based ruthenium (II) dyes for application in dye-sensitized solar cells with detailed spectroscopic and theoretical evaluation. J. Mol. Struct. 2023, 1289, 135860. [Google Scholar] [CrossRef]
- Aldusi, A.M.; Fadda, A.A.; Ismail, M.A.; Elmorsy, M.R. Simple organic dyes containing multiple anchors as effective co-sensitizers for DSSCs loaded with Ru (II) complex N-719. Appl. Organomet. Chem. 2022, 36, e6893. [Google Scholar] [CrossRef]
- Badawy, S.A.; Abdel-Latif, E.; Mohamed, W.H.; Elmorsy, M.R. Unleashing synergistic co-sensitization of BOA dyes and Ru(II) complexes for dye-sensitized solar cells: Achieving remarkable efficiency exceeding 10% through comprehensive characterization, advanced modeling, and performance analysis. RSC Adv. 2024, 14, 25549–25560. [Google Scholar] [CrossRef]
- Jain, N.; Mary, A.; Manjunath, V.; Sakla, R.; Devan, R.S.; Jose, D.A.; Naziruddin, A.R. Ruthenium complexes bearing N-heterocyclic carbene based CNC and CN^CH2C’ pincer ligands: Photophysics, electrochemistry, and solar energy conversion. J. Organomet. Chem. 2022, 959, 122203. [Google Scholar] [CrossRef]
- Jain, N.; Mary, A.; Singh, T.; Gadiyaram, S.; Joshi, J.; Jose, D.A.; Naziruddin, A.R. Terpyridine Based Heteroleptic Ruthenium Complexes: Influence of Donor and Acceptor Ligands in Photosensitization. Eur. J. Inorg. Chem. 2023, 26, e202300210. [Google Scholar] [CrossRef]
- Vega, N.C.; Tomas, F.M.A.; Katz, N.E.; Comedi, D.; Fagalde, F. Sensitizing TiO2 nanoparticles with Ru(II) polypyridyl complexes containing anchoring nitrile groups for applications in solar cells. Mater. Lett. 2024, 371, 136916. [Google Scholar] [CrossRef]
- Sruthi, M.M.; Sourava, C.P.; George, A.S.; Nitha, P.R.; Rakesh, K.M.; Jubi, J.; Suraj, S. Exploring the synergy between copper electrolytes and molecularly engineered thiocyanate-free cyclometalated ruthenium sensitizers for dye-sensitized solar cells. Photochem. Photobiol. 2024, 100, 1127–1139. [Google Scholar]
- Fetouh, H.A.; Dissouky, A.E.; Salem, H.A.; Fathy, M.; Anis, B.; Kashyout, A.E.H. Synthesis, characterization and evaluation of new alternative ruthenium complex for dye sensitized solar cells. Sci. Rep. 2024, 14, 16718. [Google Scholar] [CrossRef]
- Cheng, J.D.; He, C.X.; Chen, D.; Gu, X.Y.; Wang, S.K.; Gao, X.P.; Sun, G.Z.; Zhang, X.Z.; Pan, X.J.; Pan, X.B.; et al. Effect of ruthenium(II)-bipyridine complex photosensitizer on the panchromatic light absorption and electron transfer in N719-dye sensitized photoanodes. Opt. Mater. 2022, 133, 112924. [Google Scholar] [CrossRef]



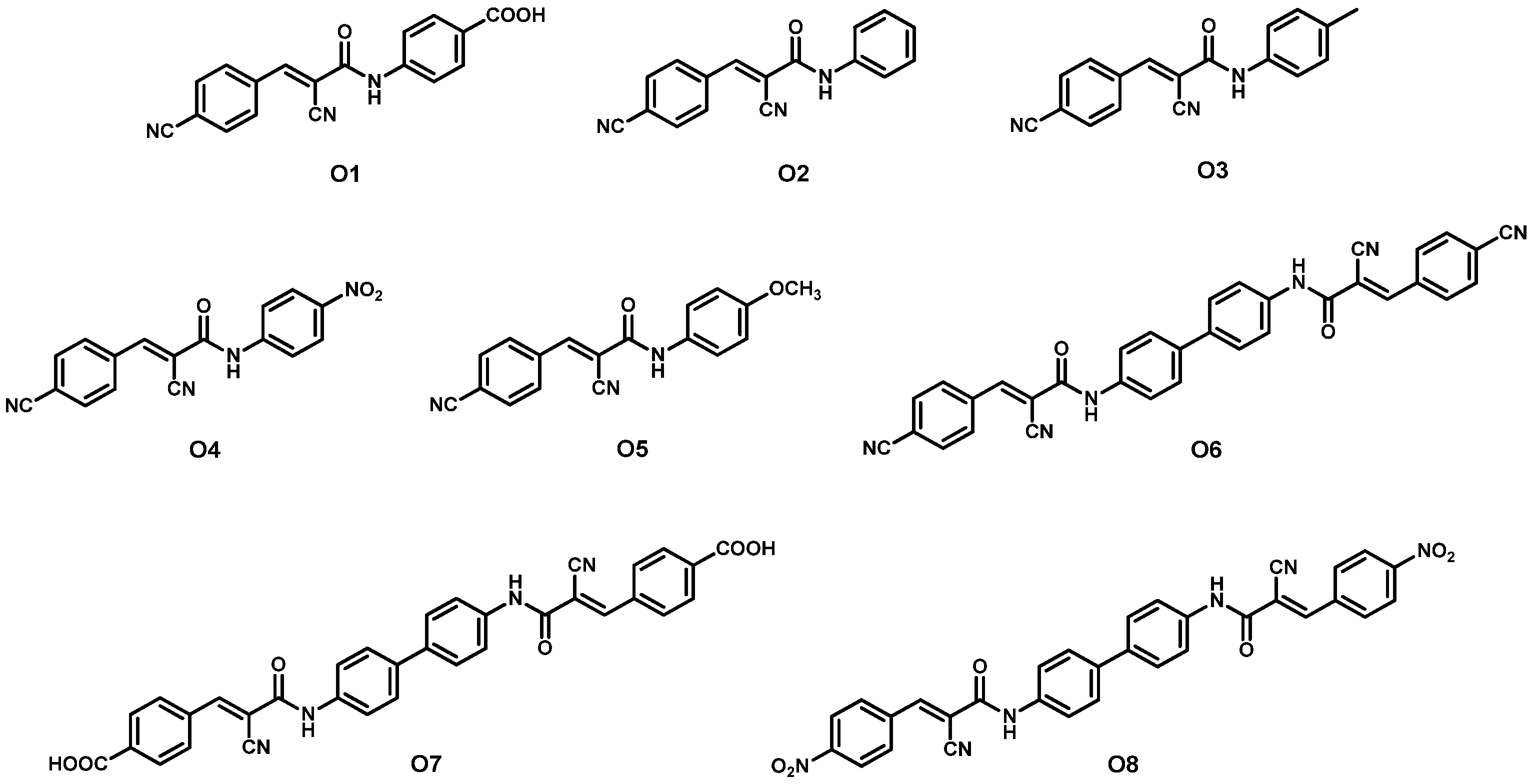
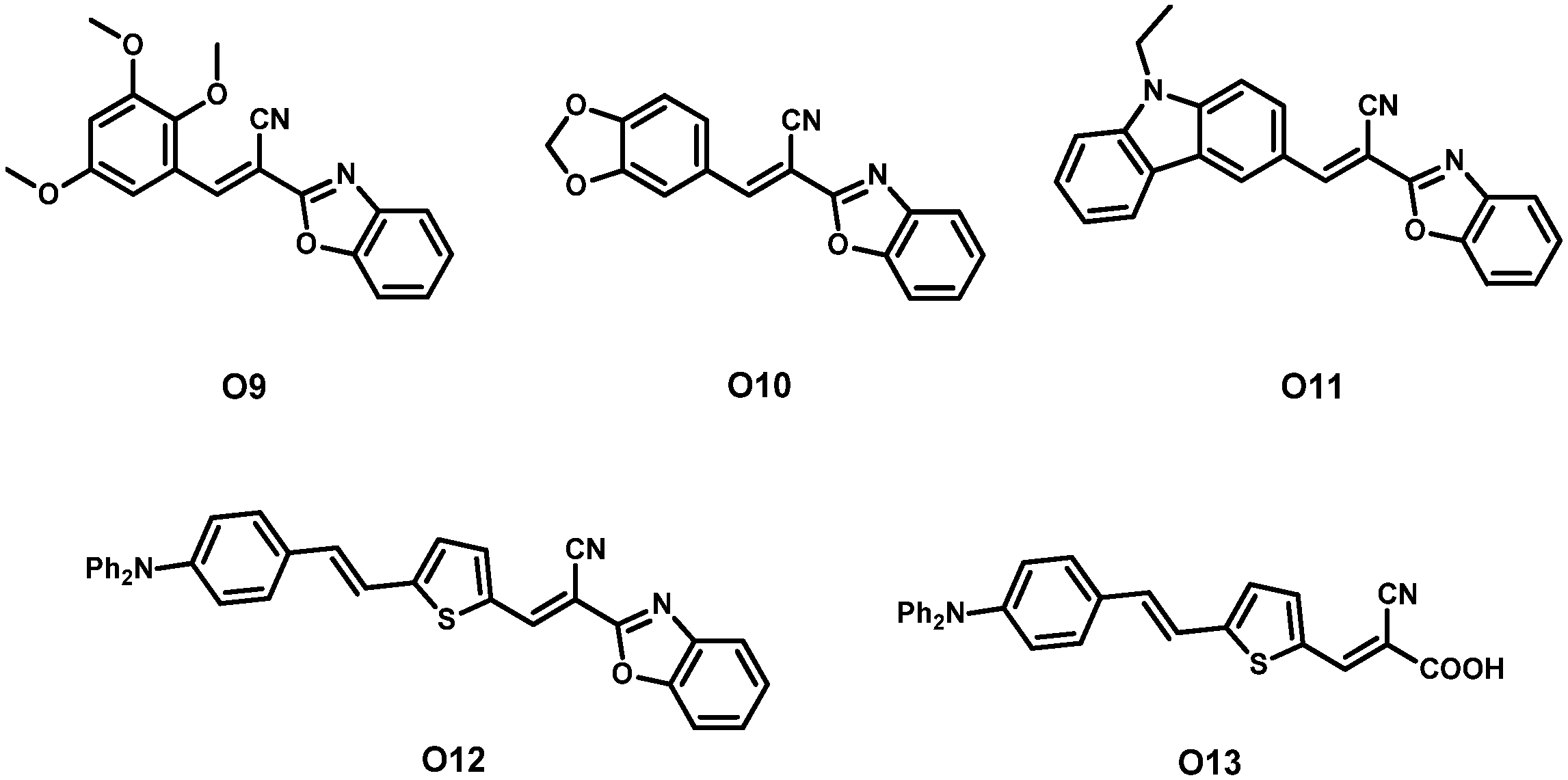
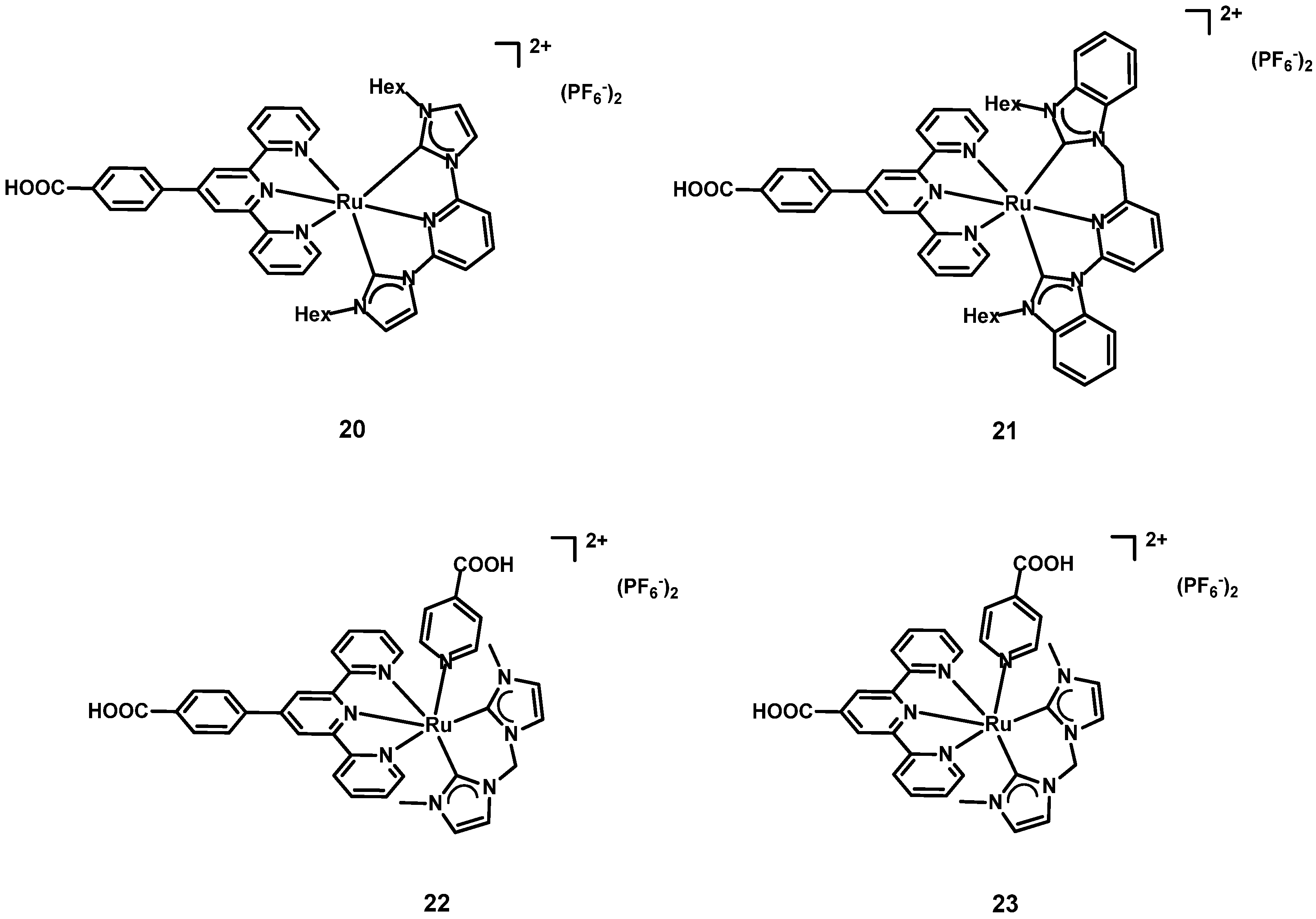


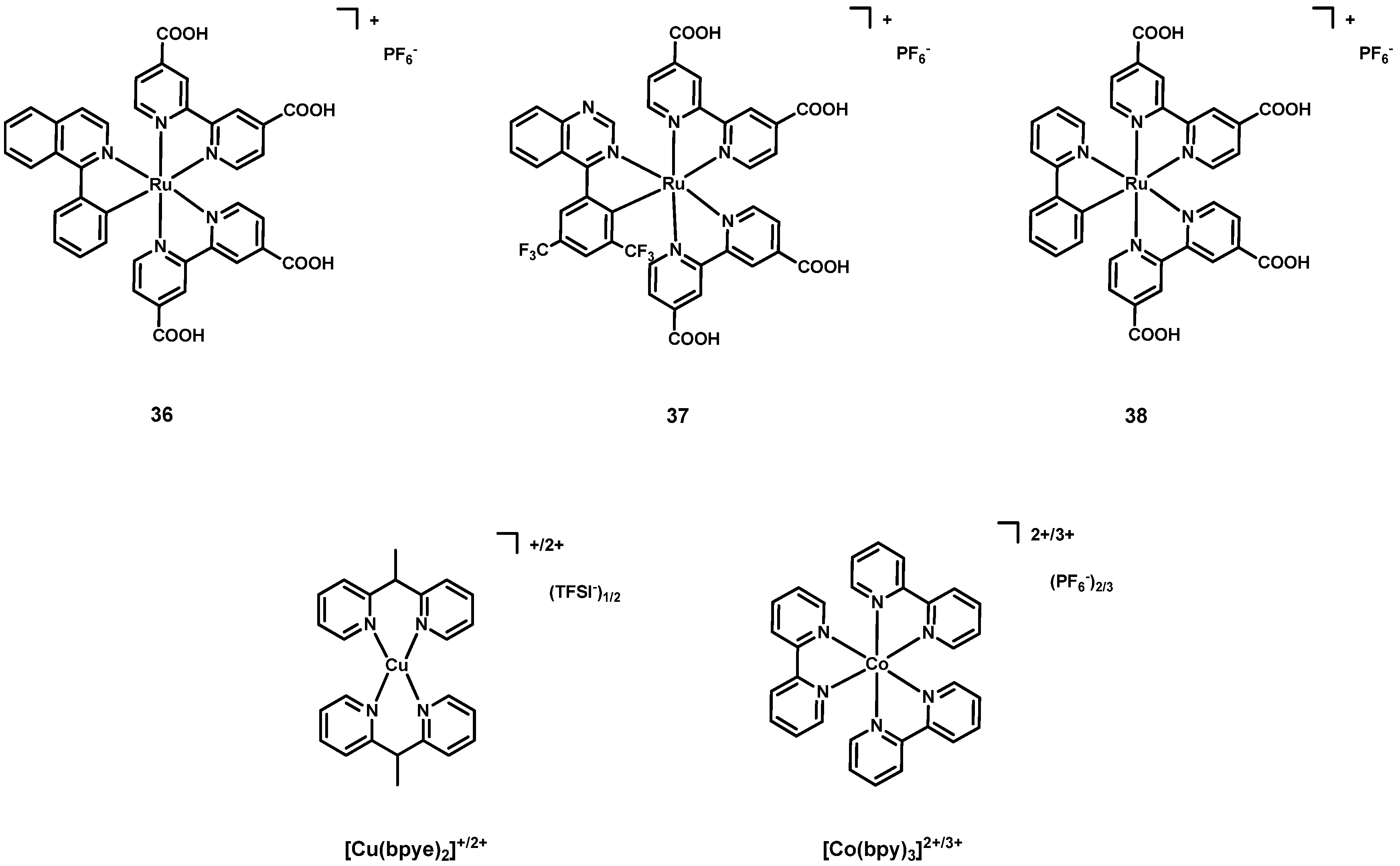


| Dye | λmax, abs/nm [ε/103 M−1 cm−1] | Jsc (mA cm−2) | Voc (V) | FF | η (%) | Refs. | |
|---|---|---|---|---|---|---|---|
| 1 | 1 2 | 283 [14.227] 316 [7.878] 486 [1.615] | 0.525 | 0.577 | 63 | 0.191 | [37] |
| 2 | 2 2 | 233 [25.945] 280 [23.143] 321 [17.292] 482 [4.180] | 2.245 | 0.598 | 73 | 0.980 | |
| 3 | 3 2 | 281 [25.352] 318 [14.852] 485 [3.466] | 0.786 | 0.574 | 65 | 0.294 | |
| 4 | 4 2 | 233 [53.720] 277 [47.545] 320 [36.568] 479 [8.971] | 7.982 | 0.643 | 67 | 3.440 | |
| 5 | N3 2 | - | 16.832 | 0.559 | 53 | 4.977 | |
| 6 | 5 2 | 279 [48.4] 324 [21.1] 496 [4.7] | 6.70 | 0.64 | 54 | 2.32 | [38] |
| 7 | 6 2 | 281 [84.5] 322 [50.5] 497 [11.0] | 3.10 | 0.65 | 75 | 1.51 | |
| 8 | 7 2 | 283 [55.3] 324 [32.0] 507 [6.1] | 1.21 | 0.47 | 73 | 0.42 | |
| 9 | 8 2 | 280 [73.7] 323 [46.1] 505 [8.9] | 3.55 | 0.54 | 54 | 1.04 | |
| 10 | N3 2 | - | 12.0 | 0.67 | 58 | 4.66 | |
| 11 | 9 2 | 283 [109.120] 318 [52.080] 499 [14.600] | 1.602 | 0.648 | 44.0 | 0.460 | [39] |
| 12 | 10 2 | 280 [75.960] 321 [50.920] 492 [10.760] | 3.792 | 0.678 | 55.1 | 1.410 | |
| 13 | N3 2 | - | 16.832 | 0.559 | 52.9 | 4.980 |
| Entry | Dye | λmax, abs/nm [ε/103 M−1 cm−1] | Redox Couple | Jsc (mA cm−2) | Voc (V) | FF | η (%) | Refs. |
|---|---|---|---|---|---|---|---|---|
| 1 | 11 2 | 297 [63.1] 372 [15.3] 516 [10.1] | I-/I3- 3 | 13.94 | 0.594 | 60.3 | 4.99 | [40] |
| 2 | 12 2 | 297 [66.6] 371 [15.3] 525 [14.6] | 19.21 | 0.675 | 62.7 | 8.14 | ||
| 3 | N719 2 | - | 19.11 | 0.693 | 58.5 | 7.74 | ||
| 4 | 17 4 | 377 [66.9] 569 [33.4] | I-/I3- 5 | 17.13 | 0.714 | 70.57 | 8.63 | [41] |
| 5 | 18 4 | 352 [49.4] 563 [27.6] | 14.08 | 0.646 | 73.05 | 6.64 | ||
| 6 | 19 4 | 353 [54.5] 563 [28.6] | 16.32 | 0.722 | 71.11 | 8.38 | ||
| 7 | N719 4 | - | 13.34 | 0.787 | 74.37 | 7.75 |
| Entry | Dye | λmax, abs/nm [ε/103 M−1 cm−1] | Jsc (mA cm−2) | Voc (V) | FF | η (%) | Refs. |
|---|---|---|---|---|---|---|---|
| 1 | O1 + N719 2 | - | 19.17 | 0.62 | 65.40 | 7.78 | [43] |
| 2 | O2 + N719 2 | - | 16.09 | 0.60 | 63.01 | 6.08 | |
| 3 | O3 + N719 2 | - | 17.25 | 0.60 | 63.56 | 6.57 | |
| 4 | O4 + N719 2 | - | 18.49 | 0.65 | 60.32 | 7.25 | |
| 5 | O5 + N719 2 | - | 17.95 | 0.61 | 65.03 | 7.12 | |
| 6 | O6 + N719 2 | - | 20.29 | 0.69 | 62.14 | 8.70 | |
| 7 | O7 + N719 2 | - | 20.96 | 0.73 | 60.78 | 9.30 | |
| 8 | O8 + N719 2 | - | 19.68 | 0.66 | 64.99 | 8.38 | |
| 9 | N719 3 | - | 18.68 | 0.64 | 61.00 | 7.30 | |
| 10 | O9 3 | 321 [21.6] 460 [50.9] | 10.49 | 0.575 | 52.02 | 3.13 | [44] |
| 11 | O10 3 | 324 [23.3] 480 [56.8] | 11.17 | 0.625 | 54.85 | 3.82 | |
| 12 | O11 3 | 322 [23.5] 495 [63.4] | 11.65 | 0.610 | 57.43 | 4.08 | |
| 13 | O12 3 | 325 [26.2] 546 [79.2] | 13.60 | 0.651 | 70.82 | 6.27 | |
| 14 | O13 3 | - | 12.68 | 0.632 | 74.10 | 5.93 | |
| 15 | N719 3 | - | 19.13 | 0.771 | 50.85 | 7.50 | |
| 16 | O11 + N719 2 | - | 21.96 | 0.791 | 58.72 | 10.20 |
| Entry | Dye | λmax, abs/nm [ε/103 M−1 cm−1] | Jsc (mA cm−2) | Voc (V) | FF | η (%) | Refs. |
|---|---|---|---|---|---|---|---|
| 1 | 20 2 | 280 [17.78] 469 [0.85] | 0.075 | 0.28864 | 27 | 0.06 | [45] |
| 2 | 21 2 | 281 [4.49] 464 [1.27] | 0.167 | 0.35254 | 44 | 0.28 | |
| 3 | N3 2 | - | 2.49 | 0.51610 | 56 | 8.04 | |
| 4 | 22 3 | 283 [108.160] 319 [50.402] 490 [13.320] | 1.040 | 1.040 | 1.040 | 1.040 | [39] |
| 5 | 23 3 | 280 [77.960] 319 [42.600] 479 [7.920] | 0.615 | 0.615 | 0.615 | 0.615 | |
| 6 | 24 3 | 282 [25.77] 310 [18.72] 492 [10.75] | 1.397 | 0.479 | 48.0 | 0.321 | [46] |
| 7 | 25 3 | 281 [13.74] 307 [13.74] 491 [6.41] | 0.225 | 0.535 | 72.0 | 0.087 | |
| 8 | 26 3 | 285 [18.36] 310 [18.72] 490 [8.02] | 1.542 | 0.512 | 56.3 | 0.441 | |
| 9 | 27 3 | 281 [23.21] 310 [25.18] 491 [8.02] | 1.750 | 0.565 | 72.1 | 0.713 | |
| 10 | 28 3 | 274 [45.8] 313 [52.6] 493 [24.5] | 0.375 | 0.603 | 57.1 | 0.131 | |
| 11 | 29 3 | 275 [25.7] 309 [28.3] 494 [14.1] | 0.379 | 0.632 | 69.3 | 0.164 | |
| 12 | 30 3 | 274 [14.0] 310 [16.6] 494 [6.9] | 0.358 | 0.597 | 61.1 | 0.130 | |
| 13 | N3 3 | - | 7.725 | 0.804 | 68.1 | 4.065 |
| Entry | Dye | λmax, abs/nm [ε/103 M−1 cm−1] | Redox Couple | Jsc (mA cm−2) | Voc (V) | FF | η (%) | Refs. |
|---|---|---|---|---|---|---|---|---|
| 1 | 31 2 | 444, 478 | I-/I3- 3 | 0.0404 | 0.171 | 28 | 0.00194 | [47] |
| 2 | 32 2 | 446, 488 | 0.123 | 0.068 | 30 | 0.00252 | ||
| 3 | 33 2 | 448, 530 | 0.325 | 0.332 | 47 | 0.054 | ||
| 4 | 34 2 | 453, 489 | 0.0142 | 0.038 | 25 | 0.00013 | ||
| 5 | 35 2 | 454, 509 | 0.427 | 0.344 | 45 | 0.0656 | ||
| 6 | 36 4,5,6 | 564 [9.437] | Cu(I)/Cu(II) 7 | 2.74 | 0.57 | 62.22 | 0.98 | [48] |
| 7 | 36 4,5,8 | 3.34 | 0.56 | 61.93 | 1.16 | |||
| 8 | 36 4,5,9 | 2.93 | 0.55 | 62.63 | 1.02 | |||
| 9 | 36 4,5,10 | 2.93 | 0.54 | 62.62 | 1.00 | |||
| 10 | 37 4,5,8 | 513 [13.688] | 0.76 | 0.44 | 51.2 | 0.17 | ||
| 11 | 38 4,5,8 | 562 [11.120] | 1.75 | 0.49 | 51.2 | 0.43 | ||
| 12 | 36 4,5,8 | 564 [9.437] | Co(II)/Co(III) 11 | 1.62 | 0.21 | 37.0 | 0.13 | |
| 13 | 39 12 | 246 [17.650] 307 [29.550] 371 [7.050] 492 [6.750] | I-/I3- 13 | 6.670 | 0.6004 | 77.29 | 3.09 | [49] |
| 14 | N3 12,14 | - | 17.813 | 0.675 | 60.73 | 7.3 | ||
| 15 | N3 12,15 | - | 11.2 | 0.650 | 68.06 | 5 |
| Entry | Dye | λmax, abs/nm [ε/103 M−1 cm−1] | Jsc (mA cm−2) | Voc (V) | FF | η (%) | Refs. |
|---|---|---|---|---|---|---|---|
| 1 | 40 2 | 475 [17.5] | 2.5 | 0.585 | 63.35 | 0.94 | [50] |
| 2 | N719 3 | - | 9.8 | 0.790 | 69.40 | 5.3 | |
| 3 | 40 + N719 4 | - | 11.5 | 0.727 | 69.06 | 5.8 | |
| 4 | 40 + N719 5 | - | 11.9 | 0.736 | 67.87 | 6.0 | |
| 5 | 40 + N719 6 | - | 12.8 | 0.733 | 65.02 | 6.3 | |
| 6 | 40 + N719 7 | - | 11.5 | 0.737 | 64.11 | 5.6 | |
| 7 | 40 + N719 8 | - | 12.2 | 0.723 | 63.39 | 5.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Colombo, A.; Dragonetti, C.; Fagnani, F.; Roberto, D. Recent Developments of Ruthenium Complexes for Dye-Sensitized Solar Cells. Electronics 2025, 14, 1639. https://doi.org/10.3390/electronics14081639
Colombo A, Dragonetti C, Fagnani F, Roberto D. Recent Developments of Ruthenium Complexes for Dye-Sensitized Solar Cells. Electronics. 2025; 14(8):1639. https://doi.org/10.3390/electronics14081639
Chicago/Turabian StyleColombo, Alessia, Claudia Dragonetti, Francesco Fagnani, and Dominique Roberto. 2025. "Recent Developments of Ruthenium Complexes for Dye-Sensitized Solar Cells" Electronics 14, no. 8: 1639. https://doi.org/10.3390/electronics14081639
APA StyleColombo, A., Dragonetti, C., Fagnani, F., & Roberto, D. (2025). Recent Developments of Ruthenium Complexes for Dye-Sensitized Solar Cells. Electronics, 14(8), 1639. https://doi.org/10.3390/electronics14081639








