Abstract
A series of modified tetraphenylporphyrins varying only in the electron-donating or electron-withdrawing character of the substituents in the para-phenyl position have been blended into the active layer of MEH-PPV:PCBM bulk heterojunction solar cells. Increasing the electron-withdrawing ability of the substituents, as quantified by the Hammett constant, systematically alters the device efficiency of ternary poly[2-methoxy-5-(2′-ethylhexyloxy)-1,4-phenylenevinylene]:porphyrin:[6,6]-phenylC61-butyric acid methyl ester (MEH-PPV:porphyrin:PCBM) bulk heterojunction organic solar cells through alteration of the HOMO/LUMO levels and, thereby, the open-circuit voltage of the cell. We show that the porphyrin concentrates at the MEH-PPV:PCBM interface in these blends and that the devices operate via a cascade mechanism when the highest occupied molecular orbital (HOMO) of the porphyrin is higher in energy that that of MEH-PPV, but via a parallel/alloy device mechanism, when the HOMO of the porphyrin is lower in energy than that of MEH-PPV. As such, this work highlights how the energetics of the ternary component can determine device performance by switching between charge generation models simply by altering the electron-withdrawing character of the porphyrin ternary additive.
1. Introduction
Over the last decade, the field of organic photovoltaics (OPVs) has matured as an important area for scientific research. In particular, OPV materials development has transitioned from the use of poly(3-hexylthiophene) (P3HT) in P3HT:fullerene blends, to more complex, higher efficiency polymer donors, and more recently to non-fullerene acceptors (NFAs) [1]. As a result, laboratory-scale device power conversion efficiencies (PCEs) have increased with the most efficient devices now exceeding 19% [2,3] and tandem device structures reported in excess of 20% [4]. However, this rapid growth in material development has not, as yet, resulted in OPV becoming a viable commercial energy alternative, due in large part to the prohibitive synthetic complexity and associated cost of the new generation of materials [5]. Consequently, in recent years, recognition of efficiency, lifetime and cost as limiting cofactors in commercial OPV development has arisen [6] and a focus on low-cost, up-scalable OPV materials has appeared in the literature, in parallel with the more efficiency-focussed reports [7]. This research has led to the successful implementation of large-scale OPV. Within the Centre for Organic Electronics (COE) at the University of Newcastle, for example, we have demonstrated several OPV installations based upon roll-to-roll (R2R) printed solar, utilizing P3HT:fullerene in a polyethylene terephthalate/silver grid/active layer/sputtered aluminium (PET/Ag/active layer/Al) architecture (Figure 1). These demonstrators have highlighted the need for inexpensive new OPV materials and/or an improvement in the efficiency of existing low-cost options.
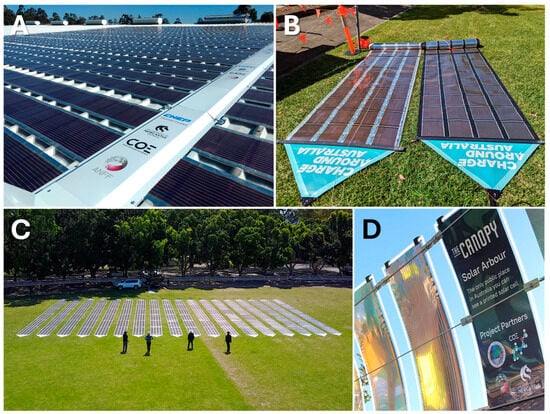
Figure 1.
Examples of COE manufactured OPV demonstrators highlighting the low weight and flexibility of the printed modules. (A) Two-hundred-meter-squared printed OPV installation at CHEP, Beresfield facility near Newcastle, Australia; (B) close up of the “roll-up” printed OPV modules used for electric vehicle charging in the Charge Around Australia circumnavigation of Australia in an electric vehicle; (C) a demonstration of the full charging array used in Charge Around Australia; (D) a detail of The Canopy, a printed OPV installation and integrated light display located at Lane Cove in Sydney, Australia.
One potential approach to improving OPV efficiencies is the use of a third component in the active layer to enhance its photovoltaic parameters [8]. The development of these ternary organic cells (TOSCs) has increased rapidly in recent years, assisted by the introduction of non-fullerene acceptors, with efficiencies above 17% reported [9,10]. These improvements have led to the area of TOSCs becoming a hot topic in photovoltaic research, with numerous impressive performance and stability results reported [11]. However, despite progress in performance and mechanistic understanding, there is still no comprehensive model for the charge transfer process. As such, recent publications have called for a return to more fundamental studies that probe mechanisms [12,13].
Historically, there have been a number of publications that have reported the use of porphyrins as ternary components in bulk heterojunction organic solar cells [14,15,16,17], with these materials often used to broaden the spectral response of the device [18]. For example, meso-substituted porphyrins have been used as donors (due to their high molar extinction coefficients in the visible part of the spectrum) to achieve efficiencies approaching 10% in binary devices [19]. However, despite their apparent suitability as optoelectronic materials, the uptake of porphyrins in organic solar applications has been slow, with these early studies reporting only modest efficiencies [20]. More recently, porphyrin dimer materials have shown greater than 11% efficiencies when mixed with another donor material [21] and up to 15% when combined with dual acceptor materials in ternary devices [22].
Porphyrins are an attractive choice as a ternary blend material for fundamental studies since they can be readily synthesised with substituents that alter their electronic behaviour. However, adding a third component, such as a porphyrin, to an OPV further complicates what is already a highly complex and not well-understood nanostructure [23,24]. Consequently, it is recognised that understanding both this new morphology and its effect on electronic behaviour is crucially important for further improving device performance [25]. However, few studies have investigated the effect of systematic structural [26] or electronic [27] changes to the porphyrin in ternary blend devices.
Structurally, for many of these porphyrin blends, aggregation of the porphyrin species is a limiting factor to efficient devices and consequently very small amounts of the porphyrin are typically added to the device active layer. None the less, these vanishingly small amounts of ternary material (typically <5% by weight) disproportionately affect the active layer nanostructure and thereby dictate device performance [12,28,29]. A key challenge in studying these systems is to be able to change just a single parameter without simultaneously affecting the properties of another. For example, we have previously shown that we can improve charge separation efficiency and thus increase device performance in ternary poly[2-methoxy-5-(2′-ethylhexyloxy)-1,4-phenylenevinylene]:porphyrin:[6,6]-phenylC61-butyric acid methyl ester (MEH-PPV:porphyrin:PCBM) by changing the steric bulk of the porphyrin species [26]. We have also shown that minor structural modification of porphyrinogens by metalation or methylation of pyrrolic nitrogen results in a change in ionisation potential, which changes both the open circuit voltage (VOC) and the degree of charge recombination of the device [27].
Electronically, four models have been established for the behaviour of a ternary material in OPV devices [12,30]:
(1) A cascade or charge transfer model, where the ternary material sits at the primary donor:acceptor interface and acts as a conduit for charge between the two species [31,32]. For this mechanism to occur, the energy levels of the ternary material must be aligned such that both electron and hole transfer is facilitated.
(2) A parallel device model, in which the ternary material acts as a secondary donor, independent of the primary donor [33]. In this case, both donor materials must interface with the acceptor material, and device efficiency will potentially be limited by the relative interfacial coverage of the two donor species [34].
(3) An alloy model, in which the ternary material and primary donor form an intimate blend, or alloy, with new physical properties and highest/lowest occupied molecular orbital (HOMO/LUMO) energy levels [35,36].
(4) An energy transfer model, in which energy transfer occurs between the primary donor and ternary materials prior to charge separation [37,38]. For this mechanism to occur, the ternary material must act as a sensitizer, extending the active layer absorption range. In these cases, the emission of the sensitizer and absorption of the donor or acceptor must overlap significantly to allow for efficient energy transfer.
Despite these conceptual advances, fully capitalizing on the advantages of the ternary blend strategy requires a deeper understanding of morphology formation and aging, blend component miscibility, charge transfer dynamics, and charge carrier transport than is currently available [23]. Indeed, despite decades of research, accurately predicting the optimal substituent design to achieve the desired bulk heterojunction morphology remains a significant challenge, particularly for molecules like porphyrins with highly extended π-conjugated frameworks that require multiple solubilizing substituents. Consequently, studying simple model systems is of considerable importance, as it can unveil fundamental rules concealed within the complexity of experimental data [39].
Here, we utilise a series of simple model tetraphenylporphyrins, in combination with the well-studied MEH-PPV:PCBM active layer blend to probe changes in the charge generation mechanism of ternary OPV devices. The key to this work is the ability to modify the HOMO level of the minority porphyrin additive without altering other properties of the device, such as recombination and morphology. Consequently, we provide insight into how changing the electronic structure of the minority ternary additive can dramatically influence the charge generation mechanism and thus be used to control device electronic properties.
2. Experimental
Materials: Chlorobenzene, obtained from Sigma-Aldrich (Darmstadt, Germany) was of high-performance liquid chromatography (HPLC) grade. Porphyrins were sourced from Frontier Scientific (Newark, DE, USA) and utilized without further modification. MEH-PPV, with a molecular weight of 16,000 g mol−1, was acquired from Jenpolymer Materials (Jena, Germany) and stored under nitrogen conditions. PCBM, with a purity of 99.5%, was purchased from Luminescence Technology Corp. (New Taipei City, Taiwan) and also stored under nitrogen. Poly(2,3-dihydrothieno-1,4-dioxin)-poly(styrenesulfonate) (PEDOT:PSS) was procured from H.C. Starck (Goslar, Germany) as Clevios P VP Al 4083, featuring a solid content of 1.3–1.7% by weight and a PEDOT:PSS ratio of 1:6 by weight, and was used as received. Patterned indium tin oxide (ITO) slides, with a sheet resistance of 15 Ω, were supplied by Xinyan Technology Ltd. (Kowloon, Hong Kong).
Device manufacture: The preparation of the studied photovoltaic devices involved the following steps: patterned ITO slides, approximately 125 nm thick, were cleaned through sonication using detergent, hexane, acetone, and isopropyl alcohol sequentially. A 50 nm-thick layer of PEDOT:PSS was applied by spin coating from an aqueous solution and subsequently annealed at 125 °C for 10 min in a nitrogen environment. MEH-PPV:porphyrinoid:PCBM solutions (with weight ratios of 8:1:32 or 4:4:32) were dissolved in chlorobenzene and spin coated to create an active layer with a thickness of roughly 90 nm. The substrates were then placed into a vacuum deposition chamber (base pressure ~10−7 mbar), where 20 nm of calcium and a 100 nm-thick aluminium cathode were deposited thermally.
Device testing: The devices’ current-voltage (IV) characteristics under air mass 1.5 (AM1.5) illumination were measured using a Keithley 2400 (Tektronik, OR, USA) source meter. External quantum efficiency (EQE) was determined using illumination from a tungsten halogen lamp passed through an Oriel Cornerstone 130 monochromator. Reference signals were obtained using a Thorlabs (Newton, NJ, USA) PDA55 silicon diode and an Ithaco (Ithaca, NY, USA) Dynatrac 395 analog lock-in amplifier, while the device’s current was recorded with a Stanford Research Systems (Sunnyvale, CA, USA) SR830 DSP digitizing lock-in amplifier. The wavelength range was restricted to above 375 nm due to the transmission limitations of the patterned ITO glass slides.
UV–Visible Absorption Spectroscopy: Spin-coated films were formed on pre-cleaned quartz slides and examined using a Varian Cary 6000i (Agilent, CA, USA) spectrophotometer. Film thickness was measured with a Tencor Alpha-Step 500 (KLA, CA, USA) surface profilometer.
Optical Microscopy: Device films were analyzed under tungsten lamp illumination using a Zeiss (Oberkochen, Germany) Axioplan2 microscope. Images were captured with an Olympus (Tokyo, Japan) DP70 CCD camera and converted to JPEG format using Olympus DP Controller software (version 2.1.1.183). The films, created through spin coating chlorobenzene solutions as previously detailed, were approximately 125 nm thick.
Near Edge X-ray Absorption Fine Structure (NEXAFS) Optical Density Mapping: NEXAFS optical density maps of device films were collected at the Advanced Light Source using beamline 5.3.2.2 [40]. The samples were deposited onto silicon nitride and rastered relative to the X-ray beam, within a sample chamber backfilled with helium at 0.33 atm. The transmitted X-ray beam was detected using a scintillator and a photomultiplier tube. NEXAFS spectra of pristine samples of MEH-PPV, porphyrin, and PCBM were analysed to identify key and orthogonal absorption energies that maximized X-ray intensity contrast among the three components. For this study, energies of 284.4 eV, 285.3 eV, and 287.4 eV were selected, corresponding to dominant absorptions in PCBM, porphyrin, and MEH-PPV, respectively.
Surface Tension Measurements: Contact angle measurements were conducted using an Attension Theta optical tensiometer (Biolin Scientific, Gothenberg, Sweden). The contact angle was determined for a ~3 μL water droplet placed on solid uniform films of MEH-PPV, PCBM, and the porphyrin series.
Cyclic voltammetry: The porphyrin HOMO levels were determined experimentally via cyclic voltammetry, based on the approach reported in previous publications [41,42]. The experimental arrangement involved a platinum (Pt) disc working electrode (WE, diameter = 1.6 mm), a Pt-coil counter electrode (CE), and a home-made Ag/Ag+ reference electrode (RE). The electrolyte contained the porphyrinoid chromophore (1 mM) and tetrabutylammonium hexafluorophosphate (TBAPF6; 0.1 M) in dichloromethane (DCM) solvent dried over molecular sieves and purged with nitrogen gas. The Pt disk was polished with a 0.05 μm alumina slurry on a polishing pad, washed thoroughly with de-ionised water, and dried with lint-free paper between each experiment. To construct the Ag/Ag+ RE, a ~7 cm length of Ag wire (Sigma-Aldrich (Darmstadt, Germany), 99.999%, Φ = 1 mm) was polished with a fine-grade garnet paper, before being inserted into Luggin capillary tube, which housed the RE and the internal electrolyte. Electrolyte (0.01 M AgNO3 + 0.1 M TBAPF6 in dry CH3CN) was filled to a 3–4 cm depth, and the open end sealed was with Teflon tape. The RE contact was provided by 1–2 cm of Ag wire. An Autolab PGSTAT12 potentiostat (Methrohm, Herisau, Switzerland) was used to generate the voltammograms by scanning at 50 mV·s−1 between 1.0 and −1.8 V vs. Ag/Ag+, starting from the solution open-circuit potential with the initial sweep in the anodic direction. Following the completion of the initial voltammetry regime, ferrocene was added to the porphyrin solution in an amount sufficient to achieve an electrolyte concentration of approximately 1 mM, and the mixture was stirred to dissolve the solid. The cyclic voltammetry regime was then repeated for one additional cycle, with the potentials adjusted using the experimentally determined standard reduction potential of the reversible ferrocene (Fc/Fc+) couple. As per convention for non-aqueous voltametric methods, all potentials reported here are referenced to this internal standard [42]. Uncompensated resistance was corrected for using a typical value for a 0.1 M TBAPF6 solution in CH2Cl2 of 2.5 kΩ [43]. Due to the low currents observed because of both the small WE area and low concentration of electroactive species, this correction was typically ~4 mV. The determined onset voltage vs. the Fc/Fc+ couple was converted to a normal hydrogen electrode (NHE) scale by adding 630 mV [42], from which the energy can be converted from the electrochemical potential scale to an absolute energy vs. vacuum with
where EHOMO is energy of the HOMO in the absolute energy level scale, q is the number of electrons in the process, Von is the potential (vs. NHE) at which the faradaic current onsets, and 4.5 is a conversion constant between electrochemical potential vs. NHE and vacuum scales.
EHOMO = −q(Von + 4.5)
Photoelectron spectroscopy in air (PESA): The HOMO energies of the MEH-PPV and PCBM components were determined using PESA. A Riken Keiki AC2 (Nara, Japan) apparatus was employed to measure the photoelectron yield as a function of excitation energy. The photoelectron yield is characterized by the following empirical formula [44]:
where E is the excitation energy, IE is the ionisation energy and Y the photoelectron emission yield. When the cube root of the photoelectron yield is plotted against excitation energy, the ionisation energy of the sample can be determined by the intersection of linear fits against the two straight parts of the curve. A standard deviation can be calculated from the linear fits.
Y ∝ (E − IE)3
Optical band gap and LUMO determination: The optical band gap of each active layer component was determined by fitting a straight line to the steepest section of the absorption edge from a pristine film of the material. The point where the linear fits of this absorption edge intersect with the absorption baseline defines the optical band gap [45]. The LUMO of each material was then determined by adding the band gap to the measured HOMO.
3. Results and Discussion
Figure 2 shows the series of porphyrins introduced into MEH-PPV:PCBM OPV devices in this work.
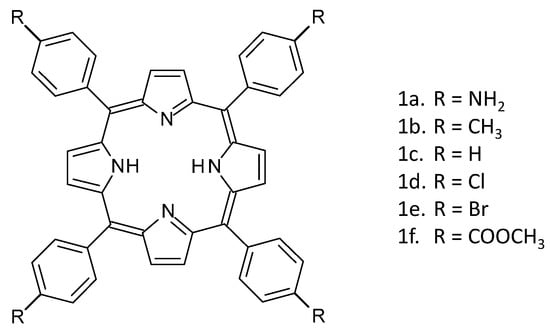
Figure 2.
Molecular structures of tetraphenylporphyrins with the para-phenyl substituents: (a) NH2, (b) CH3, (c) H, (d) Cl, (e) Br, and (f) COOCH3, used in this study.
MEH-PPV was chosen as a model donor polymer for this series of experiments for two main reasons. Firstly, MEH-PPV provides a well-studied donor system which demonstrates high blend miscibility for the porphyrin materials chosen. By contrast, similar experiments conducted with P3HT as the polymer donor material resulted in aggregation of several of the porphyrin materials, severely limiting the scope of the study [46]. Secondly, the highest occupied molecular orbital (HOMO) of MEH-PPV (−5.49 eV) lies such that the HOMO levels of the chosen porphyrins span this value, allowing a change in the charge transfer mechanism of the device to be observed. Again, by contrast, the HOMO of P3HT (~5.10 eV) [47] is lower, or approximately equal to, all of the porphyrins studied, and, consequently, a cascade model would occur for charge transfer in devices containing all of the porphyrins within the studied range.
A small amount of the porphyrin was added (<2.5%) to a standard MEH-PPV:PCBM 1:4 by weight device, producing ternary blend devices with an MEH-PPV:porphyrin:PCBM ratio of 8:1:32 by weight. Each porphyrin compound is a freebase tetraphenylporphyrin differing only in the functional group present at the 4- or para-phenyl position. The porphyrins and the abbreviations used in this report are as follows:
- (a)
- tetra(4-aminophenyl)porphyrin (TpNH2PP)
- (b)
- tetratolylporphyrin (TTP)
- (c)
- tetraphenylporphyrin (TPP)
- (d)
- tetra(4-chlorophenyl)porphyrin (TpClPP)
- (e)
- tetra(4-bromophenyl)porphyrin (TpBrPP)
- (f)
- tetra(4-methoxycarbonylphenyl)porphyrin (TpCOOCH3PP)
In addition the bis ortho-substituted porphyrin, 5,10,15,20-tetra(3,5-di-t-butylphenyl)porphyrin (tBuPP) was also incorporated into ternary blend devices for comparison purposes.
In the primary investigation, the core tetraphenylporphyrin framework remains unchanged, with variations introduced solely at the para-phenyl position. The selected substituents span a range of electronic functional properties: electron-donating (amino and methyl), neutral (hydrogen), and electron-withdrawing (chloro, bromo, and acetate methyl ester) (Figure 2).
Perturbing the conjugated π system by the addition of meso-phenyl substituent groups enables the electro-optical properties of tetraphenylporphyrins to be varied in a straightforward manner. Although the porphine and phenyl planes are generally considered not to be coplanar, rotation of the porphyrins meso-phenyl rings allows direct conjugation with the porphyrin core at least some of the time [48]. Modifying the peripheral phenyl groups with various electron-donating or electron-withdrawing substituents alters the electron density within the porphyrin core, thereby affecting its optical characteristics, including the position and intensity of UV–visible absorption peaks. [49].
The electron-donating and electron-withdrawing characteristics of tetraphenylporphyrin substituents can be quantitatively assessed using the Hammett parameter (σ) [50]. This parameter accounts for both the resonance and field effects of a substituent on a benzene ring, as measured by its influence on the ionization rate of benzoic acid in water.

The Hammett equation is as follows:
where KH is the ionisation constant for benzoic acid in water at 25 °C and KX is the ionisation constant for the substituted benzoic acid. Hammett parameters are generally determined for meta- and para-phenyl substituents; however, this work focuses exclusively on substituents at the para-phenyl position of the porphyrin molecules. Table 1 lists the Hammett parameters for these substituents [50,51], which are used as a quantitative measure of the electron-donating and withdrawing ability of the pendant functional groups in this study. A positive σpara value indicates that a substituent group is electron withdrawing, a negative σpara indicates that it is electron donating, and the size of σpara is dependent on the magnitude of the effect.

Table 1.
Hammett parameters for the substituents in the para-phenyl position of the porphyrins used in this study from [50,51]. Also listed are the experimentally determined highest occupied molecular orbitals (HOMOs) and lowest unoccupied molecular orbitals (LUMOs) for the para-substituted tetraphenylporphyrins, tBuPP, MEH-PPV polymer and PCBM used in this study.
In order to establish what effect varying the electron donating ability of the pendant functional groups has upon the electronic structure of the porphyrins, the highest occupied molecular orbital (HOMO) and lowest unoccupied molecular orbital (LUMO) of each porphyrin were determined experimentally via cyclic voltammetry and UV–visible absorption spectroscopy. These values are also presented in Table 1.
The relationship between the Hammett σpara parameter and the HOMO and LUMO energy levels are linear, with R2 values of 0.97 and 0.99, respectively. An increase in the electron-withdrawing nature of the porphyrin substituents leads to a concurrent reduction in the energies of both the HOMO and LUMO levels of the porphyrins. For para-phenyl substituted freebase tetraphenylporphyrins, oxidation typically involves a single electron transfer from the porphyrin π system. However, in cases where the substituent is highly electron-donating (e.g., alkoxy and amino groups), the initial oxidation is thought to occur at the phenyl rings rather than the porphyrin core [52]. Incorporating substituents with greater electron-withdrawing capabilities at the para-phenyl position reduces electron density in the porphyrin core, consequently lowering the HOMO energy level [49]. Since the porphyrin is likely to act as an electron donor molecule in this system, the porphyrin HOMO energy is altered such that the charge transfer energy (ECT), the HOMOdonor–LUMOacceptor energy gap, is widened.
Figure 3 shows the overall energy diagram of the devices prepared in this study. We note that the energy level for calcium metal is higher than the LUMO of PCBM, leading to an apparent energy barrier for electron transfer to the calcium/aluminium (Ca/Al) cathode. However, it has been shown that the thin (≤30 nm) Ca interfacial layers, the driving force for electron transport, across the Ca/Al electrode interface are not dictated by the work function of the Ca layer [53]. By contrast, devices fabricated with thick (>30 nm) Ca layers do show a considerable reduction in performance due to the lower Ca work function dominating the interfacial energetics. Consequently, thin calcium interfacial layers were utilised in this work, since an incomplete layer is required for optimal device performance.
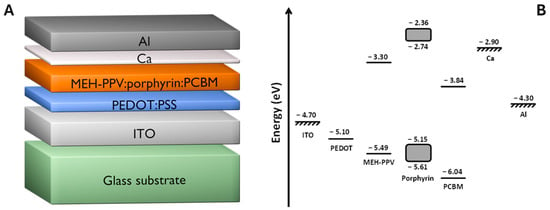
Figure 3.
(A) Cross-sectional schematic diagram of the fabricated device structure, showing the different layers. (B) HOMO-LUMO energy level schematic for the MEH-PPV:porphyrin:PCBM organic photovoltaic cells. The grey-scale boxes delineate the full range of experimentally determined HOMO and LUMO energy values for the range of porphyrins used in this study.
The LUMO levels of all the porphyrins sit above that of the polymer and are therefore well suited to electron transfer to either the polymer or the fullerene species. However, whilst the HOMO energies are placed ideally with respect to hole transfer to the PEDOT:PSS interfacial layer or directly to the electrode and to receive holes from the fullerene, the HOMO energies of the porphyrin components bracket that of the MEH-PPV donor.
Figure 4A–D show an optical microscopy image and optical density maps recorded for the characteristic NEXAFS C1s π* and σ* resonances at 284.4, 285.3 and 287.4 eV for the MEH-PPV:tetraphenylporphyrin:PCBM 8:1:32 blend film. The optical microscopy of the MEH-PPV:tetraphenylporphyrin:PCBM film shows that each ternary mixture is well blended, with no visible aggregation or phase segregation visible down to the resolution limits of the NEXAFS optical density maps (30 nm [40]). The presented microscopy is typical of that for all the porphyrin blend films investigated and demonstrates that observed trends in device performance are not associated with changes to the active layer morphology. Figure 4E shows the UV–vis spectra for all the as-spun MEH-PPV:porphyrin:PCBM 8:1:32 ternary blend films. These plots clearly show the inclusion of the porphyrin in the film with the presence of Soret bands for each porphyrin appearing as a peak at ~440 nm.
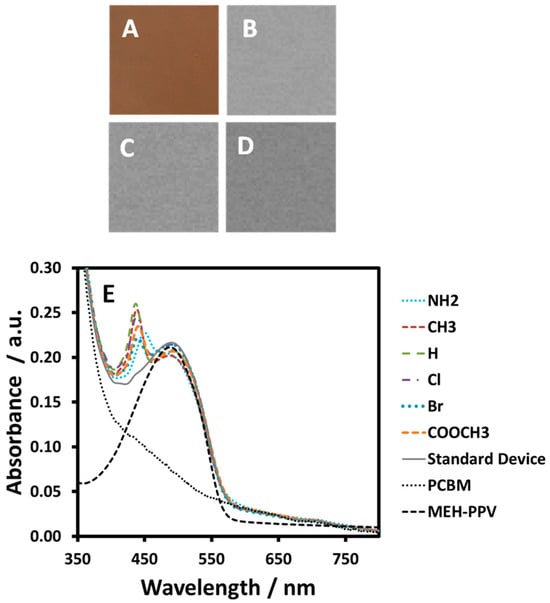
Figure 4.
(A) A 100 × 100 µm optical microscopy image of a MEH-PPV:tetraphenylporphyrin:PCBM 8:1:32 blend film and corresponding 5 × 5 µm NEXAFS optical density maps recorded at (B) 284.4 eV, (C) 285.3 eV and (D) 287.4 eV. (E) UV–vis absorption spectra of MEH-PPV:porphyrin:PCBM 8:1:32 ternary blend OPV device films and pristine MEH-PPV and PCBM films on quartz.
The performance characteristics of the 8:1:32 ratio ternary blend OPV devices are presented in Table 2. Note that these data are also listed in order of increasing Hammett σpara. The performance parameters of standard binary MEH-PPV:PCBM 8:32 devices are also presented for comparison. Typical J–V curves and EQE spectra for all device blends are shown in Figure 5. It is clear from these data that while the PCE of the MEH-PPV:porphyrin:PCBM ternary blend devices is less than that of the standard binary device, of greater significance is the fact that it systematically improves as the porphyrin para-phenyl substituents become more electron withdrawing (Hammett σpara increases). As such, this observation confirms our hypothesis that altering the HOMO levels of the ternary porphyrin component allows us to probe the charge transfer mechanism of the device; thus, it is this relative change in performance that is the focus of this study rather than the absolute difference between binary and ternary device efficiency. Indeed, the trend for the areas under the EQE plot in Figure 5B follows the same trend observed for the short circuit current-densities exhibited in Figure 5A, as expected. It is interesting to note that both HOMO and LUMO decrease with increasing device efficiency at a similar rate, an observation consistent with the UV–vis spectra (Figure 3) and EQE spectra (Figure 5B), which shows that the Soret bands of each porphyrin are of a similar wavelength (435–445 nm). The improvement in device efficiency is driven primarily by corresponding improvements in device VOC and, to a lesser extent, JSC. The systematic increase in JSC is reflected by an analogous increase in the overall EQE of the devices. Peaks in the EQE spectra at ~440 nm again demonstrate that the porphyrin additives are present in the device active layer and that they do contribute to charge generation in these devices.

Table 2.
Device characteristics for MEH-PPV:porphyrin:PCBM 8:1:32 (~2.5% porphyrin by weight) ternary blend OPV devices. Also shown are the efficiency (η), open-circuit voltage (VOC), short-circuit current density (JSC), fill factor (FF), series resistance (Rs) and shunt resistance (Rsh) device characteristics for MEH-PPV:porphyrin:PCBM 4:4:32 (10% porphyrin by weight) for devices containing tetratolylporphyrin (CH3) and tetraphenylporphyrin (H). The data presented are the average of 6 devices and the errors are presented as the standard deviation of the data. Device series resistance (Rs) and shunt resistance (Rsh) have been approximated from the inverse of the slope of the J–V curves under illumination at VOC and JSC, respectively, and agree reasonably with values for other fullerene-containing ternary blend systems [54,55,56].
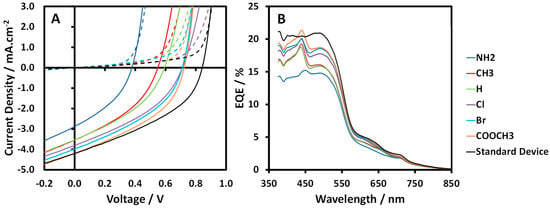
Figure 5.
(A) Light (solid line) and dark (dashed line) J–V plots and (B) external quantum efficiency spectra of MEH-PPV:porphyrin:PCBM 8:1:32 ternary blend OPV devices.
Figure 6 shows plots of device VOC as a function of device efficiency (η), the Hammett parameter of the pendant functional group (σpara) and the HOMOdonor–LUMOacceptor energy difference (ECT) for the devices containing 2.5% porphyrin by weight. All three plots exhibit good linear correlations (R2 ≥ 0.96) suggesting that the device performance is a linear function of device VOC, which is a linear function of the ECT of the added porphyrin, which is, in turn, a linear function of the Hammett parameter, σpara, of the functional groups on the periphery of the porphyrin. Thus, the performance of these devices is dictated by the electronic structure of the porphyrin, despite it being at only a 2.5% concentration in the active layer blend.
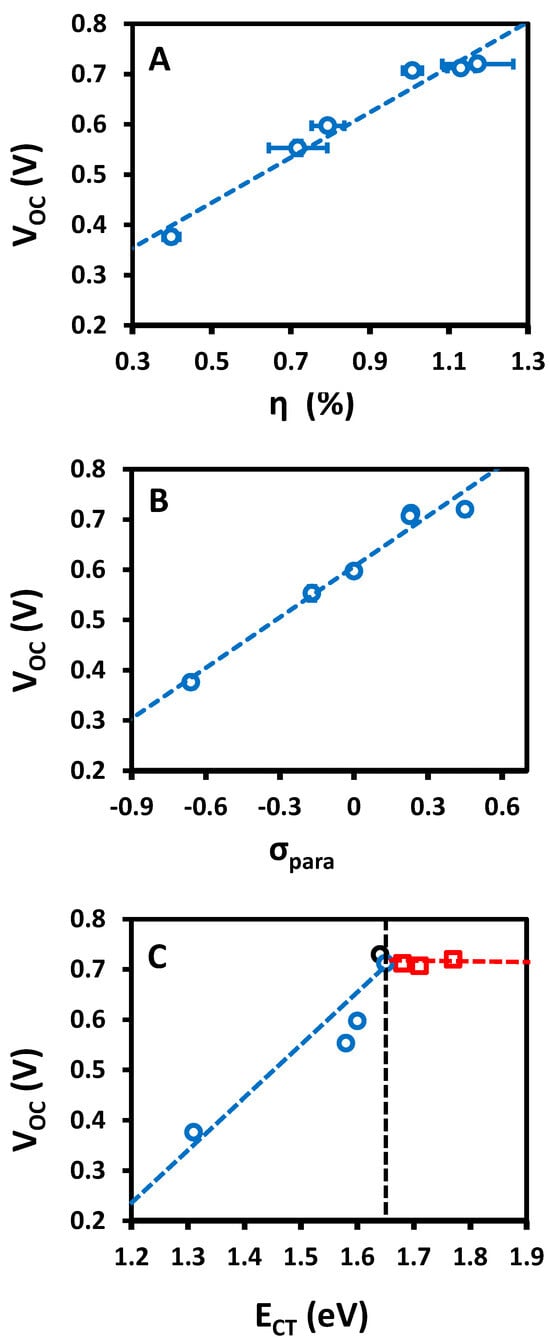
Figure 6.
Plots of (A) device efficiency, (B) Hammett σ-para constants and (C) porphyrin-PCBM ECT as a function of open-circuit voltage for MEH-PPV:porphyrin:PCBM 8:1:32 ternary blend OPV devices. The dashed lines in (A,B) are best fit lines. In (C), the vertical black dotted line indicates the ECT of MEH-PPV. Red open squares indicate the ECT if it is defined by the HOMOporphyrin–LUMOPCBM energy gap. The blue open circles indicate the ECT if it is defined by the smallest available HOMOdonor–LUMOacceptor energy gap. The blue line is a best linear fit to the blue dots. The open black circle in (C) represents data for a 5% tBuPP ternary blend device. The error bars in all three plots represent the standard deviation of the data as presented in Table 2.
Figure 6C shows that the VOC actually has a bilinear relationship with the porphyrin:PCBM charge transfer energy () where there is an inflexion point at the MEH-PPV:PCBM charge transfer energy (). In particular, we see that when , VOC is constant (red open squares). The blue open circles show the variation in VOC with ECT if the ECT is defined by the smallest available HOMOdonor–LUMOacceptor energy gap for all the data points. Now, the trend is highly linear with a slope of >0.97. Since the slope is approximately 1, the recombination does not change significantly in this series of porphyrin ternary devices. Furthermore, it is clear that the lowest ECT defines the VOC in these ternary blend devices.
Whilst we have shown that the devices remain functional upon the addition of porphyrin, with the device performance highly correlated to the HOMOdonor–LUMOacceptor gap for the porphyrin, device performance is reduced in all cases. We have previously observed that a similar reduction in device performance may arise because porphyrinoids can act as recombination sites in ternary blend devices [26,27]. However, for this series of porphyrins, the linear correlation with a slope of ~1 V/eV between ECT and VOC shows that there are no processes other than ECT changes in this series of ternary devices [57,58,59,60,61]. Therefore, recombination does not change in this series of devices, and the changes in device VOC are entirely explained by the shift in the ECT of the porphyrin. This result also indicates that for this ternary blend system, VOC is determined by the smallest HOMOdonor–LUMOacceptor gap available in the material system; this energy gap is a limiting parameter for device performance.
To further confirm that photogeneration processes in the active layer are not significantly affected by the addition of the porphyrins, the data in Figure 4E have been replotted in terms of photocurrent (Jph) and effective voltage (Veff). Jph is given by
where JL is the current density under illumination and JD the current density in the dark.
Veff is given by
where V0 is the voltage where Jph = 0 and V is the externally applied voltage.
Figure 7A shows that the Jph–Veff curves overlap irrespective of the HOMO of the porphyrin, which indicates that photogeneration processes in the photoactive layer are not significantly affected by the presence of the respective porphyrins [62]. The dashed lines in Figure 7 indicate the location of JSC and the magnitude of V0, which is approximately equal to the built-in voltage since the diffusion current can be neglected [62]. The relevant part of the voltage region (fourth quadrant in an I–V curve) is (V0 − VOC) < Veff < V0. For comparison, the J–V curves for devices from our previous work [27], in which the rate of charge recombination does change with changing porphyrinoid, has also been replotted in this manner (Figure 7B). For these devices, the Jph–Veff curves do vary with the HOMO of the porphyrin, confirming that for these porphyrinoids, photogeneration processes in the photoactive layer are affected by the presence of the porphyrinoids and, consequently, the rate of recombination is altered.
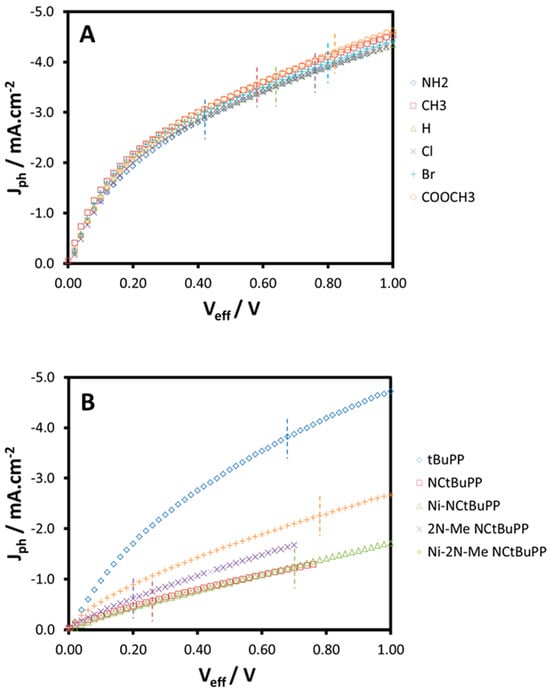
Figure 7.
Jph as a function of Veff for ternary devices consisting of (A) MEH-PPV:porphyrin:PCBM (8:1:32) and (B) the porphyrin and N-confused porphyrins presented in reference [27]. The dashed lines indicate the location of JSC. The relevant part of the voltage area (fourth quadrant in a J–V curve) is to the left of the respective dashed lines.
Based on Figure 7, we can rule out significant changes in the recombination processes of the ternary devices presented in this report as the source of the observed variations in device performance. The trends in VOC, JSC, FF and efficiency are almost entirely explained by the observed increase in V0. Consequently, we conclude that for these ternary devices, a lower porphyrin HOMO results in a higher built-in voltage, and it is this alteration of the device voltage that is dominating the device performance, despite the porphyrin being present at a level of only 2.5% by weight.
In general, the VOC is given by the quasi-Fermi levels of the hole, EF,p and electron, EF,n, populations [63]. EF,n is set by the LUMO of PCBM, while EF,p is determined by both the HOMO of MEH-PPV and the porphyrin. Hence, in a ternary system, two interfaces are of importance, in our case: MEH-PPV:PCBM and porphyrin:PCBM. Street et al. have shown that, while optical excitations are molecular in nature, the charge transfer (CT) state and Voc reflect the material averaged composition at the donor:acceptor interfaces [35]. Consequently, device Voc depends on the average MEH-PPV:porphyrinoid composition at the PCBM interface.
Honda et al. [64,65,66] have previously shown that, after annealing, porphyrinoids (silicon phthalocyanine) selectively locate at the donor:acceptor interface in ternary blend devices, as opposed to forming pure material domains, due to favourable surface energy conditions. As such, a small amount of porphyrin results in a disproportionality large interfacial coverage with only 3.4 wt % of the porphyrinoid, resulting in a 40% coverage of the donor:acceptor interface. Furthermore, the porphyrinoid:PCBM interface coverage does not change significantly with porphyrinoid loading and was found to saturate after ~5 wt% [66].
To establish the location of the porphyrin materials in our MEH-PPV:porphyrin:PCBM blends, contact angles were measured for water on pristine films of the component materials, and these were used to calculate the surface tension and wetting coefficient of the materials. The surface tension of the organic semiconductors was calculated using Neuman’s equation:
where ϴ is the contact angle, γX is the interfacial tension between the solid film and water, γlv the interfacial tension between water and air (72.86 mJm−2) and β is a constant (0.0001247) [67]. In order to determine where the porphyrin is located in the MEH-PPV:porphyrin:PCBM systems, the wetting coefficient, ωporph, was calculated using
Here, γX:Y is the interfacial tension between X and Y, which is calculated using
The results of these calculations are presented for each component material in Table 3.

Table 3.
Water/film contact angles, surface tension values and calculated wetting coefficients for each of the components in the ternary blend films.
For ωporph > 1, the porphyrin is predicted to be located in the MEH-PPV phase, whereas, if ωporph < −1, the porphyrin is predicted to be located in the PCBM phase. For values of ωporph between these limits, the porphyrin will be located at the MEH-PPV:PCBM interface. Since the wetting coefficient for all the porphyrins lie between 1 and −1, the porphyrin is located at the MEH-PPV:PCBM interface in all of the studied ternary blends.
To confirm the location of the porphyrin component at the polymer:fullerene interface in MEH-PPV:porphyrin:PCBM ternary devices, we varied the porphyrin loading for a series of devices containing an active layer of MEH-PPV, PCBM and 5,10,15,20-tetra(3,5-di-t-butylphenyl)porphyrin (tBuPP), a porphyrin with a HOMO energy of 5.48 eV, which is similar to that of MEH-PPV (5.49 eV). Ternary blend devices containing tBuPP demonstrate very similar electronic behaviour (Figure 6C) and recombination characteristics (Figure 7B) to those of the para-substituted porphyrin series. Furthermore, tBuPP has a wetting coefficient of 0.45 (Table 3), indicating that, like the para-substituted porphyrins, this porphyrin locates preferentially at the polymer:fullerene interface in ternary blend devices. Figure 8 shows the variation in VOC in MEH-PPV:tBuPP:PCBM devices as a function of porphyrin loading and reveals that the VOC rapidly drops to a constant value at ~5 wt % loading of porphyrin, following an identical trend to the interface coverage determined by Honda et al. [66]. This result indicates that these porphyrins selectively locate at the MEH-PPV:PCBM interface in MEH-PPV:porphyrin:PCBM ternary devices, leading to significant interface coverage, despite their relatively low total concentration.
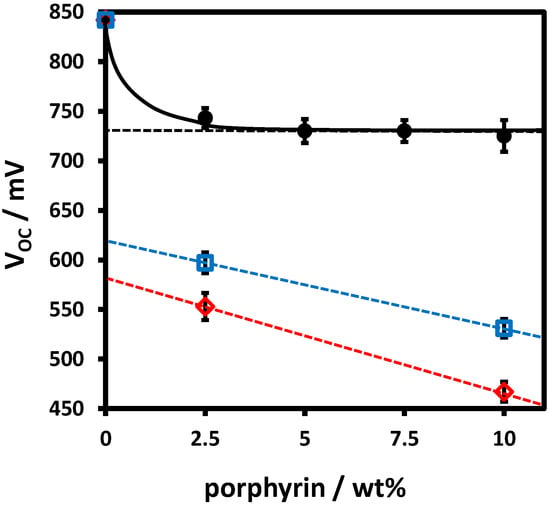
Figure 8.
VOC of MEH-PPV: tBuPP:PCBM (black filled circles), MEH-PPV:TPP:PCBM (blue open squares) and MEH-PPV:TTP:PCBM (red open diamonds) ternary devices as a function of porphyrin content. The solid and dashed trend lines have been added to guide the eye.
To establish how the ternary device mechanism varies with porphyrin HOMO level, ternary devices were also prepared and characterised with 10% by weight tetra-p-tolylporphyrin (TTP) and tetraphenylporphyrin (TPP), where both porphyrin HOMOs lie below that of MEH-PPV. The effect of changing the TTP/TPP porphyrin loading upon device VOC is also shown in Figure 8. Whereas VOC is invariant with porphyrin loading for tBuPP devices (), indicating a cascade mechanism [12], the VOC of the MEH-PPV:TPP:PCBM and MEH-PPV:TTP:PCBM ternary blends ( < ) systematically decreases with increasing porphyrin concentration. This variation aligns with either the alloy or parallel device models, whereby the two donors form an electronic alloy (with an intimately mixed morphology) or act as separate sub-cells (as physically separated domains), respectively [12]. However, the alloy model is more likely, given that the porphyrin appears to locate preferentially at the polymer:fullerene interface. Moreover, the VOC behaviour is consistent with the fact that the HOMO level for TPP and TTP lie above that of MEH-PPV, and, thus, a binary alloy of the two donors would create a pseudo-binary cell whose VOC would reduce systematically with increasing porphyrin content. Thus, the results in Figure 8 show that, for varying TPP/TTP porphyrin loadings, device VOC is determined by the relative MEH-PPV:PCBM and porphyrinoid:PCBM weighted average, consistent with the findings of Street et al. [35]. These results explain the poor relative performance of the porphyrin ternary blend devices and highlight the need to further understand the role of energetics and interface morphology in ternary blend devices.
Figure 9 summarises these results in the form of energy level diagrams for the two extreme cases studied here, the addition of (a) TpNH2PP and (b) TpCO2CH3PP. Electron transfer from the primary donor (MEH-PPV) to the acceptor (PCBM) cannot proceed via a cascade mechanism through the interfacial porphyrin in either case due to the energy mismatch of the porphyrin LUMO levels. By contrast, hole transfer can proceed via a cascade mechanism through the interfacial porphyrin when , whereas when , hole transfer from the porphyrin to MEH-PPV is energetically unfavourable. In this case, either a parallel or alloy model hole transport mechanism occurs.
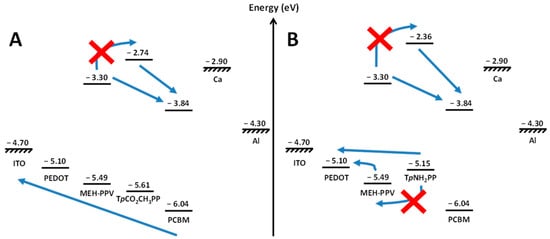
Figure 9.
Energy level diagrams for (A) TpCO2CH3PP and (B) TpNH2PP ternary OPV devices highlighting HOMO and LUMO energy level mismatches. Red crosses highlight hole/electron transfer processes that cannot proceed due to relative energy level mismatches. Blue arrows show hole/electron transfer processes that can proceed due to correct relative energy level alignments.
As such, two distinct mechanistic models operate in this series of devices. When the porphyrin HOMO is lower than that of the MEH-PPV, the device behaviour is explained by the cascade model and, consequently, ECT and VOC are defined by the MEH-PPV HOMO level. However, when the porphyrin HOMO is higher than that of the MEH-PPV, the ternary blend behaviour is explained by the parallel/alloy device model. In this case, the VOC is a function of the HOMO levels of both the porphyrin and polymer as well as their relative interfacial coverage with the acceptor material. Furthermore, the interfacial porphyrin coverage must be incomplete since the device EQEs show that MEH-PPV is the dominant contributor to current in all these devices (Figure 5B). In addition, for TpNH2PP (as well as TTP and TPP), mismatched HOMO levels mean that holes residing on the porphyrin cannot be transferred and transported directly by the primary MEH-PPV hole transport network. Given that there is a distinct porphyrin contribution to the charge output observed for these devices, we can only conclude that a secondary porphyrin/PCBM-based hole transport network (whether through a binary alloy or parallel sub cells) must exist in the devices.
4. Conclusions
Developing inexpensive active layer materials for use in OPV is a critical step in realising the commercial potential of these devices. One attractive method to achieve this goal is enhancing the efficiency of existing low-cost but relatively low-performance materials via the addition of a ternary donor material to extend the spectral response of the device. However, the effect that the addition of such materials upon device mechanism and active layer morphology is still unclear. In this study, we have systematically altered the electronic nature of a porphyrin additive to MEH-PPV:PCBM devices to probe the mechanistic and morphological effects of the addition of these materials. By varying the electron-donating or electron-withdrawing effect of functional groups in the para position of the peripheral phenyl groups in tetraphenylporphyins (−0.66 < σpara < 0.232), we have shown that we can systematically vary the HOMO (−5.61 < EHOMO < −5.15) and LUMO (−2.74 < ELUMO < −2.36) energy levels of these porphyrins. We find that these active layers generate charge with a VOC determined by the lowest donor–acceptor ECT in the blend whether the donor is the majority or minority chromophore. Thus, we can switch between cascade and parallel/alloy charge generation models simply by altering the electron-withdrawing character of the porphyrin ternary additive. Moreover, this control is enabled by the uniform nanomorphology that is exhibited by all MEH-PPV:porphyrin:PCBM blend layers wherein the porphyrin consistently forms a nanoscale interfacial layer located at the MEH-PPV:PCBM interface in all the studied ternary blends, thereby governing electronic behaviour despite being present only in trace concentrations. By demonstrating how control of both charge generation and morphology can be achieved in ternary blend OPV, this work highlights a pathway for the development of next-generation devices.
Author Contributions
Conceptualization, N.A.C., W.J.B. and P.C.D.; methodology, N.A.C.; formal analysis, N.A.C., T.W.J. and K.F.; investigation, N.A.C.; resources, P.C.D.; writing—original draft preparation, N.A.C., W.J.B. and P.C.D.; writing—review and editing, W.J.B. and P.C.D.; supervision, W.J.B. and P.C.D.; project administration, P.C.D.; funding acquisition, P.C.D. All authors have read and agreed to the published version of the manuscript.
Funding
We acknowledge travel funding provided by the International Synchrotron Access Program (ISAP) managed by the Australian Synchrotron, part of the Australian Nuclear Science and Technology Organisation (ANSTO), and funded by the Australian Government (grant number AS_IA101_ALS_03744).
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Acknowledgments
Grateful acknowledgment is extended to the University of Newcastle for providing a PhD scholarship (NC). This research was partially conducted at the Materials node of the Australian National Fabrication Facility, an organization founded under the National Collaborative Research Infrastructure Strategy to support Australia’s researchers with nano- and microfabrication facilities.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Zhang, G.; Lin, F.R.; Qi, F.; Heumüller, T.; Distler, A.; Egelhaaf, H.-J.; Li, N.; Chow, P.C.Y.; Brabec, C.J.; Jen, A.K.-Y.; et al. Renewed Prospects for Organic Photovoltaics. Chem. Rev. 2022, 122, 14180–14274. [Google Scholar] [CrossRef]
- Gao, W.; Qi, F.; Peng, Z.; Lin, F.R.; Jiang, K.; Zhong, C.; Kaminsky, W.; Guan, Z.; Lee, C.-S.; Marks, T.J.; et al. Achieving 19% Power Conversion Efficiency in Planar-Mixed Heterojunction Organic Solar Cells Using a Pseudosymmetric Electron Acceptor. Adv. Mater. 2022, 34, 2202089. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Wang, L.; Guo, C.; Xiao, J.; Liu, C.; Chen, C.; Xia, W.; Gan, Z.; Cheng, J.; Zhou, J.; et al. π-Extended Nonfullerene Acceptor for Compressed Molecular Packing in Organic Solar Cells to Achieve Over 20% Efficiency. J. Am. Chem. Soc. 2024, 146, 12011–12019. [Google Scholar] [CrossRef]
- Zheng, Z.; Wang, J.; Bi, P.; Ren, J.; Wang, Y.; Yang, Y.; Liu, X.; Zhang, S.; Hou, J. Tandem Organic Solar Cell with 20.2% Efficiency. Joule 2022, 6, 171–184. [Google Scholar] [CrossRef]
- Dastoor, P.C.; Belcher, W.J. How the West Was Won? A History of Organic Photovoltaics. Substantia 2019, 3 (Suppl. 1), 99–110. [Google Scholar]
- Al-Ahmad, A.Y.; Almayhi, F.; Al-Mudhaffer, M.F.; Griffith, M.J.; Liu, W.; Li, S.; Sivunova, K.; Elkington, D.; Cooling, N.A.; Feron, K.; et al. A Nuanced Approach for Assessing OPV Materials for Large Scale Applications. Sustain. Energy Fuels 2020, 4, 940. [Google Scholar] [CrossRef]
- Sun, C.; Pan, F.; Bin, H.; Zhang, J.; Xue, L.; Qiu, B.; Wei, Z.; Zhang, Z.-G.; Li, Y. A Low Cost and High Performance Polymer Donor Material for Polymer Solar Cells. Nat. Commun. 2018, 9, 743. [Google Scholar] [CrossRef]
- Gasparini, N.; Salleo, A.; McCulloch, I.; Baran, D. The Role of the Third Component in Ternary Organic Solar Cells. Nat. Rev. Mater. 2019, 4, 229–242. [Google Scholar] [CrossRef]
- Cui, Y.; Yao, H.; Zhang, J.; Xian, K.; Zhang, T.; Hong, L.; Wang, Y.; Xu, Y.; Ma, K.; An, C.; et al. Single-Junction Organic Photovoltaic Cells with Approaching 18% Efficiency. Adv. Mater. 2020, 32, 1908205. [Google Scholar] [CrossRef]
- Jiang, Y.; Sun, S.; Xu, R.; Liu, F.; Miao, X.; Ran, G.; Liu, K.; Yi, Y.; Zhang, W.; Zhu, X. Non-Fullerene Acceptor with Asymmetric Structure and Phenyl-Substituted Alkyl Side Chain for 20.2% Efficiency Organic Solar Cells. Nat. Energy 2024, 9, 975–986. [Google Scholar] [CrossRef]
- Doumon, N.Y.; Yang, L.; Rosei, F. Ternary Organic Solar Cells: A Review of the Role of the Third Element. Nano Energy 2022, 94, 106915. [Google Scholar] [CrossRef]
- Günther, M.; Kazerouni, N.; Blätte, D.; Perea, J.D.; Thompson, B.C.; Ameri, T. Models and Mechanisms of Ternary Organic Solar Cells. Nat. Rev. Mater. 2023, 8, 456–471. [Google Scholar] [CrossRef]
- Wang, Y.; Yu, J.; Zhang, R.; Yuan, J.; Hultmark, S.; Johnson, C.E.; Gallop, N.P.; Siegmund, B.; Qian, D.; Zhang, H.; et al. Origins of the Open-Circuit Voltage in Ternary Organic Solar Cells and Design Rules for Minimized Voltage Losses. Nat. Energy 2023, 8, 978–988. [Google Scholar] [CrossRef]
- Park, J.M.; Hong, K.-I.; Lee, H.; Jang, W.-D. Bioinspired Applications of Porphyrin Derivatives. Acc. Chem. Res. 2021, 54, 2249–2260. [Google Scholar] [CrossRef]
- Xiao, L.; Lai, T.; Liu, X.; Liu, F.; Russell, T.P.; Liu, Y.; Huang, F.; Peng, X.; Cao, Y. A Low-Bandgap Dimeric Porphyrin Molecule for 10% Efficiency Solar Cells with Small Photon Energy Loss. J. Mater. Chem. 2018, 6, 18469–18478. [Google Scholar] [CrossRef]
- Lyons, D.M.; Kesters, J.; Maes, W.; Bielawski, C.W.; Sessler, J.L. Improving Efficiencies by Modulating the Central Metal Ion in Porphyrin-Oligothiophene-Mediated P3HT/PCBM Organic Solar Cells. Synth. Met. 2013, 178, 56–61. [Google Scholar] [CrossRef]
- Rand, B.P.; Girotto, C.; Mityashin, A.; Hadipour, A.; Genoe, J.; Heremans, P. Photocurrent Enhancement in Polymer:Fullerene Bulk Heterojunction Solar Cells Doped with a Phosphorescent Molecule. Appl. Phys. Lett. 2009, 95, 173304. [Google Scholar] [CrossRef]
- Dastoor, P.C.; McNeill, C.R.; Frohne, H.; Foster, C.J.; Dean, B.; Belcher, W.J.; Fell, C.J.; Campbell, W.M.; Officer, D.L.; Blake, I.M.; et al. Understanding and Improving Solid-State Polymer/C60-Fullerene Bulk-Heterojunction Solar Cells Using Ternary Porphyrin Blends. J. Phys. Chem. C 2007, 111, 15415–15426. [Google Scholar] [CrossRef]
- Belcher, W.J.; Wagner, K.I.; Dastoor, P.C. The Effect of Porphyrin Inclusion on the Spectral Response of Ternary P3HT:Porphyrin:PCBM Bulk Heterojunction Solar Cells. Sol. Energy Mater. Sol. Cells 2007, 91, 447–452. [Google Scholar] [CrossRef]
- Park, J.M.; Lee, J.H.; Jang, W.-D. Applications of Porphyrins in Emerging Energy Conversion Technologies. Coord. Chem. Rev. 2020, 407, 213157. [Google Scholar] [CrossRef]
- Piradi, V.; Xu, W.; Wang, Z.; Ali, J.; Peng, Q.; Liu, F.; Zhu, X. Panchromatic Ternary Organic Solar Cells with Porphyrin Dimers and Absorption-Complementary Benzodithiophene-Based Small Molecules. ACS Appl. Mater. Interfaces 2019, 11, 6283–6291. [Google Scholar] [CrossRef] [PubMed]
- Dahiya, H.; Cuesta, V.; de la Cruz, P.; Langa, F.; Sharma, G.D. Highly Efficient (15.08%) All-Small-Molecule Ternary Solar Cells Constructed with a Porphyrin as a Donor and Two Acceptors. ACS Appl. Energy Mater. 2021, 4, 4498–4506. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, G. Functional Third Components in Nonfullerene Acceptor-Based Ternary Organic Solar Cells. Acc. Mater. Res. 2020, 1, 158–171. [Google Scholar] [CrossRef]
- Gao, K.; Kan, Y.; Chen, X.; Liu, F.; Kan, B.; Nian, L.; Wan, X.; Chen, Y.; Peng, X.; Russell, T.P.; et al. Low-Bandgap Porphyrins for Highly Efficient Organic Solar Cells: Materials, Morphology, and Applications. Adv. Mater. 2020, 32, 1906129. [Google Scholar] [CrossRef]
- Xu, X.; Li, Y.; Peng, Q. Ternary Blend Organic Solar Cells: Understanding the Morphology from Recent Progress. Adv. Mater. 2022, 34, 2107476. [Google Scholar] [CrossRef]
- Cooling, N.; Burke, K.B.; Zhou, X.; Lind, S.J.; Gordon, K.C.; Jones, T.W.; Dastoor, P.C.; Belcher, W.J. A Study of the Factors Influencing the Performance of Ternary MEH-PPV:Porphyrin:PCBM Heterojunction Devices: A Steric Approach to Controlling Charge Recombination. Sol. Energy Mater. Sol. Cells 2011, 95, 1767–1774. [Google Scholar] [CrossRef]
- Cooling, N.A.; Zhou, X.; Sales, T.A.; Sauer, S.E.; Lind, S.J.; Gordon, K.C.; Jones, T.W.; Burke, K.B.; Dastoor, P.C.; Belcher, W.J. A Study of the Factors Influencing the Performance of Ternary MEH-PPV:Porphyrin:PCBM Heterojunction Devices: Electronic Effects in Porphyrinoid Ternary Blend Bulk Heterojunction Photovoltaic Devices. Sol. Energy Mater. Sol. Cells 2012, 98, 308–316. [Google Scholar] [CrossRef]
- Mohapatra, A.A.; Tiwari, V.; Patil, S. Energy Transfer in Ternary Blend Organic Solar Cells: Recent Insights and Future Directions. Energy Environ. Sci. 2021, 14, 302–319. [Google Scholar] [CrossRef]
- Li, S.; Zhan, L.; Li, Y.; He, C.; Zuo, L.; Shi, M.; Chen, H. Achieving and Understanding of Highly Efficient Ternary Organic Photovoltaics: From Morphology and Energy Loss to Working Mechanism. Small Methods 2022, 6, 2200828. [Google Scholar] [CrossRef]
- Lui, L.; Kelly, M.A.; You, W.; Yu, L. Status and Prospects for Ternary Organic Photovoltaics. Nat. Photonics 2015, 9, 491–500. [Google Scholar] [CrossRef]
- Ameri, T.; Min, J.; Li, N.; Machui, F.; Baran, D.; Forster, M.; Schottler, K.J.; Dolfen, D.; Scherf, U.; Brabec, C.J. Performance Enhancement of the P3HT/PCBM Solar Cells through NIR Sensitization Using a Small-Bandgap Polymer. Adv. Energy Mater. 2012, 2, 1198–1202. [Google Scholar] [CrossRef]
- Lu, L.; Xu, T.; Chen, W.; Landry, E.S.; Yu, L. Ternary Blend Polymer Solar Cells with Enhanced Power Conversion Efficiency. Nat. Photonics 2014, 8, 716–722. [Google Scholar] [CrossRef]
- Yang, L.; Zhou, H.; Price, S.C.; You, W. Parallel-Like Bulk Heterojunction Polymer Solar Cells. J. Am. Chem. Soc. 2012, 134, 5432–5435. [Google Scholar] [CrossRef] [PubMed]
- Duck, B.; Vaughan, B.; Cooling, N.; Zhou, X.; Holdsworth, J.L.; Wen, L.L.; Rasmussen, S.C.; Dastoor, P.C.; Belcher, W.J. An Equivalent Circuit Model for Ternary Blend P3HT:pC6TP:PCBM Low Band Gap Devices. Sol. Energy Mater. Sol. Cells 2013, 114, 65–70. [Google Scholar] [CrossRef]
- Street, R.A.; Davies, D.; Khlyabich, P.P.; Burkhart, B.; Thompson, B.C. Origin of the Tuneable Open-Circuit Voltage in Ternary Blend Bulk Heterojunction Organic Solar Cells. J. Am. Chem. Soc. 2013, 135, 986–989. [Google Scholar] [CrossRef]
- Khlyabich, P.P.; Burkhart, B.; Thompson, B.C. Compositional Dependence of the Open-Circuit Voltage in Ternary Blend Bulk Heterojunction Solar Cells Based on Two Donor Polymers. J. Am. Chem. Soc. 2012, 134, 9074–9077. [Google Scholar] [CrossRef]
- Huang, J.-S.; Goh, T.; Li, X.; Sfeir, M.Y.; Bielnski, E.A.; Tomasulo, S.; Lee, M.L.; Hazari, N.; Taylor, A.D. Polymer Bulk Heterojunction Solar Cells Employing Förster Resonance Energy Transfer. Nat. Photonics 2013, 7, 479–485. [Google Scholar] [CrossRef]
- Feron, K.; Belcher, W.J.; Fell, C.J.; Dastoor, P.C. Organic Solar Cells: Understanding the Role of Förster Resonance Energy Transfer. Int. J. Mol. Sci. 2012, 13, 17019–17047. [Google Scholar] [CrossRef]
- Suzuki, M.; Suzuki, K.; Won, T.; Yamada, H. Impact of Substituents on the Performance of Small-Molecule Semiconductors in Organic Photovoltaic Devices via Regulating Morphology. J. Mater. Chem. C 2022, 10, 1162–1195. [Google Scholar] [CrossRef]
- Kilcoyne, A.L.D.; Tyliszczak, T.; Steele, W.F.; Fakra, S.; Hitchcock, P.; Franck, K.; Anderson, E.; Harteneck, B.; Rightor, E.G.; Mitchell, G.E.; et al. Interferometer-Controlled Scanning Transmission X-Ray Microscopes at the Advanced Light Source. J. Synchrotron Radiat. 2003, 10, 125–136. [Google Scholar] [CrossRef]
- Paliteiro, C.; Sobral, A. Electrochemical and Spectroelectrochemical Characterization of Meso-Tetra-Alkyl Porphyrins. Electrochim. Acta 2005, 50, 2445–2451. [Google Scholar] [CrossRef][Green Version]
- Pavlishchuk, V.V.; Addison, A.W. Conversion Constants for Redox Potentials Measured versus Different Reference Electrodes in Acetonitrile Solutions at 25 °C. Inorg. Chim. Acta 2000, 298, 97–102. [Google Scholar] [CrossRef]
- Bond, A.M.; Oldham, K.B.; Snook, G.A. Use of the Ferrocene Oxidation Process to Provide Both Reference Electrode Potential Calibration and a Simple Measurement (via Semiintegration) of the Uncompensated Resistance in Cyclic Voltammetric Studies in High-Resistance Organic Solvents. Anal. Chem. 2000, 72, 3492–3496. [Google Scholar] [CrossRef]
- Jasieniak, J.; Califano, M.; Watkins, S.E. Size-Dependent Valence and Conduction Band-Edge Energies of Semiconductor Nanocrystals. ACS Nano 2011, 5, 5888–5902. [Google Scholar] [CrossRef]
- Costa, J.C.; Taveira, R.J.; Lima, C.F.; Mendes, A.; Santos, L.M. Optical Band Gaps of Organic Semiconductor Materials. Opt. Mater. 2016, 58, 51–60. [Google Scholar] [CrossRef]
- Cooling, N. Ternary Porphyrinoid:Polymer:Fullerene Bulk Heterojunction Organic Solar Cells. Ph.D. Thesis, University of Newcastle, Newcastle, NSW, Australia, 2013. [Google Scholar]
- Acevedo-Peña, P.; Baray-Calderón, A.; Hu, H.; González, I.; Ugalde-Saldivar, V.M. Measurements of HOMO-LUMO Levels of Poly(3-Hexylthiophene) Thin Films by a Simple Electrochemical Method. J. Solid State Electrochem. 2017, 21, 2407–2414. [Google Scholar] [CrossRef]
- Balke, V.L.; Walker, F.A.; West, J.T. Electronic Effects in Transition-Metal Porphyrins. 6. The Effect of Unsymmetrical Phenyl Substitution on the Formation Constants of a Series of (Tetraphenylporphinato)Iron(III)-Bis(N-Methylimidazole) Complexes. J. Am. Chem. Soc. 1985, 107, 1226–1233. [Google Scholar] [CrossRef]
- Meot-Ner, M.; Adler, A.D. Substituent Effects in Noncoplanar π Systems, Meso-Porphyrins. J. Am. Chem. Soc. 1975, 97, 5107–5111. [Google Scholar] [CrossRef]
- Hammett, L.P. The Effect of Structure upon the Reactions of Organic Compounds. Benzene Derivatives. J. Am. Chem. Soc. 1937, 59, 96–103. [Google Scholar] [CrossRef]
- Hansch, C.; Leo, A.; Taft, R.W. A Survey of Hammett Substituent Constants and Resonance and Field Parameters. Chem. Rev. 1991, 91, 165–195. [Google Scholar] [CrossRef]
- Ransdell, R.A.; Wamser, C.C. Solvent and Substituent Effects on the Redox Properties of Free-Base Tetraphenylporphyrins in DMSO and Aqueous DMSO. J. Phys. Chem. 1992, 96, 10572–10575. [Google Scholar] [CrossRef]
- Nicolaidis, N.; Vaughan, B.; Mulligan, C.J.; Bryant, G.; Zillger, T.; Tmovec, B.; Hübler, A.C.; Holmes, N.; Cooling, N.A.; Griffith, M.; et al. Solution Processable Interface Materials for Nanoparticulate Organic Photovoltaic Devices. Appl. Phys. Lett. 2014, 104, 043902. [Google Scholar] [CrossRef]
- Tarikhum, B.H.; Ali, B.; Almyahi, F. Role of Fullerene ICxA and Non-Fullerene Y6 in P3HT-Based Ternary Organic Photovoltaics. Solid State Commun. 2023, 372, 115319. [Google Scholar] [CrossRef]
- Srivastava, S.B.; Srivastava, S.K.; Singh, S.P. Molecular-Shape-Induced Efficiency Enhancement in PC61BM and PC71BM Based Ternary Blend Organic Solar Cells. J. Phys. Chem. C 2017, 121, 17104–17111. [Google Scholar] [CrossRef]
- Farinhas, J.; Oliveira, R.; Hansson, R.; Ericsson, L.K.; Moons, E.; Morgado, J.; Charas, A. Efficient Ternary Organic Solar Cells Based on Immiscible Blends. Org. Electron. 2017, 41, 130–136. [Google Scholar] [CrossRef]
- Brabec, C.J.; Cravino, A.; Meissner, D.; Sariciftci, N.S.; Fromherz, T.; Rispens, M.T.; Sanchez, L.; Hummelen, J.C. Origin of the Open Circuit Voltage of Plastic Solar Cells. Adv. Funct. Mater. 2001, 11, 374–380. [Google Scholar] [CrossRef]
- Widmer, J.; Tietze, M.; Leo, K.; Riede, M. Open-Circuit Voltage and Effective Gap of Organic Solar Cells. Adv. Funct. Mater. 2013, 23, 5814–5821. [Google Scholar] [CrossRef]
- Graham, K.R.; Erwin, P.; Nordlund, D.; Vandewal, K.; Li, R.; Ngongang Ndjawa, G.O.; Hoke, E.T.; Salleo, A.; Thompson, M.E.; McGehee, M.D.; et al. Re-Evaluating the Role of Sterics and Electronic Coupling in Determining the Open-Circuit Voltage of Organic Solar Cells. Adv. Mater. 2013, 25, 6076–6082. [Google Scholar] [CrossRef]
- Rand, B.P.; Burk, D.P. Offset Energies at Organic Semiconductor Heterojunctions and Their Influence on the Open-Circuit Voltage of Thin-Film Solar Cells. Phys. Rev. B 2007, 75, 115327. [Google Scholar] [CrossRef]
- Scharber, M.C.; Mühlbacher, D.; Koppe, M.; Denk, P.; Waldauf, C.; Heeger, A.J.; Brabec, C.J. Design Rules for Donors in Bulk-Heterojunction Solar Cells–Towards 10% Energy-Conversion Efficiency. Adv. Mater. 2006, 18, 789–794. [Google Scholar] [CrossRef]
- Blom, P.W.M.; Mihailetchi, V.D.; Koster, L.J.A.; Markov, D.E. Device Physics of Polymer: Fullerene Bulk Heterojunction Solar Cells. Adv. Mater. 2007, 19, 1551–1566. [Google Scholar] [CrossRef]
- Gregg, B.A.; Hanna, M.C. Comparing Organic to Inorganic Photovoltaic Cells: Theory, Experiment, and Simulation. J. Appl. Phys. 2003, 93, 3605–3614. [Google Scholar] [CrossRef]
- Honda, S.; Nogami, T.; Ohkita, H.; Benten, H.; Ito, S. Improvement of the Light-Harvesting Efficiency in Polymer.Fullerene Bulk Heterojunction Solar Cells by Interfacial Dye Modification. ACS Appl. Mater. Interfaces 2009, 1, 804–810. [Google Scholar] [CrossRef] [PubMed]
- Honda, S.; Yokoya, S.; Ohkita, H.; Benten, H.; Ito, S. Light-Harvesting Mechanism in Polymer/Fullerene/Dye Ternary Blends Studied by Transient Absorption Spectroscopy. J. Phys. Chem. C 2011, 115, 11306–11317. [Google Scholar] [CrossRef]
- Honda, S.; Ohkita, H.; Benten, H.; Ito, S. Selective Dye Loading at the Heterojunction in Polymer/Fullerene Solar Cells. Adv. Energy Mater. 2011, 1, 588–598. [Google Scholar] [CrossRef]
- Li, D.; Neumann, A.W. A Reformulation of the Equation of State for Interfacial Tensions. J. Colloid Interface Sci. 1990, 137, 304–307. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).