A Review of Wearable Electroceutical Devices for Chronic Wound Healing
Abstract
1. Introduction
1.1. Types of Chronic Wounds
1.2. Electroceutical Therapy and Mechanisms of Action
1.3. Role of Wearable Technologies for Chronic Wound Healing
1.4. Research-Grade Devices
1.5. Standard of Care and Gaps
1.6. Review Objective
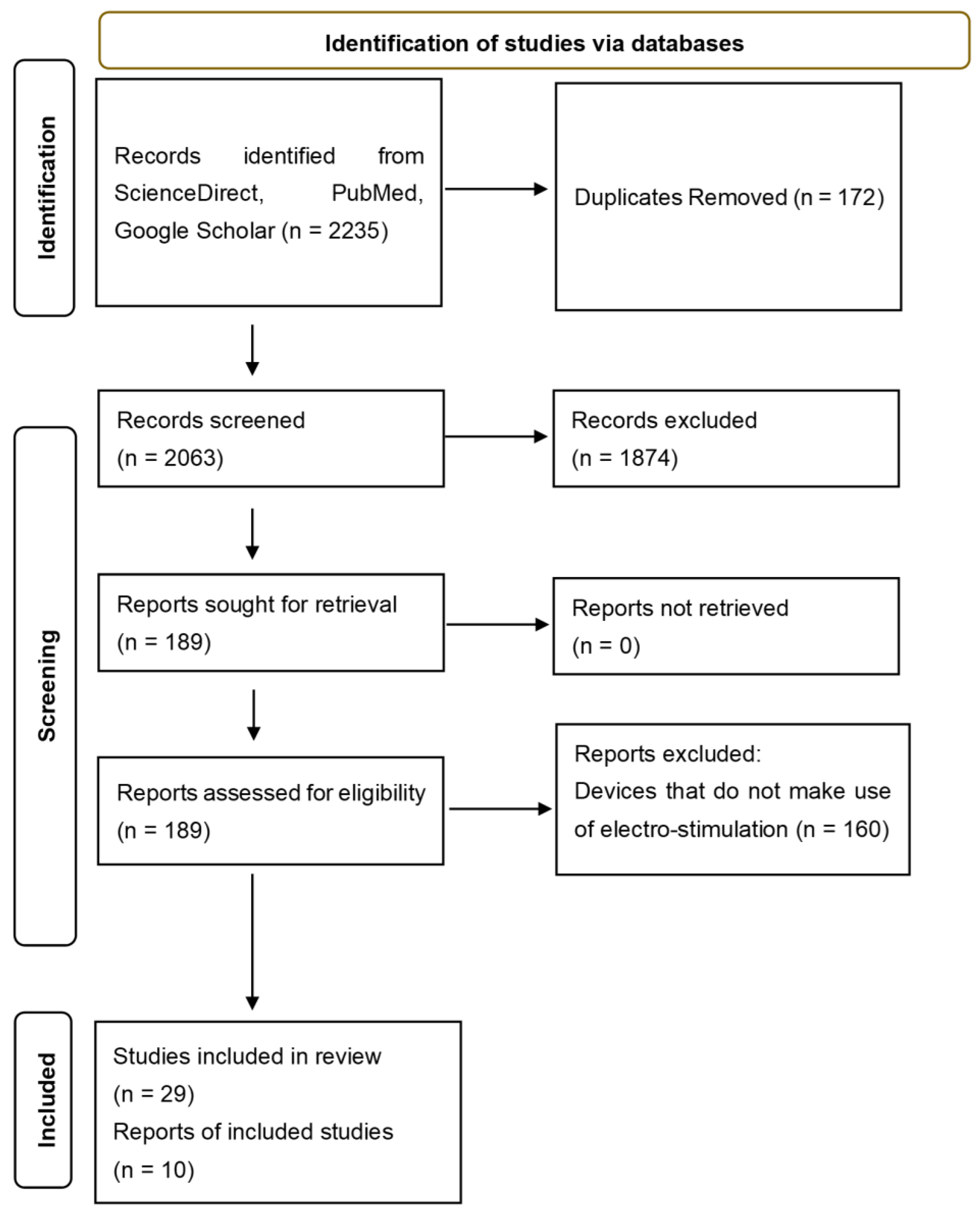
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions and Future Directions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gonzalez, A.C.D.O.; Costa, T.F.; de Araújo Andrade, Z.; Medrado, A.R.A.P. Wound Healing—A Literature Review. An. Bras. Dermatol. 2016, 91, 614–620. [Google Scholar] [CrossRef] [PubMed]
- Sanshita, H.; Chopra, H.; Singh, I.; Bin Emran, T. Wearable technology based smart dressing for effective wound monitoring– correspondence. Int. J. Surg. Open 2023, 55, 100628. [Google Scholar] [CrossRef]
- Garraud, O.; Hozzein, W.N.; Badr, G. Wound healing: Time to look for intelligent, ‘natural’ immunological approaches? BMC Immunol. 2017, 18, 23. [Google Scholar] [CrossRef]
- Kolimi, P.; Narala, S.; Nyavanandi, D.; Youssef, A.A.A.; Dudhipala, N. Innovative Treatment Strategies to Accelerate Wound Healing: Trajectory and Recent Advancements. Cells 2022, 11, 2439. [Google Scholar] [CrossRef] [PubMed]
- Järbrink, K.; Ni, G.; Sönnergren, H.; Schmidtchen, A.; Pang, C.; Bajpai, R.; Car, J. The humanistic and economic burden of chronic wounds: A protocol for a systematic review. Syst. Rev. 2017, 6, 15. [Google Scholar] [CrossRef]
- Rodrigues, M.; Kosaric, N.; Bonham, C.A.; Gurtner, G.C. Wound Healing: A Cellular Perspective. Physiol. Rev. 2019, 99, 665–706. [Google Scholar] [CrossRef]
- Nussbaum, S.R.; Carter, M.J.; Fife, C.E.; DaVanzo, J.; Haught, R.; Nusgart, M.; Cartwright, D. An Economic Evaluation of the Impact, Cost, and Medicare Policy Implications of Chronic Nonhealing Wounds. Value Health 2018, 21, 27–32. [Google Scholar] [CrossRef]
- Shiff, J.; Schwartz, K.; Hausman, B.; Seshadri, D.R.; Bogie, K.M. Development and use of a porcine model with clinically relevant chronic infected wounds. J. Tissue Viability 2023, 32, 527–535. [Google Scholar] [CrossRef]
- Yaşayan, G.; Nejati, O.; Ceylan, A.F.; Karasu, Ç.; Ugur, P.K.; Bal-Öztürk, A.; Zarepour, A.; Zarrabi, A.; Mostafavi, E. Tackling chronic wound healing using nanomaterials: Advancements, challenges, and future perspectives. Appl. Mater. Today 2023, 32, 101829. [Google Scholar] [CrossRef]
- Rijal, N.P.; Bath, J.M.; Kogan, A.B.; Narmoneva, D.A. 8—Electric field and wound healing. In Principles and Technologies for Electromagnetic Energy Based Therapies; Elsevier Inc: Amsterdam, The Netherlands, 2022; pp. 255–280. [Google Scholar]
- Sharma, A.; Shankar, R.; Yadav, A.K.; Pratap, A.; Ansari, M.A.; Srivastava, V. Burden of Chronic Nonhealing Wounds: An Overview of the Worldwide Humanistic and Economic Burden to the Healthcare System. Int. J. Low. Extremity Wounds 2024, 15347346241246339. [Google Scholar] [CrossRef]
- Houghton, P.E. Electrical stimulation therapy to promote healing of chronic wounds: A review of reviews. Chronic Wound Care Manag. Res. 2017, 4, 25–44. [Google Scholar] [CrossRef]
- Raziyeva, K.; Kim, Y.; Zharkinbekov, Z.; Kassymbek, K.; Jimi, S.; Saparov, A. Immunology of Acute and Chronic Wound Healing. Biomolecules 2021, 11, 700. [Google Scholar] [CrossRef] [PubMed]
- Tai, G.; Tai, M.; Zhao, M. Electrically stimulated cell migration and its contribution to wound healing. Burn. Trauma 2018, 6, 20. [Google Scholar] [CrossRef]
- Kloth, L.C. Electrical Stimulation Technologies for Wound Healing. Adv. Wound Care 2014, 3, 81–90. [Google Scholar] [CrossRef]
- Divya, S.; Hajra, S.; Panda, S.; Vivekananthan, V.; Mistewicz, K.; Kim, H.J.; Oh, T.H. A review on the next generation of healing: Exploring the use of triboelectric nanogenerators in wound care. Chem. Phys. Lett. 2023, 826, 140648. [Google Scholar] [CrossRef]
- Cheah, Y.J.; Buyong, M.R.; Yunus, M.H.M. Wound Healing with Electrical Stimulation Technologies: A Review. Polymers 2021, 13, 3790. [Google Scholar] [CrossRef] [PubMed]
- Piipponen, M.; Li, D.; Landén, N.X. The Immune Functions of Keratinocytes in Skin Wound Healing. Int. J. Mol. Sci. 2020, 21, 8790. [Google Scholar] [CrossRef]
- Rajendran, S.B.; Challen, K.; Wright, K.L.; Hardy, J.G. Electrical Stimulation to Enhance Wound Healing. J. Funct. Biomater. 2021, 12, 40. [Google Scholar] [CrossRef]
- Mathew-Steiner, S.S.; Roy, S.; Sen, C.K. Collagen in Wound Healing. Bioengineering 2021, 8, 63. [Google Scholar] [CrossRef]
- Tomic-Canic, M.; Burgess, J.L.; O’Neill, K.E.; Strbo, N.; Pastar, I. Skin Microbiota and its Interplay with Wound Healing. Am. J. Clin. Dermatol. 2020, 21 (Suppl. S1), 36–43. [Google Scholar] [CrossRef]
- Xu, J.; Jia, Y.; Huang, W.; Shi, Q.; Sun, X.; Zheng, L.; Wang, M.; Li, P.; Fan, Y. Non-contact electrical stimulation as an effective means to promote wound healing. Bioelectrochemistry 2022, 146, 108108. [Google Scholar] [CrossRef]
- Wan, D.; Yang, J.; Cui, X.; Ma, N.; Wang, Z.; Li, Y.; Li, P.; Zhang, Y.; Lin, Z.-H.; Sang, S.; et al. Human body-based self-powered wearable electronics for promoting wound healing driven by biomechanical motions. Nano Energy 2021, 89, 106465. [Google Scholar] [CrossRef]
- Luo, R.; Dai, J.; Zhang, J.; Li, Z. Accelerated Skin Wound Healing by Electrical Stimulation. Adv. Health Mater. 2021, 10, 2100557. [Google Scholar] [CrossRef]
- Zhao, M. Electrical fields in wound healing—An overriding signal that directs cell migration. Semin. Cell Dev. Biol. 2009, 20, 674–682. [Google Scholar] [CrossRef]
- Rouabhia, M.; Park, H.J.; Abedin-Do, A.; Douville, Y.; Méthot, M.; Zhang, Z. Electrical stimulation promotes the proliferation of human keratinocytes, increases the production of keratin 5 and 14, and increases the phosphorylation of ERK1/2 and p38 MAP kinases. J. Tissue Eng. Regen. Med. 2020, 14, 909–919. [Google Scholar] [CrossRef]
- Guo, A.; Song, B.; Reid, B.; Gu, Y.; Forrester, J.V.; Jahoda, C.A.; Zhao, M. Effects of Physiological Electric Fields on Migration of Human Dermal Fibroblasts. J. Investig. Dermatol. 2010, 130, 2320–2327. [Google Scholar] [CrossRef]
- Nazari, M.; Taremi, S.; Elahi, R.; Mostanadi, P.; Esmeilzadeh, A. Therapeutic Properties of M2 Macrophages in Chronic Wounds: An Innovative Area of Biomaterial-Assisted M2 Macrophage Targeted Therapy. Stem Cell Rev. Rep. 2024, 21, 390. [Google Scholar] [CrossRef]
- Thakral, G.; LaFontaine, J.; Najafi, B.; Talal, T.K.; Kim, P.; Lavery, L.A. Electrical stimulation to accelerate wound healing. Diabet. Foot Ankle 2013, 4, 22081. [Google Scholar] [CrossRef]
- Polak, A.; Kloth, L.C.; Blaszczak, E.; Taradaj, J.; Nawrat-Szoltysik, A. Evaluation of the Healing Progress of Pressure Ulcers Treated Evaluation of the Healing Progress of Pressure Ulcers Treated with Cathodal High-Voltage Monophasic Pulsed Current: Results with Cathodal High-Voltage Monophasic Pulsed Current: Results of a Prospective, Double-blind, Randomized Clinical Trial of a Prospective, Double-blind, Randomized Clinical Trial. Adv. Ski. Wound Care 2016, 29, 447–459. [Google Scholar]
- Krzyszczyk, P.; Schloss, R.; Palmer, A.; Berthiaume, F. The Role of Macrophages in Acute and Chronic Wound Healing and Interventions to Promote Pro-wound Healing Phenotypes. Front. Physiol. 2018, 9, 419. [Google Scholar] [CrossRef]
- Ma, T.; Zhai, X.; Huang, Y.; Zhang, M.; Zhao, X.; Du, Y.; Yan, C. A Smart Nanoplatform with Photothermal Antibacterial Capability and Antioxidant Activity for Chronic Wound Healing. Adv. Health Mater. 2021, 10, 2100033. [Google Scholar] [CrossRef]
- Rabbani, M.; Rahman, E.; Powner, M.B.; Triantis, I.F. Making Sense of Electrical Stimulation: A Meta-analysis for Wound Healing. Ann. Biomed. Eng. 2023, 52, 153–177. [Google Scholar] [CrossRef]
- Jiang, Y.; Trotsyuk, A.A.; Niu, S.; Henn, D.; Chen, K.; Shih, C.-C.; Larson, M.R.; Mermin-Bunnell, A.M.; Mittal, S.; Lai, J.-C.; et al. Wireless, closed-loop, smart bandage with integrated sensors and stimulators for advanced wound care and accelerated healing. Nat. Biotechnol. 2022, 41, 652–662. [Google Scholar] [CrossRef] [PubMed]
- Sani, E.S.; Xu, C.; Wang, C.; Song, Y.; Min, J.; Tu, J.; Solomon, S.A.; Li, J.; Banks, J.L.; Armstrong, D.G.; et al. A stretchable wireless wearable bioelectronic system for multiplexed monitoring and combination treatment of infected chronic wounds. Sci. Adv. 2023, 9, eadf7388. [Google Scholar] [CrossRef] [PubMed]
- Kao, F.-C.; Ho, H.-H.; Chiu, P.-Y.; Hsieh, M.-K.; Liao, J.; Lai, P.-L.; Huang, Y.-F.; Dong, M.-Y.; Tsai, T.-T.; Lin, Z.-H. Self-assisted wound healing using piezoelectric and triboelectric nanogenerators. Sci. Technol. Adv. Mater. 2022, 23, 1–16. [Google Scholar] [CrossRef]
- Wang, X.-F.; Li, M.-L.; Fang, Q.-Q.; Zhao, W.-Y.; Lou, D.; Hu, Y.-Y.; Chen, J.; Tan, W.-Q. Flexible electrical stimulation device with Chitosan-Vaseline® dressing accelerates wound healing in diabetes. Bioact. Mater. 2020, 6, 230–243. [Google Scholar] [CrossRef]
- Lu, S.-H.; Samandari, M.; Li, C.; Li, H.; Song, D.; Zhang, Y.; Tamayol, A.; Wang, X. Multimodal sensing and therapeutic systems for wound healing and management: A review. Sens. Actuators Rep. 2022, 4, 100075. [Google Scholar] [CrossRef]
- Farber, P.L.; Hochman, B.; Furtado, F.; Ferreira, L.M. Electricity and colloidal stability: How charge distribution in the tissue can affects wound healing. Med. Hypotheses 2014, 82, 199–204. [Google Scholar] [CrossRef]
- Mamalis, A.; Koo, E.; Garcha, M.; Murphy, W.J.; Isseroff, R.R.; Jagdeo, J. High fluence light emitting diode-generated red light modulates characteristics associated with skin fibrosis. J. Biophotonics 2016, 9, 1167–1179. [Google Scholar] [CrossRef]
- Gao, Y.; Nguyen, D.T.; Yeo, T.; Bin Lim, S.; Tan, W.X.; Madden, L.E.; Jin, L.; Long, J.Y.K.; Aloweni, F.A.B.; Liew, Y.J.A.; et al. A flexible multiplexed immunosensor for point-of-care in situ wound monitoring. Sci. Adv. 2021, 7, eabg9614. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, J.; Liu, J.; Hao, S.; Song, H.; Zhang, J. 3D Printable, Highly Stretchable, Superior Stable Ionogels Based on Poly(ionic liquid) with Hyperbranched Polymers as Macro-cross-linkers for High-Performance Strain Sensors. ACS Appl. Mater. Interfaces 2021, 13, 5614–5624. [Google Scholar] [CrossRef]
- Zhao, L.; Ren, Z.; Liu, X.; Ling, Q.; Li, Z.; Gu, H. A Multifunctional, Self-Healing, Self-Adhesive, and Conductive Sodium Alginate/Poly(vinyl alcohol) Composite Hydrogel as a Flexible Strain Sensor. ACS Appl. Mater. Interfaces 2021, 13, 11344–11355. [Google Scholar] [CrossRef]
- Li, M.; Chen, X.; Li, X.; Dong, J.; Zhao, X.; Zhang, Q. Wearable and Robust Polyimide Hydrogel Fiber Textiles for Strain Sensors. ACS Appl. Mater. Interfaces 2021, 13, 43323–43332. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zheng, H.; Tan, Y.J.; Tor, S.B.; Zhou, K. Development of an Ultrastretchable Double-Network Hydrogel for Flexible Strain Sensors. ACS Appl. Mater. Interfaces 2021, 13, 12814–12823. [Google Scholar] [CrossRef] [PubMed]
- Henricson, J.; Sandh, J.; Iredahl, F. Moisture sensor for exudative wounds—A pilot study. Ski. Res. Technol. 2021, 27, 918–924. [Google Scholar] [CrossRef] [PubMed]
- Milne, S.D.; Seoudi, I.; Al Hamad, H.; Talal, T.K.; Anoop, A.A.; Allahverdi, N.; Zakaria, Z.; Menzies, R.; Connolly, P. A wearable wound moisture sensor as an indicator for wound dressing change: An observational study of wound moisture and status. Int. Wound J. 2016, 13, 1309–1314. [Google Scholar] [CrossRef]
- Patel, S.; Ershad, F.; Zhao, M.; Isseroff, R.R.; Duan, B.; Zhou, Y.; Wang, Y.; Yu, C. Wearable electronics for skin wound monitoring and healing. Soft Sci. 2022, 2, 9. [Google Scholar] [CrossRef]
- Brown, M.S.; Ashley, B.; Koh, A. Wearable Technology for Chronic Wound Monitoring: Current Dressings, Advancements, and Future Prospects. Front. Bioeng. Biotechnol. 2018, 6, 47. [Google Scholar] [CrossRef]
- Magisetty, R.; Park, S.-M. New Era of Electroceuticals: Clinically Driven Smart Implantable Electronic Devices Moving towards Precision Therapy. Micromachines 2022, 13, 161. [Google Scholar] [CrossRef]
- Derakhshandeh, H.; Kashaf, S.S.; Aghabaglou, F.; Ghanavati, I.O.; Tamayol, A. Smart Bandages: The Future of Wound Care. Trends Biotechnol. (Regular Ed.) 2018, 36, 1259–1274. [Google Scholar] [CrossRef]
- Dargaville, T.R.; Farrugia, B.L.; Broadbent, J.A.; Pace, S.; Upton, Z.; Voelcker, N.H. Sensors and imaging for wound healing: A review. Biosens. Bioelectron. 2013, 41, 30–42. [Google Scholar] [CrossRef] [PubMed]
- Tang, N.; Zheng, Y.; Jiang, X.; Zhou, C.; Jin, H.; Jin, K.; Wu, W.; Haick, H. Wearable Sensors and Systems for Wound Healing-Related pH and Temperature Detection. Micromachines 2021, 12, 430. [Google Scholar] [CrossRef]
- Han, G.; Ceilley, R. Chronic Wound Healing: A Review of Current Management and Treatments. Adv. Ther. 2017, 34, 599–610. [Google Scholar] [CrossRef]
- Marston, W.A.; Hanft, J.; Norwood, P.; Pollak, R.; for the Dermagraft Diabetic Foot Ulcer Study Group. The Efficacy and Safety of Dermagraft in Improving the Healing of Chronic Diabetic Foot Ulcers Results of a prospective randomized trial. Diabetes Care 2003, 26, 1701–1705. [Google Scholar] [CrossRef]
- Hanft, J.R.; Surprenant, M.S. Healing of chronic foot ulcers in diabetic patients treated with a human fibroblast-derived dermis. J. Foot Ankle Surg. 2002, 41, 291–299. [Google Scholar] [CrossRef]
- Liljestrand, J. Reproductive health beyond Cairo and Beijing. Acta Obstet. Gynecol. Scand. 1997, 76, 291–293. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization; Organisation for Economic Co-Operation and Development; The World Bank. Delivering Quality Health Services: A Global Imperative for Universal Health Coverage. Geneva: World Health Organization, 2018. Available online: https://creativecommons.org/licenses/by-nc-sa/3.0/igo (accessed on 13 February 2025).
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [PubMed]
- Houghton, P.E.; Kincaid, C.B.; Lovell, M.; Campbell, K.E.; Keast, D.H.; Woodbury, M.G.; A Harris, K. Effect of Electrical Stimulation on Chronic Leg Ulcer Size and Appearance. Phys. Ther. 2003, 83, 17–28. [Google Scholar] [CrossRef]
- Adunsky, A.; Ohry, A. Decubitus direct current treatment (DDCT) of pressure ulcers: Results of a randomized double-blinded placebo controlled study. Arch. Gerontol. Geriatr. 2005, 41, 261–269. [Google Scholar] [CrossRef]
- Barman, S.R.; Chan, S.-W.; Kao, F.-C.; Ho, H.-Y.; Khan, I.; Pal, A.; Huang, C.-C.; Lin, Z.-H. A self-powered multifunctional dressing for active infection prevention and accelerated wound healing. Sci. Adv. 2023, 9, eadc8758. [Google Scholar] [CrossRef]
- Sharma, A.; Panwar, V.; Mondal, B.; Prasher, D.; Bera, M.K.; Thomas, J.; Kumar, A.; Kamboj, N.; Mandal, D.; Ghosh, D. Electrical stimulation induced by a piezo-driven triboelectric nanogenerator and electroactive hydrogel composite, accelerate wound repair. Nano Energy 2022, 99, 107419. [Google Scholar] [CrossRef]
- Jia, Y.; Xu, J.; Shi, Q.; Zheng, L.; Liu, M.; Wang, M.; Li, P.; Fan, Y. Study on the effects of alternating capacitive electric fields with different frequencies on promoting wound healing. Med. Nov. Technol. Devices 2022, 16, 100142. [Google Scholar] [CrossRef]
- Nguyen, N.; Lin, Z.-H.; Barman, S.R.; Korupalli, C.; Cheng, J.-Y.; Song, N.-X.; Chang, Y.; Mi, F.-L.; Song, H.-L.; Sung, H.-W.; et al. Engineering an integrated electroactive dressing to accelerate wound healing and monitor noninvasively progress of healing. Nano Energy 2022, 99, 107393. [Google Scholar] [CrossRef]
- Wang, C.; Jiang, X.; Kim, H.-J.; Zhang, S.; Zhou, X.; Chen, Y.; Ling, H.; Xue, Y.; Chen, Z.; Qu, M.; et al. Flexible patch with printable and antibacterial conductive hydrogel electrodes for accelerated wound healing. Biomaterials 2022, 285, 121479. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, L.; Zhang, Q.; Li, H.; Xiang, Y.; Wang, X.; Hu, X. Effective electrical stimulation by a Poly(l-lactic acid)/Vitamin B2-Based piezoelectric generator promotes wound healing. Eur. Polym. J. 2023, 189, 111962. [Google Scholar] [CrossRef]
- Fraccalvieri, M.; Salomone, M.; Zingarelli, E.M.; Rivarossa, F.; Bruschi, S. Electrical stimulation for difficult wounds: Only an alternative procedure? Int. Wound J. 2014, 12, 669–673. [Google Scholar] [CrossRef]
- Dabiri, G.; Damstetter, E.; Phillips, T. Choosing a Wound Dressing Based on Common Wound Characteristics. Adv. Wound Care 2016, 5, 32–41. [Google Scholar] [CrossRef]
- Sood, A.; Granick, M.S.; Tomaselli, N.L. Wound Dressings and Comparative Effectiveness Data. Adv. Wound Care 2014, 3, 511–529. [Google Scholar] [CrossRef]
- Tan, S.T.; Winarto, N.; Dosan, R.; Aisyah, P.B. The Benefits of Occlusive Dressings in Wound Healing. Open Dermatol. J. 2019, 13, 27–33. [Google Scholar] [CrossRef]
- Nayeri, F. Occlusive bandaging of wounds with decreased circulation promotes growth of anaerobic bacteria and necrosis: Case report. BMC Res. Notes 2016, 9, 1–3. [Google Scholar] [CrossRef]
- Laurano, R.; Boffito, M.; Ciardelli, G.; Chiono, V. Wound dressing products: A translational investigation from the bench to the market. Eng. Regen. 2022, 3, 182–200. [Google Scholar] [CrossRef]
- Jünger, M.; Arnold, A.; Zuder, D.; Stahl, H.; Heising, S. Local therapy and treatment costs of chronic, venous leg ulcers with electrical stimulation (Dermapulse®): A prospective, placebo controlled, double blind trial. Wound Repair Regen. 2008, 16, 480. [Google Scholar] [CrossRef] [PubMed]
- Kitajo, K.; Nozaki, D.; Ward, L.M.; Yamamoto, Y. Behavioral Stochastic Resonance within the Human Brain. Phys. Rev. Lett. 2003, 90, 218103. [Google Scholar] [CrossRef] [PubMed]
- Kloth, L.C. Electrical Stimulation for Wound Healing: A Review of Evidence From In Vitro Studies, Animal Experiments, and Clinical Trials. Int. J. Low. Extrem. Wounds 2005, 4, 23–44. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.; Wang, L.; Song, C.; Yao, L.; Xiao, J. Recent progresses of collagen dressings for chronic skin wound healing. Collagen Leather 2023, 5, 31. [Google Scholar] [CrossRef]
- Lampiasi, N. Macrophage Polarization: Learning to Manage It 2.0. IJMS 2023, 24, 17409. [Google Scholar] [CrossRef]
- Roy Barman, S.; Jhunjhunwala, S. Electrical Stimulation for Immunomodulation. ACS Omega 2023, 9, 52. [Google Scholar] [CrossRef]
- Schnapp, W.D.; Delcroix, G.J.-R. Improved Sensation Resulting from Spinal Cord Stimulation for the Treatment of Painful Diabetic Neuropathy: The Possible Role of Stochastic Resonance. Pain Physician 2022, 25, E1399–E1403. [Google Scholar]
- Ud-Din, S.; Sebastian, A.; Giddings, P.; Colthurst, J.; Whiteside, S.; Morris, J.; Nuccitelli, R.; Pullar, C.; Baguneid, M.; Bayat, A. Angiogenesis Is Induced and Wound Size Is Reduced by Electrical Stimulation in an Acute Wound Healing Model in Human Skin. PLoS ONE 2015, 10, e0124502. [Google Scholar] [CrossRef]
- Ren, X.; Sun, H.; Liu, J.; Guo, X.; Huang, J.; Jiang, X.; Zhang, Y.; Huang, Y.; Fan, D.; Zhang, J. Keratinocyte Electrotaxis Induced by Physiological Pulsed Direct Current Electric Fields. Bioelectrochemistry 2019, 127, 113–124. [Google Scholar] [CrossRef]
- Ganesan, O.; Morris, M.X.; Guo, L.; Orgill, D. A review of artificial intelligence in wound care. Artif. Intell. Surg. 2024, 4, 364–375. [Google Scholar] [CrossRef]
- Griffa, D.; Natale, A.; Merli, Y.; Starace, M.; Curti, N.; Mussi, M.; Castellani, G.; Melandri, D.; Piraccini, B.M.; Zengarini, C. Artificial Intelligence in Wound Care: A Narrative Review of the Currently Available Mobile Apps for Automatic Ulcer Segmentation. BioMedInformatics 2024, 4, 2321–2337. [Google Scholar] [CrossRef]
- Chen, M.; Cao, M.; Xu, T. Progress in the application of artificial intelligence in skin wound assessment and prediction of healing time. Am. J. Transl. Res. 2024, 16, 2765. [Google Scholar] [CrossRef] [PubMed]
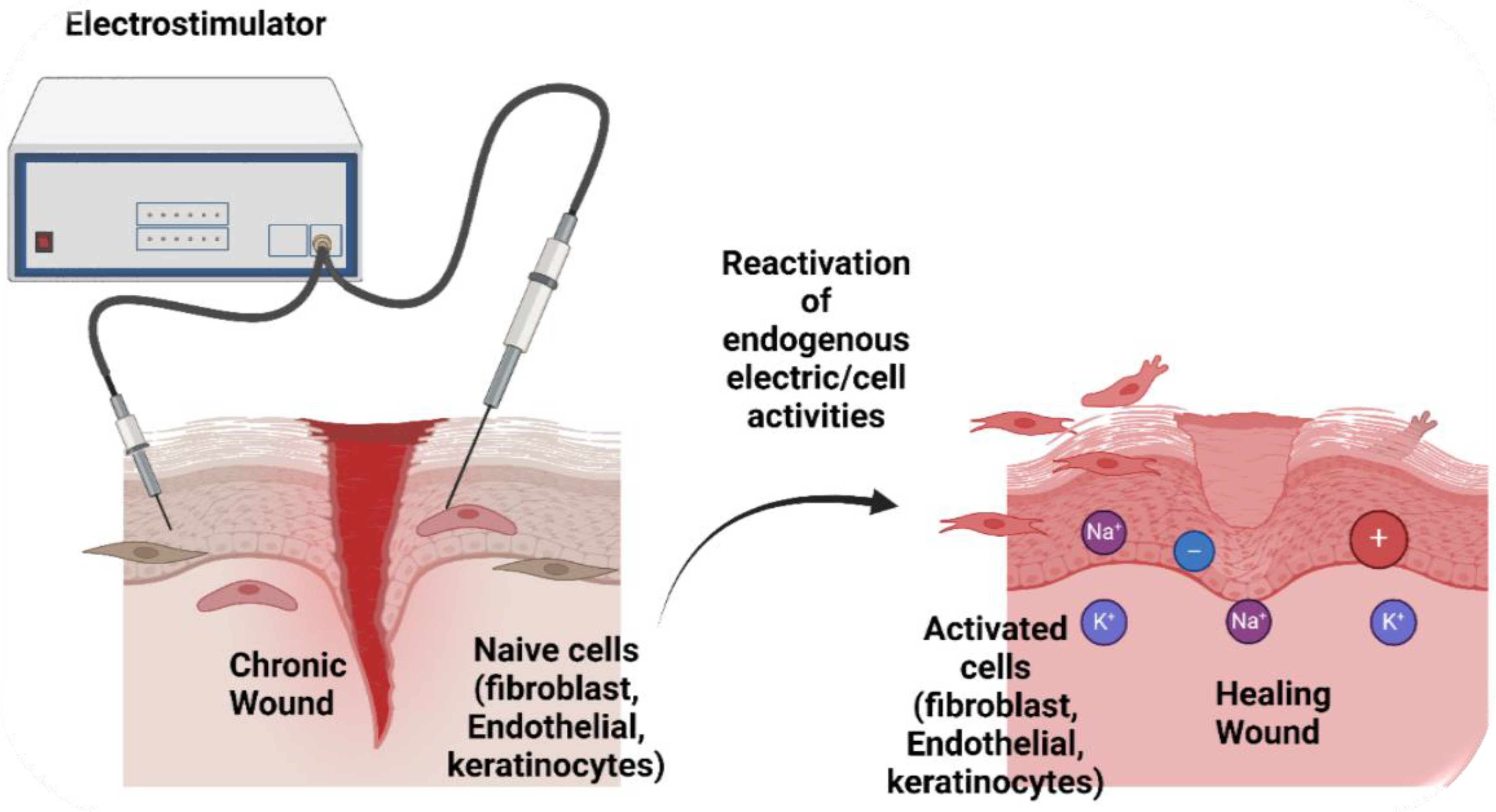
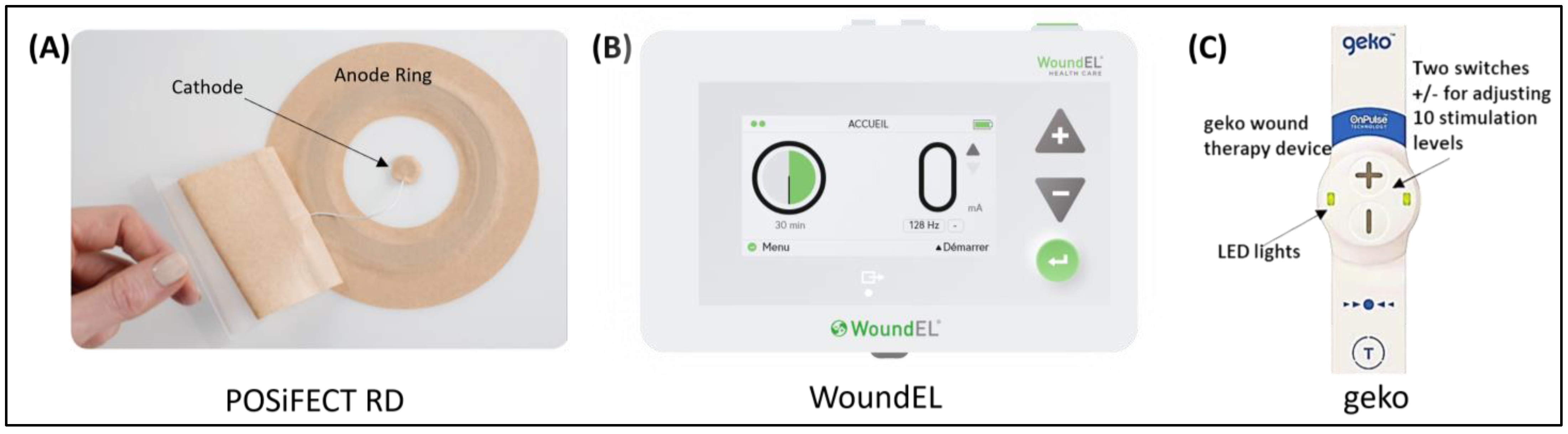
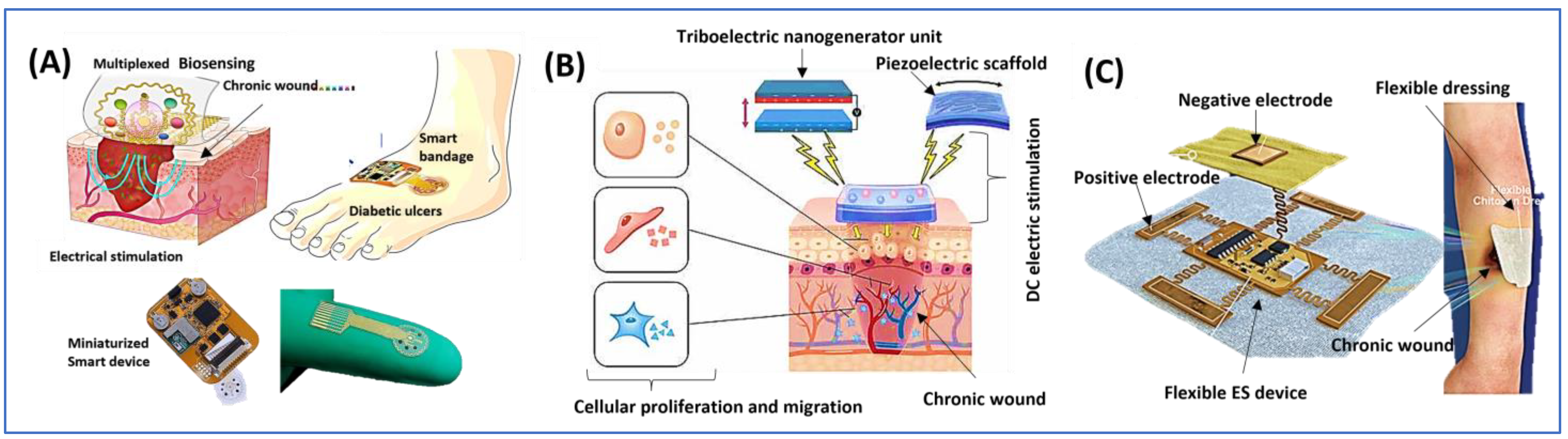
| Phase | Effects of Electrostimulation |
|---|---|
| Inflammatory | Enhanced phagocytosis by neutrophils and macrophages Inhibited bacteria proliferation and limited sepsis formation A short inflammatory phase |
| Proliferative | Promotion of the migration, proliferation, and differentiation of keratinocytes, fibroblasts, and endothelial cells and enabling of granulation tissue formation Extracellular matrix deposition with proteins such as collagen, proteoglycans, and glycosaminoglycans to facilitate repair |
| Remodeling | Collagen fibril alignment Transformation of type III collagen to type I collagen |
| Wound Type | Pathophysiology |
|---|---|
| Diabetic foot ulcers | Loss of sensation (diabetic neuropathy) in the lower extremities of the body |
| Pressure ulcers | Prolonged pressure, compression, or friction of a body part leading to skin and tissue necrosis |
| Vascular ulcers | Emboli and ischemic ulcers narrow the arterial lumen (arterial ulcers). Venous hypertension is caused by faulty valves, which cause blood to backflow and increase the pressure of the venous wall (venous ulcer). |
| Types | Definition | Number of Mentions |
|---|---|---|
| Smart bandage devices | Medication delivery/wound care and debridement | 208 |
| Smart polymer devices | Wound care, medication delivery | 317 |
| Electric stimulators | Uses (direct, pulsed, alternating) electric current to stimulate healing | 29 |
| Light stimulators | Photo-stimulation | 382 |
| Negative pressure wound therapy | Regulates air pressure on wound surfaces | 2 |
| Wound physical parameters sensors | Physiologic parameter monitoring | 1283 |
| Thermo-responsive devices | Utilizes heat energy | 2 |
| Chemical sensor devices | Biochemical parameter monitoring | 12 |
| Total | 2235 |
| Type of Device | Type of Current Generated | Stimulation Pattern | Effects | Results | Ref |
|---|---|---|---|---|---|
| EGS Model 300 Electrical Stimulators | High-voltage pulsed current | Microstimulation of cells, cell proliferation and migration to wound sites | Cell electrotaxis, wound contraction | Reduction in chronic wound surface area | [60] |
| Decubitus Direct Current Treatment (DDCT) | Direct/alternating current | Wound electric activity stimulation and re-activation | Wound contraction and remodeling | Wound contraction rate significantly improved | [61] |
| Wireless closed-loop, smart bandage with sensors and stimulators | Direct current | Re-activation of vascular endothelial growth factor (VEGF) | Pro-regenerative | Significant increase in wound impedance, angiogenesis | [34] |
| Self-powered TENG | Direct current | Endogenous electric field stimulation and re-activation from micro-electric current generated from friction by materials | Inhibition of bacterial proliferation/cellular activation for wound healing | Fibroblast/EC migration, proliferation, angiogenesis | [62] |
| Wearable piezo-triboelectric nanogenerator device | Piezo and triboelectric (direct current) | Biomechanical electric impulse generator | Endothelial cell and fibroblast activation | Endothelial cell/fibroblast migration | [63] |
| Alternative capacitive electric field (ACEF) exposure system | ACEF | Alternative capacitive electric field | Keratinocytes, dermal fibroblast, macrophages | Cell polarization, enhanced cell activity, proliferation, and migration | [64] |
| Interdigital array (IDA) electrode (TENG) | Triboelectric nanogenerator | A direct current electric stimulator from frictional forces generated from materials | Vascular endothelial growth factor (VEGF), CD31 marker | Enhanced wound vascularization | [65] |
| Flexible ePatch, silver nanowire (AgNW) methacrylate alginate | Pulsed current | Current stimulated by compressional forces | Antibacterial, angiogenesis, cell proliferation, re-epithelization | Fibroblast, endothelial cell electrotaxis, immune cell modulation | [66] |
| Poly (l-lactic acid)/Vit. B2-based piezoelectric device | Pulsed current | Piezoelectric generator activation by mechanical dynamics of body movement | Collagen deposition, re-epithelization, neovascularization, increased growth factor concentration in wounds | Fibroblast proliferation and migration | [67] |
| Bioelectric Signal Therapy (BST) device | Alternating current | Low-frequency (2 Hz) periodic pulse sequence | Pain relief, wound contraction | Activation of cutaneous sensory nerves | [68] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mutah, A.A.; Amitrano, J.; Seeley, M.A.; Seshadri, D. A Review of Wearable Electroceutical Devices for Chronic Wound Healing. Electronics 2025, 14, 1376. https://doi.org/10.3390/electronics14071376
Mutah AA, Amitrano J, Seeley MA, Seshadri D. A Review of Wearable Electroceutical Devices for Chronic Wound Healing. Electronics. 2025; 14(7):1376. https://doi.org/10.3390/electronics14071376
Chicago/Turabian StyleMutah, Ali Abba, Joseph Amitrano, Mark A. Seeley, and Dhruv Seshadri. 2025. "A Review of Wearable Electroceutical Devices for Chronic Wound Healing" Electronics 14, no. 7: 1376. https://doi.org/10.3390/electronics14071376
APA StyleMutah, A. A., Amitrano, J., Seeley, M. A., & Seshadri, D. (2025). A Review of Wearable Electroceutical Devices for Chronic Wound Healing. Electronics, 14(7), 1376. https://doi.org/10.3390/electronics14071376





