Development of Green Lignin–MWCNTs Hybrids for Sustainable Conductive Materials
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of MWCNTs–Soda Lignin Hybrids
2.3. Experimental Methods
2.3.1. Dynamic Light Scattering (DLS)
2.3.2. Scanning Electron Microscopy (SEM)
2.3.3. Transmission Electron Microscopy (TEM)
2.3.4. Broadband Dielectric Spectriscopy (BDS)
2.3.5. Four-Point Probe (4PP)
2.3.6. Raman Spectroscopy
2.3.7. Electrochemical Measurements
Determination of Electro-Active Surface Area
3. Results and Discussion
3.1. Particle Size and Surface Charge of Hybrids
3.2. Stability Test
3.3. Microscopic Imaging
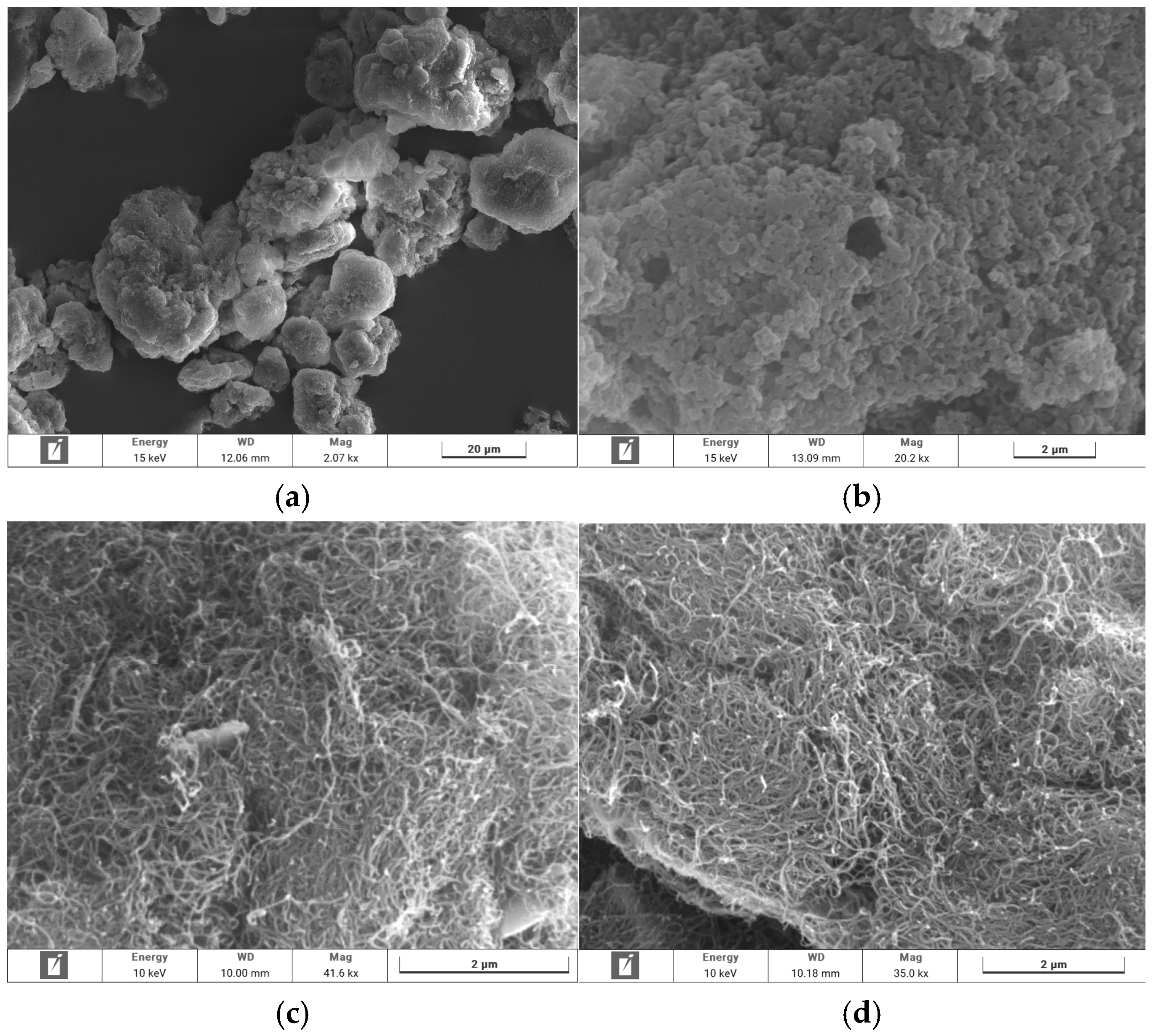
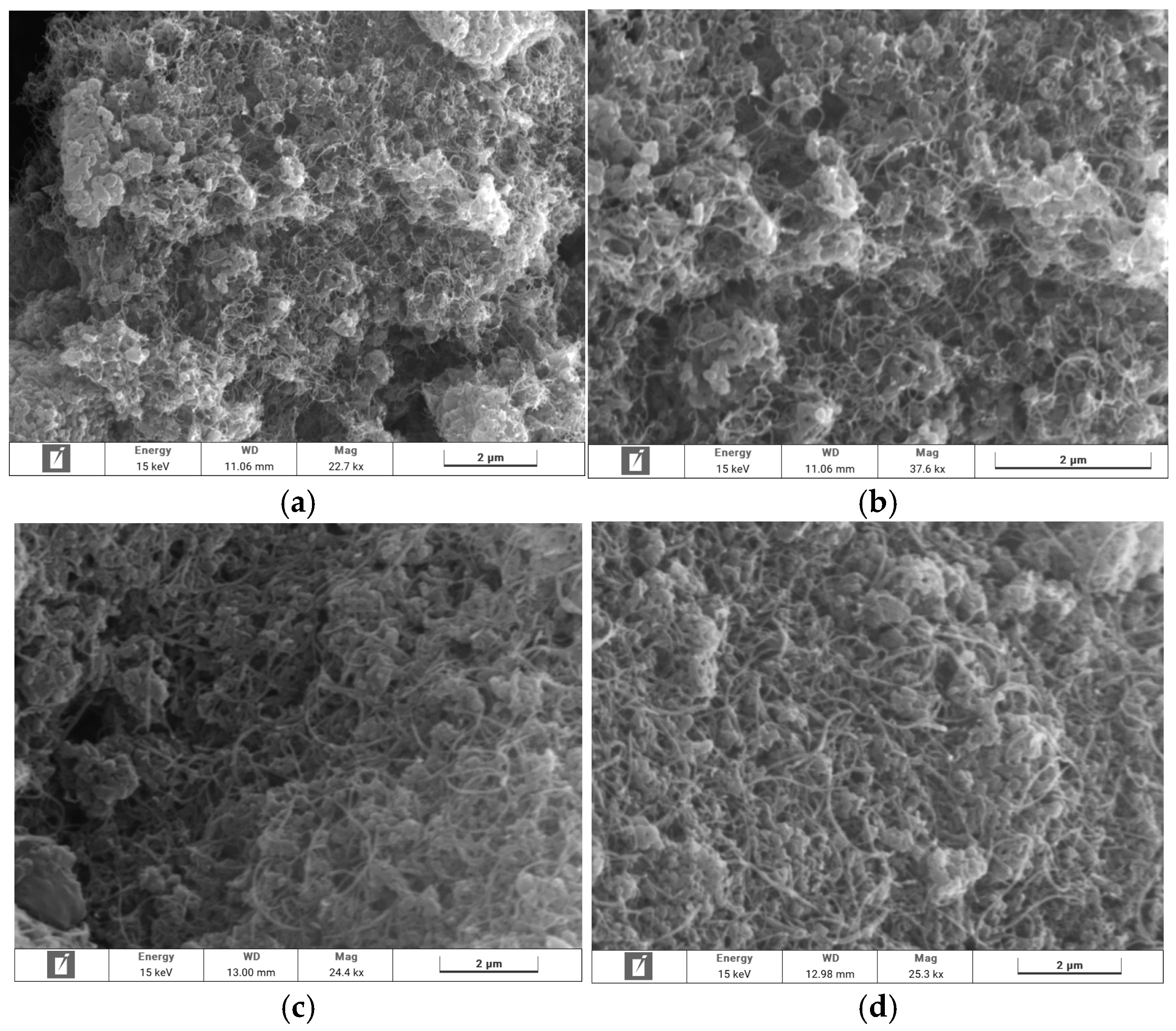
3.3.1. SEM Imaging
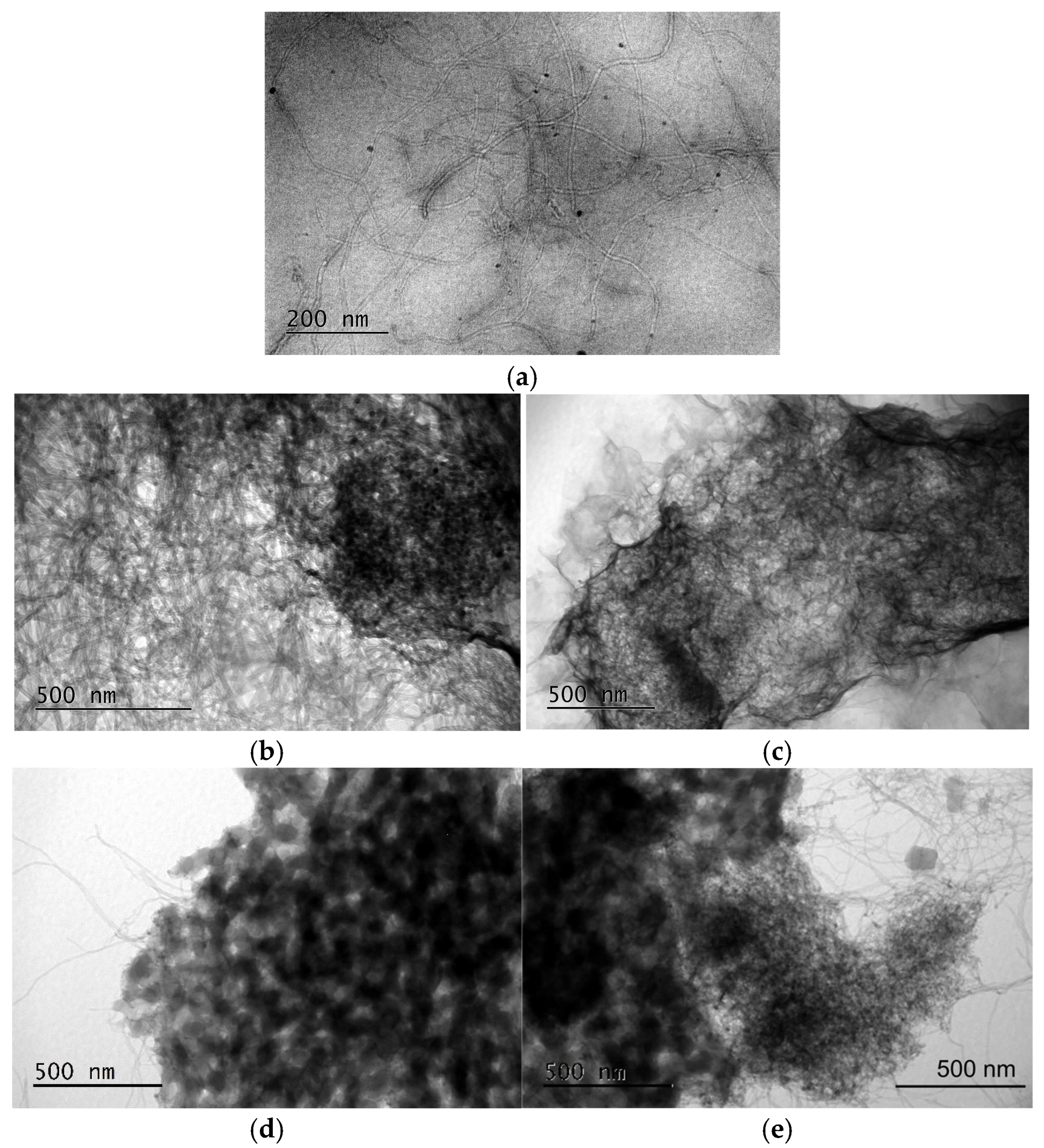
3.3.2. TEM Imaging
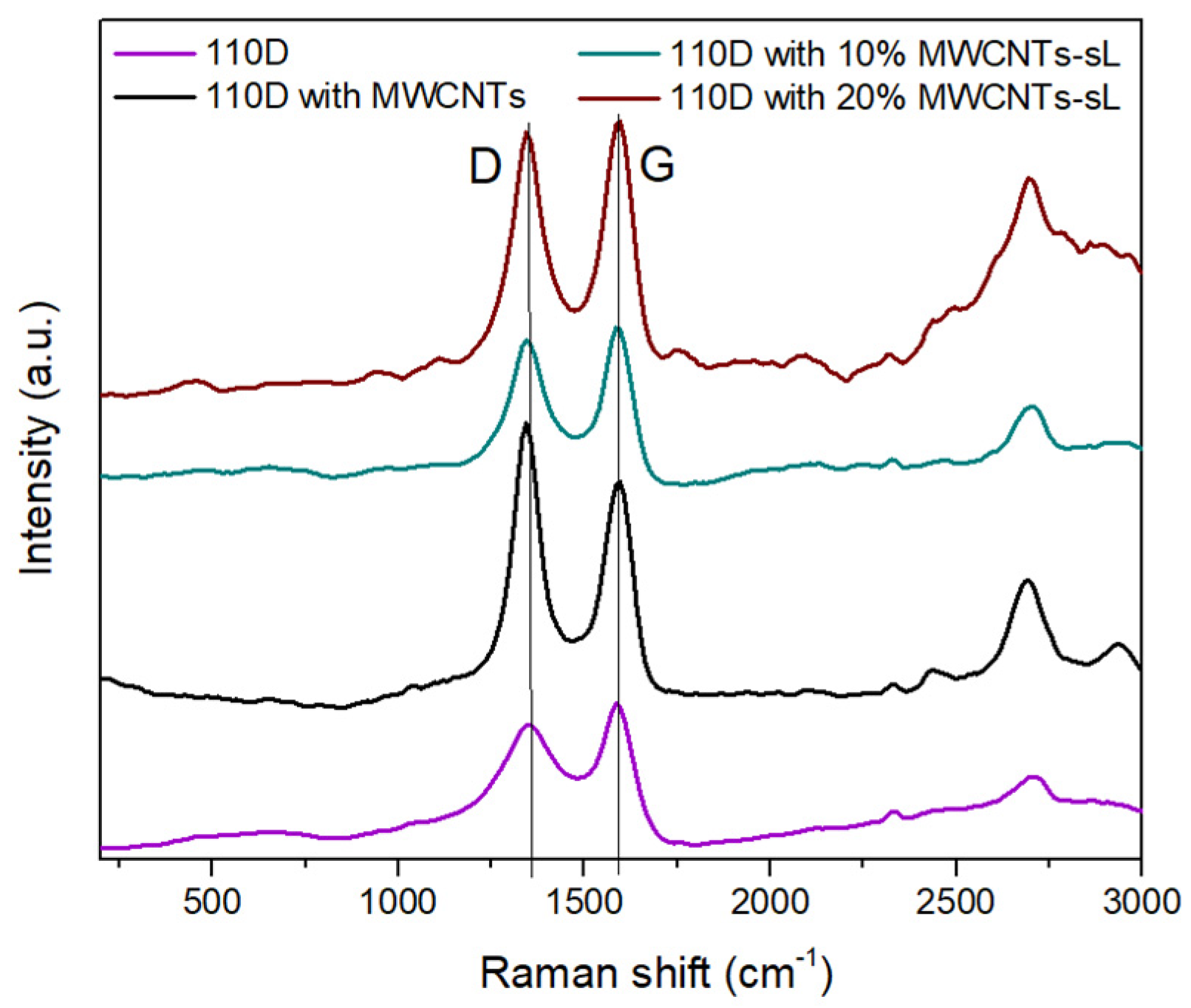
3.4. Raman Results and Interpretation
3.5. Electrical Characterization
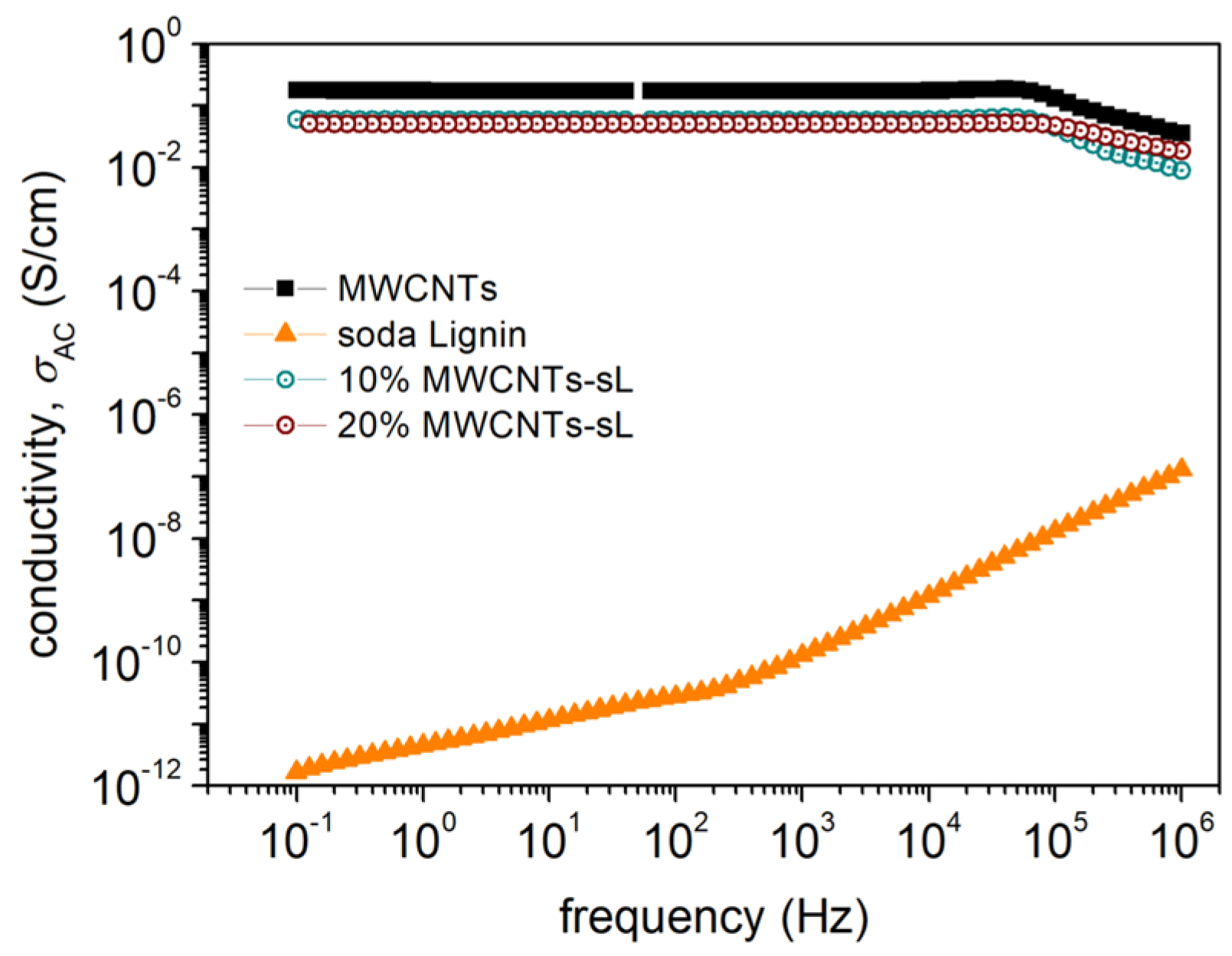
3.5.1. BDS Analysis
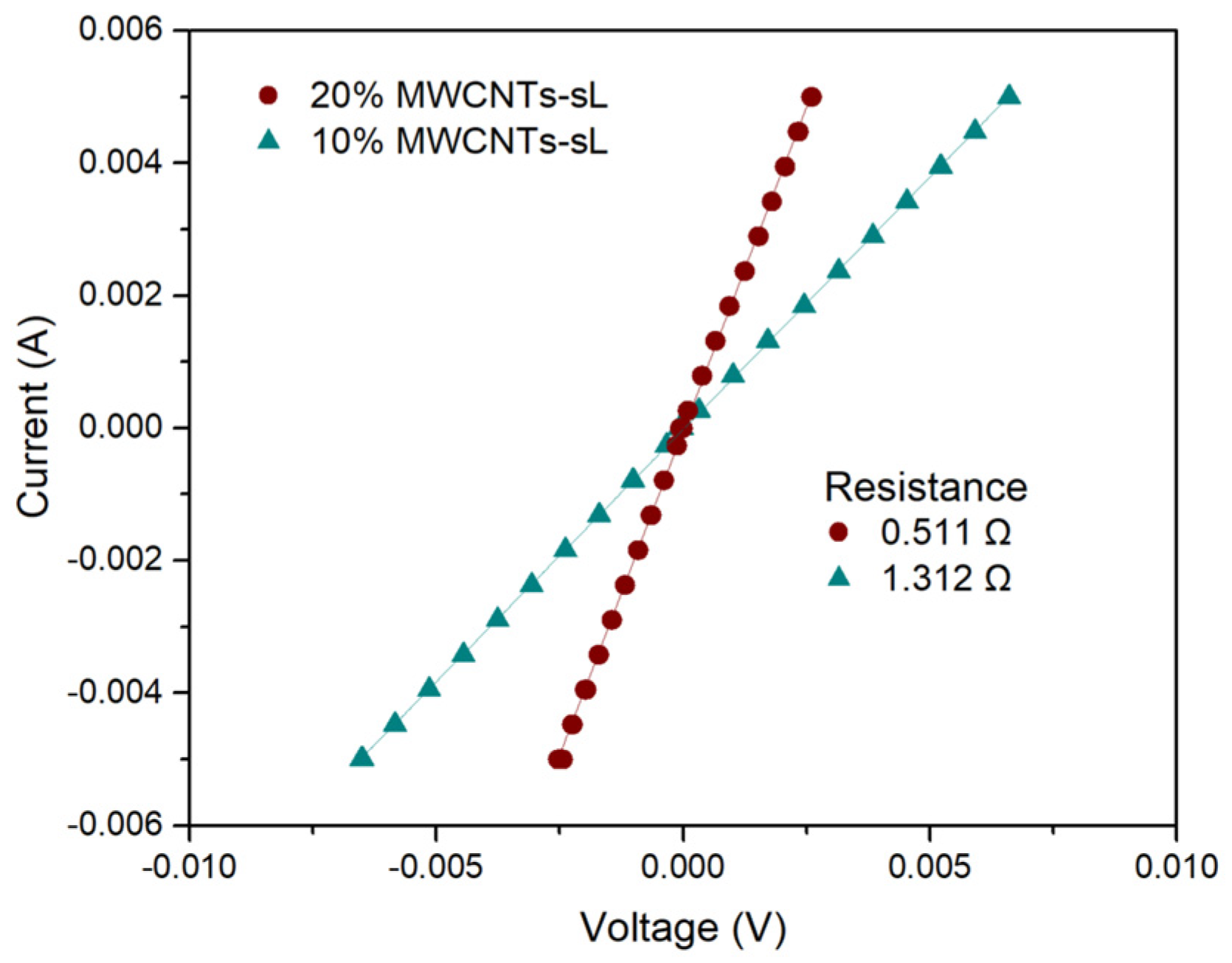
3.5.2. Four-Point Probe Analysis
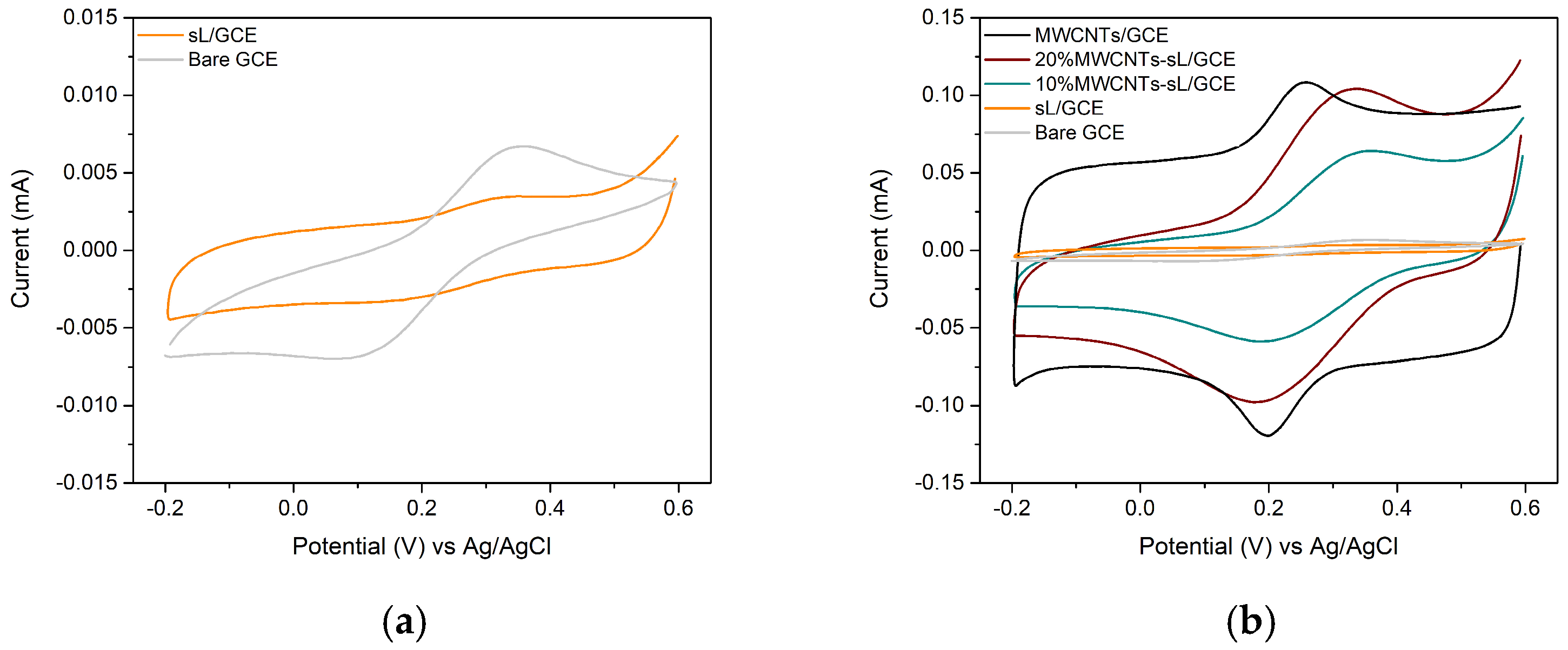
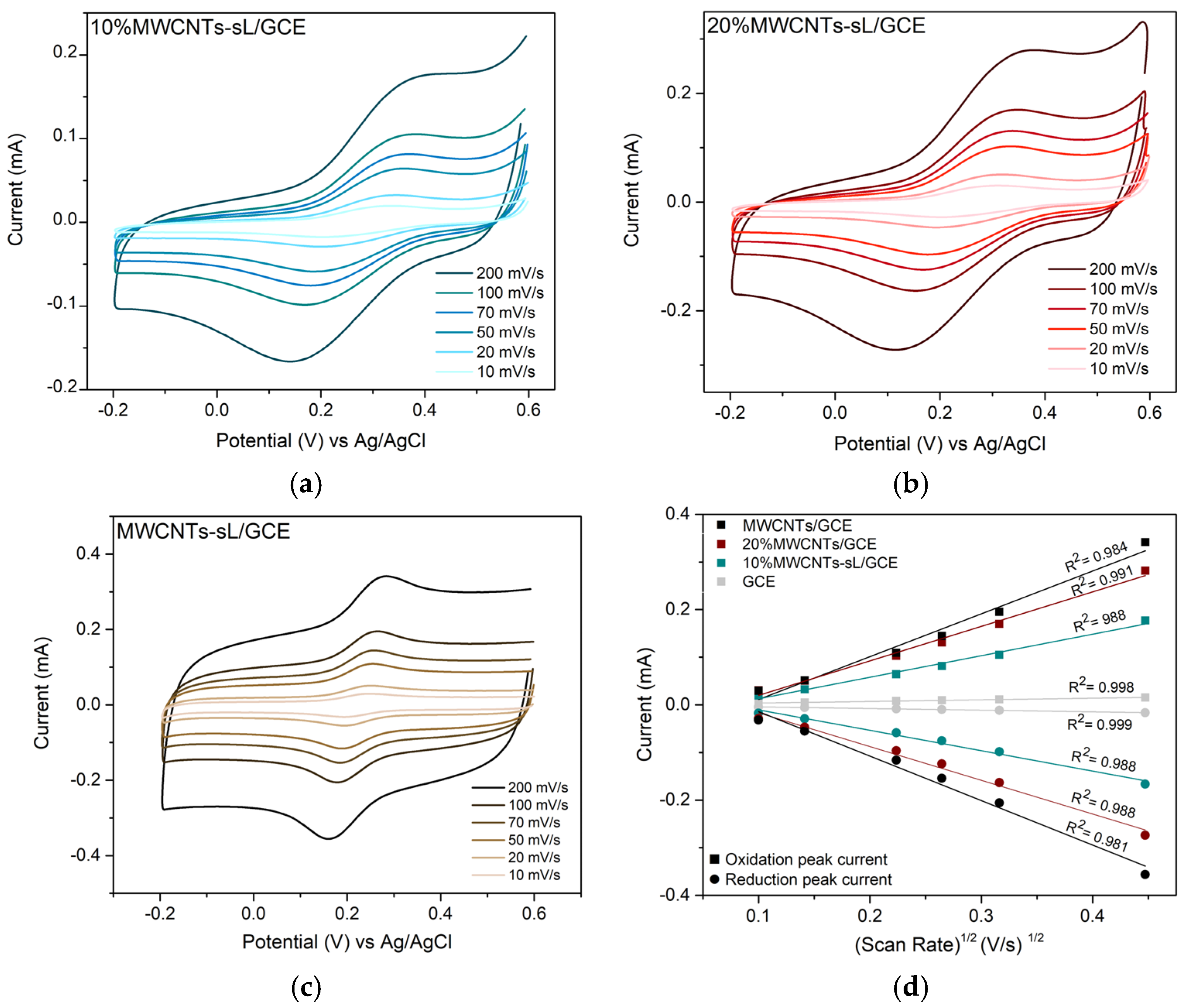
3.6. Electrochemical Performance
Determination of Electro-Active Surface Area
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BDS | Broadband dielectric spectroscopy |
| CNTs | Carbon nanotubes |
| CTAB | Cetrimonium bromide |
| CGA | Chlorogenic acid |
| CE | Counter electrode |
| DI | Deionized |
| DLS | Dynamic light scattering |
| DMF | Dimethylformamide |
| DMSO | Dimethylsulfoxide |
| EASA | Electroactive surface area |
| EUG | Eucommia ulmoides gum |
| GCE | Glassy carbon electrode |
| HDD | Hydrodynamic diameter |
| I-V | Current–voltage |
| MTMS | Methyl trimethoxysilane |
| MWCNTs | Multi-walled carbon nanotubes |
| PAN | Polyacrylonitrile |
| PDMS | Polydimethylsiloxane |
| PLA | Polylactic acid |
| PP-g-MA | Polypropylene-grafted-maleic anhydride |
| RE | Reference electrode |
| Rs | Sheet resistance |
| SDS | Sodium dodecyl sulfate |
| SEM | Scanning electron microscopy |
| SHE | Standard hydrogen electrode |
| sL | Soda lignin |
| TEM | Transmission electron microscopy |
| Triton X-100 | Octyl phenol ethoxylate |
| US | Ultrasonication |
| WE | Working electrode |
| ZP | Zeta potential |
| 4PP | Four-point probe |
References
- Su, Z.; Yang, Y.; Huang, Q.; Chen, R.; Ge, W.; Fang, Z.; Huang, F.; Wang, X. Designed Biomass Materials for “Green” Electronics: A Review of Materials, Fabrications, Devices, and Perspectives. Prog. Mater. Sci. 2022, 125, 100917. [Google Scholar] [CrossRef]
- Jain, M.; Kumar, D.; Chaudhary, J.; Kumar, S.; Sharma, S.; Singh Verma, A. Review on E-Waste Management and Its Impact on the Environment and Society. Waste Manag. Bull. 2023, 1, 34–44. [Google Scholar] [CrossRef]
- Georgs, V.; Piili, H.; Gustafsson, J.; Xu, C. A Critical Review on Lignin Structure, Chemistry, and Modification towards Utilisation in Additive Manufacturing of Lignin-Based Composites. Ind. Crops Prod. 2025, 233, 121416. [Google Scholar] [CrossRef]
- Ali, S.; Rani, A.; Dar, M.; Qaisrani, M.; Noman, M.; Yoganathan, K.; Asad, M.; Berhanu, A.; Barwant, M.; Zhu, D. Recent Advances in Characterization and Valorization of Lignin and Its Value-Added Products: Challenges and Future Perspectives. Biomass 2024, 4, 947–977. [Google Scholar] [CrossRef]
- Libretti, C.; Santos Correa, L.; Meier, M.A.R. From Waste to Resource: Advancements in Sustainable Lignin Modification. Green Chem. 2024, 26, 4358–4386. [Google Scholar] [CrossRef]
- Ruwoldt, J.; Blindheim, F.H.; Chinga-Carrasco, G. Functional Surfaces, Films, and Coatings with Lignin—A Critical Review. RSC Adv. 2023, 13, 12529–12553. [Google Scholar] [CrossRef]
- Zhang, H.; Li, H.; Wang, W.; Li, P.; Liu, S.; Yang, M.; He, C. Biomass Lignin as Dispersion to Substantially Enhance Carbon Nanotubes Thermoelectric Converter for Energy Harvesting. Carbon 2024, 229, 119489. [Google Scholar] [CrossRef]
- Beg, S.; Rahman, M.; Jain, A.; Saini, S.; Hasnain, M.S.; Swain, S.; Imam, S.; Kazmi, I.; Akhter, S. Emergence in the Functionalized Carbon Nanotubes as Smart Nanocarriers for Drug Delivery Applications. In Fullerens, Graphenes and Nanotubes; Elsevier: Amsterdam, The Netherlands, 2018; pp. 105–133. ISBN 978-0-12-813691-1. [Google Scholar]
- Manzetti, S.; Andersen, O. Carbon Nanotubes in Electronics: Background and Discussion for Waste-Handling Strategies. Challenges 2013, 4, 75–85. [Google Scholar] [CrossRef]
- Tu, C.; Luo, W.; Peng, Y.; Yu, P.; Shi, C.; Wu, Z.; Shao, L.; Zhan, P. Preparation of Lignin-Based Carbon Nanotubes Using Micelles as Soft Template. Ind. Crops Prod. 2023, 191, 116009. [Google Scholar] [CrossRef]
- Zhou, M.; Wang, Z.; Wang, X. Carbon Nanotubes for Sensing Applications. In Industrial Applications of Carbon Nanotubes; Elsevier: Amsterdam, The Netherlands, 2017; pp. 129–150. ISBN 978-0-323-41481-4. [Google Scholar]
- Khan, F.S.A.; Mubarak, N.M.; Khalid, M.; Walvekar, R.; Abdullah, E.C.; Ahmad, A.; Karri, R.R.; Pakalapati, H. Functionalized Multi-Walled Carbon Nanotubes and Hydroxyapatite Nanorods Reinforced with Polypropylene for Biomedical Application. Sci. Rep. 2021, 11, 843. [Google Scholar] [CrossRef] [PubMed]
- Yoon, H.; Thompson, R.; Hwang, B. Dispersibility Study of Carbon Nanotubes Using Multiple Light Scattering: A Mini-Review. Colloid Interface Sci. Commun. 2023, 52, 100686. [Google Scholar] [CrossRef]
- Li, S.; Yan, J.; Zhang, Y.; Qin, Y.; Zhang, Y.; Du, S. Comparative Investigation of Carbon Nanotubes Dispersion Using Surfactants: A Molecular Dynamics Simulation and Experimental Study. J. Mol. Liq. 2023, 377, 121569. [Google Scholar] [CrossRef]
- Choi, Y.J.; Nacpil, E.J.C.; Han, J.; Zhu, C.; Kim, I.S.; Jeon, I. Recent Advances in Dispersant Technology for Carbon Nanotubes toward Energy Device Applications. Adv. Energy Sustain. Res. 2024, 5, 2300219. [Google Scholar] [CrossRef]
- Yang, H.; Neal, L.; Flores, E.E.; Adronov, A.; Kim, N.Y. Role and Impact of Surfactants in Carbon Nanotube Dispersions and Sorting. J. Surfactants Deterg. 2023, 26, 607–622. [Google Scholar] [CrossRef]
- Abdulhameed, A.; Halin, I.A.; Mohtar, M.N.; Hamidon, M.N. Optimization of Surfactant Concentration in Carbon Nanotube Solutions for Dielectrophoretic Ceiling Assembly and Alignment: Implications for Transparent Electronics. ACS Omega 2022, 7, 3680–3688. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Qiu, Y. Dispersion, Sedimentation and Aggregation of Multi-Walled Carbon Nanotubes as Affected by Single and Binary Mixed Surfactants. R. Soc. Open Sci. 2019, 6, 190241. [Google Scholar] [CrossRef]
- Goodman, S.M.; Ferguson, N.; Dichiara, A.B. Lignin-Assisted Double Acoustic Irradiation for Concentrated Aqueous Dispersions of Carbon Nanotubes. RSC Adv. 2017, 7, 5488–5496. [Google Scholar] [CrossRef]
- Rochez, O.; Zorzini, G.; Amadou, J.; Claes, M.; Richel, A. Dispersion of Multiwalled Carbon Nanotubes in Water by Lignin. J. Mater. Sci. 2013, 48, 4962–4964. [Google Scholar] [CrossRef]
- Teng, N.-Y.; Dallmeyer, I.; Kadla, J.F. Effect of Softwood Kraft Lignin Fractionation on the Dispersion of Multiwalled Carbon Nanotubes. Ind. Eng. Chem. Res. 2013, 52, 6311–6317. [Google Scholar] [CrossRef]
- Culebras, M.; Ren, G.; O’Connell, S.; Vilatela, J.J.; Collins, M.N. Lignin Doped Carbon Nanotube Yarns for Improved Thermoelectric Efficiency. Adv. Sustain. Syst. 2020, 4, 2000147. [Google Scholar] [CrossRef]
- Kamarudin, S.F.; Ismail, N.H.; Mariatti, M. Lignin-Assisted Carbon Nanotube Dispersion for Conductive Ink Application. In Proceedings of the 3rd International Postgraduate Conference on Materials, Minerals & Polymer (MAMIP) 2019, Penang, Malaysia, 31 October–1 November 2019; p. 020003. [Google Scholar]
- Wang, E.; Huang, W.; Miao, Y.; Jia, L.; Liang, Y.; Wang, S.; Zhang, W.; Zou, L.-H.; Zhong, Y.; Huang, J. Conductive and Superhydrophobic Lignin/Carbon Nanotube Coating with Nest-like Structure for Deicing, Oil Absorption and Wearable Piezoresistive Sensor. Int. J. Biol. Macromol. 2024, 278, 134886. [Google Scholar] [CrossRef]
- Onfray, C.; Thiam, A. Biomass-Derived Carbon-Based Electrodes for Electrochemical Sensing: A Review. Micromachines 2023, 14, 1688. [Google Scholar] [CrossRef]
- Madikere Raghunatha Reddy, A.K.; Darwiche, A.; Reddy, M.V.; Zaghib, K. Review on Advancements in Carbon Nanotubes: Synthesis, Purification, and Multifaceted Applications. Batteries 2025, 11, 71. [Google Scholar] [CrossRef]
- Degefu, H.; Amare, M.; Tessema, M.; Admassie, S. Lignin Modified Glassy Carbon Electrode for the Electrochemical Determination of Histamine in Human Urine and Wine Samples. Electrochim. Acta 2014, 121, 307–314. [Google Scholar] [CrossRef]
- Bounegru, A.V.; Bounegru, I. Chitosan-Based Electrochemical Sensors for Pharmaceuticals and Clinical Applications. Polymers 2023, 15, 3539. [Google Scholar] [CrossRef]
- Shalauddin, M.; Akhter, S.; Basirun, W.J.; Bagheri, S.; Anuar, N.S.; Johan, M.R. Hybrid Nanocellulose/f-MWCNTs Nanocomposite for the Electrochemical Sensing of Diclofenac Sodium in Pharmaceutical Drugs and Biological Fluids. Electrochim. Acta 2019, 304, 323–333. [Google Scholar] [CrossRef]
- Liu, T.; Sun, S.; Song, W.; Sun, X.; Niu, Q.; Liu, H.; Ohsaka, T.; Wu, J. A Lightweight and Binder-Free Electrode Enabled by Lignin Fibers@carbon-Nanotubes and Graphene for Ultrastable Lithium–Sulfur Batteries. J. Mater. Chem. A 2018, 6, 23486–23494. [Google Scholar] [CrossRef]
- Won, K.; Kim, Y.-H.; An, S.; Lee, H.J.; Park, S.; Choi, Y.-K.; Kim, J.H.; Hwang, H.-I.; Kim, H.J.; Kim, H.; et al. Glucose Oxidase/Cellulose–Carbon Nanotube Composite Paper as a Biocompatible Bioelectrode for Biofuel Cells. Appl. Biochem. Biotechnol. 2013, 171, 1194–1202. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Yang, L.; Lu, X.; He, C. Biodegradable and Renewable Poly(Lactide)–Lignin Composites: Synthesis, Interface and Toughening Mechanism. J. Mater. Chem. A 2015, 3, 3699–3709. [Google Scholar] [CrossRef]
- Chokkareddy, R.; Redhi, G.G.; Karthick, T. A Lignin Polymer Nanocomposite Based Electrochemical Sensor for the Sensitive Detection of Chlorogenic Acid in Coffee Samples. Heliyon 2019, 5, e01457. [Google Scholar] [CrossRef]
- Klonos, P.A.; Ioannidis, R.O.; Pitsavas, A.; Bikiaris, N.D.; Makri, S.P.; Koutsourea, S.; Grigoropoulos, A.; Deligkiozi, I.; Zoikis-Karathanasis, A.; Kyritsis, A.; et al. Segmental Mobility, Interfacial Polymer, Crystallization and Conductivity Study in Polylactides Filled with Hybrid Lignin-CNT Particles. Nanomaterials 2025, 15, 660. [Google Scholar] [CrossRef]
- Asl, A.Z.; Rafati, A.A.; Khazalpour, S. Electrocatalytic Behavior of TiO2 /MWCNTs Nanocomposite Decorated on Glassy Carbon Electrode for Individual and Simultaneous Voltammetric Determination of Adenine and Guanine in Real Samples. J. Electrochem. Soc. 2022, 169, 047516. [Google Scholar] [CrossRef]
- Zhang, M.; Lu, Z.; Zhao, J.; Hasebe, Y.; Zhang, Z.; Wang, Y. Simultaneous Electrochemical Determination of Hydroquinone and Catechol by Lignocellulose Biopolymers Derived Porous Carbon. ChemistrySelect 2023, 8, e202303610. [Google Scholar] [CrossRef]
- Arrigo, R.; Teresi, R.; Gambarotti, C.; Parisi, F.; Lazzara, G.; Dintcheva, N. Sonication-Induced Modification of Carbon Nanotubes: Effect on the Rheological and Thermo-Oxidative Behaviour of Polymer-Based Nanocomposites. Materials 2018, 11, 383. [Google Scholar] [CrossRef]
- Rennhofer, H.; Zanghellini, B. Dispersion State and Damage of Carbon Nanotubes and Carbon Nanofibers by Ultrasonic Dispersion: A Review. Nanomaterials 2021, 11, 1469. [Google Scholar] [CrossRef] [PubMed]
- Price, G.J.; Nawaz, M.; Yasin, T.; Bibi, S. Sonochemical Modification of Carbon Nanotubes for Enhanced Nanocomposite Performance. Ultrason. Sonochem. 2018, 40, 123–130. [Google Scholar] [CrossRef]
- Badorrek, J.; Walter, M. Computational Study on Noncovalent Interactions between (n, n) Single-walled carbon nanotubes and Simple Lignin Model-compounds. J. Comput. Chem. 2022, 43, 340–348. [Google Scholar] [CrossRef] [PubMed]
- Łukawski, D.; Grześkowiak, W.; Lekawa-Raus, A.; Widelicka, M.; Lisiecki, F.; Dudkowiak, A. Flame Retardant Effect of Lignin/Carbon Nanotubes/Potassium Carbonate Composite Coatings on Cotton Roving. Cellulose 2020, 27, 7271–7281. [Google Scholar] [CrossRef]
- Ravindren, R.; Mondal, S.; Nath, K.; Das, N.C. Synergistic Effect of Double Percolated Co-supportive MWCNT-CB Conductive Network for High-performance EMI Shielding Application. Polym. Adv. Technol. 2019, 30, 1506–1517. [Google Scholar] [CrossRef]
- Inam, A.; Brydson, R.; Edmonds, D.V. Raman Spectroscopy Study of the Crystallinity of Graphite Formed in an Experimental Free-Machining Steel. Mater. Charact. 2020, 163, 110264. [Google Scholar] [CrossRef]
- Agarwal, U.P. Analysis of Cellulose and Lignocellulose Materials by Raman Spectroscopy: A Review of the Current Status. Molecules 2019, 24, 1659. [Google Scholar] [CrossRef]
- Datsyuk, V.; Kalyva, M.; Papagelis, K.; Parthenios, J.; Tasis, D.; Siokou, A.; Kallitsis, I.; Galiotis, C. Chemical Oxidation of Multiwalled Carbon Nanotubes. Carbon 2008, 46, 833–840. [Google Scholar] [CrossRef]
- Osswald, S.; Havel, M.; Gogotsi, Y. Monitoring Oxidation of Multiwalled Carbon Nanotubes by Raman Spectroscopy. J. Raman Spectrosc. 2007, 38, 728–736. [Google Scholar] [CrossRef]
- Monti, M.; Zaccone, M.; Frache, A.; Torre, L.; Armentano, I. Dielectric Spectroscopy of PP/MWCNT Nanocomposites: Relationship with Crystalline Structure and Injection Molding Condition. Nanomaterials 2021, 11, 550. [Google Scholar] [CrossRef] [PubMed]
- Iannarelli, A.; Ghaffarian Niasar, M.; Ross, R. Electrode Interface Polarization Formation in Dielectric Elastomer Actuators. Sens. Actuators A Phys. 2020, 312, 111992. [Google Scholar] [CrossRef]
- Jiang, M.-J.; Dang, Z.-M.; Bozlar, M.; Miomandre, F.; Bai, J. Broad-Frequency Dielectric Behaviors in Multiwalled Carbon Nanotube/Rubber Nanocomposites. J. Appl. Phys. 2009, 106, 084902. [Google Scholar] [CrossRef]
- Oliveira, F.M.; Ezquerra, T.A.; Denchev, Z.Z. Broadband Electrical Conductivity of Metal/Carbon Nanotubes Polyamide 6 Composites Fabricated by Reactive Encapsulation. J. Mater. Sci. 2024, 59, 1348–1363. [Google Scholar] [CrossRef]
- Arjmand, M.; Sundararaj, U. Broadband Dielectric Properties of Multiwalled Carbon Nanotube/Polystyrene Composites. Polym. Eng. Sci. 2015, 55, 173–179. [Google Scholar] [CrossRef]
- Lage-Rivera, S.; Ares-Pernas, A.; Becerra Permuy, J.C.; Gosset, A.; Abad, M.-J. Enhancement of 3D Printability by FDM and Electrical Conductivity of PLA/MWCNT Filaments Using Lignin as Bio-Dispersant. Polymers 2023, 15, 999. [Google Scholar] [CrossRef]
- Chinnappan, A.; Lee, J.K.Y.; Jayathilaka, W.A.D.M.; Ramakrishna, S. Fabrication of MWCNT/Cu Nanofibers via Electrospinning Method and Analysis of Their Electrical Conductivity by Four-Probe Method. Int. J. Hydrogen Energy 2018, 43, 721–729. [Google Scholar] [CrossRef]
- Hoang, A.S.; Nguyen, H.N.; Bui, H.T.; Tran, A.T.; Duong, V.A.; Nguyen, V.B. Carbon Nanotubes Materials and Their Application to Guarantee Safety from Exposure to Electromagnetic Fields. Adv. Nat. Sci Nanosci. Nanotechnol. 2013, 4, 025012. [Google Scholar] [CrossRef]
- Kang, H.; Luo, S.; Du, H.; Han, L.; Li, D.; Li, L.; Fang, Q. Bio-Based Eucommia Ulmoides Gum Composites with High Electromagnetic Interference Shielding Performance. Polymers 2022, 14, 970. [Google Scholar] [CrossRef]
- Łukawski, D.; Martin, A.; Dudkowiak, A. Cost-Effective, Electroconductive Sodium Lignosulfonate/Carbon Nanotube Composite Coating for Paper Electronics. Diam. Relat. Mater. 2023, 139, 110372. [Google Scholar]
- Madinehei, M.; Kuester, S.; Kaydanova, T.; Moghimian, N.; David, É. Influence of Graphene Nanoplatelet Lateral Size on the Electrical Conductivity and Electromagnetic Interference Shielding Performance of Polyester Nanocomposites. Polymers 2021, 13, 2567. [Google Scholar] [CrossRef] [PubMed]
- Koç, Y.; Morali, U.; Erol, S.; Avci, H. Investigation of Electrochemical Behavior of Potassium Ferricyanide/Ferrocyanide Redox Probes on Screen Printed Carbon Electrode through Cyclic Voltammetry and Electrochemical Impedance Spectroscopy. Turk. J. Chem. 2021, 45, 1895–1915. [Google Scholar] [CrossRef] [PubMed]
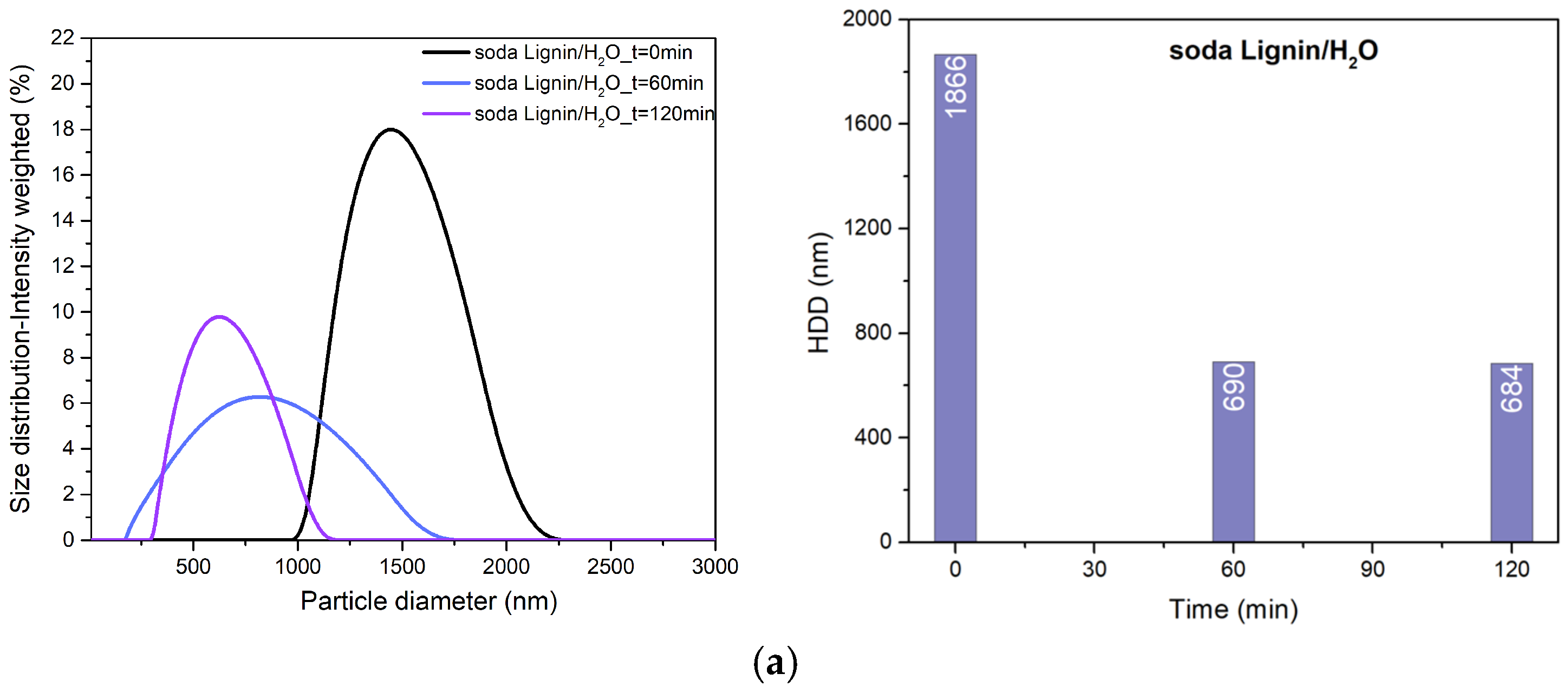
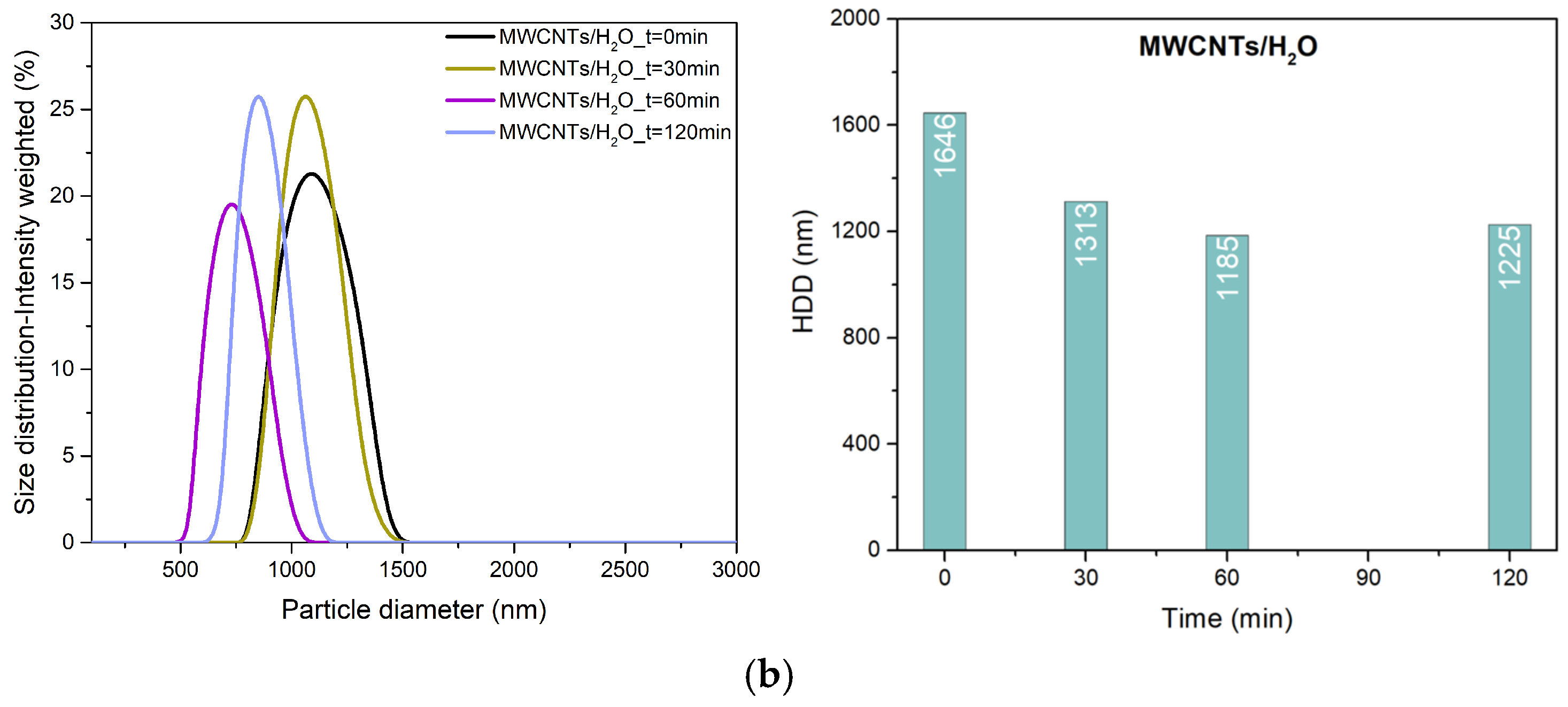
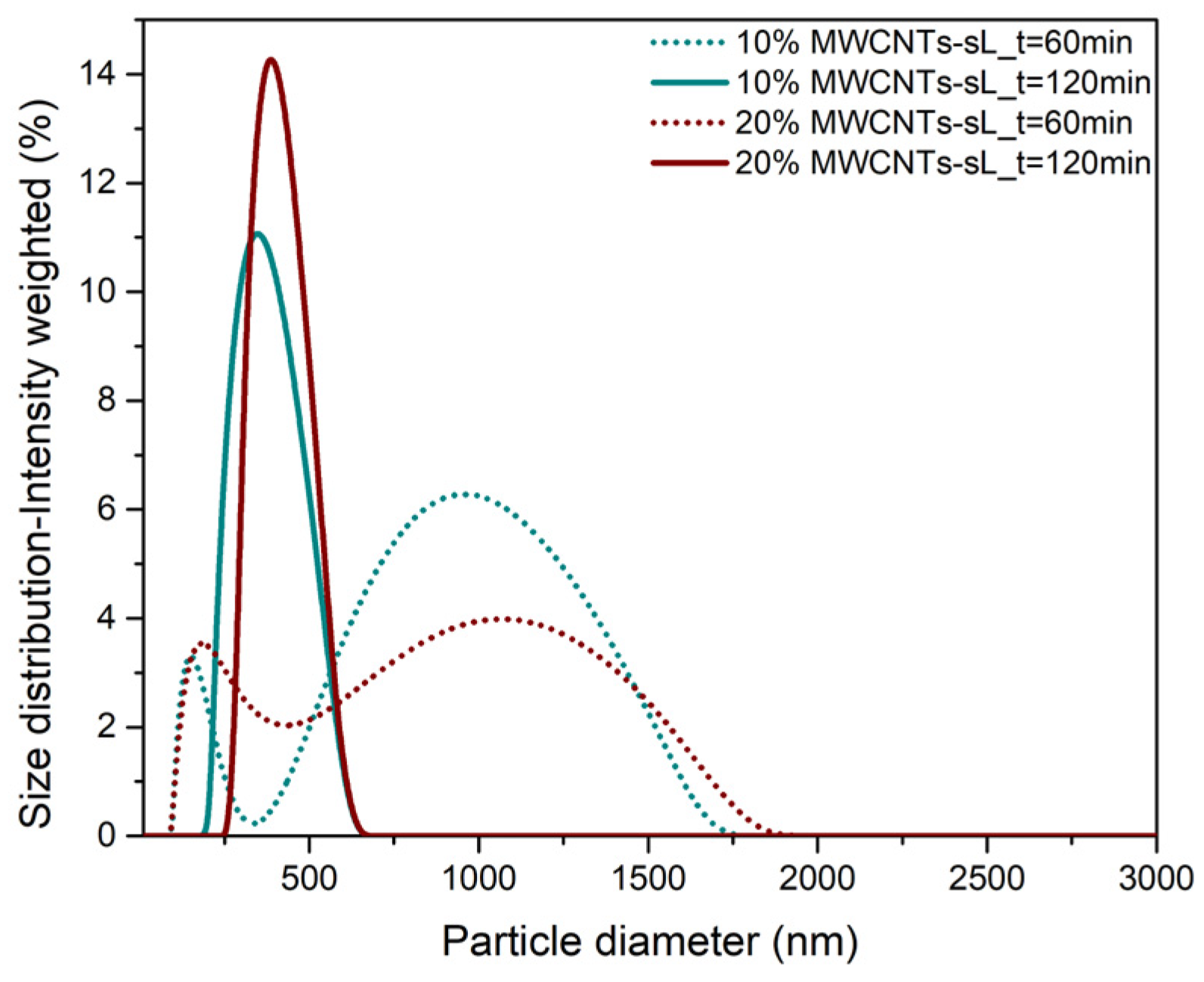

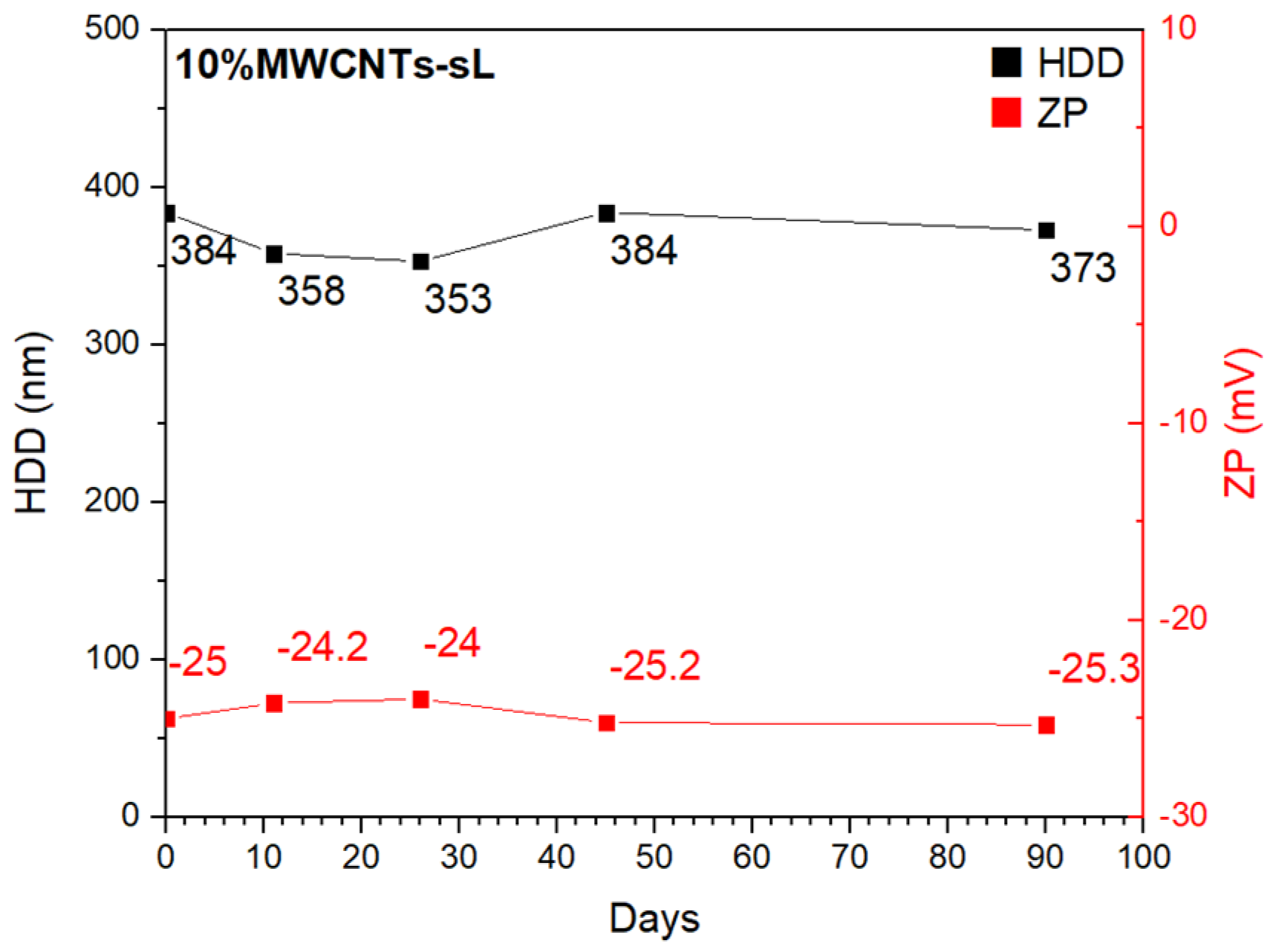
| Sample | US Time (min) | HDD (nm) | ZP (mV) |
|---|---|---|---|
| 10% MWCNTs–sL | 60 | 645 | −23 |
| 10% MWCNTs–sL | 120 | 384 | −25 |
| 20% MWCNTs–sL | 60 | 661 | −20 |
| 20% MWCNTs–sL | 120 | 470 | −21 |
| Sample Name | IG/ID |
|---|---|
| 110D | 1.95 |
| soda Lignin | 1.44 |
| MWCNTs | 0.79 |
| 10% MWCNTs–sL | 1.21 |
| 20% MWCNTs–sL | 1.09 |
| Sample Name | Measured Resistance (Ω) | Initial Sheet Resistance (Ω/sq) | Geometric Corrected Sheet Resistance (Ω/sq) | Final Sheet Resistance (Ω/sq) | Estimated Conductivity (S/cm) |
|---|---|---|---|---|---|
| 10% MWCNTs–sL | 1.312 | 5.946 | 5.155 | 5.102 | 1.960 |
| 20% MWCNTs–sL | 0.511 | 2.316 | 2.00 | 1.987 | 5.033 |
| Electrode | Slope | Electroactive Area (cm2) |
|---|---|---|
| Bare GCE | 3.43 × 10−5 | 0.0500 |
| 10% MWCNTs–sL/GCE | 4.40 × 10−4 | 0.6417 |
| 20% MWCNTs–sL/GCE | 7.16 × 10−4 | 1.0442 |
| MWCNTs/GCE | 9.17 × 10−4 | 1.3364 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Makri, S.P.; Koutsourea, S.; Grigoropoulos, A.; Berkesi, K.; Kartsinis, M.; Deligkiozi, I.; Zoikis-Karathanasis, A. Development of Green Lignin–MWCNTs Hybrids for Sustainable Conductive Materials. Electronics 2025, 14, 4539. https://doi.org/10.3390/electronics14224539
Makri SP, Koutsourea S, Grigoropoulos A, Berkesi K, Kartsinis M, Deligkiozi I, Zoikis-Karathanasis A. Development of Green Lignin–MWCNTs Hybrids for Sustainable Conductive Materials. Electronics. 2025; 14(22):4539. https://doi.org/10.3390/electronics14224539
Chicago/Turabian StyleMakri, Sofia P., Stefania Koutsourea, Alexios Grigoropoulos, Kata Berkesi, Michalis Kartsinis, Ioanna Deligkiozi, and Alexandros Zoikis-Karathanasis. 2025. "Development of Green Lignin–MWCNTs Hybrids for Sustainable Conductive Materials" Electronics 14, no. 22: 4539. https://doi.org/10.3390/electronics14224539
APA StyleMakri, S. P., Koutsourea, S., Grigoropoulos, A., Berkesi, K., Kartsinis, M., Deligkiozi, I., & Zoikis-Karathanasis, A. (2025). Development of Green Lignin–MWCNTs Hybrids for Sustainable Conductive Materials. Electronics, 14(22), 4539. https://doi.org/10.3390/electronics14224539








