Enabling Super-Resolution Quantitative Phase Imaging via OpenSRQPI—A Standardized Plug-and-Play Open-Source Tool for Digital Holographic Microscopy with Structured and Oblique Illumination
Abstract
1. Introduction
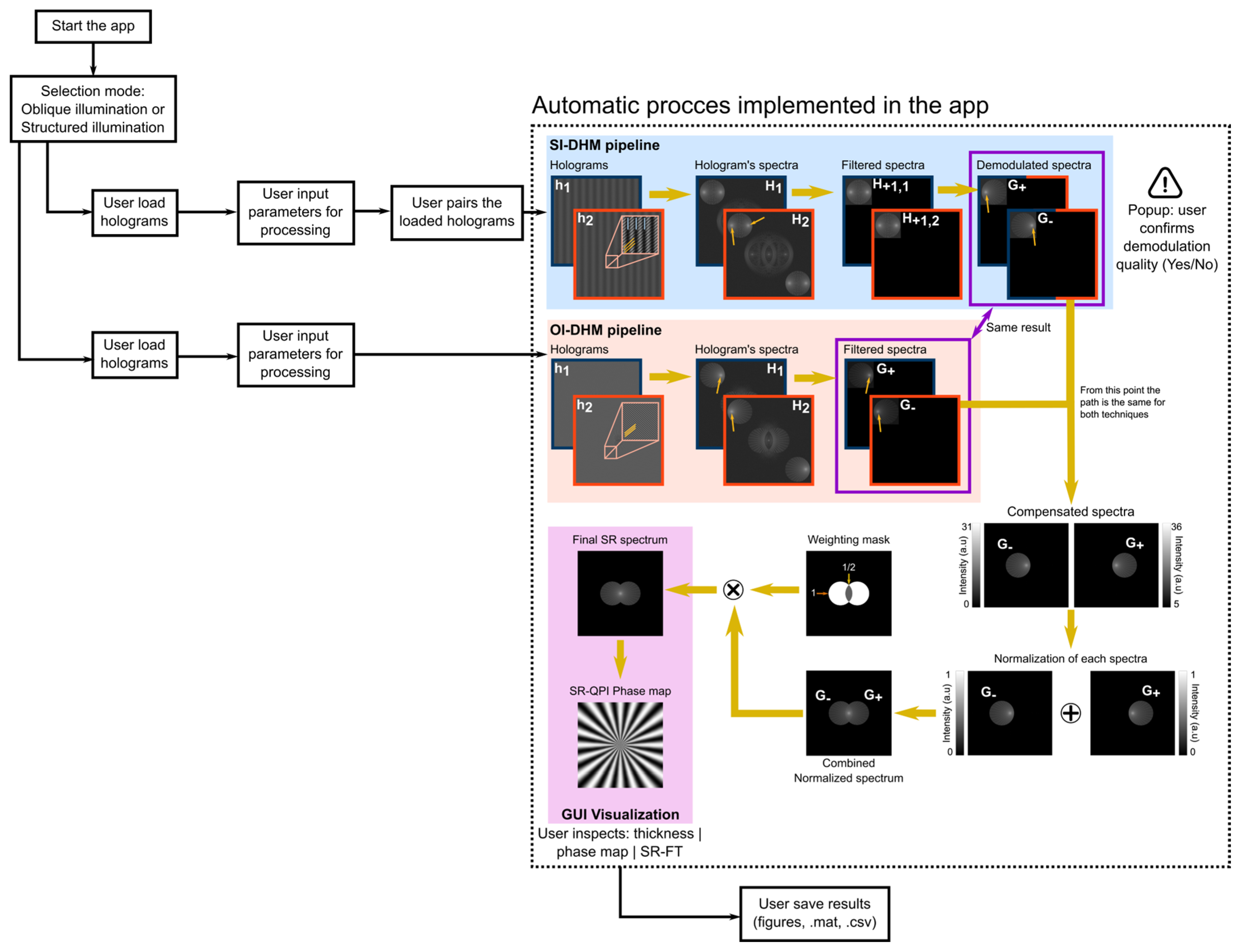
2. OpenSRQPI Tool
- Spectral Filtering for both SI- and OI-DHM: The first operation is the isolation of the +1 term in the hologram spectrum using a two-step strategy in which, firstly, a rectangular filter crops the quarter of the spectrum containing the +1 order as identified by the user earlier, and, secondly, a circular filter isolates the object spectrum frequencies. The circular filter is automatically determined using MATLAB’s findcircles function and applied to a binary spectrum generated with a threshold TH = mean + 0.8·STD, where the mean and standard deviation (STD) are estimated from a 60 × 60-px2 corner region representing the noise background of the Fourier transform. This threshold formula was empirically determined through testing with both noiseless and noisy simulated holograms. In all simulated cases, the compact support of the diffracted orders was clearly defined, and the selected threshold consistently isolated the correct spectral components. However, in experimental holograms—particularly at higher illumination angles—the visibility of the compact support decreases, and the current thresholding approach may not always ensure optimal isolation of the desired spectral region. Future releases of OpenSRQPI will address this limitation by incorporating a more robust, unsupervised spatial-filtering algorithm capable of reliably identifying and isolating spectral terms under varying illumination and noise conditions.
- Blind Demodulation for SI-DHM mode: This demodulation step is based on the generalized SI-DHM framework, which automatically demodulates each laterally shifted object spectrum independently of the other without prior knowledge of the phase shifts between the recorded holograms [43]. The selection of the correct phase shift to properly decouple the two laterally shifted spectra is based on the minimization of a cost function that quantifies the ratio of the residual order’s magnitude to the total magnitude (sum of expected and residual orders), which is defined in Ref. [43], using MATLAB’s built-in particleswarm algorithm. Iterative Particle Swarm Optimization is a population-based stochastic optimization algorithm in which a set of candidate solutions (i.e., particles) move through the search space guided by both their own experience and the collective knowledge of the swarm. Each particle updates its velocity and position based on three components: its current momentum, the best solution it has found so far, and the best solution identified by any particle in the swarm. Through an iterative process, the swarm converges toward optimal or near-optimal solutions. The optimization options of the particleswarm algorithm are a swarm size (set to 4), a maximum number of iterations allowed (set to 500), and a termination tolerance on the cost function (set to 10−8 a.u.). The swarm size is 4 since a small swarm is sufficient to explore the 1D bounded search space domain. The maximum number of iterations is 500, balancing convergence reliability and computational cost. The termination tolerance of 10−8 a.u. is adopted to provide a strict convergence criterion since small angular deviations in the estimated phase shift can lead to significant phase errors in the reconstructed holograms. The optimal phase shift ranges between 0 and 2π, preventing the optimizer from exploring non-physical values. The rest of the parameters (i.e., inertia weight, cognitive/social coefficients, and velocity limits) remain at MATLAB’s standard settings. Because demodulation performance depends strongly on the data quality, the algorithm incorporates a verification stage to confirm the uniqueness of the demodulated laterally shifted replica before proceeding with the processing pipeline.
- Phase compensation: The centering of the maximum peak in the laterally shifted object spectra to the zero frequency has been implemented using the high-order vortex-Legendre phase compensation [44], improving phase compensation accuracy (i.e., spatial invariance on the phase values) while maintaining computational efficiency. The order and mode of the Legendre polynomial to be compensated should be defined before starting the processing.
- Spectral Normalization and Fusion: The next step focuses on combining the centered object spectra into an SR spectrum. Whereas the combination of these spectra is the direct summation in fluorescence-based SIM, in QPI, this process should be carefully implemented since each individual spectrum has its own and different dynamic range. This means that each individual spectrum should be normalized before summation, ensuring super-resolved QPI measurements. More details on the effects of this normalization are found in Ref. [45], where the QPI capability of the reconstructed SR phase distribution is analyzed and compared, both with and without normalizing each demodulated spectrum. Normalization ensures balance, but overlapping spectral content across angles can still distort frequency weighting. To address this, the combined SR spectrum is multiplied pixel-wise by a weighting mask, which is automatically generated to equalize the spectral content [31]. The inverse Fourier transform of this product provides the spatial complex SR distribution, from which the quantitative phase map can be directly obtained by computing the angle of this complex field. This resultant phase map retains QPI integrity while achieving resolution enhancement beyond the diffraction limit.
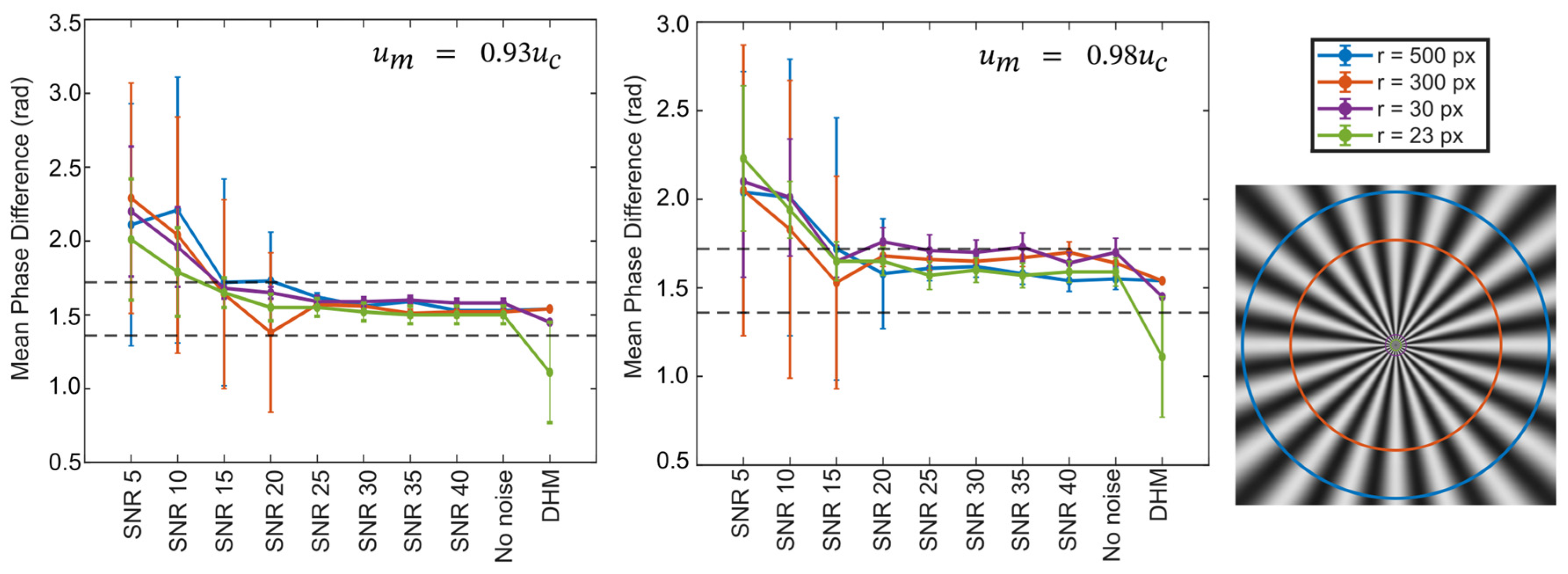
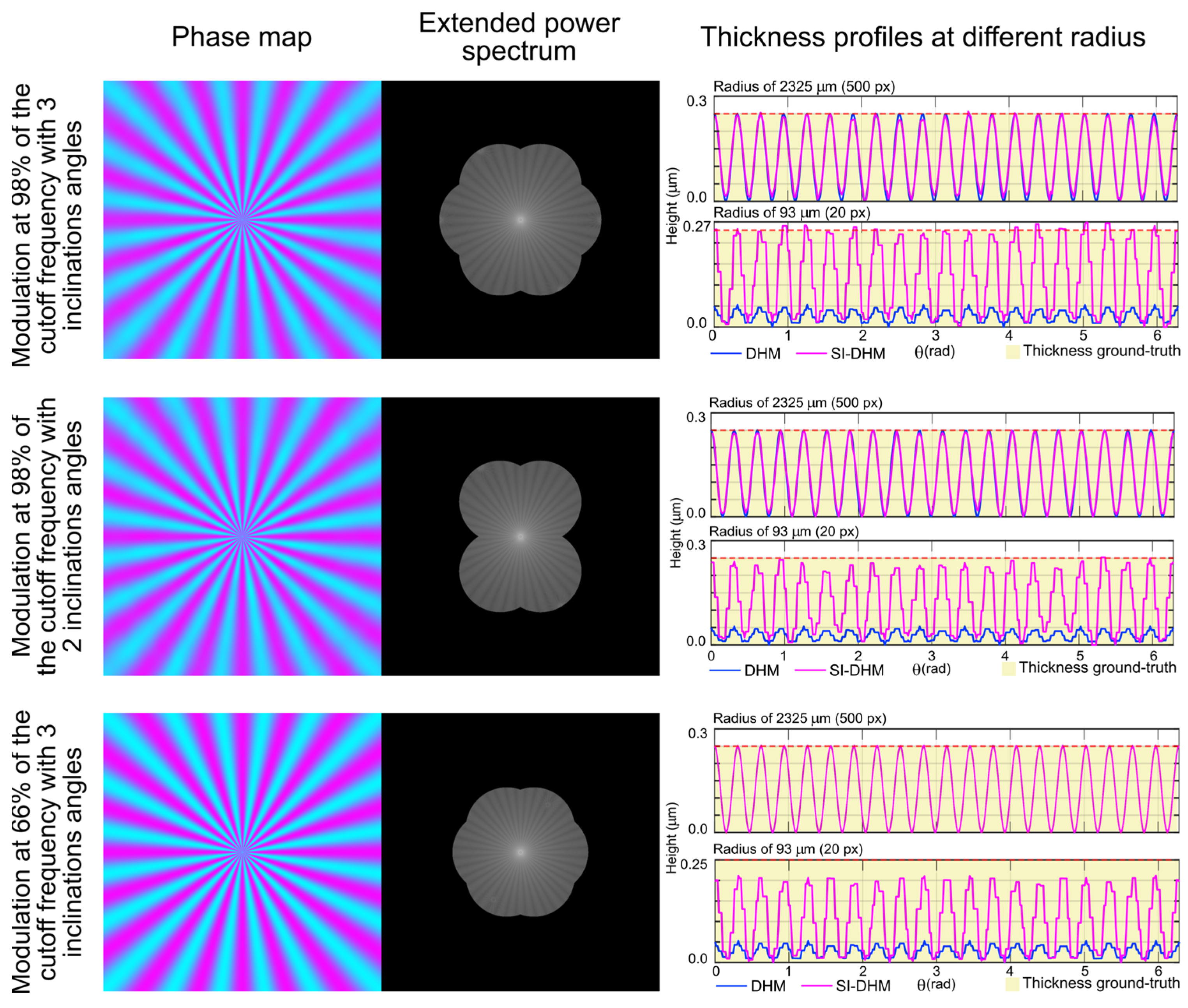
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 2D | Two-Dimensional |
| 3D | Three-Dimensional |
| DHM | Digital Holographic Microscope |
| FT | Fourier Transform |
| GUI | Graphical User Interface |
| OI | Oblique Illumination |
| QPI | Quantitative Phase Imaging |
| SI | Structured Illumination |
| SIM | Structured Illumination Microscopy |
References
- Aknoun, S.; Yonnet, M.; Djabari, Z.; Graslin, F.; Taylor, M.; Pourcher, T.; Wattellier, B.; Pognonec, P. Quantitative Phase Microscopy for Non-Invasive Live Cell Population Monitoring. Sci. Rep. 2021, 11, 4409. [Google Scholar] [CrossRef]
- Nguyen, T.L.; Pradeep, S.; Judson-Torres, R.L.; Reed, J.; Teitell, M.A.; Zangle, T.A. Quantitative Phase Imaging: Recent Advances and Expanding Potential in Biomedicine. ACS Nano 2022, 16, 11516–11544. [Google Scholar] [CrossRef]
- Wang, R.; Wang, Z.; Millet, L.; Gillette, M.U.; Levine, A.J.; Popescu, G. Dispersion-Relation Phase Spectroscopy of Intracellular Transport. Opt. Express 2011, 19, 20571–20579. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Kim, K.; Jung, J.; Heo, J.; Cho, S.; Lee, S.; Chang, G.; Jo, Y.; Park, H.; Park, Y. Quantitative Phase Imaging Techniques for the Study of Cell Pathophysiology: From Principles to Applications. Sensors 2013, 13, 4170–4191. [Google Scholar] [CrossRef]
- Liu, Y.; Uttam, S. Perspective on Quantitative Phase Imaging to Improve Precision Cancer Medicine. J. Biomed. Opt. 2024, 29, S22705. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.; Matemba, L.E.; Lee, K.; Kazyoba, P.E.; Yoon, J.; Massaga, J.J.; Kim, K.; Kim, D.-J.; Park, Y. Optical Characterization of Red Blood Cells from Individuals with Sickle Cell Trait and Disease in Tanzania Using Quantitative Phase Imaging. Sci. Rep. 2016, 6, 31698. [Google Scholar] [CrossRef]
- Kim, Y.; Shim, H.; Kim, K.; Park, H.; Jang, S.; Park, Y. Profiling Individual Human Red Blood Cells Using Common-Path Diffraction Optical Tomography. Sci. Rep. 2014, 4, 6659. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.; Jo, Y.; Cho, H.; Min, H.; Park, Y. Learning-Based Screening of Hematologic Disorders Using Quantitative Phase Imaging of Individual Red Blood Cells. Biosens. Bioelectron. 2019, 123, 69–76. [Google Scholar] [CrossRef]
- Song, G.; Steelman, Z.A.; Finkelstein, S.; Yang, Z.; Martin, L.; Chu, K.K.; Farsiu, S.; Arshavsky, V.Y.; Wax, A. Multimodal Coherent Imaging of Retinal Biomarkers of Alzheimer’s Disease in a Mouse Model. Sci. Rep. 2020, 10, 7912. [Google Scholar] [CrossRef]
- Polanco, E.R.; Moustafa, T.E.; Butterfield, A.; Scherer, S.D.; Cortes-Sanchez, E.; Bodily, T.; Spike, B.T.; Welm, B.E.; Bernard, P.S.; Zangle, T.A. Multiparametric Quantitative Phase Imaging for Real-Time, Single Cell, Drug Screening in Breast Cancer. Commun. Biol. 2022, 5, 794. [Google Scholar] [CrossRef]
- Marzi, A.; Eder, K.M.; Barroso, Á.; Kemper, B.; Schnekenburger, J. Quantitative Phase Imaging as Sensitive Screening Method for Nanoparticle-Induced Cytotoxicity Assessment. Cells 2024, 13, 697. [Google Scholar] [CrossRef] [PubMed]
- de Groot, P.J.; Deck, L.L.; Su, R.; Osten, W. Contributions of Holography to the Advancement of Interferometric Measurements of Surface Topography. Light Adv. Manuf. 2022, 3, 258–277. [Google Scholar] [CrossRef]
- Ferraro, P.; Grilli, S.; Alfieri, D.; De Nicola, S.; Finizio, A.; Pierattini, G.; Javidi, B.; Coppola, G.; Striano, V. Extended Focused Image in Microscopy by Digital Holography. Opt. Express 2005, 13, 6738–6749. [Google Scholar] [CrossRef]
- Yu, Z.; Chen, W.; Liu, J.; Zhang, J.; Guo, Y.; Gu, H.; Dong, Z.; Nie, L.; Jiang, H.; Deng, H.; et al. Characterizing Lateral Distribution of Complex Refractive Index Based on Quantitative Amplitude-Phase Imaging (QAPI). Opt. Lasers Eng. 2025, 194, 109199. [Google Scholar] [CrossRef]
- Kemper, B.; Bally, G. von Digital Holographic Microscopy for Live Cell Applications and Technical Inspection. Appl. Opt. 2008, 47, A52–A61. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Cao, Z.; Yu, S. A Review on Optical Characterization of Refractive Index in Photonic Related Devices and Applications. J. Phys. D Appl. Phys. 2024, 57, 483002. [Google Scholar] [CrossRef]
- Wang, Z.; Millet, L.; Mir, M.; Ding, H.; Unarunotai, S.; Rogers, J.; Gillette, M.U.; Popescu, G. Spatial Light Interference Microscopy (SLIM). Opt. Express 2011, 19, 1016–1026. [Google Scholar] [CrossRef]
- Cuche, E.; Bevilacqua, F.; Depeursinge, C. Digital Holography for Quantitative Phase-Contrast Imaging. Opt. Lett. 1999, 24, 291–293. [Google Scholar] [CrossRef]
- Popescu, G.; Ikeda, T.; Dasari, R.R.; Feld, M.S. Diffraction Phase Microscopy for Quantifying Cell Structure and Dynamics. Opt. Lett. 2006, 31, 775–777. [Google Scholar] [CrossRef]
- Ou, X.; Horstmeyer, R.; Yang, C.; Zheng, G. Quantitative Phase Imaging via Fourier Ptychographic Microscopy. Opt. Lett. 2013, 38, 4845–4848. [Google Scholar] [CrossRef]
- Streibl, N. Phase Imaging by the Transport Equation of Intensity. Opt. Commun. 1984, 49, 6–10. [Google Scholar] [CrossRef]
- Javidi, B.; Carnicer, A.; Anand, A.; Barbastathis, G.; Chen, W.; Ferraro, P.; Goodman, J.W.; Horisaki, R.; Khare, K.; Kujawinska, M.; et al. Roadmap on Digital Holography [Invited]. Opt. Express 2021, 29, 35078–35118. [Google Scholar] [CrossRef]
- Yu, X.; Hong, J.; Liu, C.; Kim, M.K. Review of Digital Holographic Microscopy for Three-Dimensional Profiling and Tracking. Opt. Eng. 2014, 53, 112306. [Google Scholar] [CrossRef]
- Marquet, P.; Depeursinge, C.; Magistretti, P.J. Review of Quantitative Phase-Digital Holographic Microscopy: Promising Novel Imaging Technique to Resolve Neuronal Network Activity and Identify Cellular Biomarkers of Psychiatric Disorders. Neurophotonics 2014, 1, 20901. [Google Scholar] [CrossRef]
- Tahara, T.; Quan, X.; Otani, R.; Takaki, Y.; Matoba, O. Digital Holography and Its Multidimensional Imaging Applications: A Review. Microscopy 2018, 67, 55–67. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Ortiga, E.; Doblas, A.; Saavedra, G.; Martínez-Corral, M.; Garcia-Sucerquia, J. Off-Axis Digital Holographic Microscopy: Practical Design Parameters for Operating at Diffraction Limit. Appl. Opt. 2014, 53, 2058–2066. [Google Scholar] [CrossRef] [PubMed]
- Hussain, A.; Mudassar, A.A. Optical Super Resolution Using Tilted Illumination Coupled with Object Rotation. Opt. Commun. 2015, 339, 34–40. [Google Scholar] [CrossRef]
- Mico, V.; Zalevsky, Z.; García-Martínez, P.; García, J. Superresolved Imaging in Digital Holography by Superposition of Tilted Wavefronts. Appl. Opt. 2006, 45, 822–828. [Google Scholar] [CrossRef]
- Ma, Y.; Wen, K.; Liu, M.; Zheng, J.; Chu, K.; Smith, Z.J.; Liu, L.; Gao, P. Recent Advances in Structured Illumination Microscopy. J. Phys. Photonics 2021, 3, 024009. [Google Scholar] [CrossRef]
- Yaghoubi, S.H.S.; Ebrahimi, S.; Dashtdar, M. Structured Illumination in Fresnel Biprism-Based Digital Holographic Microscopy. Opt. Lasers Eng. 2022, 159, 107215. [Google Scholar] [CrossRef]
- Sánchez-Ortiga, E.; Martínez-Corral, M.; Saavedra, G.; Garcia-Sucerquia, J. Enhancing Spatial Resolution in Digital Holographic Microscopy by Biprism Structured Illumination. Opt. Lett. 2014, 39, 2086–2089. [Google Scholar] [CrossRef]
- Müller, M.; Mönkemöller, V.; Hennig, S.; Hübner, W.; Huser, T. Open-Source Image Reconstruction of Super-Resolution Structured Illumination Microscopy Data in ImageJ. Nat. Commun. 2016, 7, 10980. [Google Scholar] [CrossRef] [PubMed]
- Křížek, P.; Lukeš, T.; Ovesný, M.; Fliegel, K.; Hagen, G.M. SIMToolbox: A MATLAB Toolbox for Structured Illumination Fluorescence Microscopy. Bioinformatics 2016, 32, 318–320. [Google Scholar] [CrossRef]
- Cao, R.; Li, Y.; Chen, X.; Ge, X.; Li, M.; Guan, M.; Hou, Y.; Fu, Y.; Xu, X.; Leterrier, C. Open-3DSIM: An Open-Source Three-Dimensional Structured Illumination Microscopy Reconstruction Platform. Nat. Methods 2023, 20, 1183–1186. [Google Scholar] [CrossRef] [PubMed]
- Dong, C.; Loy, C.C.; He, K.; Tang, X. Image Super-Resolution Using Deep Convolutional Networks. IEEE Trans. Pattern Anal. Mach. Intell. 2015, 38, 295–307. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Wang, M.; Huang, Q.; Liu, X. Robust Multi-Frame Super-Resolution Based on Adaptive Half-Quadratic Function and Local Structure Tensor Weighted Btv. Sensors 2021, 21, 5533. [Google Scholar] [CrossRef]
- Jin, L.; Liu, B.; Zhao, F.; Hahn, S.; Dong, B.; Song, R.; Elston, T.C.; Xu, Y.; Hahn, K.M. Deep Learning Enables Structured Illumination Microscopy with Low Light Levels and Enhanced Speed. Nat. Commun. 2020, 11, 1934. [Google Scholar] [CrossRef]
- Song, L.; Liu, X.; Xiong, Z.; Ahamed, M.; An, S.; Zheng, J.; Ma, Y.; Gao, P. Super-Resolution Reconstruction of Structured Illumination Microscopy Using Deep-Learning and Sparse Deconvolution. Opt. Lasers Eng. 2024, 174, 107968. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, Q.; Zhang, J.; Ye, Z.; Ye, H.; Zhu, Y.; Kuang, C.; Chen, Y.; Liu, W. Deep Learning Enables Contrast-Robust Super-Resolution Reconstruction in Structured Illumination Microscopy. Opt. Express 2024, 32, 3316–3328. [Google Scholar] [CrossRef]
- Liu, T.; Liu, J.; Li, D.; Tan, S. Bayesian Deep-Learning Structured Illumination Microscopy Enables Reliable Super-Resolution Imaging with Uncertainty Quantification. Nat. Commun. 2025, 16, 5027. [Google Scholar] [CrossRef]
- Zhang, Q.; Liang, C.; Tang, M.; Yang, X.; Lin, M.; Han, Y.; Liu, X.; Yang, Q. Alternative Deep Learning Method for Fast Spatial-Frequency Shift Imaging Microscopy. Opt. Express 2023, 31, 3719–3730. [Google Scholar] [CrossRef]
- Huo, Y.; Gang, S.; Guan, C. FCIHMRT: Feature Cross-Layer Interaction Hybrid Method Based on Res2Net and Transformer for Remote Sensing Scene Classification. Electronics 2023, 12, 4362. [Google Scholar] [CrossRef]
- Obando-Vásquez, S.; Castaneda, R.; Restrepo, R.; Trujillo, C.; Doblas, A. Generalized Computational Framework for Phase Image Reconstruction in Structured Illumination Digital Holographic Microscopy. Opt. Express 2025, 33, 16454–16467. [Google Scholar] [CrossRef]
- Ortega, K.; Restrepo, R.; Padilla-Vivanco, A.; Castaneda, R.; Doblas, A.; Trujillo, C. Intricate Quantitative Phase Imaging via Vortex-Legendre High-Order Phase Compensation. Opt. Lasers Eng. 2025, 195, 109318. [Google Scholar] [CrossRef]
- Schenider, A.; Obando-Vasquez, S.; Doblas, A. SI-QPI-Stitcher—A User-Friendly Computational Tool to Stitch the Super-Resolved Spectral Components for Accurate Quantitative Phase Imaging (QPI) in Digital Holographic Microscopy (DHM) with Structured Illumination (SI). Proc. SPIE 2025, 13604, Optics and Photonics for Information Processing XIX, 136040U. [Google Scholar]
- Goodman, J.W. Statistical Optics; John Wiley & Sons: Hoboken, NJ, USA, 2015; ISBN 1119009456. [Google Scholar]
- Obando-Vásquez, S.; Lopera, M.J.; Restrepo, R.; Trujillo, C. Comparative Analysis of Digital Holographic Microscopy and Digital Lensless Holographic Microscopy for Quantitative Phase Imaging. Opt. Contin. 2024, 3, 309–323. [Google Scholar] [CrossRef]
- Doblas, A.; Buitrago-Duque, C.; Robinson, A.; Garcia-Sucerquia, J. Phase-Shifting Digital Holographic Microscopy with an Iterative Blind Reconstruction Algorithm. Appl. Opt. 2019, 58, G311–G317. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Obando-Vasquez, S.; Schneider, A.; Doblas, A. Enabling Super-Resolution Quantitative Phase Imaging via OpenSRQPI—A Standardized Plug-and-Play Open-Source Tool for Digital Holographic Microscopy with Structured and Oblique Illumination. Electronics 2025, 14, 4513. https://doi.org/10.3390/electronics14224513
Obando-Vasquez S, Schneider A, Doblas A. Enabling Super-Resolution Quantitative Phase Imaging via OpenSRQPI—A Standardized Plug-and-Play Open-Source Tool for Digital Holographic Microscopy with Structured and Oblique Illumination. Electronics. 2025; 14(22):4513. https://doi.org/10.3390/electronics14224513
Chicago/Turabian StyleObando-Vasquez, Sofia, Alan Schneider, and Ana Doblas. 2025. "Enabling Super-Resolution Quantitative Phase Imaging via OpenSRQPI—A Standardized Plug-and-Play Open-Source Tool for Digital Holographic Microscopy with Structured and Oblique Illumination" Electronics 14, no. 22: 4513. https://doi.org/10.3390/electronics14224513
APA StyleObando-Vasquez, S., Schneider, A., & Doblas, A. (2025). Enabling Super-Resolution Quantitative Phase Imaging via OpenSRQPI—A Standardized Plug-and-Play Open-Source Tool for Digital Holographic Microscopy with Structured and Oblique Illumination. Electronics, 14(22), 4513. https://doi.org/10.3390/electronics14224513







