Retiplus: Augmented Reality Rehabilitation System to Enhance Autonomy and Quality of Life in Individuals with Low Vision
Abstract
1. Introduction
2. Related Work
3. Methodology
3.1. Patient Interaction with the System
3.2. Visual Condition Assessment
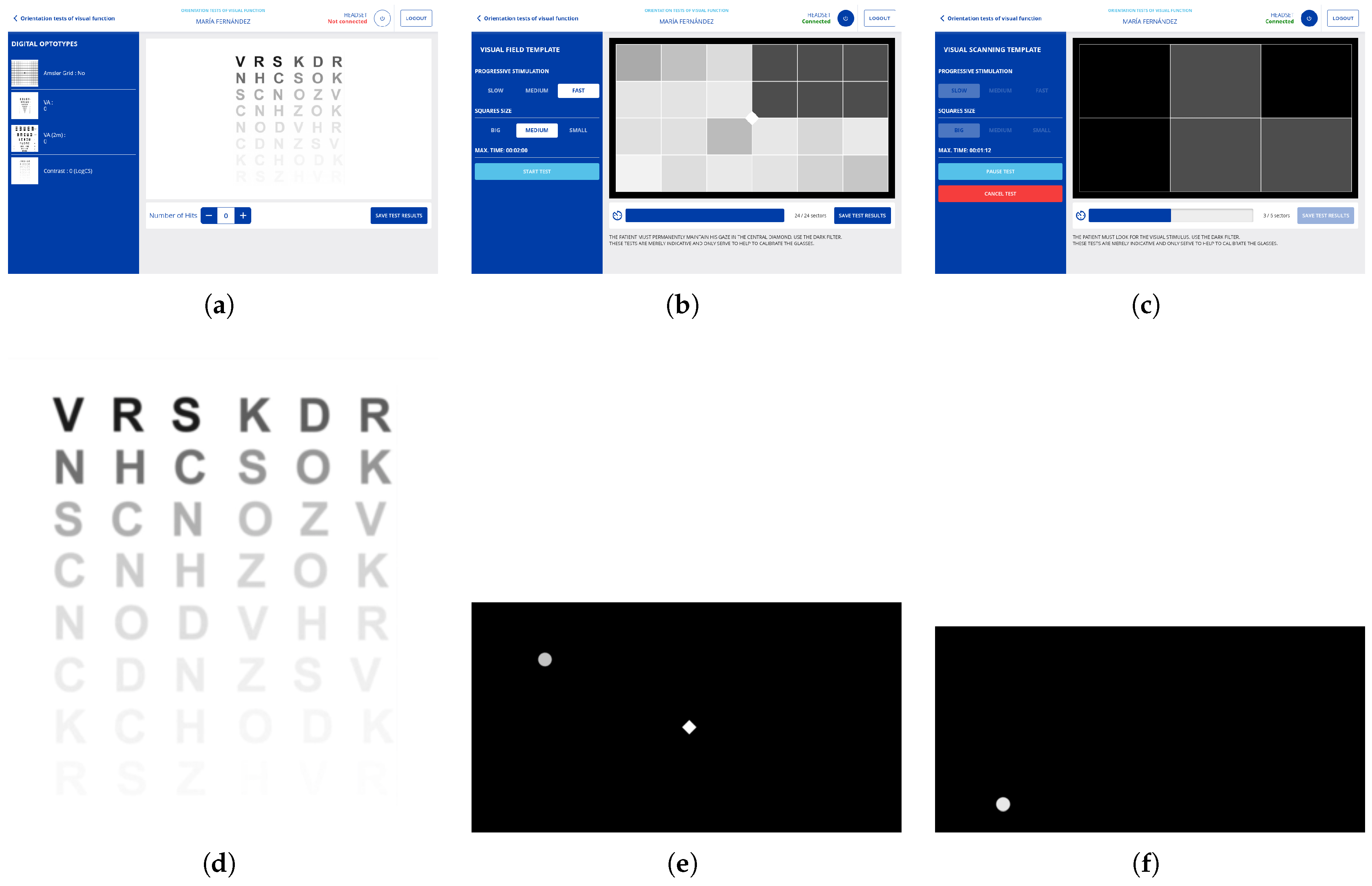
3.2.1. Digitization of Optometric Evaluation Instruments
3.2.2. Visual Field Measurements
3.2.3. Determine Visual Scanning Capability
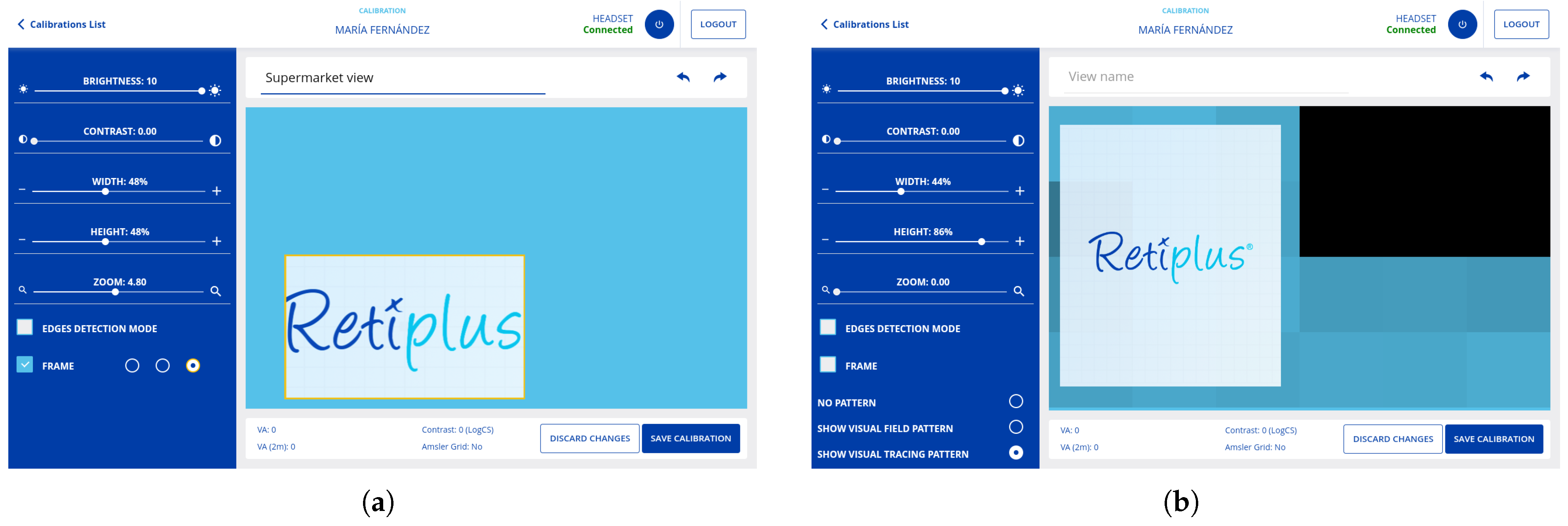
3.3. Personalization of Visual Aids and Rehabilitation
3.4. Patient Usage Data
3.5. Compilation of Key Features
3.6. Retiplus: A Method to Address the Identified System Requirements
3.6.1. Implementation
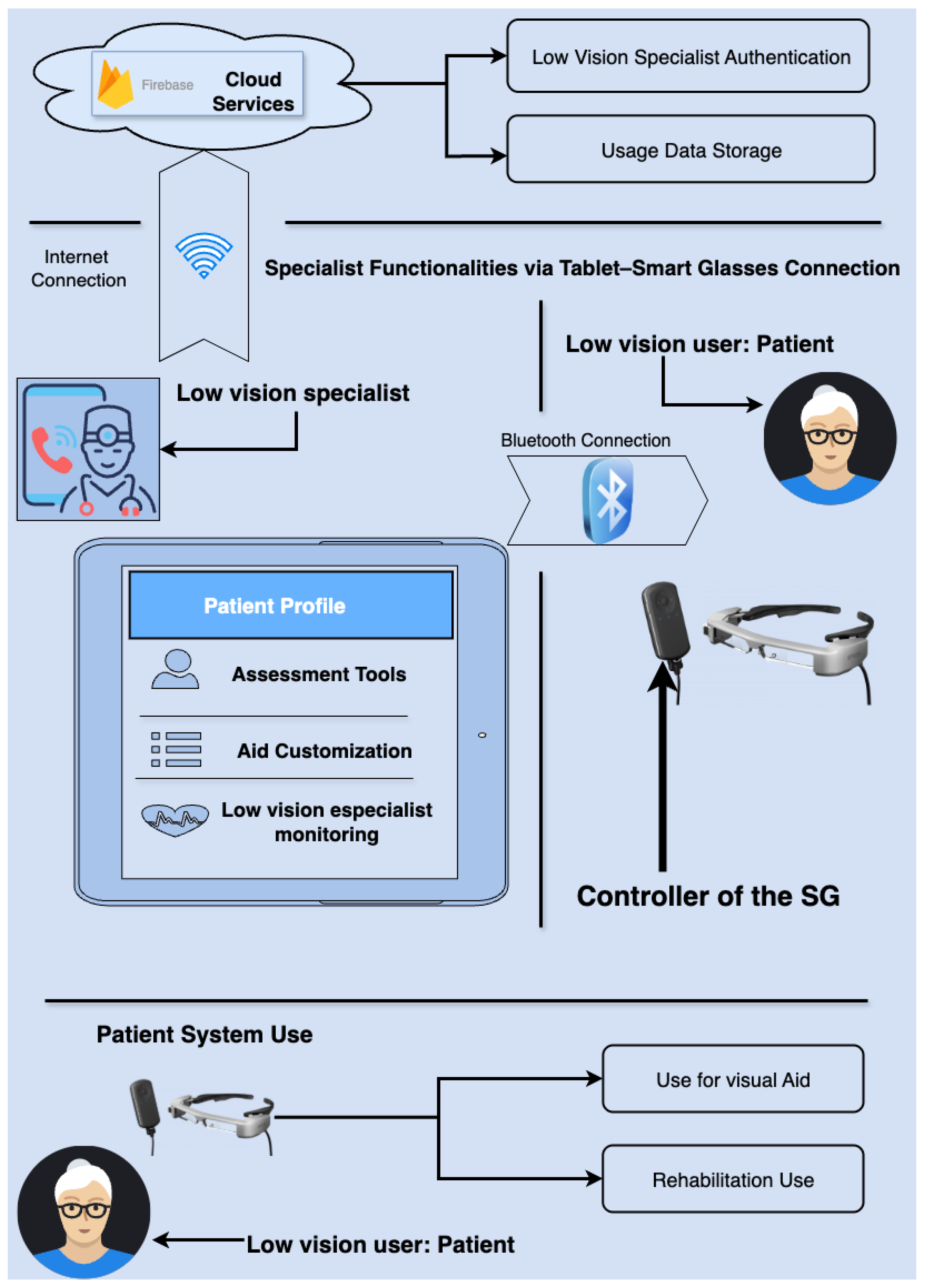
3.6.2. System Hardware and Functional Scheme
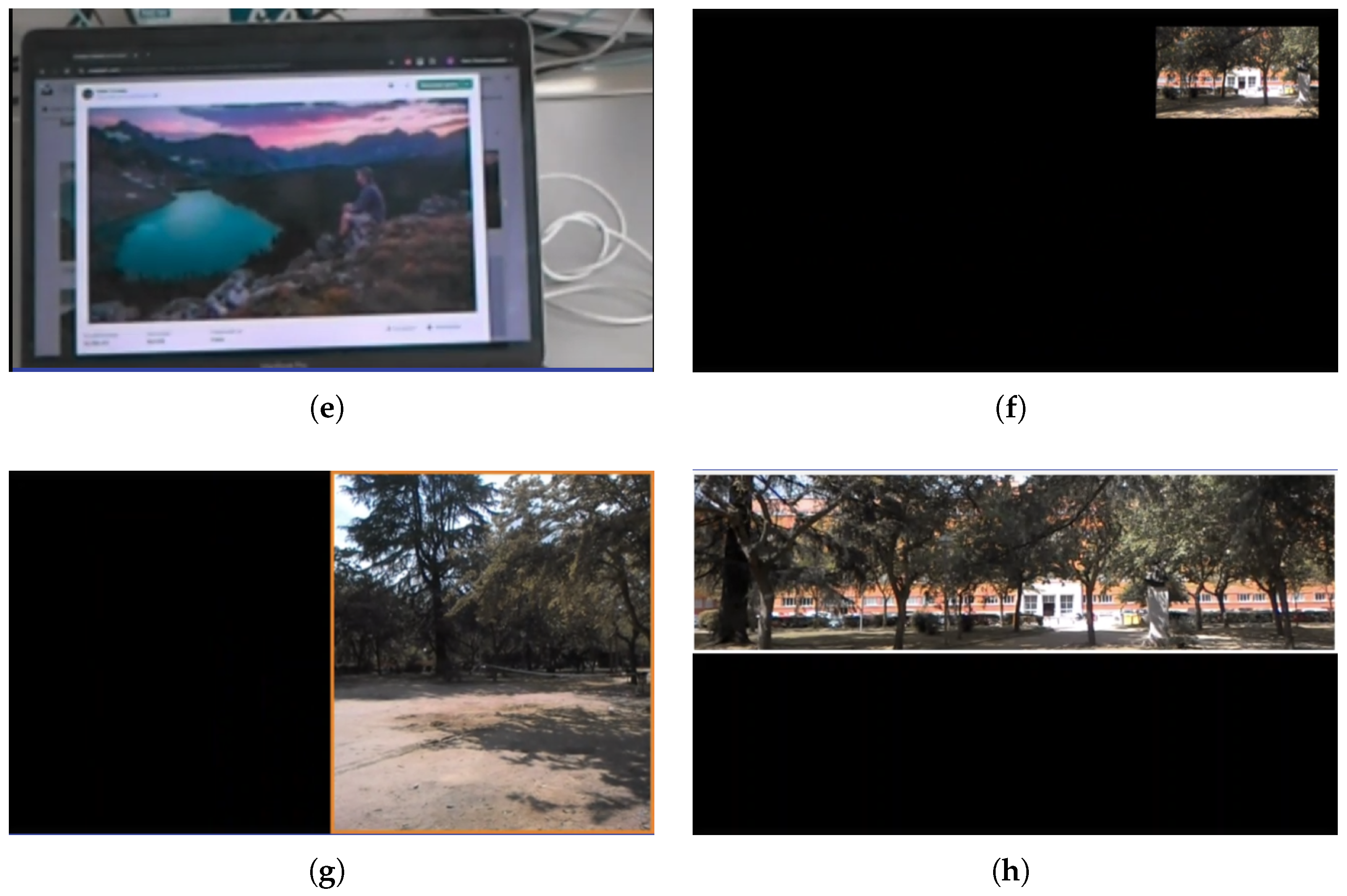
3.7. System Performance and Real-Time Pipeline
- 1.
- Acquisition: frames captured by the smart-glasses camera (Epson Moverio BT-350) (Seiko Epson Corporation, Suwa, Nagano, Japan).
- 2.
- Pre-processing: lightweight resizing/format conversion to reduce computing and memory bandwidth.
- 3.
- Real-time processing: application of the minification/rescaling and overlay rendering on the onboard controller (Intel® Atom™ x5, Quad-Core 1.44 GHz, 2 GB RAM) (Intel Corporation, Santa Clara, CA, USA).
- 4.
- Composition and projection: immediate display of processed frames on the binocular see-through screens.
3.8. Development and Use of the System
3.8.1. Interaction
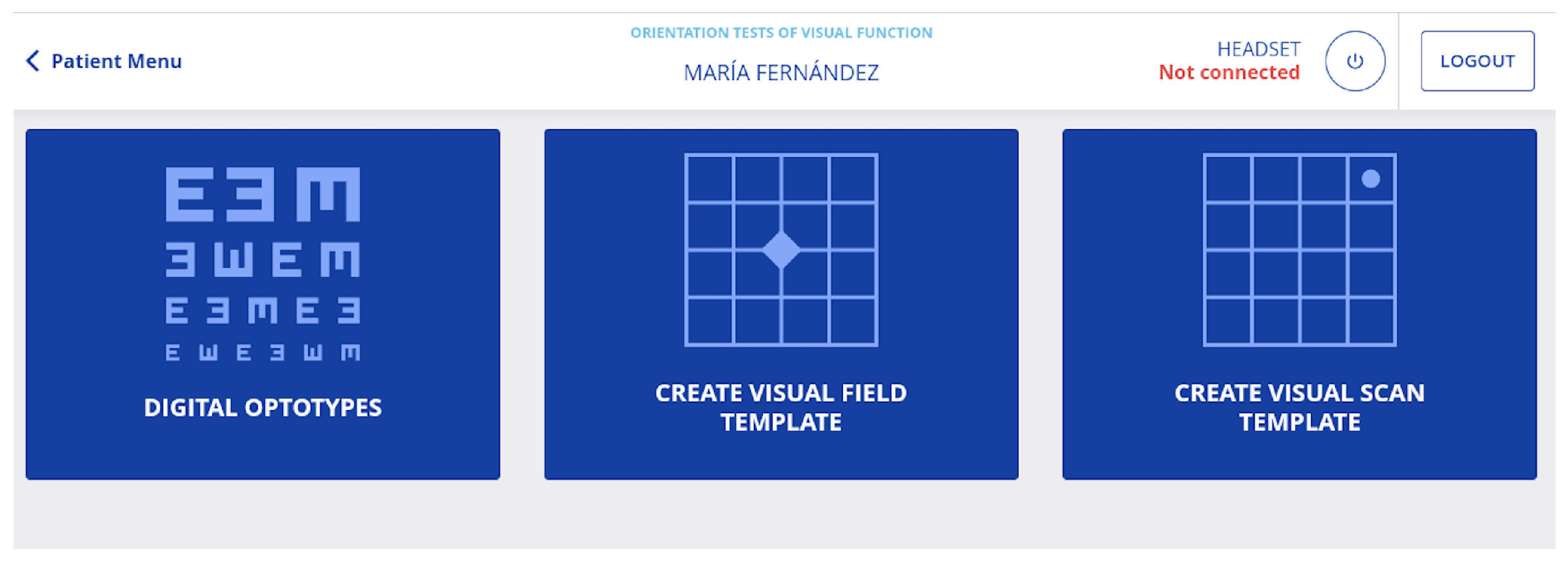
3.8.2. Evaluate Mode
3.8.3. Calibrate Mode
3.8.4. Patient Profile
3.8.5. Use of the System
3.9. Achievements and Benefits for Users
4. Validation & Results
4.1. Clinical Study Methodology
4.1.1. Binocular Visual Acuity
4.1.2. Binocular Contrast Sensitivity Function: Measurement with and Without Retiplus
4.1.3. Binocular Visual Field Measurement: With and Without Retiplus
4.1.4. Measurements in Dynamic Ambulation
4.2. Results of the Clinical Study
4.3. User Experience Evaluation
4.3.1. Eikholt Report
4.3.2. UCMR: Introduction
- 1.
- Evaluation of visual function n = 20: Visual field, visual acuity, and contrast sensitivity assessments were conducted in a manner consistent with the methodology described in the previous section of the clinical study. Additionally, a basic method for measuring the visual field was devised to capture patient impressions. This included a test referred to as the “Practical exercise at 3 m,” accompanied by a corresponding questionnaire “Questionnaire for the practical visual field at 3 m exercise” (PVF3MQ).
- 2.
- Training phase n = 16: Five training sessions were conducted to facilitate interaction with the augmented reality aid, with each session evaluated using a questionnaire “Peripheral Vision and Mobility Questionnaire” (PVMQ). The aim of this training was to achieve progressive adaptation to the augmented reality aid across different stages until it could be effectively used by patients in any daily situation—indoors, outdoors, and in controlled or uncontrolled environments. A survey was also provided to the specialists to assess the evolution of the users’ training sessions, which we called the “training evolution questionnaire” (TEQ).
- 3.
- Pre-training and post-training assessment n = 16: The PVMQ questionnaire was administered both before and after each training session, utilizing a Likert scale [87] to measure responses.
4.3.3. UCMR: Practical Exercise of VF at 3 m
- 1.
- Have you been able to describe more objects to your right, left, and around you?
- 2.
- Has your overall vision improved?
- 3.
- Has your vision improved in low light?
- 4.
- Do you think your orientation can improve?
- 5.
- Do you think that after your training, the system can help you with ambulation?
4.3.4. UCMR: Training
4.3.5. UCMR: Peripheral Vision and Mobility Questionnaire
- 1.
- Do you have difficulty getting around in crowded areas?
- 2.
- Do you have difficulty getting around in unfamiliar places?
- 3.
- Do you only take the bus or the subway?
- 4.
- Do you have difficulty at dusk or in low-light conditions?
- 5.
- What degree of difficulty do you have going down steps, stairs, or curbs in low light or at night?
- 6.
- Crossing streets
- 7.
- Locating traffic lights
- 8.
- Locating objects
- 9.
- Seeing billboards, signs, etc.
- 10.
- What degree of difficulty do you have noticing objects to the side while walking?
- 11.
- When you become disoriented, do you have difficulty regaining your bearings?
4.3.6. UCMR: Results of Protocol
4.3.7. Compilation of Video Testimonials: Reactions to the Use of the Aid
4.3.8. Conclusions of User Experience Evaluations
5. Conclusions
Limitations of Retiplus System
6. Future Work
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Blindness and Vision Impairment. 2023. Available online: https://www.who.int/news-room/fact-sheets/detail/blindness-and-visual-impairment (accessed on 13 October 2023).
- Chan, T.; Friedman, D.S.; Bradley, C.; Massof, R. Estimates of incidence and prevalence of visual impairment, low vision, and blindness in the United States. JAMA Ophthalmol. 2018, 136, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Bourne, R.; Steinmetz, J.D.; Flaxman, S.; Briant, P.S.; Taylor, H.R.; Resnikoff, S.; Casson, R.J.; Abdoli, A.; Abu-Gharbieh, E.; Afshin, A.; et al. Trends in prevalence of blindness and distance and near vision impairment over 30 years: An analysis for the Global Burden of Disease Study. Lancet Glob. Health 2021, 9, e130–e143. [Google Scholar] [CrossRef] [PubMed]
- Wong, W.L.; Su, X.; Li, X.; Cheung, C.M.G.; Klein, R.; Cheng, C.Y.; Wong, T.Y. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: A systematic review and meta-analysis. Lancet Glob. Health 2014, 2, e106–e116. [Google Scholar] [CrossRef] [PubMed]
- Enoch, J.; Jones, L.; Taylor, D.J.; Bronze, C.; Kirwan, J.F.; Jones, P.R.; Crabb, D.P. How do different lighting conditions affect the vision and quality of life of people with glaucoma? A systematic review. Eye 2020, 34, 138–154. [Google Scholar] [CrossRef]
- Tham, Y.C.; Li, X.; Wong, T.Y.; Quigley, H.A.; Aung, T.; Cheng, C.Y. Global prevalence of glaucoma and projections of glaucoma burden through 2040: A systematic review and meta-analysis. Ophthalmology 2014, 121, 2081–2090. [Google Scholar] [CrossRef]
- Teo, Z.L.; Tham, Y.C.; Yu, M.; Cheng, C.Y.; Wong, T.Y.; Sabanayagam, C. Do we have enough ophthalmologists to manage vision-threatening diabetic retinopathy? A global perspective. Eye 2020, 34, 1255–1261. [Google Scholar] [CrossRef]
- Cho, N.H.; Shaw, J.; Karuranga, S.; Huang, Y.; da Rocha Fernandes, J.; Ohlrogge, A.; Malanda, B. IDF Diabetes Atlas: Global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res. Clin. Pract. 2018, 138, 271–281. [Google Scholar] [CrossRef]
- Khalil, H. Diabetes microvascular complications—A clinical update. Diabetes Metab. Syndr. Clin. Res. Rev. 2017, 11, S133–S139. [Google Scholar] [CrossRef]
- Rainey, L.; Elsman, E.B.M.; van Nispen, R.M.A.; van Leeuwen, L.M.; van Rens, G.H.M.B. Comprehending the impact of low vision on the lives of children and adolescents: A qualitative approach. Qual. Life Res. 2016, 25, 2633–2643. [Google Scholar] [CrossRef]
- Binns, A.M.; Bunce, C.; Dickinson, C.; Harper, R.; Tudor-Edwards, R.; Woodhouse, M.; Linck, P.; Suttie, A.; Jackson, J.; Lindsay, J.; et al. How effective is low vision service provision? A systematic review. Surv. Ophthalmol. 2012, 57, 34–65. [Google Scholar] [CrossRef]
- Demmin, D.L.; Silverstein, S.M. Visual impairment and mental health: Unmet needs and treatment options. Clin. Ophthalmol. 2020, 2020, 4229–4251. [Google Scholar] [CrossRef]
- Dhital, A.; Pey, T.; Stanford, M.R. Visual loss and falls: A review. Eye 2010, 24, 1437–1446. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Kupferstein, E.; Tal, D.; Azenkot, S. “It Looks Beautiful but Scary” How Low Vision People Navigate Stairs and Other Surface Level Changes. In Proceedings of the 20th International ACM SIGACCESS Conference on Computers and Accessibility, Galway, Ireland, 22–24 October 2018; pp. 307–320. [Google Scholar]
- Mehra, D.; Le, P.H. Physiology, night vision. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2019. [Google Scholar]
- Rivera-Romero, O.; Gabarron, E.; Ropero, J.; Denecke, K. Designing personalised mHealth solutions: An overview. J. Biomed. Inform. 2023, 146, 104500. [Google Scholar] [CrossRef]
- Ro, Y.K.; Brem, A.; Rauschnabel, P.A. Augmented reality smart glasses: Definition, concepts and impact on firm value creation. In Augmented Reality and Virtual Reality: Empowering Human, Place and Business; Springer: Cham, Switzerland, 2018; pp. 169–181. [Google Scholar]
- Coughlan, J.M.; Miele, J. AR4VI: AR as an accessibility tool for people with visual impairments. In Proceedings of the 2017 IEEE International Symposium on Mixed and Augmented Reality (ISMAR-Adjunct), Nantes, France, 9–13 October 2017; pp. 288–292. [Google Scholar]
- Chen, Y.; Wang, Q.; Chen, H.; Song, X.; Tang, H.; Tian, M. An overview of augmented reality technology. J. Phys. Conf. Ser. 2019, 1237, 022082. [Google Scholar] [CrossRef]
- Ortiz-Escobar, L.M.; Chavarria, M.A.; Schönenberger, K.; Hurst, S.; Stein, M.A.; Mugeere, A.; Rivas Velarde, M. Assessing the implementation of user-centred design standards on assistive technology for persons with visual impairments: A systematic review. Front. Rehabil. Sci. 2023, 4, 1238158. [Google Scholar] [CrossRef] [PubMed]
- Ruffieux, S.; Hwang, C.; Junod, V.; Caldara, R.; Lalanne, D.; Ruffieux, N. Tailoring assistive smart glasses according to pathologies of visually impaired individuals: An exploratory investigation on social needs and difficulties experienced by visually impaired individuals. Univers. Access Inf. Soc. 2023, 22, 463–475. [Google Scholar] [CrossRef]
- Deemer, A.D.; Bradley, C.K.; Ross, N.C.; Natale, D.M.; Itthipanichpong, R.; Werblin, F.S.; Massof, R.W. Low vision enhancement with head-mounted video display systems: Are we there yet? Optom. Vis. Sci. Off. Publ. Am. Acad. Optom. 2018, 95, 694. [Google Scholar] [CrossRef]
- Scheiman, M. Understanding and Managing Vision Deficits: A Guide for Occupational Therapists; Routledge: Oxford, UK, 2011. [Google Scholar]
- Altinbay, D.; Taskin, I. Evaluation of vision-related quality of life in Retinitis Pigmentosa patients with low vision. Jpn. J. Ophthalmol. 2021, 65, 777–785. [Google Scholar] [CrossRef]
- Brown, M.M.; Brown, G.C.; Sharma, S.; Landy, J.; Bakal, J. Quality of life with visual acuity loss from diabetic retinopathy and age-related macular degeneration. Arch. Ophthalmol. 2002, 120, 481–484. [Google Scholar] [CrossRef]
- Minto, H.; Butt, I.A. Low vision devices and training. Community Eye Health 2004, 17, 6. [Google Scholar]
- Agarwal, R.; Tripathi, A. Current modalities for low vision rehabilitation. Cureus 2021, 13, e16561. [Google Scholar] [CrossRef]
- Apfelbaum, H.; Peli, E. Tunnel vision prismatic field expansion: Challenges and requirements. Transl. Vis. Sci. Technol. 2015, 4, 8. [Google Scholar] [CrossRef] [PubMed]
- Massof, R.W.; Rickman, D.L.; Lalle, P.A. Low vision enhancement system. Johns Hopkins APL Tech. Dig. 1994, 15, 120–125. [Google Scholar]
- Harper, R.; Culham, L.; Dickinson, C. Head mounted video magnification devices for low vision rehabilitation: A comparison with existing technology. Br. J. Ophthalmol. 1999, 83, 495–500. [Google Scholar] [CrossRef]
- Vargas-Martin, F.; Peli, E. Augmented-view for restricted visual field: Multiple device implementations. Optom. Vis. Sci. 2002, 79, 715–723. [Google Scholar] [CrossRef]
- Culham, L.E.; Chabra, A.; Rubin, G.S. Clinical performance of electronic, head-mounted, low-vision devices. Ophthalmic Physiol. Opt. 2004, 24, 281–290. [Google Scholar] [CrossRef] [PubMed]
- Sivakumar, P.; Vedachalam, R.; Kannusamy, V.; Odayappan, A.; Venkatesh, R.; Dhoble, P.; Moutappa, F.; Narayana, S. Barriers in utilisation of low vision assistive products. Eye 2020, 34, 344–351. [Google Scholar] [CrossRef]
- Hoogsteen, K.M.P.; Osinga, S.A.; Steenbekkers, B.L.P.A.; Szpiro, S.F.A. Functionality versus inconspicuousness: Attitudes of people with low vision towards OST smart glasses. In Proceedings of the 22nd International ACM SIGACCESS Conference on Computers and Accessibility, Virtual, 26–28 October 2020; pp. 1–4. [Google Scholar]
- Lam, N.; Leat, S.J. Barriers to accessing low-vision care: The patient’s perspective. Can. J. Ophthalmol. 2013, 48, 458–462. [Google Scholar] [CrossRef] [PubMed]
- Ehrlich, J.R.; Ojeda, L.V.; Wicker, D.; Day, S.; Howson, A.; Lakshminarayanan, V.; Moroi, S.E. Head-mounted display technology for low-vision rehabilitation and vision enhancement. Am. J. Ophthalmol. 2017, 176, 26–32. [Google Scholar] [CrossRef]
- Irisvision. 2024. Available online: https://irisvision.com/irisvision-inspire/ (accessed on 7 June 2024).
- Yeo, J.H.; Bae, S.H.; Lee, S.H.; Kim, K.W.; Moon, N.J. Clinical performance of a smartphone-based low vision aid. Sci. Rep. 2022, 12, 10752. [Google Scholar] [CrossRef]
- Orcam. 2024. Available online: https://www.orcam.com/es-es/home (accessed on 8 September 2024).
- Xu, D.; Yu, M.; Zheng, C.; Ji, S.; Dai, J. The effects of an electronic head-mounted display in vision rehabilitation for patients with tunnel vision. Int. Ophthalmol. 2024, 44, 109. [Google Scholar] [CrossRef]
- Esight. 2024. Available online: https://www.esighteyewear.com/how-it-works/ (accessed on 9 November 2024).
- Wittich, W.; Lorenzini, M.C.; Markowitz, S.N.; Tolentino, M.; Gartner, S.A.; Goldstein, J.E.; Dagnelie, G. The effect of a head-mounted low vision device on visual function. Optom. Vis. Sci. 2018, 95, 774. [Google Scholar] [CrossRef]
- Younis, O.; Al-Nuaimy, W.; Alomari, M.H.; Rowe, F. A hazard detection and tracking system for people with peripheral vision loss using smart glasses and augmented reality. Int. J. Adv. Comput. Sci. Appl. 2019, 10, 1–9. [Google Scholar] [CrossRef]
- Parker, A.T.; Swobodzinski, M.; Wright, J.D.; Hansen, K.; Morton, B.; Schaller, E. Wayfinding tools for people with visual impairments in real-world settings: A literature review of recent studies. Front. Educ. 2021, 6, 723816. [Google Scholar] [CrossRef]
- Palanker, D.; Le Mer, Y.; Mohand-Said, S.; Sahel, J.A. Simultaneous perception of prosthetic and natural vision in AMD patients. Nat. Commun. 2022, 13, 513. [Google Scholar] [CrossRef] [PubMed]
- Stelmack, J. Quality of life of low-vision patients and outcomes of low-vision rehabilitation. Optom. Vis. Sci. 2001, 78, 335–342. [Google Scholar] [CrossRef] [PubMed]
- Cross, N.; van Steen, C.; Zegaoui, Y.; Satherley, A.; Angelillo, L. Current and future treatment of Retinitis Pigmentosa. Clin. Ophthalmol. 2022, 16, 2909. [Google Scholar] [CrossRef]
- Verbakel, S.K.; van Huet, R.A.; Boon, C.J.; den Hollander, A.I.; Collin, R.W.; Klaver, C.C.; Hoyng, C.B.; Roepman, R.; Klevering, B.J. Non-syndromic Retinitis Pigmentosa. Prog. Retin. Eye Res. 2018, 66, 157–186. [Google Scholar] [CrossRef]
- Chun, R.; Cucuras, M.; Jay, W.M. Current perspectives of bioptic driving in low vision. Neuro-Ophthalmology 2016, 40, 53–58. [Google Scholar] [CrossRef]
- Wang, S.; Moharrer, M.; Baliutaviciute, V.; Dougherty, B.E.; Cybis, W.; Bowers, A.R.; Luo, G. Bioptic telescope use in naturalistic driving by people with visual impairment. Transl. Vis. Sci. Technol. 2020, 9, 11. [Google Scholar] [CrossRef]
- Berger, S. 5 Activities to Improve Visual Scanning and Tracking. 2024. Available online: https://napacenter.org/visual-scanning-activities/ (accessed on 17 March 2024).
- Kasneci, E.; Black, A.A.; Wood, J.M. Eye-tracking as a tool to evaluate functional ability in everyday tasks in glaucoma. J. Ophthalmol. 2017, 2017, 6425913. [Google Scholar] [CrossRef] [PubMed]
- Deemer, A.D.; Swenor, B.K.; Fujiwara, K.; Deremeik, J.T.; Ross, N.C.; Natale, D.M.; Bradley, C.K.; Werblin, F.S.; Massof, R.W. Preliminary evaluation of two digital image processing strategies for head-mounted magnification for low vision patients. Transl. Vis. Sci. Technol. 2019, 8, 23. [Google Scholar] [CrossRef] [PubMed]
- Vargas-Martín, F.; Peli, E. Eye movements of patients with tunnel vision while walking. Investig. Ophthalmol. Vis. Sci. 2006, 47, 5295–5302. [Google Scholar] [CrossRef][Green Version]
- Luo, G.; Woods, R.L.; Peli, E. Collision judgment when using an augmented-vision head-mounted display device. Investig. Ophthalmol. Vis. Sci. 2009, 50, 4509–4515. [Google Scholar] [CrossRef]
- de Jong, P.T. A history of visual acuity testing and optotypes. Eye 2022, 38, 13–24. [Google Scholar] [CrossRef]
- PrecisionVison. Snellen Eye Chart—A Description and Explanation. 2025. Available online: https://precision-vision.com/snellen-eye-chart-a-description-and-explanation/?srsltid=AfmBOorg1f9pd0gl-nPgPTvExsVmzGT7JtCZ7722LN000zZrFOG5k-6G, (accessed on 3 February 2025).
- Retiplus. Sistema Retiplus. 2023. Available online: https://retiplus.com/ (accessed on 25 July 2023).
- Sharma, S.; Soni, S.; Kaushik, S.; Kalaivani, M.; Dadhwal, V.; Sharma, K.A.; Sharma, D. SwasthGarbh: A smartphone App for improving the quality of antenatal care and ameliorating maternal-fetal health. IEEE J. Biomed. Health Inform. 2022, 27, 2729–2738. [Google Scholar] [CrossRef] [PubMed]
- Ionic. Ionicframework. 2024. Available online: https://ionicframework.com/ (accessed on 7 March 2024).
- Waranashiwar, J.; Ukey, M. Ionic framework with angular for hybrid app development. Int. J. New Technol. Res. 2018, 4, 263068. [Google Scholar]
- Firebase Google. Firebase Real Time Database. 2024. Available online: https://firebase.google.com/docs/database?hl=es-419 (accessed on 15 June 2024).
- Goswami, L.; Agrawal, P. Iot based diagnosing of fault detection in power line transmission through google firebase database. In Proceedings of the 2020 4th International Conference on Trends in Electronics and Informatics (ICOEI) (48184), Tirunelveli, India, 15–17 June 2020; pp. 415–420. [Google Scholar]
- Ahmed, T.; Nuruddin, A.T.B.; Latif, A.B.; Arnob, S.S.; Rahman, R. A real-time controlled closed loop IoT based home surveillance system for Android using Firebase. In Proceedings of the 2020 6th International Conference on Control, Automation and Robotics (ICCAR), Singapore, 20–23 April 2020; pp. 601–606. [Google Scholar]
- Epson. Moverio BT 350 Specifications. 2023. Available online: https://neoverio.com/wp-content/uploads/2019/10/EPSON-MOVERIO-RANGE-BRO1119-MIDRES.pdf (accessed on 6 September 2023).
- Unsplash; Anderson, N. Dos Hombres Riendose el Uno del Otro. 2024. Available online: https://unsplash.com/es/fotos/dos-hombres-riendose-el-uno-del-otro-FHiJWoBodrs (accessed on 9 October 2024).
- Bjerager, J.; Schneider, M.; Potapenko, I.; van Dijk, E.H.; Faber, C.; Grauslund, J.; Pfau, K.; Huemer, J.; Muttuvelu, D.V.; Rasmussen, M.L.; et al. Diagnostic accuracy of the Amsler grid test for detecting neovascular age-related macular degeneration: A systematic review and meta-analysis. JAMA Ophthalmol. 2023, 141, 315–323. [Google Scholar] [CrossRef]
- Rubin, G.S. Measuring reading performance. Vis. Res. 2013, 90, 43–51. [Google Scholar] [CrossRef]
- Hu, C.X.; Zangalli, C.; Hsieh, M.; Gupta, L.; Williams, A.L.; Richman, J.; Spaeth, G.L. What do patients with glaucoma see? Visual symptoms reported by patients with glaucoma. Am. J. Med. Sci. 2014, 348, 403–409. [Google Scholar] [CrossRef]
- Kaiser, P.K. Prospective evaluation of visual acuity assessment: A comparison of snellen versus ETDRS charts in clinical practice (An AOS Thesis). Trans. Am. Ophthalmol. Soc. 2009, 107, 311. [Google Scholar] [PubMed]
- Njeru, S.M.; Osman, M.; Brown, A.M. The effect of test distance on visual contrast sensitivity measured using the Pelli–Robson chart. Transl. Vis. Sci. Technol. 2021, 10, 32. [Google Scholar] [CrossRef]
- Fuller, M.L.; Briceño, C.A.; Nelson, C.C.; Bradley, E.A. Tangent screen perimetry in the evaluation of visual field defects associated with ptosis and dermatochalasis. PLoS ONE 2017, 12, e0174607. [Google Scholar] [CrossRef]
- Goñi, F.J.; Maja, K. Standard Automated Perimetry. In Glaucoma Imaging; Springer: Cham, Switzerland, 2016; pp. 1–26. [Google Scholar]
- Phu, J.; Khuu, S.K.; Zangerl, B.; Kalloniatis, M. A comparison of Goldmann III, V and spatially equated test stimuli in visual field testing: The importance of complete and partial spatial summation. Ophthalmic Physiol. Opt. 2017, 37, 160–176. [Google Scholar] [CrossRef]
- Field, A. Discovering Statistics Using IBM SPSS Statistics; Sage Publications Limited: London, UK, 2024. [Google Scholar]
- Optician Certification. Isopter. 2024. Available online: https://opticiancertification.org/isopter/ (accessed on 14 December 2024).
- Sakai, D.; Maeda, T.; Yamamoto, M.; Yokota, S.; Maeda, A.; Hirami, Y.; Nakamura, M.; Kurimoto, Y.; Mandai, M. Relationship between residual visual field and full-field stimulus testing in patients with late-stage retinal degenerative diseases. Sci. Rep. 2024, 14, 2793. [Google Scholar] [CrossRef]
- Lilliefors, H.W. On the Kolmogorov–Smirnov test for normality with mean and variance unknown. J. Am. Stat. Assoc. 1967, 62, 399–402. [Google Scholar] [CrossRef]
- Miller, R.G., Jr. Beyond ANOVA: Basics of Applied Statistics; CRC Press: Boca Raton, FL, USA, 1997. [Google Scholar]
- NKBD. National Competence Service for the Deafblind. 2025. Available online: https://www.dovblindhet.no/ (accessed on 28 January 2025).
- Eikholt. National Resource Center for the Deafblind. 2025. Available online: https://www.eikholt.no/ (accessed on 28 January 2025).
- Retiplus. YouTube Retiplus Channel. 2025. Available online: https://www.youtube.com/@retiplus1103 (accessed on 5 February 2025).
- Retiplus. Retiplus Testimonials. 2025. Available online: https://retiplus.com/en/testimonios/ (accessed on 6 January 2025).
- Johansson, A.-B.; Lund, R.; Retiplus. An Innovation Project. 2025. Available online: https://www.eikholt.no/app/uploads/2023/06/Eikholt-rapport-02_22-web.pdf (accessed on 22 January 2025).
- Edwards, A.; Fishman, G.A.; Anderson, R.J.; Grover, S.; Derlacki, D.J. Visual acuity and visual field impairment in Usher syndrome. Arch. Ophthalmol. 1998, 116, 165–168. [Google Scholar] [CrossRef] [PubMed]
- Fundación Retina España. 2025. Available online: https://www.retina.es/ (accessed on 3 February 2025).
- Nemoto, T.; Beglar, D. Likert-scale questionnaires. In Proceedings of the JALT 2013 Conference Proceedings, Kobe, Japan, 25–29 October 2013; Volume 108, pp. 1–6. [Google Scholar]
- Shapiro, S.S.; Wilk, M.B. An analysis of variance test for normality (complete samples). Biometrika 1965, 52, 591–611. [Google Scholar] [CrossRef]
- UEQ Org. User Experience Questionnaire. 2025. Available online: https://www.ueq-online.org/ (accessed on 29 January 2025).
- Laugwitz, B.; Held, T.; Schrepp, M. Construction and evaluation of a user experience questionnaire. In Proceedings of the HCI and Usability for Education and Work: 4th Symposium of the Workgroup Human-Computer Interaction and Usability Engineering of the Austrian Computer Society, USAB 2008, Graz, Austria, 20–21 November 2008; Proceedings 4. Springer: Berlin/Heidelberg, Germany, 2008; pp. 63–76. [Google Scholar]
- Pur, D.R.; Lee-Wing, N.; Bona, M.D. The use of augmented reality and virtual reality for visual field expansion and visual acuity improvement in low vision rehabilitation: A systematic review. Graefe’s Arch. Clin. Exp. Ophthalmol. 2023, 261, 1743–1755. [Google Scholar] [CrossRef]
- Csapó, Á.; Wersényi, G.; Nagy, H.; Stockman, T. A survey of assistive technologies and applications for blind users on mobile platforms: A review and foundation for research. J. Multimodal User Interfaces 2015, 9, 275–286. [Google Scholar] [CrossRef]




| REF. | Statistician | gl | Sig p-Value |
|---|---|---|---|
| VD0 | 0.133 | 29 | 0.183 |
| VDSR | 0.152 | 29 | 0.075 |
| VDCR | 0.103 | 29 | 0.200 * |
| AGE | 0.100 | 29 | 0.200 * |
| VA | 0.149 | 29 | 0.086 |
| CS | 0.133 | 29 | 0.183 |
| VF | 0.159 | 29 | 0.051 |
| VF2 | 0.156 | 29 | 0.059 |
| Sum of Squares | gl | Mean Square | F | Sig | ||
|---|---|---|---|---|---|---|
| VA by Disease | Between groups | 0.028 | 1 | 0.028 | 0.198 | 0.660 |
| Within groups | 3.936 | 28 | 0.141 | - | - | |
| Total | 3.964 | 29 | - | - | - | |
| CS by Disease | Between groups | 0.112 | 1 | 0.112 | 0.348 | 0.560 |
| Within groups | 8.993 | 28 | 0.321 | - | - | |
| Total | 9.150 | 29 | - | - | - | |
| VF by Disease | Between groups | 2.023 | 1 | 2.023 | 0.088 | 0.769 |
| Within groups | 642.386 | 28 | 22.942 | - | - | |
| Total | 644.409 | 29 | - | - | - | |
| VF2 by Disease | Between groups | 31.788 | 1 | 31.788 | 1.473 | 0.235 |
| Within groups | 604.228 | 28 | 21.580 | - | - | |
| Total | 636.016 | 29 | - | - | - | |
| VA by Age | Between groups | 0.434 | 2 | 0.217 | 1.661 | 0.209 |
| Within groups | 3.530 | 27 | 0.131 | - | - | |
| Total | 3.964 | 29 | - | - | - | |
| CS by Age | Between groups | 0.066 | 2 | 0.033 | 0.098 | 0.907 |
| Within groups | 0.039 | 27 | 0.335 | - | - | |
| Total | 9.150 | 29 | - | - | - | |
| VF by Age | Between groups | 31.380 | 2 | 15.690 | 0.691 | 0.510 |
| Within groups | 613.028 | 27 | 22.705 | - | - | |
| Total | 644.409 | 29 | - | - | - | |
| VF2 by Age | Between groups | 2.066 | 2 | 1.033 | 0.044 | 0.957 |
| Within groups | 633.950 | 27 | 23.480 | - | - | |
| Total | 636.016 | 29 | - | - | - |
| N | Correlation | Sig p Factor | Sig p of Two Factors | |
|---|---|---|---|---|
| Pair VF and VF2 | 30 | 0.608 | <0.001 | <0.001 |
| Measure | Value |
|---|---|
| Mean (m) | −6.37815 |
| Standard deviation (SD) | 4.16194 |
| Standard error of the mean (SEM) | 0.75986 |
| 95% CI Lower bound | −7.93224 |
| 95% CI Upper bound | −4.82405 |
| t statistic (t) | −8.394 |
| Degrees of freedom (gl) | 29 |
| Significance one factor (Sig of OF) | <0.001 |
| Significance factors (Sig of F) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiménez, J.J.; Bayón, J.; Guijarro, M.; Bernárdez-Vilaboa, R.; Cámara, R.; Recas, J. Retiplus: Augmented Reality Rehabilitation System to Enhance Autonomy and Quality of Life in Individuals with Low Vision. Electronics 2025, 14, 3589. https://doi.org/10.3390/electronics14183589
Jiménez JJ, Bayón J, Guijarro M, Bernárdez-Vilaboa R, Cámara R, Recas J. Retiplus: Augmented Reality Rehabilitation System to Enhance Autonomy and Quality of Life in Individuals with Low Vision. Electronics. 2025; 14(18):3589. https://doi.org/10.3390/electronics14183589
Chicago/Turabian StyleJiménez, Jonathan José, Juan Bayón, María Guijarro, Ricardo Bernárdez-Vilaboa, Rafael Cámara, and Joaquín Recas. 2025. "Retiplus: Augmented Reality Rehabilitation System to Enhance Autonomy and Quality of Life in Individuals with Low Vision" Electronics 14, no. 18: 3589. https://doi.org/10.3390/electronics14183589
APA StyleJiménez, J. J., Bayón, J., Guijarro, M., Bernárdez-Vilaboa, R., Cámara, R., & Recas, J. (2025). Retiplus: Augmented Reality Rehabilitation System to Enhance Autonomy and Quality of Life in Individuals with Low Vision. Electronics, 14(18), 3589. https://doi.org/10.3390/electronics14183589








