Post Hoc Event-Related Potential Analysis of Kinesthetic Motor Imagery-Based Brain-Computer Interface Control of Anthropomorphic Robotic Arms
Abstract
1. Introduction
2. Materials and Methods
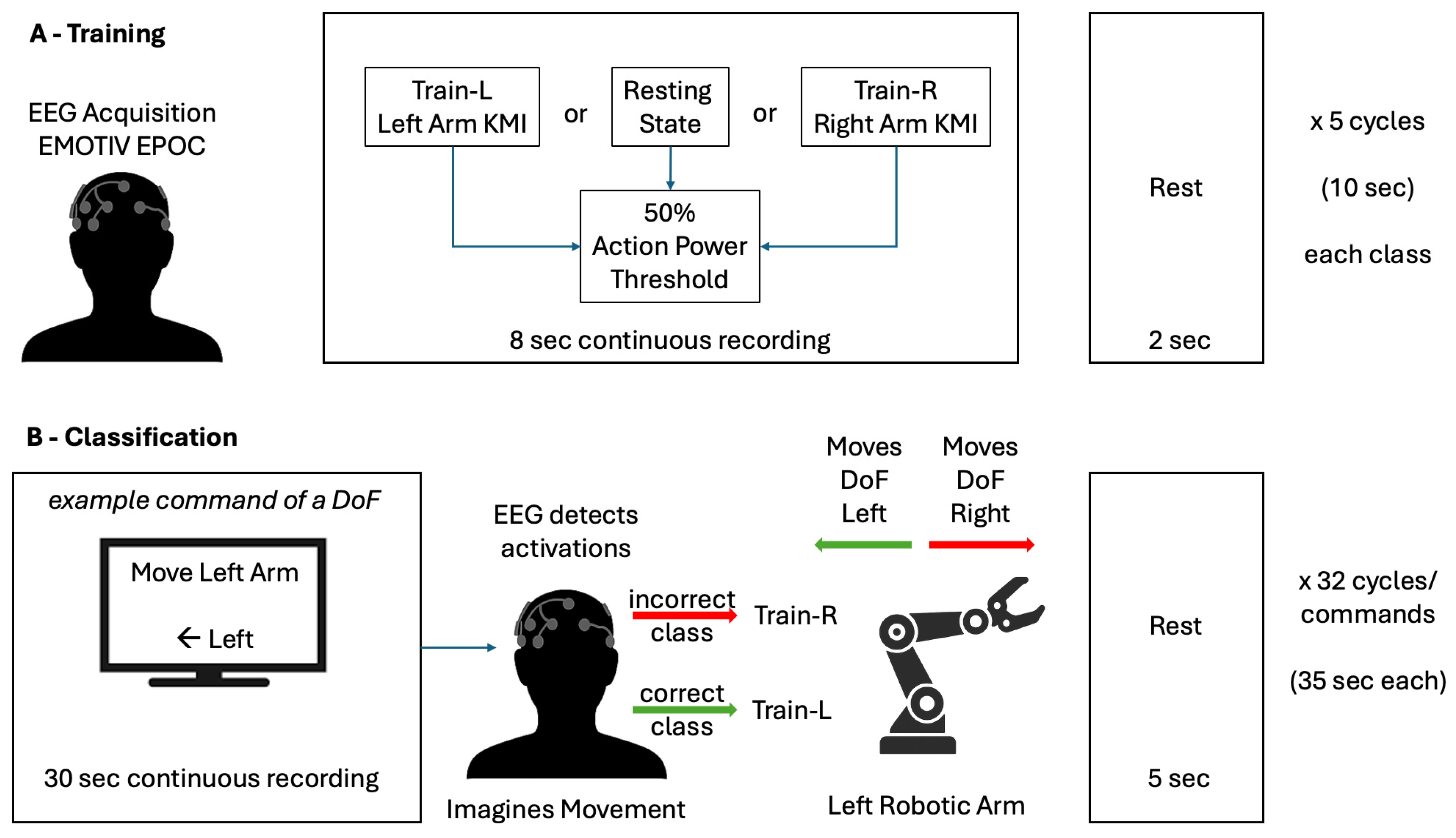
2.1. Study Design and Data
2.2. Experimental Procedures
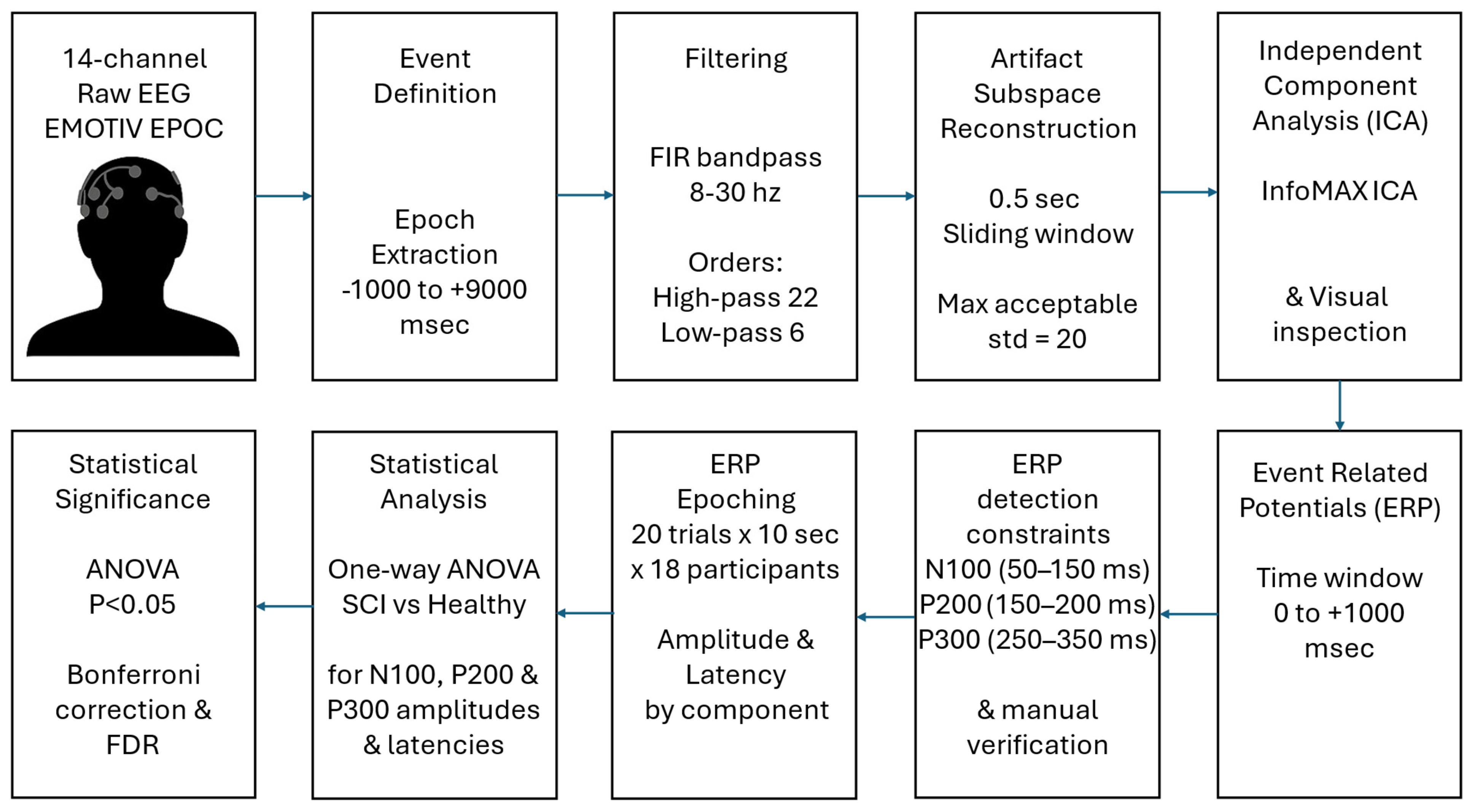
2.3. Signal Pre-Processing
- Event Definition and Epoch Extraction: The epochs of each subject, corresponding to 20 events of 10 s duration each (epochs from −1 to +9 s relative to event onset), were manually extracted to capture brain activity associated with the tasks.
- Artifact Data Removal: The Clean Rawdata tool in EEGLAB, which utilizes artifact subspace reconstruction (ASR), was used to eliminate artifacts and bad data from the EEG recordings [29,30]. The ASR algorithm uses linear algebra methods, such as singular value decomposition (SVD) or principal component analysis (PCA), to decompose data into components (subspaces), isolating brain-related activity from artifact-related components. For burst artifact correction, the maximum acceptable standard deviation in a 0.5 s sliding window was set to 20. These settings enabled automated, data-driven denoising, improving the reliability of subsequent ERP component estimation.
- Independent Component Analysis (ICA): ICA was used to identify and remove artifacts from the EEG signal, implemented via the runica.m function, utilizing InfoMax ICA [31]. However, no independent components were removed, as visual inspection did not reveal any components clearly attributable to artifacts (e.g., eye blinks, muscle activity).
2.4. ERP Analysis
- Time Window for ERP Component Analysis: The time window for ERP analysis was set from 0 to +1000 ms after event onset, allowing for capturing early sensory processing, as well as later cognitive components.
- ERP Component Definition and Peak Detection: Specific ERP components (N100, P200, P300) were identified based on their amplitude and latency within defined time windows. Peaks were detected using a hybrid approach combining derivative-based extremum detection and temporal window constraints. For any voltage time series V(t), candidate peaks satisfied the following:
- a.
- First derivative zero-crossing V′(t) = 0.
- b.
- Second derivative test:
- i.
- V″(t) < 0 for positive peaks (P200, P300);
- ii.
- V″(t) > 0 for negative peaks (N100).
- c.
- Amplitude threshold: |V(t)| > 2.5 × baseline root mean square (RMS) (0–50 ms post-stimulus).
- d.
- Component-specific temporal windows constrained search ranges: N100: 50–150 ms (negative peak), P200: 150–250 ms (positive peak), and P300: 250–400 ms (positive peak).
- e.
- Manual (visual) verification of ERP peak detection, where V(t): voltage amplitude as a function of time t (measured in microvolts, μV); V′(t): first derivative of voltage with respect to time (μV/ms); V″(t): second derivative of voltage with respect to time (μV/ms2); t: time in milliseconds (ms) relative to stimulus onset; RMS_baseline: root mean square amplitude of the baseline period (μV).
- 3.
- ERP Epoching: From the 18 participants analyzed, the total epochs included 20 trials × 10 s × 18 subjects = 3.600 s, defined as N_trials: number of experimental trials per participant; T_duration: duration of each trial (seconds); N_subjects: total number of study participants.
- 4.
- Statistical Analysis: For each ERP component (N100, P200, P300), a separate one-way analysis of variance (ANOVA) [32] was conducted, comparing amplitude and latency measures between groups across 14 EEG channels. The ANOVA model for amplitude comparisons was specified as
- 5.
- Statistical Testing: A one-way analysis of variance (ANOVA) was performed at a significance level of 0.05 for the 3 ERP components of interest across time points for each channel to assess differences in ERP amplitudes between groups. Significant results prompted post hoc analyses to further explore specific group differences [32].
- 6.
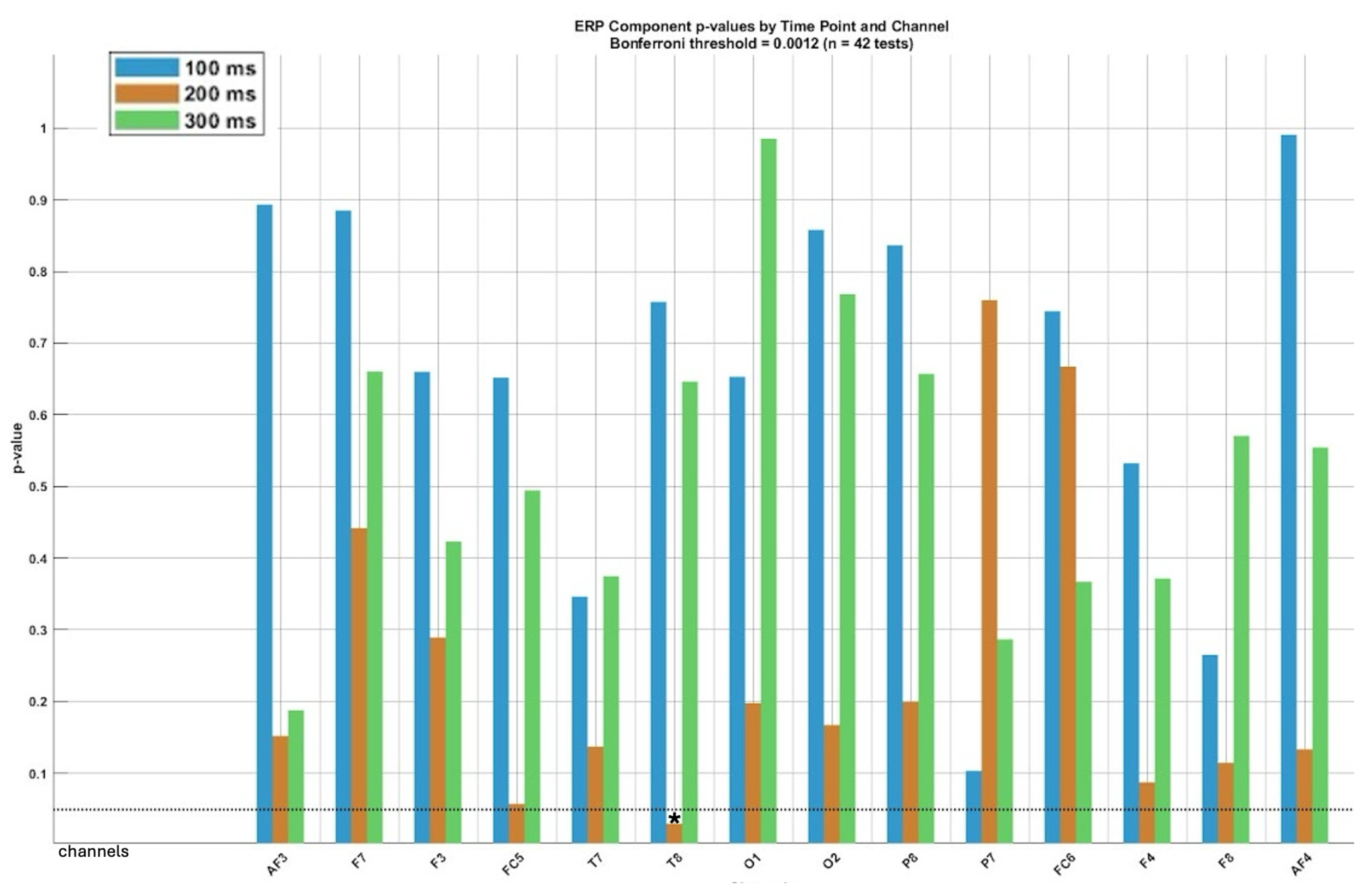
- Correction for multiple comparisons: To control for the increased risk of false positives resulting from multiple comparisons across electrode channels and time points, we applied two widely accepted statistical correction methods: the Bonferroni correction and the false discovery rate (FDR) correction (Benjamini–Hochberg procedure). The Bonferroni correction is a conservative method that controls the family-wise error rate (FWER) by adjusting the significance threshold proportionally to the number of comparisons. Although effective in minimizing Type I errors, it increases the risk of Type II errors, particularly in high-dimensional datasets like EEG results. To balance this limitation, we also applied the FDR correction, which controls the expected false discovery proportion among significant findings. FDR is less conservative and more suitable for ERP studies involving multiple channels and temporal comparisons, as it allows for greater sensitivity while still controlling false positives. We used a p-value threshold of 0.05, indicating that at most, 5% of reported significant results are expected to be false discoveries. Results were reported as significant only when they passed both correction thresholds, thereby reinforcing the robustness of the statistical inferences.
3. Results
3.1. EEG Wavelength Amplitude Ranges and Comparisons
- 50 ms: channel FC6;
- 100 ms: channels FC6, F4, and T8;
- 150 ms: channels FC5 and T8;
- 200 ms: channels P7 and T8;
- 300 ms: channels F3 and P8;
- 400 ms: channels F3, F7, P8, FC6, and AF4;
- 450–500 ms: channels F8, P8, T8, FC6, AF3, and O2;
- 550 ms: channels F4, FC6, and O2;
- 600 ms: channels FC4, F8, FC6, O1, and O2;
- 700 ms: channel AF3;
- 800 ms: channels FC5 and P7;
- 850: channel P7.
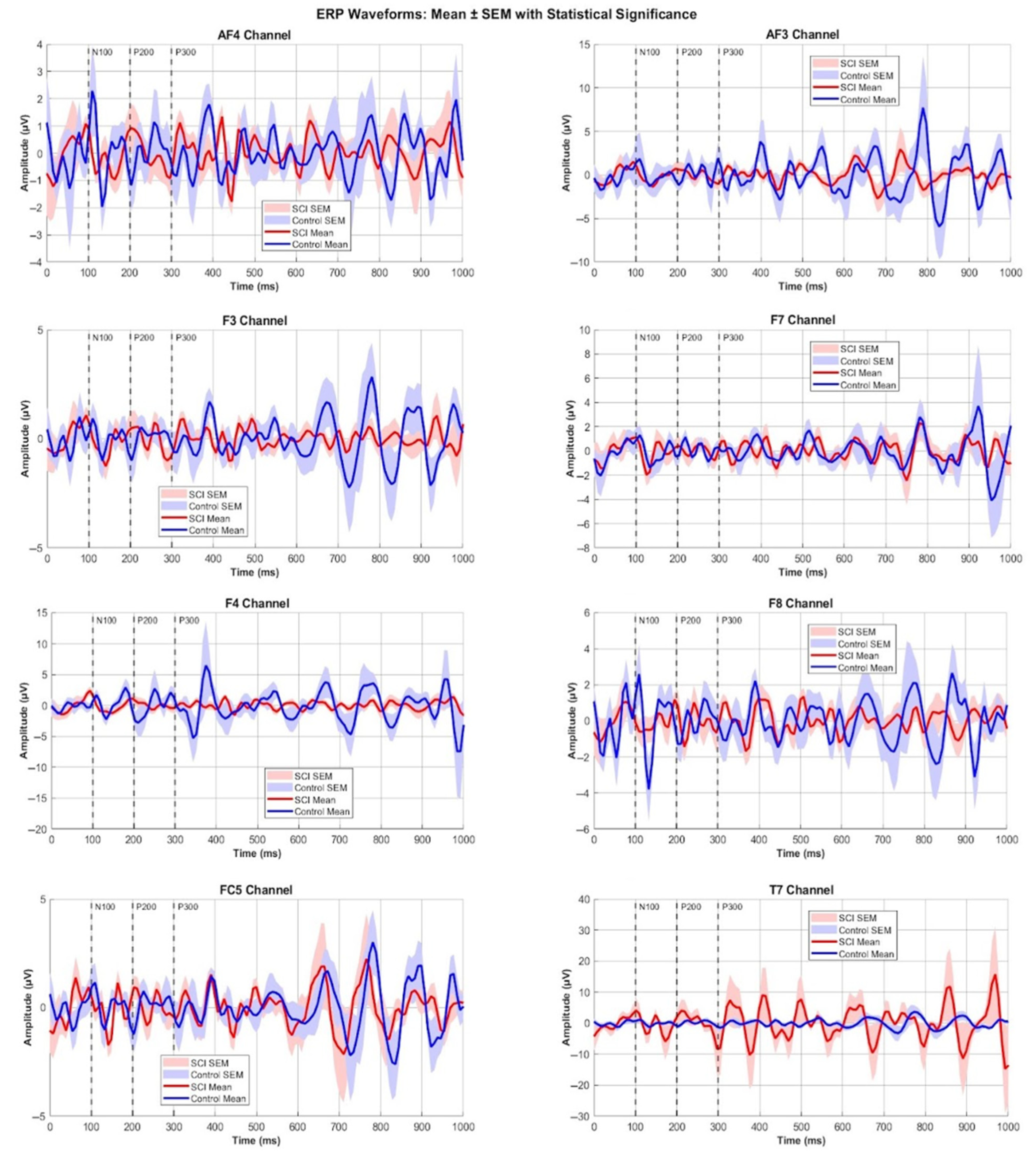
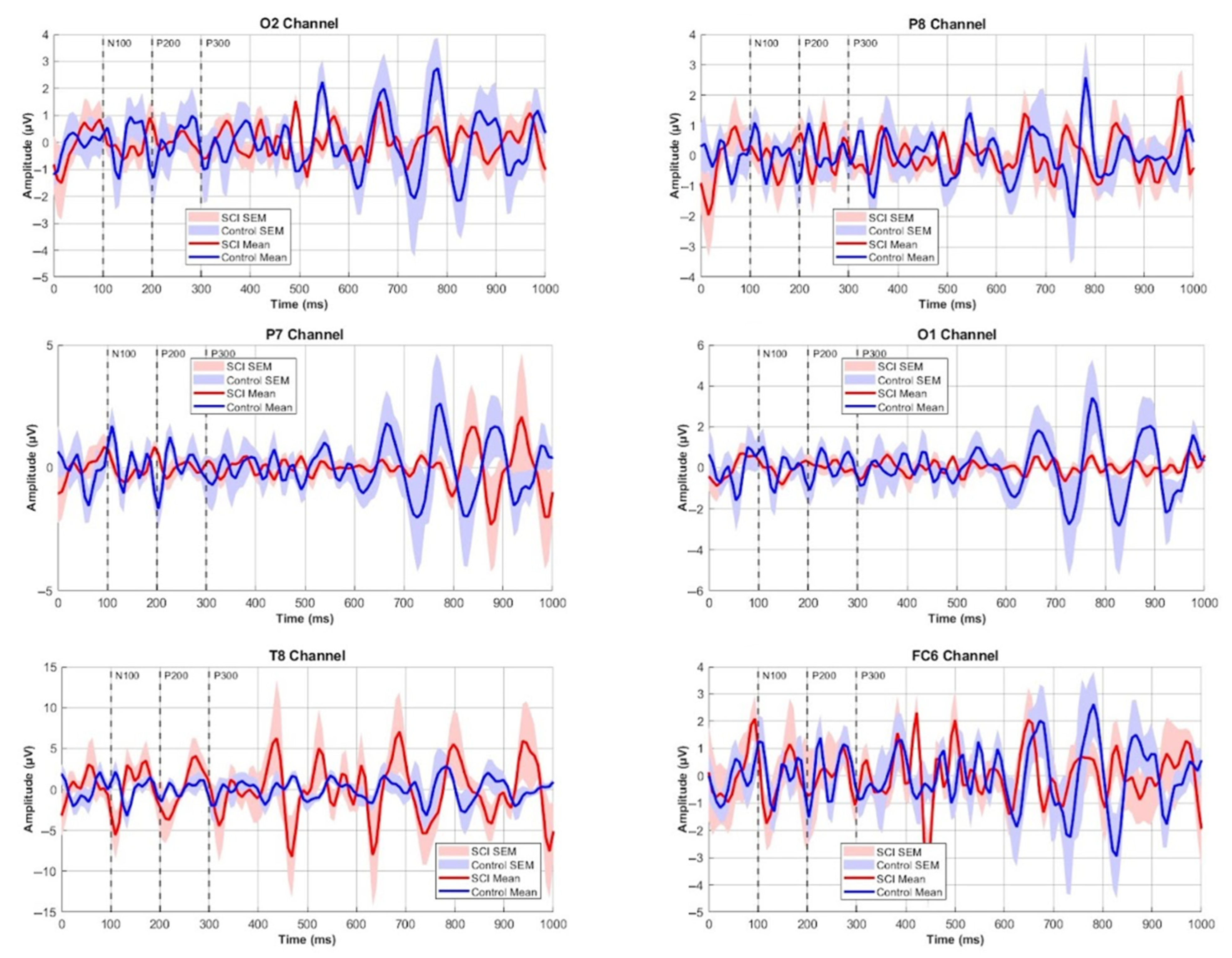
3.2. ERP Components Comparisons
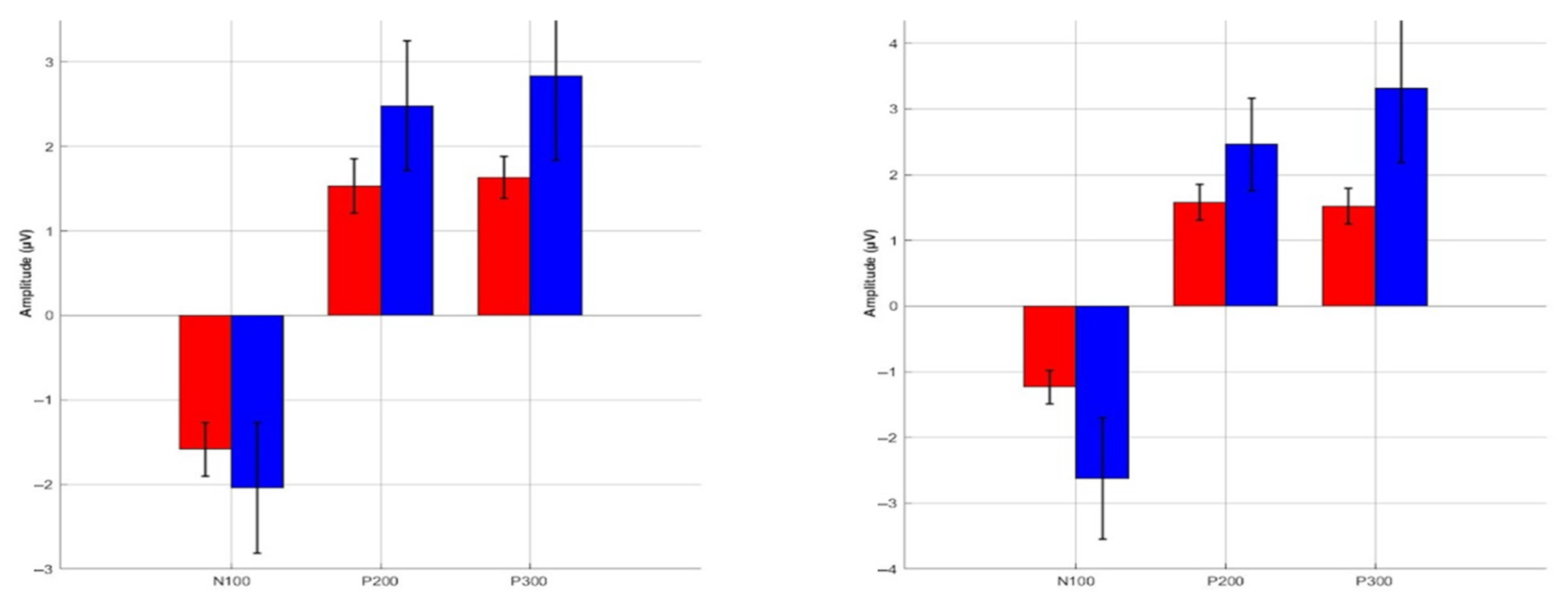
3.3. ERP Component Amplitudes
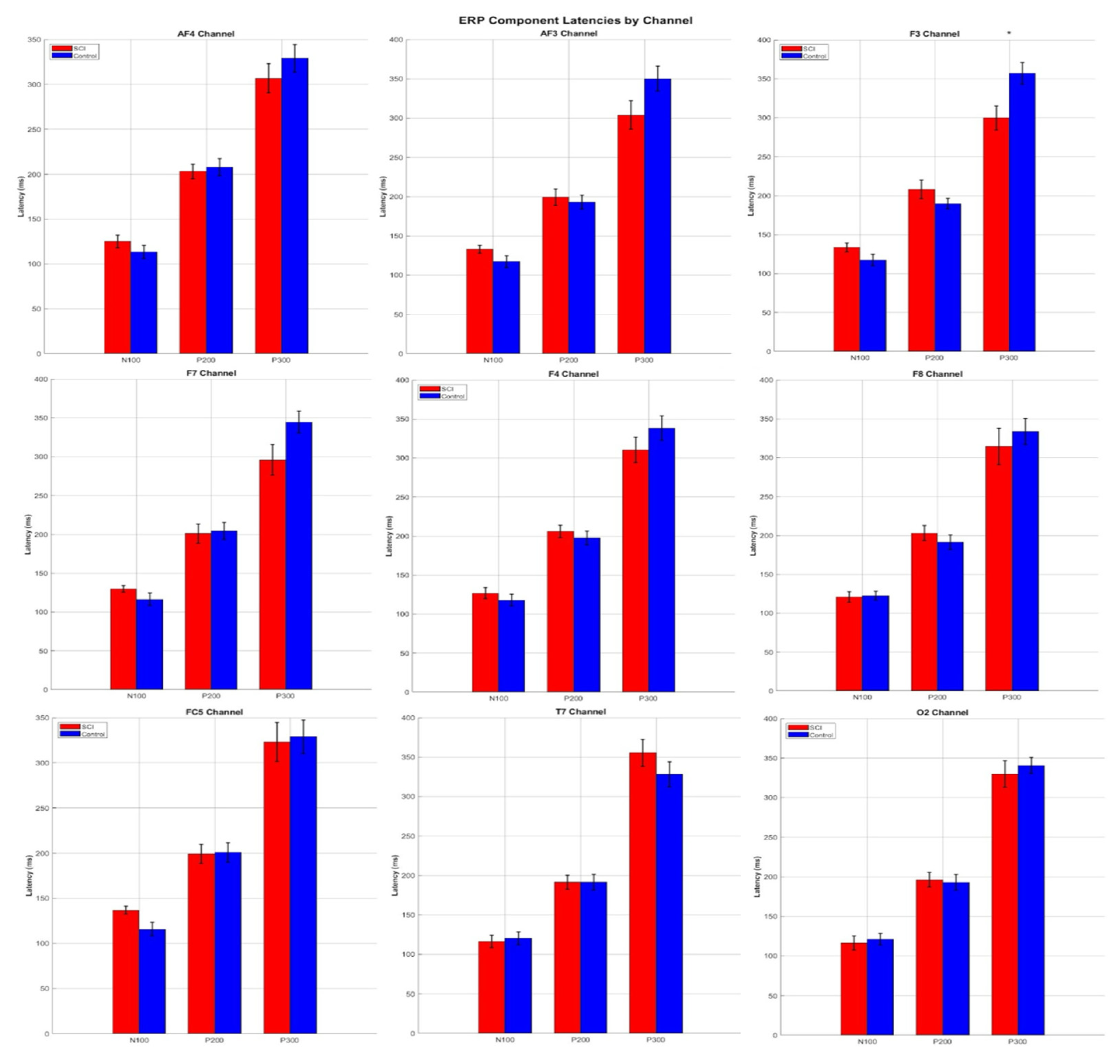
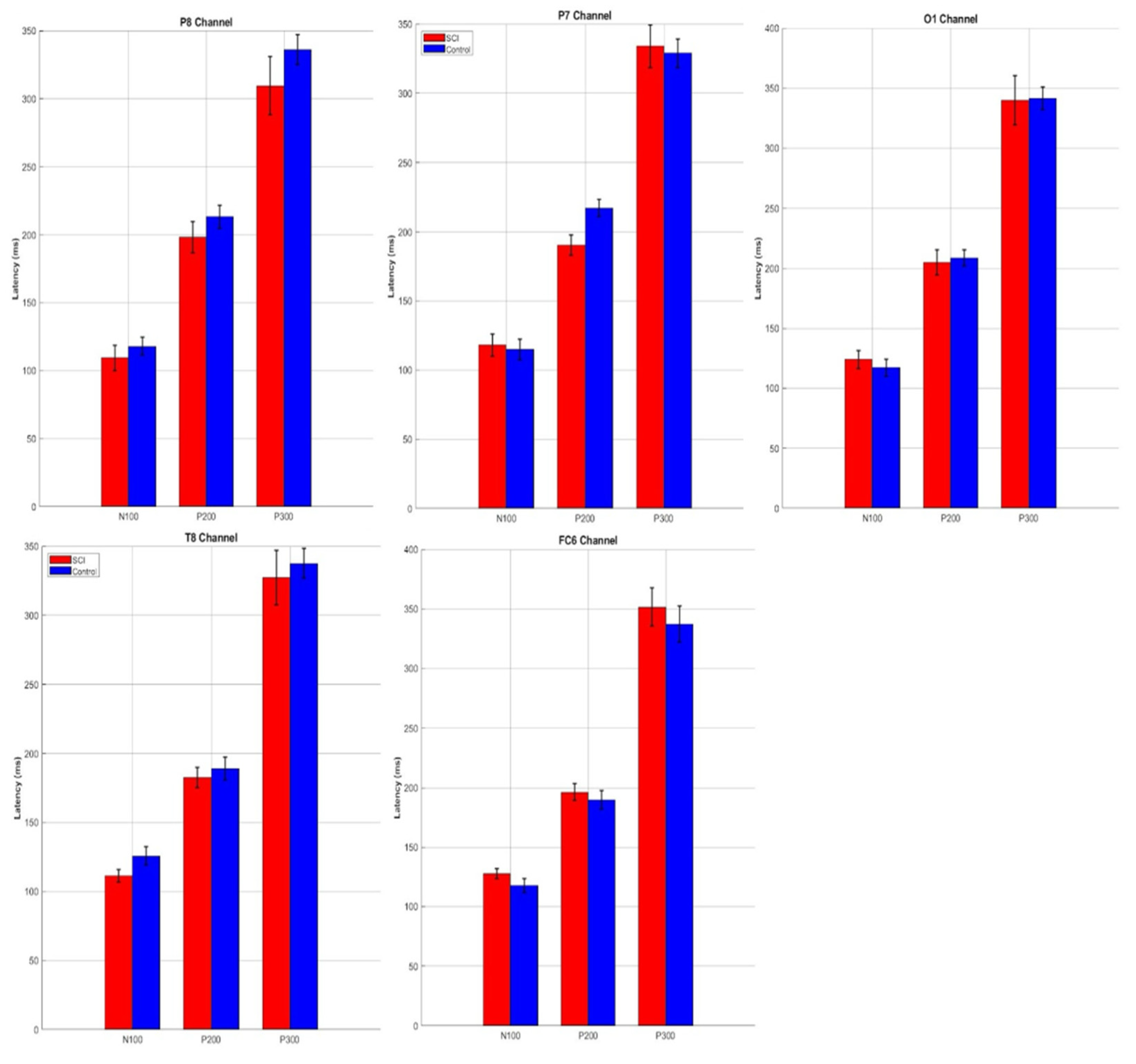
3.4. ERP Component Latencies
4. Discussion
4.1. Key Contributions and Analysis Pipeline
4.2. Neurophysiological Context for ERPs in BCI After Spinal Cord Injury
4.3. Limitations and Future Work
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ANOVA | Analysis of Variance |
| ASR | Artificial Subspace Reconstruction |
| BCI | Brain–Computer Interface |
| BERD | Biomedical Electronics Robotics and Devices Group |
| ECG | Electrocardiography |
| EEG | Electroencephalography |
| EMG | Electromyography |
| ERP | Event Related Potential |
| FDR | False Discovery Rate |
| FIR | Finite Impulse Response |
| FWER | Family-Wise Error Rate |
| HFRI | Hellenic Foundation for Research and Innovation |
| ICA | Independent Component Analysis |
| IIR | Infinite Impulse Response |
| KMI | Kinesthetic Motor Imagery |
| MI | Motor Imagery |
| MoU | Memorandum of Understanding |
| RMS | Root Mean Square |
| SCI | Spinal Cord Injury |
| SD | Standard Deviation |
References
- Athanasiou, A.; Klados, M.A.; Styliadis, C.; Foroglou, N.; Polyzoidis, K.; Bamidis, P.D. Investigating the Role of Alpha and Beta Rhythms in Functional Motor Networks. Neuroscience 2018, 378, 54–70. [Google Scholar] [CrossRef]
- Athanasiou, A.; Klados, M.A.; Pandria, N.; Foroglou, N.; Kavazidi, K.R.; Polyzoidis, K.; Bamidis, P.D. A Systematic Review of Investigations into Functional Brain Connectivity Following Spinal Cord Injury. Front. Hum. Neurosci. 2017, 11, 517. [Google Scholar] [CrossRef]
- Ruggiero, F.; Ferrucci, R.; Bocci, T.; Nigro, M.; Vergari, M.; Marceglia, S.; Barbieri, S.; Priori, A. Spino-Cerebellar tDCS Modulates N100 Components of the P300 Event Related Potential. Neuropsychologia 2019, 135, 107231. [Google Scholar] [CrossRef] [PubMed]
- Polich, J. Updating P300: An Integrative Theory of P3a and P3b. Clin. Neurophysiol. 2007, 118, 2128–2148. [Google Scholar] [CrossRef]
- Aguilar, J.; Humanes-Valera, D.; Alonso-Calviño, E.; Yague, J.G.; Moxon, K.A.; Oliviero, A.; Foffani, G. Spinal Cord Injury Immediately Changes the State of the Brain. J. Neurosci. 2010, 30, 7528–7537. [Google Scholar] [CrossRef]
- Kotchoubey, B.; Lang, S.; Winter, S.; Birbaumer, N. Cognitive Processing in Completely Paralyzed Patients with Amyotrophic Lateral Sclerosis. Eur. J. Neurol. 2003, 10, 551–558. [Google Scholar] [CrossRef]
- López-Larraz, E.; Trincado-Alonso, F.; Rajasekaran, V.; Pérez-Nombela, S.; del-Ama, A.J.; Aranda, J.; Minguez, J.; Gil-Agudo, A.; Montesano, L. Control of an Ambulatory Exoskeleton with a Brain–Machine Interface for Spinal Cord Injury Gait Rehabilitation. Front. Neurosci. 2016, 10, 359. [Google Scholar] [CrossRef]
- Vastano, R.; Widerstrom-Noga, E. Event-Related Potentials during Mental Rotation of Body-Related Stimuli in Spinal Cord Injury Population. Neuropsychologia 2023, 179, 108447. [Google Scholar] [CrossRef] [PubMed]
- Lyu, Y.; Guo, X.; Bekrater-Bodmann, R.; Flor, H.; Tong, S. An Event-Related Potential Study on the Time Course of Mental Rotation in Upper-Limb Amputees. Clin. Neurophysiol. 2017, 128, 744–750. [Google Scholar] [CrossRef]
- Ament, P.A.; Cohen, M.J.; Schandler, S.L.; Sowa, M.; Vulpe, M. Auditory P3 Event Related Potentials (ERP) and Brainstem Auditory Evoked Responses (BAER) after Spinal Cord Injury in Humans. J. Spinal Cord Med. 1995, 18, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Cohen, M.J.; Ament, P.A.; Schandler, S.L.; Vulpe, M. Changes in the P300 Component of the Tactile Event-Related Potential Following Spinal Cord Injury. Spinal Cord 1996, 34, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Lacourse, M. Cortical Potentials during Imagined Movements in Individuals with Chronic Spinal Cord Injuries. Behavioural. Brain Res. 1999, 104, 73–88. [Google Scholar] [CrossRef]
- Di Russo, F.; Aprile, T.; Spitoni, G.; Spinelli, D. Impaired Visual Processing of Contralesional Stimuli in Neglect Patients: A Visual-Evoked Potential Study. Brain 2008, 131, 842–854. [Google Scholar] [CrossRef] [PubMed]
- Lazzaro, I.; Tran, Y.; Wijesuriya, N.; Craig, A. Central Correlates of Impaired Information Processing in People with Spinal Cord Injury. J. Clin. Neurophysiol. 2013, 30, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Wang, Q.; Li, K.; He, S.; Qin, S.; Feng, Z.; Chen, Y.; Song, P.; Yang, T.; Zhang, Y.; et al. A BCI-Based Environmental Control System for Patients With Severe Spinal Cord Injuries. IEEE Trans. Biomed. Eng. 2017, 64, 1959–1971. [Google Scholar] [CrossRef]
- Rupp, R. Challenges in Clinical Applications of Brain Computer Interfaces in Individuals with Spinal Cord Injury. Front. Neuroeng. 2014, 7, 38. [Google Scholar] [CrossRef]
- Mondini, V.; Sburlea, A.-I.; Müller-Putz, G.R. Towards Unlocking Motor Control in Spinal Cord Injured by Applying an Online EEG-Based Framework to Decode Motor Intention, Trajectory and Error Processing. Sci. Rep. 2024, 14, 4714. [Google Scholar] [CrossRef]
- Bamidis, P. HEROES: Human Extremity Robotic Rehabilitation and Outcome Enhancement for Stroke; Clinicaltrials.Gov: Bethesda, MD, USA, 2024.
- Bamidis, P. Brainwave Control of a Wearable Robotic Arm for Rehabilitation and Neurophysiological Study in Cervical Spine Injury; Clinicaltrials.Gov: Bethesda, MD, USA, 2020.
- Arfaras, G.; Athanasiou, A.; Pandria, N.; Kavazidi, K.R.; Kartsidis, P.; Astaras, A.; Bamidis, P.D. Visual Versus Kinesthetic Motor Imagery for BCI Control of Robotic Arms (Mercury 2.0). In Proceedings of the 2017 IEEE 30th International Symposium on Computer-Based Medical Systems (CBMS), Thessaloniki, Greece, 22–24 June 2017; IEEE: Piscataway, NJ, USA, 2017; pp. 440–445. [Google Scholar]
- Athanasiou, A.; Xygonakis, I.; Pandria, N.; Kartsidis, P.; Arfaras, G.; Kavazidi, K.R.; Foroglou, N.; Astaras, A.; Bamidis, P.D. Towards Rehabilitation Robotics: Off-the-Shelf BCI Control of Anthropomorphic Robotic Arms. BioMed. Res. Int. 2017, 2017, 1–17. [Google Scholar] [CrossRef]
- Athanasiou, A.; Arfaras, G.; Pandria, N.; Xygonakis, I.; Foroglou, N.; Astaras, A.; Bamidis, P.D. Wireless Brain-Robot Interface: User Perception and Performance Assessment of Spinal Cord Injury Patients. Wirel. Commun. Mob. Comput. 2017, 2017, 1–16. [Google Scholar] [CrossRef]
- Xygonakis, I.; Athanasiou, A.; Pandria, N.; Kugiumtzis, D.; Bamidis, P.D. Decoding Motor Imagery through Common Spatial Pattern Filters at the EEG Source Space. Comput. Intell. Neurosci. 2018, 2018, 1–10. [Google Scholar] [CrossRef]
- Moustakas, N.; Kartsidis, P.; Athanasiou, A.; Astaras, A.; Bamidis, P.D. Development of MERCURY Version 2.0 Robotic Arms for Rehabilitation Applications. In Proceedings of the 8th ACM International Conference on PErvasive Technologies Related to Assistive Environments, Corfu, Greece, 1–3 July 2015; pp. 1–4. [Google Scholar]
- Delorme, A.; Makeig, S. EEGLAB: An Open Source Toolbox for Analysis of Single-Trial EEG Dynamics Including Independent Component Analysis. J. Neurosci. Methods 2004, 134, 9–21. [Google Scholar] [CrossRef] [PubMed]
- CIMeC Wiki|Data Pre-Processing. Available online: http://wiki.cimec.unitn.it/tiki-index.php?page=Data+pre-processing (accessed on 12 June 2025).
- Nounou, M.N.; Bakshi, B.R. Multiscale Methods for Denoising and Compression. In Data Handling in Science and Technology; Elsevier: Amsterdam, The Netherlands, 2000; Volume 22, pp. 119–150. ISBN 978-0-444-50111-0. [Google Scholar]
- Mitra, S.K. Digital Signal Processing: A Computer-Based Approach, 2nd ed.; McGraw-Hill Series in Electrical and Computer Engineering; McGraw-Hill/Irwin: Boston, MA, USA, 2001; ISBN 978-0-07-232105-0. [Google Scholar]
- Miyakoshi, M. Artifact Subspace Reconstruction: A Candidate for a Dream Solution for EEG Studies, Sleep or Awake. Sleep 2023, 46, zsad241. [Google Scholar] [CrossRef] [PubMed]
- Kothe, C.A.; Makeig, S. BCILAB: A Platform for Brain–Computer Interface Development. J. Neural Eng. 2013, 10, 056014. [Google Scholar] [CrossRef] [PubMed]
- Hyvärinen, A.; Oja, E. Independent Component Analysis: Algorithms and Applications. Neural Netw. 2000, 13, 411–430. [Google Scholar] [CrossRef]
- Kim, H.-Y. Analysis of Variance (ANOVA) Comparing Means of More than Two Groups. Restor. Dent. Endod. 2014, 39, 74. [Google Scholar] [CrossRef]
- Athanasiou, A.; Terzopoulos, N.; Pandria, N.; Xygonakis, I.; Foroglou, N.; Polyzoidis, K.; Bamidis, P.D. Functional Brain Connectivity during Multiple Motor Imagery Tasks in Spinal Cord Injury. Neural Plast. 2018, 2018, 1–20. [Google Scholar] [CrossRef]
- Herbert, B.M.; Pollatos, O.; Schandry, R. Interoceptive Sensitivity and Emotion Processing: An EEG Study. Int. J. Psychophysiol. 2007, 65, 214–227. [Google Scholar] [CrossRef]
- Dagdevir, E.; Tokmakci, M. Optimization of Preprocessing Stage in EEG Based BCI Systems in Terms of Accuracy and Timing Cost. Biomed. Signal Process. Control 2021, 67, 102548. [Google Scholar] [CrossRef]
- Widmann, A.; Schröger, E.; Maess, B. Digital Filter Design for Electrophysiological Data—A Practical Approach. J. Neurosci. Methods 2015, 250, 34–46. [Google Scholar] [CrossRef]
- De Cheveigné, A.; Nelken, I. Filters: When, Why, and How (Not) to Use Them. Neuron 2019, 102, 280–293. [Google Scholar] [CrossRef]
- Kappenman, E.S.; Luck, S.J. The Effects of Electrode Impedance on Data Quality and Statistical Significance in ERP Recordings. Psychophysiology 2010. [Google Scholar] [CrossRef]
- Luck, S.J.; Gaspelin, N. How to Get Statistically Significant Effects in Any ERP Experiment (and Why You Shouldn’t). Psychophysiology 2017, 54, 146–157. [Google Scholar] [CrossRef] [PubMed]
- Näätänen, R.; Picton, T. The N1 Wave of the Human Electric and Magnetic Response to Sound: A Review and an Analysis of the Component Structure. Psychophysiology 1987, 24, 375–425. [Google Scholar] [CrossRef] [PubMed]
- Freund, P.; Weiskopf, N.; Ashburner, J.; Wolf, K.; Sutter, R.; Altmann, D.R.; Friston, K.; Thompson, A.; Curt, A. MRI Investigation of the Sensorimotor Cortex and the Corticospinal Tract after Acute Spinal Cord Injury: A Prospective Longitudinal Study. Lancet Neurol. 2013, 12, 873–881. [Google Scholar] [CrossRef] [PubMed]
- Collignon, O.; Vandewalle, G.; Voss, P.; Albouy, G.; Charbonneau, G.; Lassonde, M.; Lepore, F. Functional Specialization for Auditory–Spatial Processing in the Occipital Cortex of Congenitally Blind Humans. Proc. Natl. Acad. Sci. USA 2011, 108, 4435–4440. [Google Scholar] [CrossRef]
- Ritter, W.; Simson, R.; Vaughan, H.G. Event-Related Potential Correlates of Two Stages of Information Processing in Physical and Semantic Discrimination Tasks. Psychophysiology 1983, 20, 168–179. [Google Scholar] [CrossRef]
- Crowley, K.E.; Colrain, I.M. A Review of the Evidence for P2 Being an Independent Component Process: Age, Sleep and Modality. Clin. Neurophysiol. 2004, 115, 732–744. [Google Scholar] [CrossRef]
- Rademacher, J.; Morosan, P.; Schormann, T.; Schleicher, A.; Werner, C.; Freund, H.-J.; Zilles, K. Probabilistic Mapping and Volume Measurement of Human Primary Auditory Cortex. NeuroImage 2001, 13, 669–683. [Google Scholar] [CrossRef]
- Driver, J.; Noesselt, T. Multisensory Interplay Reveals Crossmodal Influences on ‘Sensory-Specific’ Brain Regions, Neural Responses, and Judgments. Neuron 2008, 57, 11–23. [Google Scholar] [CrossRef]
- Soltani, M.; Knight, R.T. Neural Origins of the P300. Crit. Rev. Neurobiol. 2000, 14, 199–224. [Google Scholar] [CrossRef]
- Comerchero, M.D.; Polich, J. P3a and P3b from Typical Auditory and Visual Stimuli. Clin. Neurophysiol. 1999, 110, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Pfurtscheller, G.; Neuper, C. Motor Imagery and Direct Brain-Computer Communication. Proc. IEEE 2001, 89, 1123–1134. [Google Scholar] [CrossRef]
- López-Larraz, E.; Antelis, J.M.; Montesano, L.; Gil-Agudo, A.; Minguez, J. Continuous Decoding of Motor Attempt and Motor Imagery from EEG Activity in Spinal Cord Injury Patients. In Proceedings of the 2012 Annual International Conference of the IEEE Engineering in Medicine and Biology Society, San Diego, CA, USA, 28 August–1 September 2012; IEEE: Piscataway, NJ, USA, 2012; pp. 1798–1801. [Google Scholar]
- Blight, A.R.; Hsieh, J.; Curt, A.; Fawcett, J.W.; Guest, J.D.; Kleitman, N.; Kurpad, S.N.; Kwon, B.K.; Lammertse, D.P.; Weidner, N.; et al. The Challenge of Recruitment for Neurotherapeutic Clinical Trials in Spinal Cord Injury. Spinal Cord 2019, 57, 348–359. [Google Scholar] [CrossRef] [PubMed]
- Barbeau, H.; Norman, K.; Fung, J.; Visintin, M.; Ladouceur, M. Does Neurorehabilitation Play a Role in the Recovery of Walking in Neurological Populations? Ann. N. Y. Acad. Sci. 1998, 860, 377–392. [Google Scholar] [CrossRef]
- Gomez-Tapia, C.; Bozic, B.; Longo, L. Evaluation of EEG Pre-Processing and Source Localization in Ecological Research. Front. Neuroimaging 2025, 4, 1479569. [Google Scholar] [CrossRef]
- Salisbury, D.B.; Parsons, T.D.; Monden, K.R.; Trost, Z.; Driver, S.J. Brain–Computer Interface for Individuals after Spinal Cord Injury. Rehabil. Psychol. 2016, 61, 435–441. [Google Scholar] [CrossRef]
- Tarara, P.; Przybył, I.; Schöning, J.; Gunia, A. Motor Imagery-Based Brain-Computer Interfaces: An Exploration of Multiclass Motor Imagery-Based Control for Emotiv EPOC X. Front. Neuroinform. 2025, 19, 1625279. [Google Scholar]
- Fakhruzzaman, M.N.; Riksakomara, E.; Suryotrisongko, H. EEG Wave Identification in Human Brain with Emotiv EPOC for Motor Imagery. Procedia Comput. Sci. 2015, 72, 269–276. [Google Scholar] [CrossRef]
- Winkler, I.; Haufe, S.; Tangermann, M. Automatic Classification of Artifactual ICA-Components for Artifact Removal in EEG Signals. Behav. Brain Funct. 2011, 7, 30. [Google Scholar] [CrossRef]
- Acunzo, D.J.; MacKenzie, G.; Van Rossum, M.C.W. Systematic Biases in Early ERP and ERF Components as a Result of High-Pass Filtering. J. Neurosci. Methods 2012, 209, 212–218. [Google Scholar] [CrossRef]
- Tanner, D.; Morgan-Short, K.; Luck, S.J. How Inappropriate High-pass Filters Can Produce Artifactual Effects and Incorrect Conclusions in ERP Studies of Language and Cognition. Psychophysiology 2015, 52, 997–1009. [Google Scholar] [CrossRef] [PubMed]
- Michel, C.M.; Murray, M.M. Towards the Utilization of EEG as a Brain Imaging Tool. Neuroimage 2012, 61, 371–385. [Google Scholar] [CrossRef]
- Zoefel, B.; Huster, R.J.; Herrmann, C.S. Neurofeedback Training of the Upper Alpha Frequency Band in EEG Improves Cognitive Performance. Neuroimage 2011, 54, 1427–1431. [Google Scholar] [CrossRef]
- Babiloni, C.; Blinowska, K.; Bonanni, L.; Cichocki, A.; De Haan, W.; Del Percio, C.; Dubois, B.; Escudero, J.; Fernández, A.; Frisoni, G.; et al. What Electrophysiology Tells Us about Alzheimer’s Disease: A Window into the Synchronization and Connectivity of Brain Neurons. Neurobiol. Aging 2020, 85, 58–73. [Google Scholar] [CrossRef] [PubMed]
- Reuderink, B.; Mühl, C.; Poel, M. Valence, Arousal and Dominance in the EEG during Game Play. IJAACS 2013, 6, 45. [Google Scholar] [CrossRef]
| Channels | SCI Group Min (μV) | Control Group Min (μV) | SCI Group Min (μV) | Control Group Max (μV) |
|---|---|---|---|---|
| AF3 | −6.70 | −6.56 | 4.28 | 7.77 |
| F7 | −8.76 | −7.19 | 7.19 | 6.26 |
| F3 | −4.82 | −5.74 | 2.79 | 5.35 |
| FC5 | −5.89 | −6.56 | 4.37 | 5.25 |
| T7 | −21.62 | −5.60 | 19.72 | 5.55 |
| P7 | −5.15 | −6.25 | 4.00 | 5.74 |
| O1 | −5.07 | −5.66 | 3.2 | 5.49 |
| O2 | −7.29 | −4.94 | 6.03 | 4.85 |
| P8 | −7.99 | −5.96 | 7.05 | 5.02 |
| T8 | −8.67 | −4.19 | 9.00 | 5.20 |
| FC6 | −4.60 | −5.76 | 3.34 | 6.15 |
| F4 | −5.94 | −14.19 | 3.98 | 11.83 |
| F8 | −7.08 | −4.88 | 5.41 | 4.31 |
| AF4 | −5.68 | −6.66 | 3.57 | 4.76 |
| Channel | SCI Group Min (ms) | Control Group Min (ms) | SCI Group Max (ms) | Control Group Max (ms) |
|---|---|---|---|---|
| AF3 | 0 | 0 | 320.31 | 343.75 |
| F7 | 0 | 0 | 304.69 | 375.00 |
| F3 | 0 | 0 | 398.44 | 312.50 |
| FC5 | 0 | 0 | 382.81 | 382.81 |
| T7 | 0 | 0 | 375.00 | 390.62 |
| P7 | 0 | 0 | 351.56 | 328.12 |
| O1 | 0 | 0 | 328.12 | 367.19 |
| O2 | 0 | 0 | 398.44 | 312.50 |
| P8 | 0 | 0 | 398.44 | 390.62 |
| T8 | 0 | 0 | 375.00 | 312.50 |
| FC6 | 0 | 0 | 328.12 | 370.00 |
| F4 | 0 | 0 | 380.00 | 375.00 |
| F8 | 0 | 0 | 367.19 | 310.00 |
| AF4 | 0 | 0 | 310.00 | 390.00 |
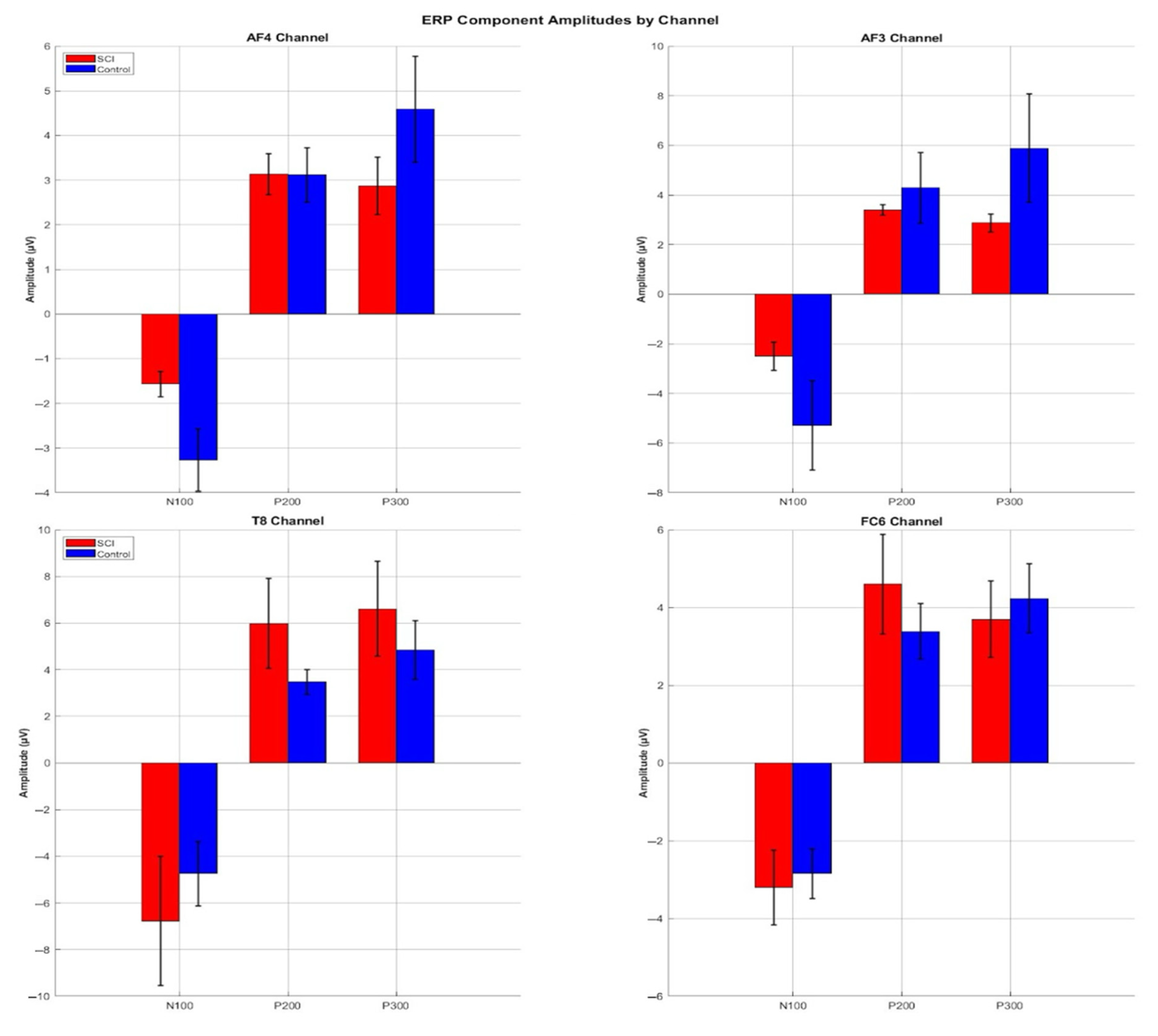
| Channels | Components | SCI Min (μV) | SCI Max (μV) | SCI Mean (μV) | Control Min (μV) | Control Max (μV) | Control Mean (mV) |
|---|---|---|---|---|---|---|---|
| AF3 | N100 | −5.24 | −1.35 | −2.97 | −17.27 | −0.75 | −5.68 |
| P200 | 2.26 | 4.19 | 3.41 | 0.70 | 15.25 | 4.29 | |
| P300 | 1.23 | 4.30 | 2.82 | 0.44 | 16.55 | 4.43 | |
| F7 | N100 | −6.06 | −1.15 | −3.27 | −10.46 | −0.47 | −2.80 |
| P200 | 2.14 | 4.62 | 3.44 | 0.53 | 7.61 | 2.76 | |
| P300 | 1.91 | 5.28 | 2.82 | 0.76 | 4.34 | 2.61 | |
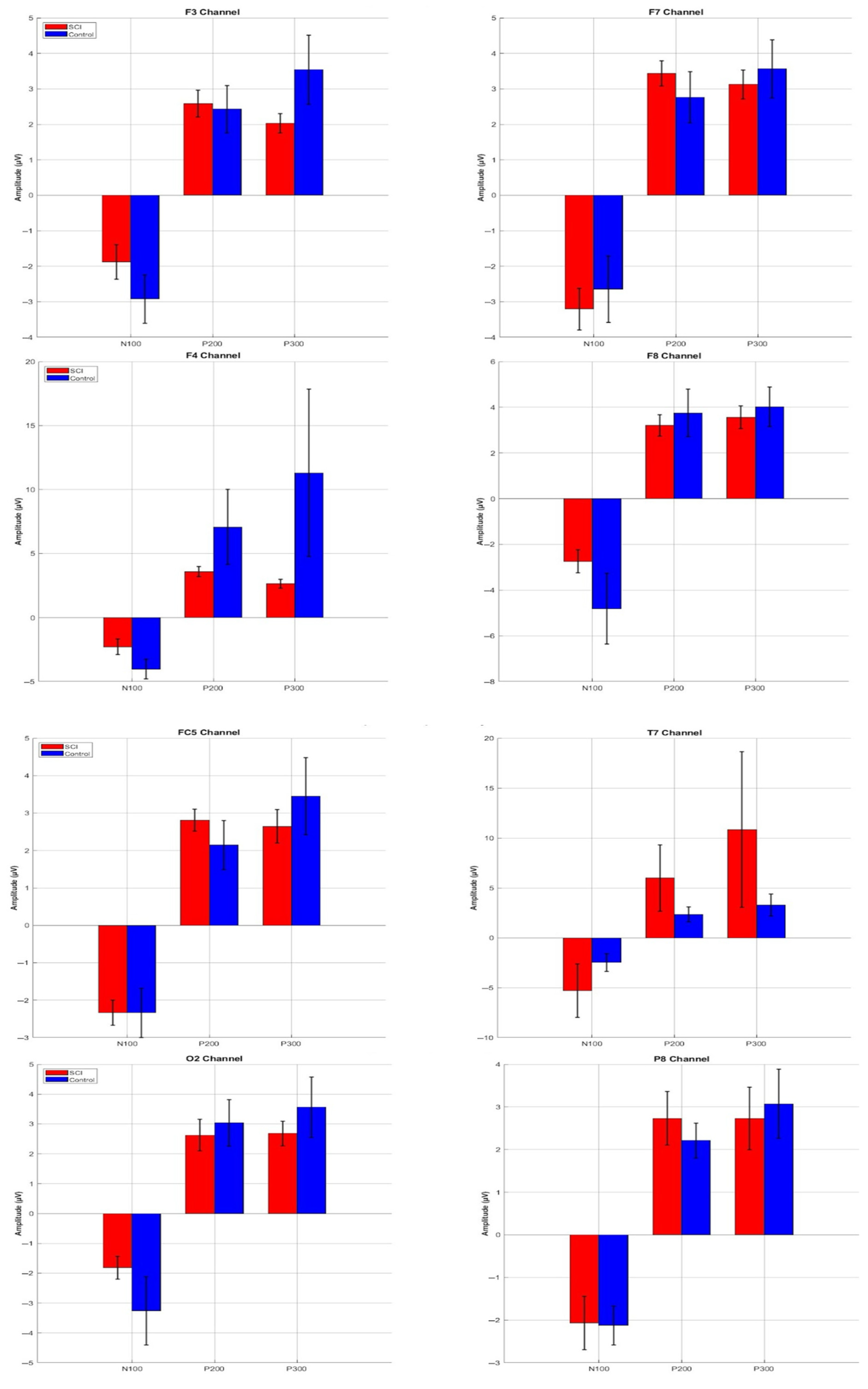
| F3 | N100 | −4.35 | −0.67 | −2.13 | −11.20 | −0.65 | −3.29 |
| P200 | 0.98 | 3.99 | 2.58 | 0.71 | 6.98 | 2.43 | |
| P300 | 0.85 | 3.30 | 2.01 | 0.49 | 6.59 | 2.21 | |
| FC5 | N100 | −3.85 | −1.40 | −2.36 | −8.98 | −0.26 | −2.65 |
| P200 | 1.50 | 4.21 | 2.81 | 0.28 | 6.01 | 2.15 | |
| P300 | 0.20 | 4.45 | 2.14 | 0.23 | 9.60 | 2.72 | |
| T7 | N100 | −23.94 | −1.69 | −5.52 | −13.27 | −0.47 | −2.92 |
| P200 | 1.03 | 29.14 | 6.00 | 0.40 | 8.02 | 2.34 | |
| P300 | 0.62 | 65.07 | 10.04 | 0.76 | 9.89 | 2.60 | |
| P7 | N100 | −2.77 | −0.65 | −1.63 | −10.16 | −0.19 | −2.76 |
| P200 | 0.49 | 3.35 | 1.53 | 0.19 | 6.57 | 2.48 | |
| P300 | 0.61 | 1.73 | 1.26 | 0.54 | 8.47 | 2.30 | |
| O1 | N100 | −2.35 | −0.58 | −1.27 | −15.08 | −0.52 | −3.19 |
| P200 | 0.49 | 2.58 | 1.58 | 0.76 | 8.03 | 2.46 | |
| P300 | 0.16 | 1.77 | 1.11 | 0.94 | 11.00 | 2.69 | |
| O2 | N100 | −3.98 | −1.15 | −1.98 | −12.86 | −0.69 | −3.80 |
| P200 | 0.70 | 4.96 | 2.62 | 0.96 | 9.35 | 3.03 | |
| P300 | 0.78 | 3.84 | 2.41 | 1.01 | 10.08 | 2.94 | |
| P8 | N100 | −4.86 | −0.55 | −2.38 | −6.66 | −0.71 | −2.79 |
| P200 | 0.90 | 6.68 | 2.73 | 0.85 | 5.22 | 2.21 | |
| P300 | 0.52 | 6.68 | 2.58 | 0.46 | 4.95 | 2.21 | |
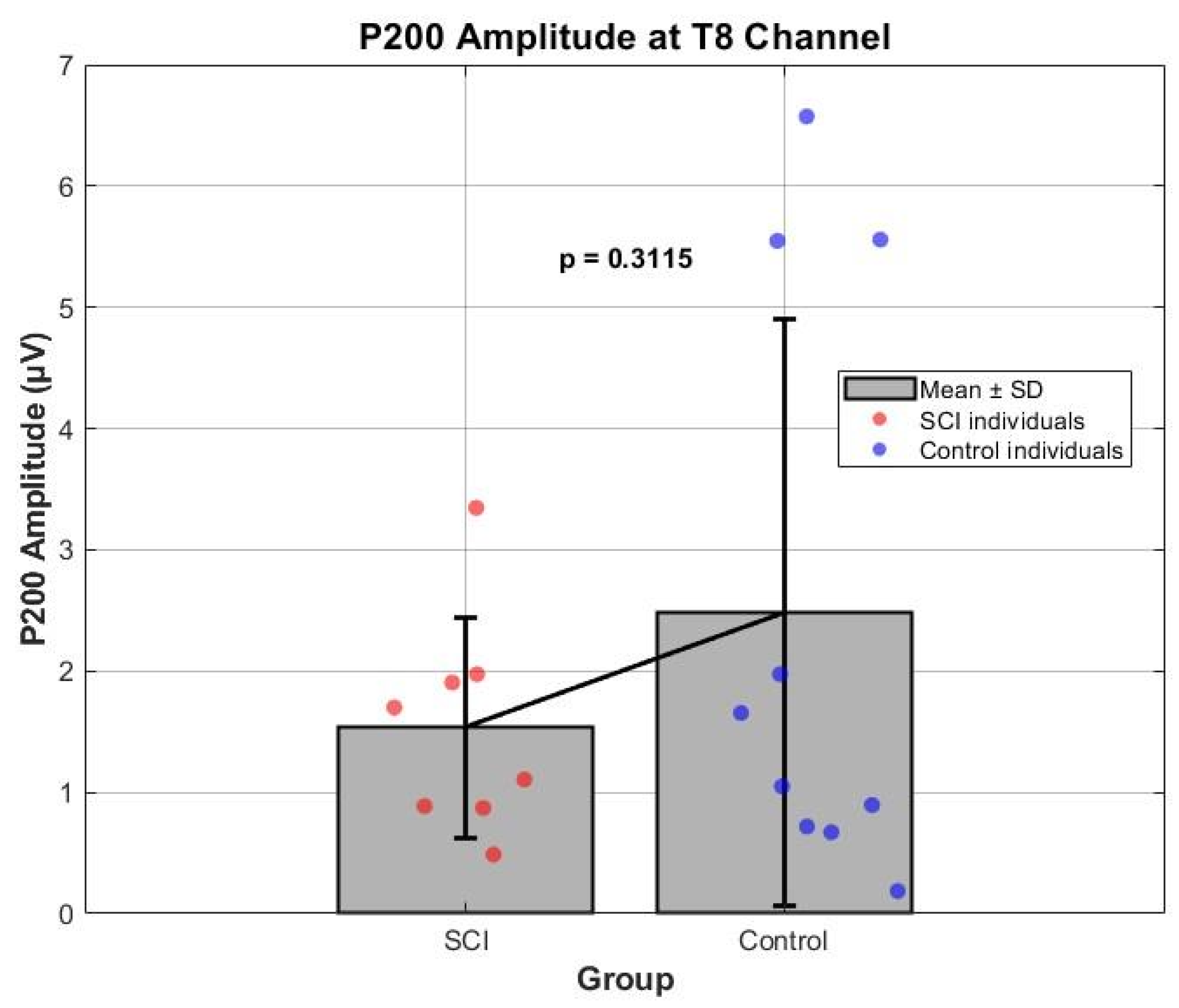
| T8 | N100 | −19.55 | −1.30 | −7.31 | −14.98 | −0.93 | −4.74 |
| P200 | 1.65 | 15.95 | 5.98 | 1.66 | 6.00 | 3.47 | |
| P300 | 0.53 | 19.38 | 5.56 | 0.99 | 13.66 | 4.00 | |
| FC6 | N100 | −8.48 | −0.75 | −3.51 | −7.19 | −0.43 | −3.30 |
| P200 | 1.83 | 12.27 | 4.60 | 0.99 | 7.89 | 3.39 | |
| P300 | 1.05 | 10.05 | 3.29 | 0.90 | 6.33 | 2.79 | |
| F4 | N100 | −5.98 | −1.15 | −2.69 | −10.74 | −1.20 | −4.53 |
| P200 | 1.66 | 4.77 | 3.57 | 0.91 | 30.48 | 7.07 | |
| P300 | 0.19 | 3.55 | 2.41 | 0.85 | 30.48 | 6.39 | |
| F8 | N100 | −5.05 | −1.47 | −3.09 | −14.89 | −1.36 | −4.98 |
| P200 | 1.24 | 4.98 | 3.21 | 0.91 | 10.44 | 3.75 | |
| P300 | 1.32 | 6.12 | 3.15 | 0.87 | 8.68 | 3.56 | |
| AF4 | N100 | −4.11 | −0.59 | −2.41 | −20.84 | −0.67 | −4.78 |
| P200 | 0.60 | 4.69 | 3.13 | 0.68 | 5.82 | 3.11 | |
| P300 | 0.55 | 5.71 | 2.80 | 0.97 | 12.34 | 3.31 |
| Channels | Components | SCI Min (ms) | SCI Max (ms) | SCI Mean (ms) | Control Min (ms) | Control Max (ms) | Control Mean (ms) |
|---|---|---|---|---|---|---|---|
| AF3 | N100 | 54.69 | 148.44 | 109.38 | 54.69 | 148.44 | 109.38 |
| P200 | 156.25 | 250.00 | 199.22 | 156.25 | 226.56 | 192.97 | |
| P300 | 250.00 | 320.31 | 281.25 | 257.81 | 320.31 | 284.72 | |
| F7 | N100 | 70.31 | 148.44 | 124.02 | 54.69 | 148.44 | 101.56 |
| P200 | 156.25 | 250.00 | 201.17 | 156.25 | 250.00 | 204.69 | |
| P300 | 250.00 | 304.69 | 274.41 | 250.00 | 343.75 | 300.78 | |
| F3 | N100 | 54.69 | 148.44 | 116.21 | 54.69 | 148.44 | 109.38 |
| P200 | 156.25 | 250.00 | 208.01 | 156.25 | 218.75 | 189.84 | |
| P300 | 257.81 | 328.12 | 290.04 | 250.00 | 343.75 | 288.28 | |
| FC5 | N100 | 70.31 | 148.44 | 130.86 | 54.69 | 148.44 | 99.22 |
| P200 | 164.06 | 250.00 | 199.22 | 156.25 | 250.00 | 200.78 | |
| P300 | 250.00 | 328.12 | 289.06 | 250.00 | 343.75 | 292.19 | |
| T7 | N100 | 54.69 | 132.81 | 103.52 | 54.69 | 140.62 | 93.75 |
| P200 | 156.25 | 218.75 | 191.41 | 156.25 | 250.00 | 191.41 | |
| P300 | 257.81 | 328.12 | 294.92 | 250.00 | 343.75 | 296.88 | |
| P7 | N100 | 62.50 | 148.44 | 112.30 | 54.69 | 148.44 | 95.31 |
| P200 | 156.25 | 226.56 | 190.43 | 179.69 | 242.19 | 217.19 | |
| P300 | 250.00 | 335.94 | 288.09 | 257.81 | 343.75 | 230.31 | |
| O1 | N100 | 54.69 | 148.44 | 112.30 | 54.69 | 140.62 | 100.00 |
| P200 | 171.88 | 250.00 | 205.08 | 164.06 | 234.38 | 208.59 | |
| P300 | 250.00 | 328.12 | 284.18 | 273.44 | 343.75 | 314.06 | |
| O2 | N100 | 70.31 | 148.44 | 98.63 | 62.50 | 148.44 | 107.03 |
| P200 | 156.25 | 234.38 | 196.29 | 156.25 | 234.38 | 102.97 | |
| P300 | 250.00 | 343.75 | 302.73 | 257.81 | 343.75 | 312.50 | |
| P8 | N100 | 70.31 | 148.44 | 98.63 | 62.50 | 148.44 | 105.47 |
| P200 | 156.25 | 250.00 | 198.24 | 156.25 | 250.00 | 213.28 | |
| P300 | 250.00 | 296.88 | 267.58 | 257.81 | 343.75 | 303.12 | |
| T8 | N100 | 54.69 | 117.19 | 98.84 | 85.94 | 148.44 | 125.78 |
| P200 | 156.25 | 210.94 | 182.68 | 164.06 | 226.56 | 189.06 | |
| P300 | 250.00 | 312.50 | 282.23 | 257.81 | 343.75 | 299.22 | |
| FC6 | N100 | 62.50 | 140.62 | 110.35 | 54.69 | 140.62 | 107.81 |
| P200 | 164.06 | 226.56 | 196.29 | 156.25 | 226.56 | 189,84 | |
| P300 | 250.00 | 320.31 | 291.02 | 250.00 | 343.75 | 283.59 | |
| F4 | N100 | 54.69 | 148.44 | 102.54 | 54.69 | 148.44 | 110.16 |
| P200 | 179.69 | 250.00 | 206.05 | 156.25 | 250.00 | 197.66 | |
| P300 | 250.00 | 328.12 | 289.06 | 250.00 | 320.31 | 282.03 | |
| F8 | N100 | 54.69 | 140.62 | 102.54 | 54.69 | 140.62 | 114.84 |
| P200 | 164.06 | 242.19 | 282.23 | 156.25 | 226.56 | 278.91 | |
| P300 | 250.00 | 320.31 | 367.19 | 257.81 | 320.31 | 312.50 | |
| AF4 | N100 | 54.69 | 140.62 | 99.61 | 54.69 | 140.62 | 100.78 |
| P200 | 179.69 | 250.00 | 203.12 | 164.06 | 250.00 | 207.81 | |
| P300 | 250.00 | 328.12 | 291.99 | 250.00 | 320.31 | 282.03 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spanos, M.; Gazea, T.; Triantafyllidis, V.; Mitsopoulos, K.; Vrahatis, A.; Hadjinicolaou, M.; Bamidis, P.D.; Athanasiou, A. Post Hoc Event-Related Potential Analysis of Kinesthetic Motor Imagery-Based Brain-Computer Interface Control of Anthropomorphic Robotic Arms. Electronics 2025, 14, 3106. https://doi.org/10.3390/electronics14153106
Spanos M, Gazea T, Triantafyllidis V, Mitsopoulos K, Vrahatis A, Hadjinicolaou M, Bamidis PD, Athanasiou A. Post Hoc Event-Related Potential Analysis of Kinesthetic Motor Imagery-Based Brain-Computer Interface Control of Anthropomorphic Robotic Arms. Electronics. 2025; 14(15):3106. https://doi.org/10.3390/electronics14153106
Chicago/Turabian StyleSpanos, Miltiadis, Theodora Gazea, Vasileios Triantafyllidis, Konstantinos Mitsopoulos, Aristidis Vrahatis, Maria Hadjinicolaou, Panagiotis D. Bamidis, and Alkinoos Athanasiou. 2025. "Post Hoc Event-Related Potential Analysis of Kinesthetic Motor Imagery-Based Brain-Computer Interface Control of Anthropomorphic Robotic Arms" Electronics 14, no. 15: 3106. https://doi.org/10.3390/electronics14153106
APA StyleSpanos, M., Gazea, T., Triantafyllidis, V., Mitsopoulos, K., Vrahatis, A., Hadjinicolaou, M., Bamidis, P. D., & Athanasiou, A. (2025). Post Hoc Event-Related Potential Analysis of Kinesthetic Motor Imagery-Based Brain-Computer Interface Control of Anthropomorphic Robotic Arms. Electronics, 14(15), 3106. https://doi.org/10.3390/electronics14153106









