Developing a Novel Muscle Fatigue Index for Wireless sEMG Sensors: Metrics and Regression Models for Real-Time Monitoring
Abstract
1. Introduction
1.1. Background and Motivation
1.2. Related Work
1.3. Contribution of This Work
2. Materials and Methods
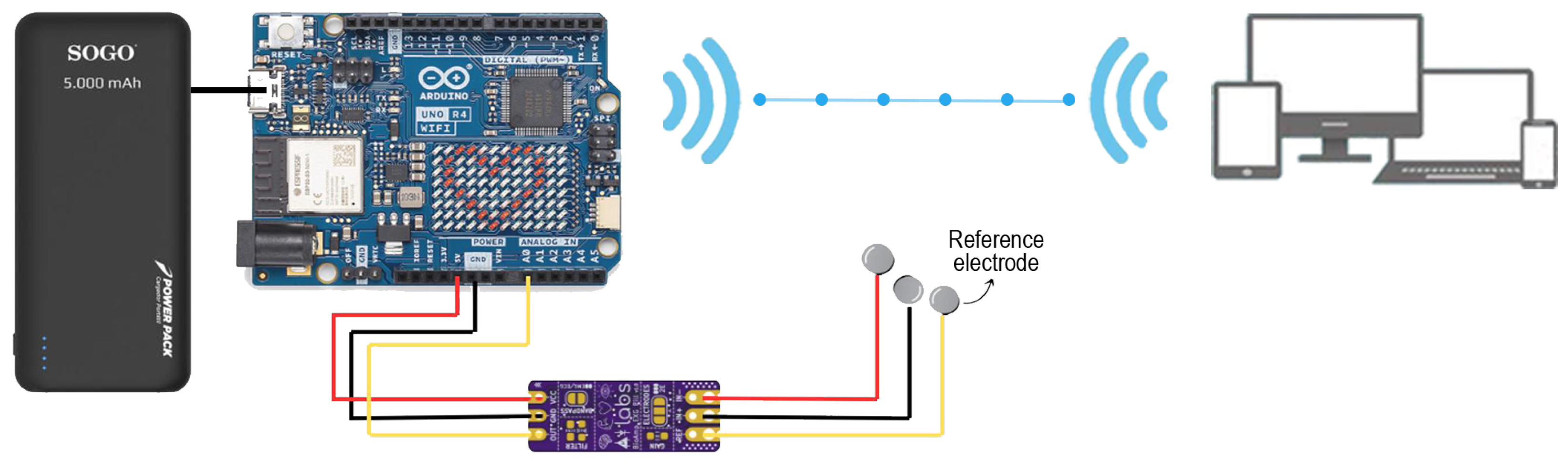
2.1. System Design
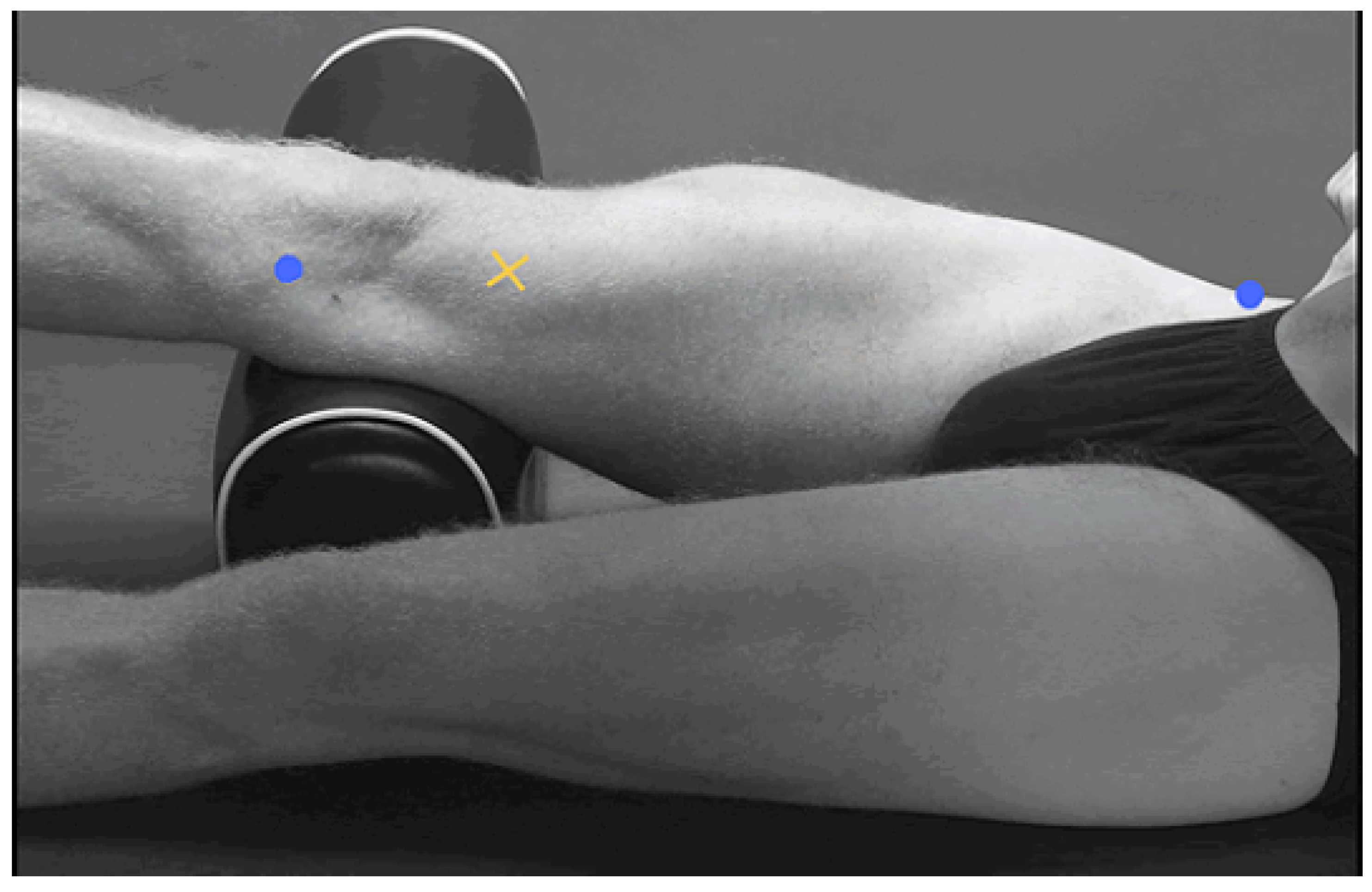
2.2. Data Collection
2.3. Signal Processing and Feature Extraction
2.3.1. Feature Evaluation
- RMS;
- MNF/ARV ratio;
- Instantaneous Mean Amplitude Difference (IMA Difference);
- EMD-based Median Frequencies (MDF1 and MDF2);
- Fluctuation Variance;
- Fluctuation Range Values;
- Fluctuation Mean Difference.
2.3.2. Window Size Analysis
3. Metric Standardization and Fatigue Modeling
3.1. Baseline Establishment
- Metric (Active/RMS (Rest))
- Metric (Active)/Metric (RMS (Rest))
- Metric (Active/RMS (1st Active))
- Metric (Active)/Metric (RMS (1st Active))
- Equal-weighted sum
- Average
- PCA
- t-SNE
3.2. Machine Learning Model Training and Evaluation
- Simple Linear Regression
- Support Vector Regression
- Random Forest Regression
- Gradient-Boosting Machine Regression
- Long Short-Term Memory (LSTM) Neural Network Regression
- Convolutional Neural Network Regression
- k-Nearest Neighbor Regression
4. Results and Discussion
4.1. Baseline and Metric Analysis
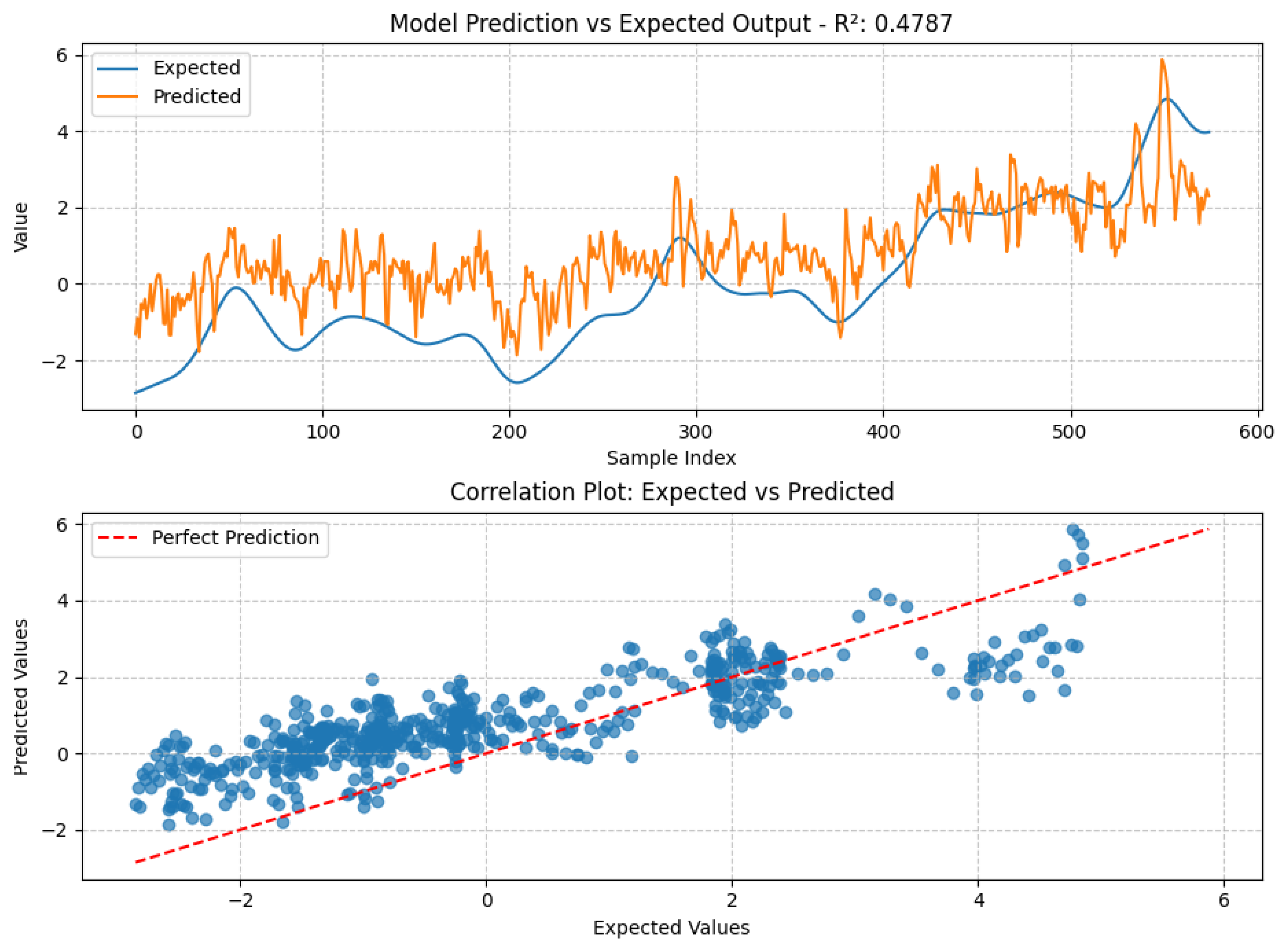
4.2. Fatigue Estimation Performance
4.3. Machine Learning Model Performance
4.4. Comparative Discussion
5. Conclusions and Future Work
5.1. Key Findings
5.2. Contributions of This Work
5.3. Limitations
5.4. Future Work
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Al-Ayyad, M.; Owida, H.A.; De Fazio, R.; Al-Naami, B.; Visconti, P. Electromyography Monitoring Systems in Rehabilitation: A Review of Clinical Applications, Wearable Devices and Signal Acquisition Methodologies. Electronics 2023, 12, 1520. [Google Scholar] [CrossRef]
- Liu, S.H.; Lin, C.B.; Chen, Y.; Chen, W.; Huang, T.S.; Hsu, C.Y. An EMG Patch for the Real-Time Monitoring of Muscle-Fatigue Conditions During Exercise. Sensors 2019, 19, 3108. [Google Scholar] [CrossRef] [PubMed]
- Wencong, X. A Surface EMG System: Local Muscle Fatigue Detection. Master’s Thesis, Delft University of Technology, Delft, The Netherlands, 2020. [Google Scholar]
- Samann, F.; Schanze, T. EMG based muscle fatigue detection using autocorrelation and k-means clustering. In Proceedings on Automation in Medical Engineering; Infinite Science: Lübeck, Germany, 2023. [Google Scholar]
- Guzu, A.; Neacşu, A.; Georgian, N. Automatic Muscle Fatigue and Movement Recognition Based on sEMG Signals. In Proceedings of the International Symposium ELMAR, Zadar, Croatia, 16–18 September 2024; pp. 259–262. [Google Scholar] [CrossRef]
- Wu, Y.D.; Ruan, S.J.; Lee, Y.H. An Ultra-Low Power Surface EMG Sensor for Wearable Biometric and Medical Applications. Biosensors 2021, 11, 411. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Liu, G.; Sun, Y.; Lin, K.; Zhou, Z.; Cai, J. Application of Surface Electromyography in Exercise Fatigue: A Review. Front. Syst. Neurosci. 2022, 16, 893275. [Google Scholar] [CrossRef] [PubMed]
- Yousif, H.; Zakaria, A.; Abdul Rahim, N.; Salleh, A.; Sabry, M.; Alfarhan, K.; Kamarudin, L.; Syed Zakaria, S.M.M.; Hasan, A.; K Hussain, M. Assessment of Muscles Fatigue Based on Surface EMG Signals Using Machine Learning and Statistical Approaches: A Review. IOP Conf. Ser. Mater. Sci. Eng. 2019, 705, 012010. [Google Scholar] [CrossRef]
- Qassim, H.; Hasan, W.; Ramli, H.; Harith, H.; Mat, L.; Ismail, L. Proposed Fatigue Index for the Objective Detection of Muscle Fatigue Using Surface Electromyography and a Double-Step Binary Classifier. Sensors 2022, 22, 1900. [Google Scholar] [CrossRef] [PubMed]
- Kinugasa, R.; Kubo, S. Development of Consumer-Friendly Surface Electromyography System for Muscle Fatigue Detection. IEEE Access 2023, 11, 6394–6403. [Google Scholar] [CrossRef]
- BioAmp EXG Pill v1.0b Schematic. Available online: https://raw.githubusercontent.com/upsidedownlabs/BioAmp-EXG-Pill/main/graphics/docs/BioAmp-EXG-Pill-v1.0b-schematic.png (accessed on 1 October 2023).
- Myoware 2.0 Muscle Sensor Advanced Guide/Technical Specifications. Available online: https://myoware.com/products/muscle-sensor/ (accessed on 3 October 2023).
- Recommendations for Sensor Locations in Vastus Medialiss. Available online: http://seniam.org/quadricepsfemorisvastusmedialis.html (accessed on 10 December 2023).
- Palumbo, A.; Vizza, P.; Calabrese, B.; Ielpo, N. Biopotential Signal Monitoring Systems in Rehabilitation: A Review. Sensors 2021, 21, 7172. [Google Scholar] [CrossRef] [PubMed]
- Granados, J.; Rahmani, A.M.; Nikander, P.; Liljeberg, P.; Tenhunen, H. Web-Enabled Intelligent Gateways for eHealth Internet-of-Things. In Internet of Things, User-Centric IoT; Springer: Cham, Switzerland, 2015; pp. 248–254. [Google Scholar] [CrossRef]
- Fernando, J.B.; Yoshioka, M.; Ozawa, J. Estimation of muscle fatigue by ratio of mean frequency to average rectified value from surface electromyography. In Proceedings of the 2016 38th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Orlando, FL, USA, 16–20 August 2016; pp. 5303–5306. [Google Scholar] [CrossRef]
- Candotti, C.; Loss, J.; La Torre, M.; Melo, M.; Araújo, L.; Marcks, V. Use of electromyography to assess pain in the upper trapezius and lower back muscles within a fatigue protocol. Braz. J. Phys. Ther. 2009, 13, 144–151. [Google Scholar] [CrossRef]
- Kiryu, T.; Sasaki, I.; Shibai, K.; Tanaka, K. Providing appropriate exercise levels for the elderly. IEEE Eng. Med. Biol. Mag. 2001, 20, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.H.; Chang, K.M.; Cheng, D.C. The Progression of Muscle Fatigue During Exercise Estimation With the Aid of High-Frequency Component Parameters Derived From Ensemble Empirical Mode Decomposition. IEEE J. Biomed. Health Inform. 2014, 18, 1647–1658. [Google Scholar] [CrossRef] [PubMed]
- Wang, C. Analyzing the influencing factors of sports fatigue based on algorithm. Rev. Bras. Med. Esporte 2021, 27, 377–380. [Google Scholar] [CrossRef]
- Molinari, F.; Knaflitz, M.; Bonato, P.; Actis, M. Electrical manifestations of muscle fatigue during concentric and eccentric isokinetic knee flexion-extension movements. IEEE Trans. Biomed. Eng. 2006, 53, 1309–1316. [Google Scholar] [CrossRef] [PubMed]
- Tsai, A.C.; Hsieh, T.H.; Luh, J.J.; Lin, T.T. A comparison of upper-limb motion pattern recognition using EMG signals during dynamic and isometric muscle contractions. Biomed. Signal Process. Control 2014, 11, 17–26. [Google Scholar] [CrossRef]
- Zhang, X.; Gu, Z. High accuracy recognition of muscle fatigue based on sEMG multifractal and LSTM. J. Theor. Appl. Mech. 2024, 62, 117–128. [Google Scholar] [CrossRef] [PubMed]
- Chalard, A.; Belle, M.; Montané, E.; Marque, P.; Amarantini, D.; Gasq, D. Impact of the EMG normalization method on muscle activation and the antagonist-agonist co-contraction index during active elbow extension: Practical implications for post-stroke subjects. J. Electromyogr. Kinesiol. 2020, 51, 102403. [Google Scholar] [CrossRef] [PubMed]
- Halaki, M.; Ginn, K. Normalization of EMG Signals: To Normalize or Not to Normalize and What to Normalize to? In Computational Intelligence in Electromyography Analysis—A Perspective on Current Applications and Future Challenges; InTechOpen: Rijeka, Croatia, 2012; pp. 175–194. [Google Scholar] [CrossRef]
- Junquera-Godoy, I.; Gomis-Tena Dolz, J.; Martínez-de-Juan, J.L.; Saiz Rodríguez, F.J.; Prats-Boluda, G. Analysis of Surface Electromyographic Parameters for the Assessment of Muscle Fatigue During Moderate Exercises; Universidad de Valladolid: Valladolid, Spain, 2022; pp. 35–38. [Google Scholar]
- Brown, N.; Bichler, S.; Fiedler, M.; Alt, W. Fatigue detection in strength training using three-dimensional accelerometry and principal component analysis. Sport. Biomech. Soc. Biomech. Sport. 2016, 15, 139–150. [Google Scholar] [CrossRef] [PubMed]
- John, C.; Omekara, C.; Okwara, G. The Principal Component Analysis Biplot Predictions versus the Ordinary Least Squares Regression Predictions: The Anthropometric Case Study. Asian J. Probab. Stat. 2019, 3, 1–10. [Google Scholar] [CrossRef]
- Rogers, D.; Macisaac, D. EMG-based muscle fatigue assessment during dynamic contractions using principal component analysis. J. Electromyogr. Kinesiol. Off. J. Int. Soc. Electrophysiol. Kinesiol. 2011, 21, 811–818. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, F.H.; Machado, A.; Andrade, A. On the Use of t-Distributed Stochastic Neighbor Embedding for Data Visualization and Classification of Individuals with Parkinson’s Disease. Comput. Math. Methods Med. 2018, 2018, 8019232. [Google Scholar] [CrossRef] [PubMed]



| Metric Category | Window Size 200 Samples (0.250 s) | Window Size 400 Samples (0.500 s) | Window Size 800 Samples (1.000 s) | Window Size 1600 Samples (2.000 s) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Step Size (Samples) | Step Size (Samples) | Step Size (Samples) | Step Size (Samples) | ||||||||||||||
| 150 | 100 | 50 | 25 | 300 | 200 | 100 | 50 | 600 | 400 | 200 | 100 | 1200 | 800 | 400 | 200 | ||
| Variance | MNF/ARV | 8.00 | 8.05 | 8.08 | 8.08 | 7.23 | 7.15 | 7.15 | 7.15 | 6.67 | 6.66 | 6.67 | 6.68 | 6.45 | 6.39 | 6.36 | 6.38 |
| IMA | 28.77 | 28.99 | 29.01 | 28.99 | 13.61 | 13.61 | 13.62 | 13.62 | 6.50 | 6.54 | 6.54 | 6.54 | 3.17 | 3.16 | 3.17 | 3.17 | |
| EMD | 767.65 | 755.85 | 755.00 | 758.81 | 506.94 | 520.80 | 519.62 | 517.02 | 391.65 | 388.95 | 384.56 | 386.74 | 315.48 | 298.99 | 298.54 | 297.91 | |
| Fluct (×) | 4.48 | 5.21 | 4.88 | 4.70 | 4.28 | 4.19 | 4.36 | 4.10 | 3.35 | 3.44 | 3.46 | 3.56 | 2.81 | 2.98 | 2.97 | 2.95 | |
| Max–Min | MNF/ARV | 25.13 | 25.27 | 33.10 | 36.39 | 19.01 | 19.04 | 19.09 | 19.41 | 16.35 | 16.56 | 18.65 | 18.65 | 16.06 | 16.44 | 16.45 | 16.57 |
| IMA | 30.57 | 30.57 | 34.86 | 34.86 | 19.56 | 18.81 | 19.56 | 19.61 | 12.28 | 12.29 | 12.30 | 12.33 | 7.94 | 7.79 | 7.94 | 7.96 | |
| EMD | 260.00 | 280.00 | 280.00 | 280.00 | 244.00 | 246.00 | 246.00 | 246.00 | 175.00 | 167.00 | 179.00 | 187.00 | 201.56 | 182.81 | 201.56 | 201.56 | |
| Fluct (×) | 62.57 | 70.08 | 70.08 | 71.25 | 47.05 | 37.76 | 47.51 | 47.51 | 22.26 | 23.20 | 23.31 | 28.36 | 19.14 | 19.93 | 19.93 | 19.93 | |
| Max Differential | MNF/ARV | 10.67 | 13.82 | 12.79 | 11.10 | 7.70 | 7.34 | 5.89 | 4.73 | 9.48 | 8.94 | 6.85 | 4.11 | 8.64 | 9.02 | 6.62 | 5.60 |
| IMA | 17.02 | 14.97 | 13.01 | 11.97 | 8.18 | 7.30 | 6.48 | 6.22 | 5.19 | 4.56 | 2.97 | 2.25 | 2.96 | 2.39 | 1.91 | 1.24 | |
| EMD | 216.00 | 196.00 | 236.00 | 240.00 | 202.00 | 204.00 | 204.00 | 188.00 | 118.00 | 129.00 | 118.00 | 150.00 | 134.38 | 139.06 | 101.56 | 113.28 | |
| Fluct (×) | 60.85 | 61.71 | 60.77 | 55.37 | 43.09 | 33.80 | 39.23 | 39.30 | 19.51 | 19.26 | 17.73 | 22.35 | 15.04 | 11.16 | 11.01 | 9.60 | |
| Computation Time (s) | 0.057 | 0.057 | 0.036 | 0.035 | 0.053 | 0.061 | 0.051 | 0.094 | 0.147 | 0.153 | 0.132 | 0.153 | 0.306 | 0.282 | 0.16209 | 0.159 | |
| Participant | MNF/ARV Ratio | IMA Difference | EMD | Fluctuation |
|---|---|---|---|---|
| Subject 1 | 20–70 | 0.1–0.35 | 30–120 | 0–17 |
| Subject 2 | 30–80 | 0.1–0.3 | 30–125 | 0–12 |
| Subject 3 | 30–80 | 0.12–0.325 | 30–110 | 0–13 |
| Subject 4 | 40–100 | 0.1–0.22 | 35–120 | 0–7 |
| Subject 5 | 30–70 | 0.125–0.3 | 35–110 | 0–10 |
| Subject 6 | 50–95 | 0.1–0.19 | 35–140 | 0–7 |
| Subject 7 | 35–90 | 0.1–0.25 | 35–140 | 0–6 |
| Subject 8 | 50–110 | 0.08–0.16 | 25–100 | 0–5 |
| Subject 9 | 35–85 | 0.1–0.22 | 35–125 | 0–6 |
| Subject 10 | 30–80 | 0.1–0.275 | 30–95 | 0–12 |
| Subject 11 | 40–90 | 0.1–0.27 | 30–115 | 0–9 |
| Model | R2 | MSE |
|---|---|---|
| Random Forest | 0.5209 | 1.4059 |
| Gradient-Boosting | 0.5198 | 1.4090 |
| LSTM | 0.4876 | 1.5037 |
| Simple Linear | 0.4718 | 1.5499 |
| SVR | 0.4704 | 1.5542 |
| KNN | 0.4598 | 1.5853 |
| CNN | 0.4303 | 1.6717 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miaoulis, D.; Stivaros, I.; Koubias, S. Developing a Novel Muscle Fatigue Index for Wireless sEMG Sensors: Metrics and Regression Models for Real-Time Monitoring. Electronics 2025, 14, 2097. https://doi.org/10.3390/electronics14112097
Miaoulis D, Stivaros I, Koubias S. Developing a Novel Muscle Fatigue Index for Wireless sEMG Sensors: Metrics and Regression Models for Real-Time Monitoring. Electronics. 2025; 14(11):2097. https://doi.org/10.3390/electronics14112097
Chicago/Turabian StyleMiaoulis, Dimitrios, Ioannis Stivaros, and Stavros Koubias. 2025. "Developing a Novel Muscle Fatigue Index for Wireless sEMG Sensors: Metrics and Regression Models for Real-Time Monitoring" Electronics 14, no. 11: 2097. https://doi.org/10.3390/electronics14112097
APA StyleMiaoulis, D., Stivaros, I., & Koubias, S. (2025). Developing a Novel Muscle Fatigue Index for Wireless sEMG Sensors: Metrics and Regression Models for Real-Time Monitoring. Electronics, 14(11), 2097. https://doi.org/10.3390/electronics14112097






