Thin Film Semiconductor Metal Oxide Oxygen Sensors: Limitations, Challenges, and Future Progress
Abstract
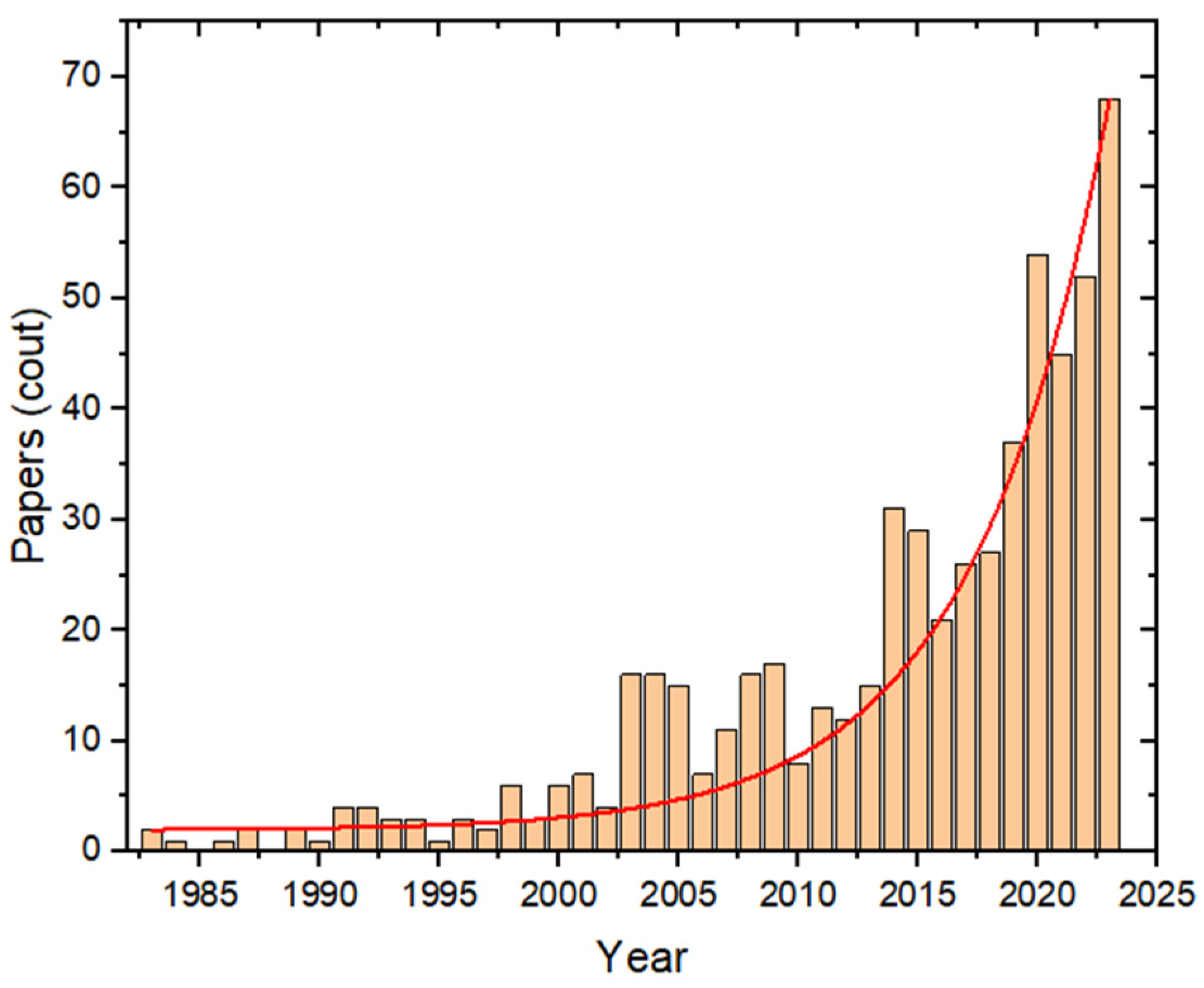
:1. Introduction
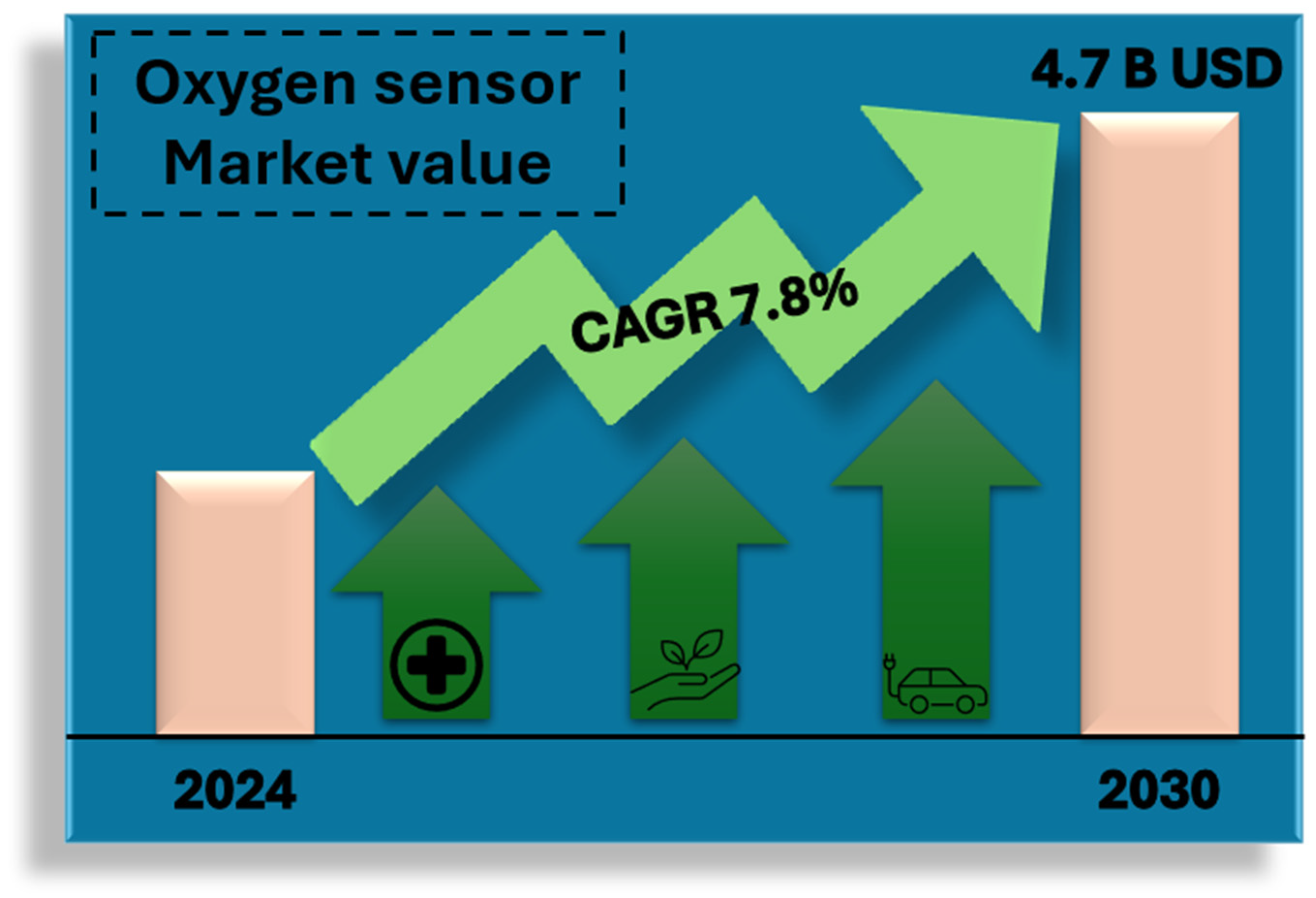
2. Oxygen Sensor Market
- Increased regulatory requirements for monitoring air quality and industrial emissions;
- Development of medical technologies and the need to monitor patients’ condition in real time;
- Growing demand for energy efficiency and optimization of industrial processes.
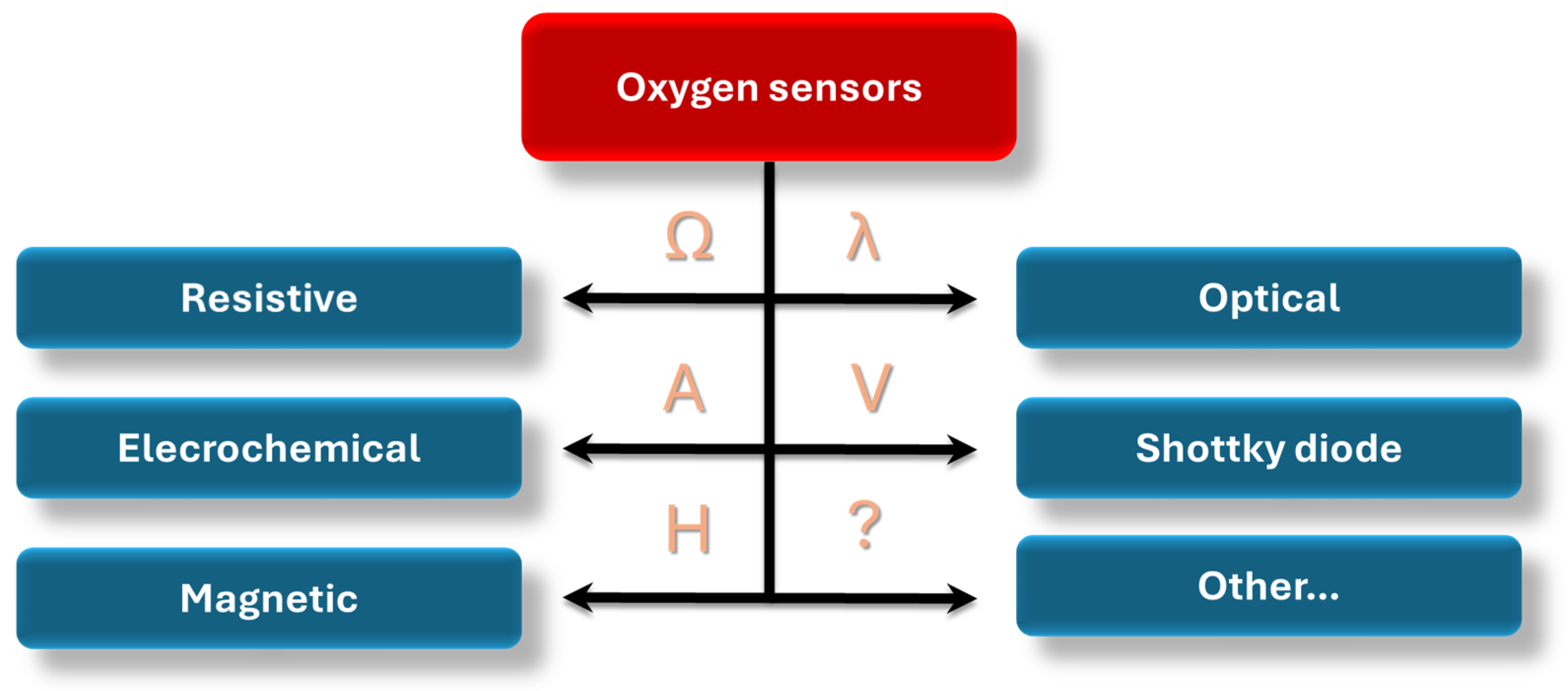
3. Classification of Oxygen Sensors in Terms of Their Sensing Mechanism
3.1. Optical Sensors
3.2. Schottky Diode
3.3. Electrochemical Devices
3.4. Magnetic Sensors
3.5. Chemiresistive Sensors
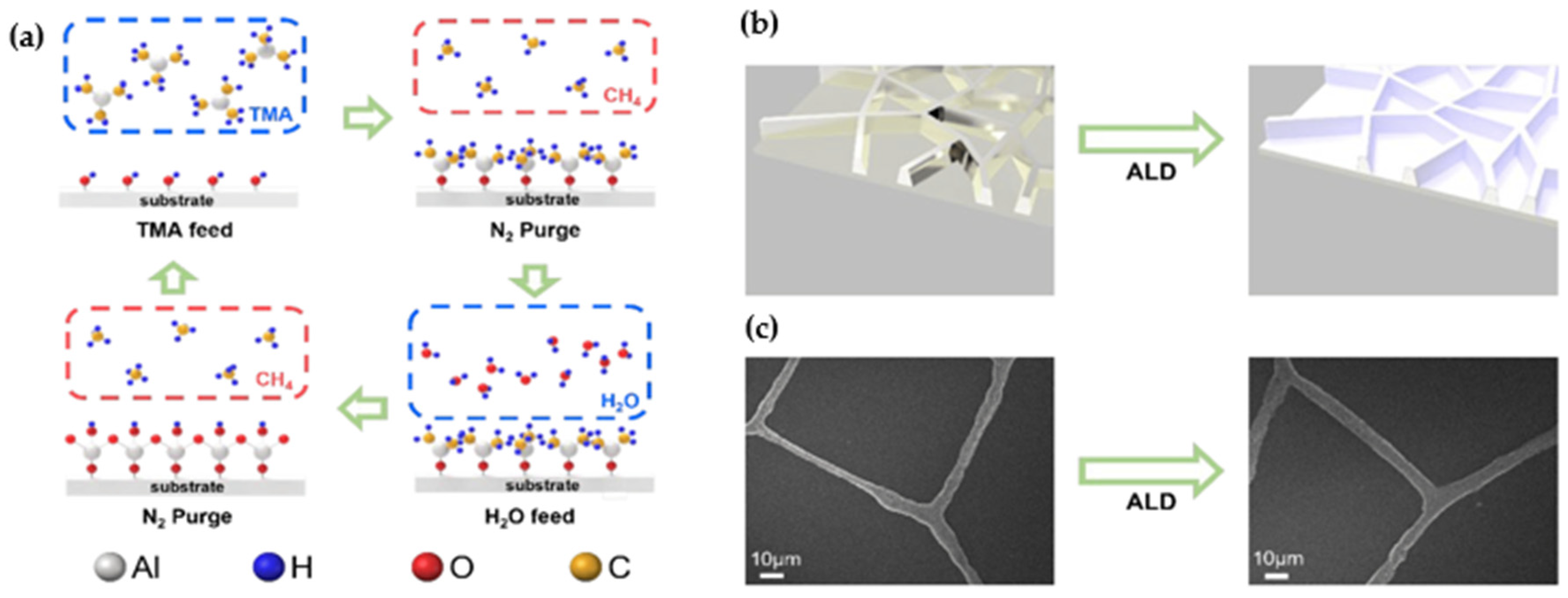
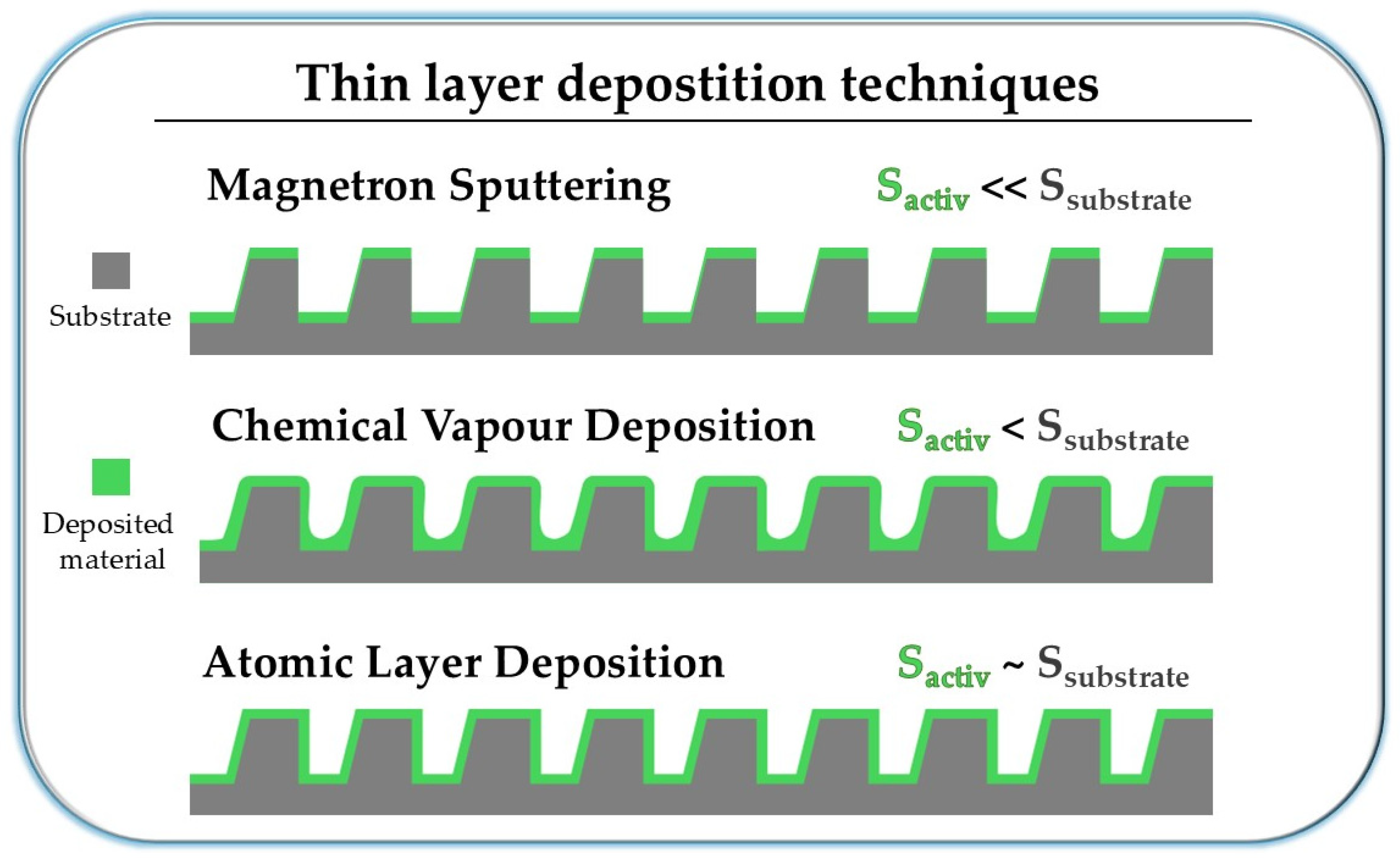
4. Thin Films Fabrication
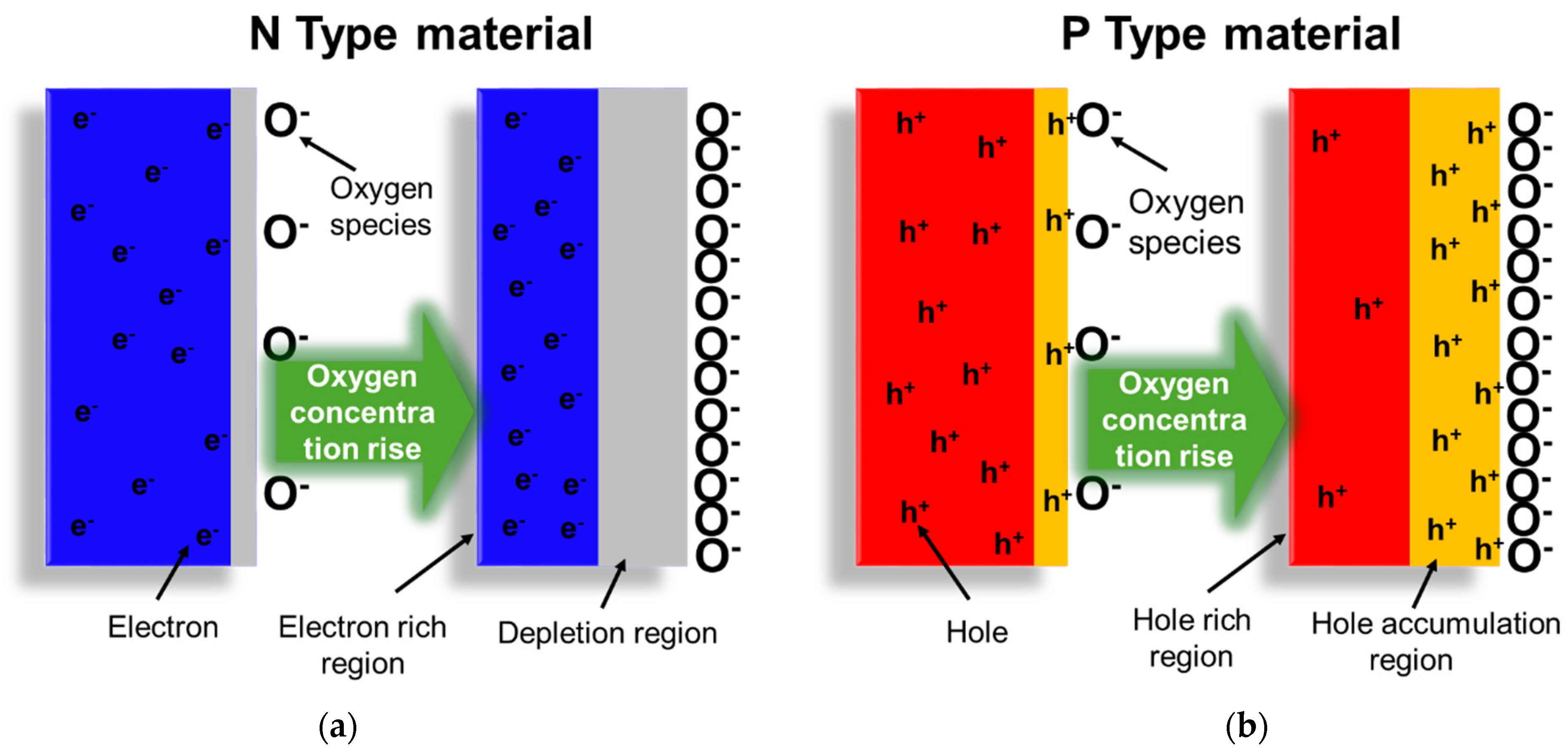
5. Resistive Oxygen Sensors
6. Critical Discussion
7. Conclusions and Perspectives
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Koley, G.; Liu, J.; Nomani, W.; Yim, M.; Wen, X.; Hsia, T.-Y. Miniaturized Implantable Pressure and Oxygen Sensors Based on Polydimethylsiloxane Thin Films. Mater. Sci. Eng. C 2009, 29, 685–690. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Kim, S.; Puligundla, P.; Ko, S. Carbon Dioxide and Oxygen Gas Sensors-Possible Application for Monitoring Quality, Freshness, and Safety of Agricultural and Food Products with Emphasis on Importance of Analytical Signals and Their Transformation. J. Korean Soc. Appl. Biol. Chem. 2014, 57, 723–733. [Google Scholar] [CrossRef]
- Moos, R.; Menesklou, W.; Schreiner, H.-J.; Härdtl, K.H. Materials for Temperature Independent Resistive Oxygen Sensors for Combustion Exhaust Gas Control. Sens. Actuators B Chem. 2000, 67, 178–183. [Google Scholar] [CrossRef]
- Tsujita, W.; Yoshino, A.; Ishida, H.; Moriizumi, T. Gas Sensor Network for Air-Pollution Monitoring. Sens. Actuators B Chem. 2005, 110, 304–311. [Google Scholar] [CrossRef]
- Genner, A.; Martín-Mateos, P.; Moser, H.; Lendl, B. A Quantum Cascade Laser-Based Multi-Gas Sensor for Ambient Air Monitoring. Sensors 2020, 20, 1850. [Google Scholar] [CrossRef] [PubMed]
- Ramamoorthy, R.; Dutta, P.K.; Akbar, S.A. Oxygen Sensors: Materials, Methods, Designs and Applications. J. Mater. Sci. 2003, 38, 4271–4282. [Google Scholar] [CrossRef]
- Banerjee, S.; Kelly, C.; Kerry, J.P.; Papkovsky, D.B. High Throughput Non-Destructive Assessment of Quality and Safety of Packaged Food Products Using Phosphorescent Oxygen Sensors. Trends Food Sci. Technol. 2016, 50, 85–102. [Google Scholar] [CrossRef]
- Tutunea, D.; Dumitru, I.; Oțăt, O.V.; Racilă, L.; Geonea, I.D.; Rotea, C.C. Oxygen Sensor Testing for Automotive Applications. Appl. Mech. Mater. 2020, 896, 249–254. [Google Scholar] [CrossRef]
- Cretescu, I.; Lutic, D.; Manea, L.R.; Cretescu, I.; Lutic, D.; Manea, L.R. Electrochemical Sensors for Monitoring of Indoor and Outdoor Air Pollution. Electrochem. Sens. Technol. 2017, 4, 65. [Google Scholar] [CrossRef]
- Kim, I.D.; Rothschild, A.; Tuller, H.L. Advances and New Directions in Gas-Sensing Devices. Acta Mater. 2013, 61, 974–1000. [Google Scholar] [CrossRef]
- Funazaki, N.; Hemmi, A.; Ito, S.; Asano, Y.; Yano, Y.; Miura, N.; Yamazoe, N. Application of Semiconductor Gas Sensor to Quality Control of Meat Freshness in Food Industry. Sens. Actuators B Chem. 1995, 25, 797–800. [Google Scholar] [CrossRef]
- Gomes, J.B.A.; Rodrigues, J.J.P.C.; Rabêlo, R.A.L.; Kumar, N.; Kozlov, S. IoT-Enabled Gas Sensors: Technologies, Applications, and Opportunities. J. Sens. Actuator Netw. 2019, 8, 57. [Google Scholar] [CrossRef]
- Wan, H.; Yin, H.; Lin, L.; Zeng, X.; Mason, A.J. Miniaturized Planar Room Temperature Ionic Liquid Electrochemical Gas Sensor for Rapid Multiple Gas Pollutants Monitoring. Sens. Actuators B Chem. 2018, 255, 638–646. [Google Scholar] [CrossRef] [PubMed]
- Rosenfeld, D.; Schmid, P.E.; Széles, S.; Lévy, F.; Demarne, V.; Grisel, A. Electrical Transport Properties of Thin-Film Metal-Oxide-Metal Nb2O5 Oxygen Sensors. Sens. Actuators B Chem. 1996, 37, 83–89. [Google Scholar] [CrossRef]
- IndustryARC. Oxygen Sensor Market—Forecast (2024–2030). 2023. Available online: https://www.industryarc.com/Report/15788/oxygen-sensor-market.html (accessed on 20 August 2024).
- Shubham Munde. Global Gas. Sensor Market. 2020. Available online: https://www.marketresearchfuture.com/reports/gas-sensors-market-5459 (accessed on 20 August 2024).
- Cirulnick, E.; Zhang, H.; Klotzkin, D. Optical Oxygen Sensors With Improved Lifetime Incorporating Titania Beads and Polydimethylsiloxane Coatings. Photonic Sens. 2022, 12, 68–73. [Google Scholar] [CrossRef]
- Wang, X.D.; Wolfbeis, O.S. Optical Methods for Sensing and Imaging Oxygen: Materials, Spectroscopies and Applications. Chem. Soc. Rev. 2014, 43, 3666–3761. [Google Scholar] [CrossRef] [PubMed]
- Koren, K.; Hutter, L.; Enko, B.; Pein, A.; Borisov, S.M.; Klimant, I. Tuning the Dynamic Range and Sensitivity of Optical Oxygen-Sensors by Employing Differently Substituted Polystyrene-Derivatives. Sens. Actuators B Chem. 2013, 176, 344–350. [Google Scholar] [CrossRef]
- Zhang, K.; Lu, S.; Qu, Z.; Feng, X. Tuning the Sensitivity and Dynamic Range of Optical Oxygen Sensing Films by Blending Various Polymer Matrices. Biosensors 2022, 12, 5. [Google Scholar] [CrossRef]
- Schierbaum, K.D.; Kirner, U.K.; Geiger, J.F.; Göpel, W. Schottky-Barrier and Conductivity Gas Sensors Based upon Pd/SnO2 and Pt/TiO2. Sens. Actuators B Chem. 1991, 4, 87–94. [Google Scholar] [CrossRef]
- Hung, S.C.; Chen, C.W.; Shieh, C.Y.; Chi, G.C.; Fan, R.; Pearton, S.J. High Sensitivity Carbon Monoxide Sensors Made by Zinc Oxide Modified Gated GaN/AlGaN High Electron Mobility Transistors under Room Temperature. Appl. Phys. Lett. 2011, 98, 223504. [Google Scholar] [CrossRef]
- Cho, I.; Sim, Y.C.; Cho, M.; Cho, Y.-H.; Park, I. Monolithic Micro Light-Emitting Diode/Metal Oxide Nanowire Gas Sensor with Microwatt-Level Power Consumption. ACS Sens. 2020, 5, 563–570. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Dai, Z.H.; Luo, Y.; Wang, X.L.; Bai, X.X.; Tang, Z.W.; Li, Y.G. Schottky Barrier Diode Oxygen Sensor Based on Silicon Carbide and Manufacturing Method Thereof. Patent CN102507704A, 2011. [Google Scholar]
- Akmal, N.; Lauer, J. Electrochemical Oxygen Sensors: Principles and Applications. In ACS Symposium Series; Oxford University Press: Oxford, UK, 1998; Volume 690, pp. 149–160. [Google Scholar]
- Han, J.H.; Kim, S.; Choi, J.; Kang, S.; Pak, Y.K.; Pak, J.J. Development of Multi-Well-Based Electrochemical Dissolved Oxygen Sensor Array. Sens. Actuators B Chem. 2020, 306, 127465. [Google Scholar] [CrossRef]
- Liang, Y.; Wu, Z.; Wei, Y.; Ding, Q.; Zilberman, M.; Tao, K.; Xie, X.; Wu, J. Self-Healing, Self-Adhesive and Stable Organohydrogel-Based Stretchable Oxygen Sensor with High Performance at Room Temperature. Nanomicro Lett. 2022, 14, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.Tecnosens.It/En/Electrochemical/Ke-50 (accessed on 24 July 2024).
- Ashley, B.K.; Brown, M.S.; Park, Y.; Kuan, S.; Koh, A. Skin-Inspired, Open Mesh Electrochemical Sensors for Lactate and Oxygen Monitoring. Biosens. Bioelectron. 2019, 132, 343–351. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhang, L.; Wu, Y.; Ding, W.; Liu, Y.; Zhao, S.; Gu, J. Research on the Influence of Core Sensing Components on the Performance of Galvanic Dissolved Oxygen Sensors. Sensors 2024, 24, 4155. [Google Scholar] [CrossRef] [PubMed]
- Adeniji, T.M.; Stine, K.J. Nanostructure Modified Electrodes for Electrochemical Detection of Contaminants of Emerging Concern. Coatings 2023, 13, 381. [Google Scholar] [CrossRef]
- Moldoveanu, I.; van Stefan-Staden, R.-I.; van Staden, J.F. Electrochemical Sensors Based on Nanostructured Materials. Handb. Nanoelectrochemistry 2015, 12, 1–15. [Google Scholar] [CrossRef]
- Miura, N.; Sato, T.; Anggraini, S.A.; Ikeda, H.; Zhuiykov, S. A Review of Mixed-Potential Type Zirconia-Based Gas Sensors. Ionics 2014, 20, 901–925. [Google Scholar] [CrossRef]
- Halley, S.; Ramaiyan, K.P.; Tsui, L.K.; Garzon, F. A Review of Zirconia Oxygen, NOx, and Mixed Potential Gas Sensors—History and Current Trends. Sens. Actuators B Chem. 2022, 370, 132363. [Google Scholar] [CrossRef]
- Hu, C.; Bai, X.; Wang, Y.; Jin, W.; Zhang, X.; Hu, S. Inkjet Printing of Nanoporous Gold Electrode Arrays on Cellulose Membranes for High-Sensitive Paper-like Electrochemical Oxygen Sensors Using Ionic Liquid Electrolytes. Anal. Chem. 2012, 84, 3745–3750. [Google Scholar] [CrossRef] [PubMed]
- Sapkota, R.; Isik, S.; Suyanto, H.; Rupiasih, N.N.; Ingles, N.; Rizal, c. Sensor with combined plasmonic and magnetic activities. Biosens. Bioelectron. X 2024, 19, 100506. [Google Scholar] [CrossRef]
- Krippner, P.; Wetzko, M.; Szasz, P.; Andres, B.; Bauer, T. MEMS Based Paramagnetic Oxygen Measurement. In Proceedings of the TRANSDUCERS 2007–2007 International Solid-State Sensors, Actuators and Microsystems Conference, Lyon, France, 10–14 June 2007; IEEE: Piscataway, NJ, USA, 2007; pp. 2393–2396. [Google Scholar]
- Schmid, U.; Seidel, H.; Mueller, G.; Becker, T. Theoretical Considerations on the Design of a Miniaturised Paramagnetic Oxygen Sensor. Sens. Actuators B Chem. 2006, 116, 213–220. [Google Scholar] [CrossRef]
- Vonderschmidt, S.; Müller, J. A Fluidic Bridge Based MEMS Paramagnetic Oxygen Sensor. Sens. Actuators B Chem. 2013, 188, 22–30. [Google Scholar] [CrossRef]
- König, F.; Müller, J. A Novel Micro Paramagnetic Oxygen Sensor Based on an Anisotropic Magneto Resistance-Device. In Proceedings of the SENSORS, 2010 IEEE, Waikoloa, HI, USA, 1–4 November 2010; pp. 1496–1499. [Google Scholar] [CrossRef]
- Jasek, K.; Pasternak, M.; Grabka, M. Paramagnetic Sensors for the Determination of Oxygen Concentration in Gas Mixtures. ACS Sens. 2022, 7, 3228–3242. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.; Yu, F.; Zhang, L.; Wang, W.; Chen, L.; Li, Y. Review of ZnO-Based Nanomaterials in Gas Sensors. Solid State Ion. 2021, 360, 115544. [Google Scholar] [CrossRef]
- Zhang, B.; Li, M.; Song, Z.; Kan, H.; Yu, H.; Liu, Q.; Zhang, G.; Liu, H. Sensitive H2S Gas Sensors Employing Colloidal Zinc Oxide Quantum Dots. Sens. Actuators B Chem. 2017, 249, 558–563. [Google Scholar] [CrossRef]
- Izu, N.; Shin, W.; Murayama, N.; Kanzaki, S. Resistive Oxygen Gas Sensors Based on CeO2 Fine Powder Prepared Using Mist Pyrolysis. Sens. Actuators B Chem. 2002, 87, 95–98. [Google Scholar] [CrossRef]
- Zhao, S.; Shen, Y.; Yan, X.; Zhou, P.; Yin, Y.; Lu, R.; Han, C.; Cui, B.; Wei, D. Complex-Surfactant-Assisted Hydrothermal Synthesis of One-Dimensional ZnO Nanorods for High-Performance Ethanol Gas Sensor. Sens. Actuators B Chem. 2019, 286, 501–511. [Google Scholar] [CrossRef]
- Kim, W.; Choi, M.; Yong, K. Generation of Oxygen Vacancies in ZnO Nanorods/Films and Their Effects on Gas Sensing Properties. Sens. Actuators B Chem. 2015, 209, 989–996. [Google Scholar] [CrossRef]
- Ciftyurek, E.; Li, Z.; Schierbaum, K. Adsorbed Oxygen Ions and Oxygen Vacancies: Their Concentration and Distribution in Metal Oxide Chemical Sensors and Influencing Role in Sensitivity and Sensing Mechanisms. Sensors 2022, 23, 29. [Google Scholar] [CrossRef] [PubMed]
- Li, G.R.; Hu, T.; Pan, G.L.; Yan, T.Y.; Gao, X.P.; Zhu, H.Y. Morphology-Function Relationship of ZnO: Polar Planes, Oxygen Vacancies, and Activity. J. Phys. Chem. C 2008, 112, 11859–11864. [Google Scholar] [CrossRef]
- Peng, S.; Wang, Z.; Liu, R.; Bi, J.; Wu, J. Controlled Oxygen Vacancies of ZnFe2O4 with Superior Gas Sensing Properties Prepared via a Facile One-Step Self-Catalyzed Treatment. Sens. Actuators B Chem. 2019, 288, 649–655. [Google Scholar] [CrossRef]
- Mokrushin, A.S.; Simonenko, T.L.; Simonenko, N.P.; Gorobtsov, P.Y.; Kadyrov, N.C.; Simonenko, E.P.; Sevastyanov, V.G.; Kuznetsov, N.T. Chemoresistive Gas-Sensing Properties of Highly Dispersed Nb2O5 Obtained by Programmable Precipitation. J. Alloys Compd. 2021, 868, 159090. [Google Scholar] [CrossRef]
- Tian, X.; Cui, X.; Lai, T.; Ren, J.; Yang, Z.; Xiao, M.; Wang, B.; Xiao, X.; Wang, Y. Gas Sensors Based on TiO2 Nanostructured Materials for the Detection of Hazardous Gases: A Review. Nano Mater. Sci. 2021, 3, 390–403. [Google Scholar] [CrossRef]
- Simonenko, E.P.; Nagornov, I.A.; Mokrushin, A.S.; Kashevsky, S.V.; Gorban, Y.M.; Simonenko, T.L.; Simonenko, N.P.; Kuznetsov, N.T. Low Temperature Chemoresistive Oxygen Sensors Based on Titanium-Containing Ti2CTx and Ti3C2Tx MXenes. Materials 2023, 16, 4506. [Google Scholar] [CrossRef] [PubMed]
- Izu, N.; Shin, W.; Matsubara, I.; Murayama, N. Development of Resistive Oxygen Sensors Based on Cerium Oxide Thick Film. J. Electroceram. 2004, 13, 703–706. [Google Scholar] [CrossRef]
- Trettnak, W.; Gruber, W.; Reininger, F.; Klimant, I. Recent Progress in Optical Oxygen Sensor Instrumentation. Sens. Actuators B Chem. 1995, 29, 219–225. [Google Scholar] [CrossRef]
- Wang, X.D.; Chen, H.X.; Zhao, Y.; Chen, X.; Wang, X.R.; Chen, X. Optical Oxygen Sensors Move towards Colorimetric Determination. TrAC Trends Anal. Chem. 2010, 29, 319–338. [Google Scholar] [CrossRef]
- Hasumoto, H.; Imazu, T.; Miura, T.; Kogure, K. Use of an Optical Oxygen Sensor to Measure Dissolved Oxygen in Seawater. J. Oceanogr. 2006, 62, 99–103. [Google Scholar] [CrossRef]
- Oeba, D.A.; Bodunrin, J.O.; Moloi, S.J. The Electrical Characteristics and Conduction Mechanisms of Zn Doped Silicon-Based Schottky Barrier Diode. Heliyon 2023, 9, e22793. [Google Scholar] [CrossRef] [PubMed]
- Alan Sibu, G.; Gayathri, P.; Akila, T.; Marnadu, R.; Balasubramani, V. Manifestation on the Choice of a Suitable Combination of MIS for Proficient Schottky Diodes for Optoelectronic Applications: A Comprehensive Review. Nano Energy 2024, 125, 109534. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, T.; Chu, Z.; Jin, W. Recent Progress on Nanomaterial-Based Electrochemical Dissolved Oxygen Sensors. Chin. J. Chem. Eng. 2024, 68, 103–119. [Google Scholar] [CrossRef]
- Rivas, L.; Dulay, S.; Miserere, S.; Pla, L.; Marin, S.B.; Parra, J.; Eixarch, E.; Gratacós, E.; Illa, M.; Mir, M.; et al. Micro-Needle Implantable Electrochemical Oxygen Sensor: Ex-Vivo and in-Vivo Studies. Biosens. Bioelectron. 2020, 153, 112028. [Google Scholar] [CrossRef]
- Mun, T.; Koo, J.Y.; Lee, J.; Kim, S.J.; Umarji, G.; Amalnerkar, D.; Lee, W. Resistive-Type Lanthanum Ferrite Oxygen Sensor Based on Nanoparticle-Assimilated Nanofiber Architecture. Sens. Actuators B Chem. 2020, 324, 128712. [Google Scholar] [CrossRef]
- Rothschild, A.; Litzelman, S.J.; Tuller, H.L.; Menesklou, W.; Schneider, T.; Ivers-Tiffée, E. Temperature-Independent Resistive Oxygen Sensors Based on SrTi1−xFexO3−δ Solid Solutions. Sens. Actuators B Chem. 2005, 108, 223–230. [Google Scholar] [CrossRef]
- Moos, R.; Izu, N.; Rettig, F.; Reiß, S.; Shin, W.; Matinfara, I. Resistive Oxygen Gas Sensors for Harsh Environments. Sensors 2011, 11, 3439–3465. [Google Scholar] [CrossRef]
- Baek, J.W.; Kim, I.D. Advances in Perovskite Oxides for Chemiresistive Sensors. Acc. Mater. Res. 2023, 4, 1108–1120. [Google Scholar] [CrossRef]
- Postica, V.; Lupan, O.; Gapeeva, A.; Hansen, L.; Khaledialidusti, R.; Mishra, A.K.; Drewes, J.; Kersten, H.; Faupel, F.; Adelung, R.; et al. Improved Long-Term Stability and Reduced Humidity Effect in Gas Sensing: SiO2 Ultra-Thin Layered ZnO Columnar Films. Adv. Mater. Technol. 2021, 6, 2001137. [Google Scholar] [CrossRef]
- Yan, H.Q.; Liu, C.C. Humidity Effects on the Stability of a Solid Polymer Electrolyte Oxygen Sensor. Sens. Actuators B Chem. 1993, 10, 133–136. [Google Scholar] [CrossRef]
- Koren, K.; Borisov, S.M.; Klimant, I. Stable Optical Oxygen Sensing Materials Based on Click-Coupling of Fluorinated Platinum(II) and Palladium(II) Porphyrins—A Convenient Way to Eliminate Dye Migration and Leaching. Sens. Actuators B Chem. 2012, 169, 173–181. [Google Scholar] [CrossRef]
- Abegunde, O.O.; Akinlabi, E.T.; Oladijo, O.P.; Akinlabi, S.; Ude, A.U.; Abegunde, O.O.; Akinlabi, E.T.; Oladijo, O.P.; Akinlabi, S.; Ude, A.U. Overview of Thin Film Deposition Techniques. AIMS Mater. Sci. 2019, 6, 174–199. [Google Scholar] [CrossRef]
- Messier, R. Thin Film Deposition Processes. MRS Bull. 1988, 13, 18–21. [Google Scholar] [CrossRef]
- Sequeda, F.O. Thin Film Deposition Techniques in Microelectronics. JOM 1986, 38, 55–65. [Google Scholar] [CrossRef]
- Bose, S. Oxidation and corrosion-resistant coatings. High Temp. Coat. 2007, 71–154. [Google Scholar] [CrossRef]
- Kotok, V.; Kovalenko, V.; Stathopoulos, V.N. Techniques for Coating Applications. Encycl. Smart Mater. 2022, 1, 243–257. [Google Scholar] [CrossRef]
- Zafar, M.S.; Farooq, I.; Awais, M.; Najeeb, S.; Khurshid, Z.; Zohaib, S. Bioactive Surface Coatings for Enhancing Osseointegration of Dental Implants. In Biomedical, Therapeutic and Clinical Applications of Bioactive Glasses; Woodhead Publishing: Cambridge, UK, 2019; pp. 313–329. [Google Scholar] [CrossRef]
- Wang, F.; Wu, J. Magnetron Sputtering. In Modern Ion Plating Technology: Fundamentals and Applications; Elsevier: Amsterdam, The Netherlands, 2023; pp. 189–228. [Google Scholar] [CrossRef]
- Carlsson, J.O.; Martin, P.M. Chemical Vapor Deposition. In Handbook of Deposition Technologies for Films and Coatings: Science, Applications and Technology; William Andrew Publishing: Norwich, CT, USA, 2010; pp. 314–363. [Google Scholar] [CrossRef]
- Xie, L.; Abliz, D.; Li, D. Thin Film Coating for Polymeric Micro Parts. Compr. Mater. Process. Thirteen Vol. Set 2014, 7, 157–170. [Google Scholar] [CrossRef]
- Urrutia, A.; Rivero, P.J.; Goicoechea, J.; Arregui, F.J. Micro/Nanodeposition Techniques for Enhanced Optical Fiber Sensors. In Handbook of Nanomaterials for Sensing Applications; Elsevier: Amsterdam, The Netherlands, 2021; pp. 531–573. [Google Scholar] [CrossRef]
- Qin, R.; Wang, Y.; Yao, L.; Yang, L.; Zhao, Q.; Ding, S.; Liu, L.L.; Pan, F. Progress in Interface Structure and Modification of Zinc Anode for Aqueous Batteries. Nano Energy 2022, 98, 107333. [Google Scholar] [CrossRef]
- Oh, J.; Seo, G.; Kim, J.; Bae, S.; Park, J.W.; Hwang, J.H. Plasma-Enhanced Atomic Layer Deposition of Zirconium Oxide Thin Films and Its Application to Solid Oxide Fuel Cells. Coatings 2021, 11, 362. [Google Scholar] [CrossRef]
- Lee, J.H.; Choi, T.Y.; Cheon, H.S.; Youn, H.Y.; Lee, G.W.; Lee, S.N.; Kim, H.K. Conformal and Transparent Al2O3 Passivation Coating via Atomic Layer Deposition for High Aspect Ratio Ag Network Electrodes. Metals 2023, 13, 528. [Google Scholar] [CrossRef]
- Mistry, K.; Allen, C.; Auth, C.; Beattie, B.; Bergstrom, D.; Bost, M.; Brazier, M.; Buehler, M.; Cappellani, A.; Chau, R.; et al. A 45nm Logic Technology with High-K+Metal Gate Transistors, Strained Silicon, 9 Cu Interconnect Layers, 193nm Dry Patterning, and 100% Pb-Free Packaging. In Proceedings of the 2007 IEEE International Electron Devices Meeting, Washington, DC, USA, 10–12 December 2007. [Google Scholar] [CrossRef]
- Kaloyeros, A.E.; Eisenbraun, E. Ultrathin Diffusion Barriers/Liners for Gigascale Copper Metallization. Annu. Rev. Mater. Sci. 2000, 30, 363–385. [Google Scholar] [CrossRef]
- Franco, M.A.; Conti, P.P.; Andre, R.S.; Correa, D.S. A Review on Chemiresistive ZnO Gas Sensors. Sens. Actuators Rep. 2022, 4, 100100. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Y.; Tian, F.; Liang, H.; Wang, K.; Zhao, X.; Lu, Z.; Jiang, K.; Yang, L.; Lou, X. From the Surface Reaction Control to Gas-Diffusion Control: The Synthesis of Hierarchical Porous SnO2 Microspheres and Their Gas-Sensing Mechanism. J. Phys. Chem. C 2015, 119, 15963–15976. [Google Scholar] [CrossRef]
- Izu, N.; Shin, W.; Matasubara, I.; Murayama, N. Kinetic Behavior of Resistive Oxygen Sensor Using Cerium Oxide. Sens. Actuators B Chem. 2004, 100, 411–416. [Google Scholar] [CrossRef]
- Wang, H.; Chen, L.; Wang, J.; Sun, Q.; Zhao, Y. A Micro Oxygen Sensor Based on a Nano Sol-Gel TiO2 Thin Film. Sensors 2014, 14, 16423–16433. [Google Scholar] [CrossRef]
- Izu, N.; Itoh, T.; Shin, W.; Matsubara, I.; Murayama, N. Evaluation of Response Characteristics of Resistive Oxygen Sensors Using Ce0.9Zr0.1O2 Thick Film by Pressure Modulation Method. Sens. Actuators B Chem. 2008, 130, 466–469. [Google Scholar] [CrossRef]
- Shin, W.; Izu, N.; Matsubara, I.; Murayama, N. Millisecond-Order Response Measurement for Fast Oxygen Gas Sensors. Sens. Actuators B Chem. 2004, 100, 395–400. [Google Scholar] [CrossRef]
- Rajavel, K.; Lalitha, M.; Radhakrishnan, J.K.; Senthilkumar, L.; Rajendra Kumar, R.T. Multiwalled Carbon Nanotube Oxygen Sensor: Enhanced Oxygen Sensitivity at Room Temperature and Mechanism of Sensing. ACS Appl. Mater. Interfaces 2015, 7, 23857–23865. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Fu, H.; Tian, Y.; Deng, T.; Cai, J.; Wu, J.; Tu, T.; Li, T.; Tan, C.; Liang, Y.; et al. Exploiting Two-Dimensional Bi2O2Se for Trace Oxygen Detection. Angew. Chem. Int. Ed. 2020, 59, 17938–17943. [Google Scholar] [CrossRef]
- Tabata, H.; Fukuda, H.; Matsushita, K.; Kubo, O.; Kikuchi, T.; Sato, T.; Kamimura, T.; Ueda, T.; Shimazaki, R.; Tanjo, H.; et al. Metal–Oxide-Layer-Coated Single-Walled Carbon Nanotubes as a Sensor for Trace Amounts of Oxygen. Appl. Phys. Express 2014, 7, 035101. [Google Scholar] [CrossRef]
- Lu, C.-Y.; Chang, S.-P.; Chang, S.-J.; Hsueh, T.-J.; Hsu, C.-L.; Chiou, Y.-Z.; Chen, I.-C. ZnO Nanowire-Based Oxygen Gas Sensor. IEEE Sens. J. 2009, 9, 485–489. [Google Scholar] [CrossRef]
- Ahmed, F.; Arshi, N.; Anwar, M.S.; Danish, R.; Koo, B.H. Mn-Doped ZnO Nanorod Gas Sensor for Oxygen Detection. Curr. Appl. Phys. 2013, 13, S64–S68. [Google Scholar] [CrossRef]
- Rajput, J.K.; Pathak, T.K.; Kumar, V.; Swart, H.C.; Purohit, L.P. CdO:ZnO Nanocomposite Thin Films for Oxygen Gas Sensing at Low Temperature. Mater. Sci. Eng. B 2018, 228, 241–248. [Google Scholar] [CrossRef]
- Cava, C.E.; Salvatierra, R.V.; Alves, D.C.B.; Ferlauto, A.S.; Zarbin, A.J.G.; Roman, L.S. Self-Assembled Films of Multi-Wall Carbon Nanotubes Used in Gas Sensors to Increase the Sensitivity Limit for Oxygen Detection. Carbon 2012, 50, 1953–1958. [Google Scholar] [CrossRef]
- Rajput, J.K.; Pathak, T.K.; Purohit, L.P. Impact of Sputtering Power on Properties of CdO:ZnO Thin Films Synthesized by Composite Method for Oxygen Gas Sensing Application. J. Electron. Mater. 2019, 48, 6640–6646. [Google Scholar] [CrossRef]
- Oztel, S.; Kaya, S.; Budak, E.; Yilmaz, E. Influences of Platinum Doping Concentrations and Operation Temperatures on Oxygen Sensitivity of Pt/SnO2/Pt Resistive Gas Sensors. J. Mater. Sci. Mater. Electron. 2019, 30, 14813–14821. [Google Scholar] [CrossRef]
- Zandi, A.; Gilani, A.; Fard, H.G.; Koohsorkhi, J. An Optimized Resistive CNT-Based Gas Sensor with a Novel Configuration by Top Electrical Contact. Diam. Relat. Mater. 2019, 93, 224–232. [Google Scholar] [CrossRef]
- Izydorczyk, W.; Izydorczyk, J. Structure, Surface Morphology, Chemical Composition, and Sensing Properties of SnO2 Thin Films in an Oxidizing Atmosphere. Sensors 2021, 21, 5741. [Google Scholar] [CrossRef]
- Tonezzer, M.; Lacerda, R.G. Integrated Zinc Oxide Nanowires/Carbon Microfiber Gas Sensors. Sens. Actuators B Chem. 2010, 150, 517–522. [Google Scholar] [CrossRef]
- Felix, A.A.; Rupp, J.L.M.; Varela, J.A.; Orlandi, M.O. Multi-Functional Properties of CaCu3Ti4O12 Thin Films. J. Appl. Phys. 2012, 112, 54512. [Google Scholar] [CrossRef]
- Kim, Y.H.; Kim, K.Y.; Choi, Y.R.; Shim, Y.-S.; Jeon, J.-M.; Lee, J.-H.; Kim, S.Y.; Han, S.; Jang, H.W. Ultrasensitive Reversible Oxygen Sensing by Using Liquid-Exfoliated MoS 2 Nanoparticles. J. Mater. Chem. A Mater. 2016, 4, 6070–6076. [Google Scholar] [CrossRef]
- Suematsu, K.; Yuasa, M.; Kida, T.; Yamazoe, N.; Shimanoe, K. Determination of Oxygen Adsorption Species on SnO2: Exact Analysis of Gas Sensing Properties Using a Sample Gas Pretreatment System. J. Electrochem. Soc. 2014, 161, B123–B128. [Google Scholar] [CrossRef]
- Mokrushin, A.S.; Simonenko, E.P.; Simonenko, N.P.; Akkuleva, K.T.; Antipov, V.V.; Zaharova, N.V.; Malygin, A.A.; Bukunov, K.A.; Sevastyanov, V.G.; Kuznetsov, N.T. Oxygen Detection Using Nanostructured TiO2 Thin Films Obtained by the Molecular Layering Method. Appl. Surf. Sci. 2019, 463, 197–202. [Google Scholar] [CrossRef]
- Ali, D.; Muneer, I.; Butt, M.Z. Influence of Aluminum Precursor Nature on the Properties of AZO Thin Films and Its Potential Application as Oxygen Sensor. Opt. Mater. 2021, 120, 111406. [Google Scholar] [CrossRef]
- Hossein-Babaei, F.; Keshmiri, M.; Kakavand, M.; Troczynski, T. A Resistive Gas Sensor Based on Undoped P-Type Anatase. Sens. Actuators B Chem. 2005, 110, 28–35. [Google Scholar] [CrossRef]
- Cao, W.; Tan, O.K.; Zhu, W.; Jiang, B.; Gopal Reddy, C.V. An Amorphous-like Xα-Fe2O3−(1−x)ZrO2 Solid Solution System for Low Temperature Resistive-Type Oxygen Sensing. Sens. Actuators B Chem. 2001, 77, 421–426. [Google Scholar] [CrossRef]
- Cao, W.; Tan, O.K.; Zhu, W.; Pan, J.S.; Bin, J. Study of Xα-Fe2O3−(1−x)ZrO2 Solid Solution for Low-Temperature Resistive Oxygen Gas Sensors. IEEE Sens. J. 2003, 3, 421–434. [Google Scholar] [CrossRef]
- Mokrushin, A.S.; Simonenko, N.P.; Simonenko, T.L.; Simonenko, E.P.; Sevast’yanov, V.G.; Kuznetsov, N.T. Synthesis and Chemoresistive Gas-Sensing Properties of Highly Dispersed Titanium-Doped Nb2O5. Russ. J. Inorg. Chem. 2021, 66, 1425–1433. [Google Scholar] [CrossRef]
- Ramshanker, N.; Ganapathi, K.L.; Varun, N.; Bhat, M.S.; Mohan, S. Development of CeO2-HfO2 Mixed Oxide Thin Films for High Performance Oxygen Sensors. IEEE Sens. J. 2021, 21, 18326–18333. [Google Scholar] [CrossRef]
- Mokrushin, A.S.; Simonenko, E.P.; Simonenko, N.P.; Bukunov, K.A.; Sevastyanov, V.G.; Kuznetsov, N.T. Gas-Sensing Properties of Nanostructured CeO2-XZrO2 Thin Films Obtained by the Sol-Gel Method. J. Alloys Compd. 2019, 773, 1023–1032. [Google Scholar] [CrossRef]
- Almaev, A.V.; Chernikov, E.V.; Kushnarev, B.O.; Yakovlev, N.N.; Korusenko, P.M.; Nesov, S.N. Oxygen Sensors Based on Thin Films of Gallium Oxide Modified with Silicon. In Proceedings of the 6th International Electronic Conference on Sensors and Applications, Online, 15–30 November 2019; MDPI: Basel, Switzerland, 2019; Volume 42, p. 4. [Google Scholar]
- Miller, J.B.; Ashok, T.; Lee, S.; Broitman, E. Zinc Oxide-Based Thin Film Functional Layers for Chemiresistive Sensors. Thin Solid Film. 2012, 520, 6669–6676. [Google Scholar] [CrossRef]
- Simonenko, T.L.; Simonenko, N.P.; Mokrushin, A.S.; Simonenko, E.P.; Glumov, O.V.; Mel’nikova, N.A.; Murin, I.V.; Kalinina, M.V.; Shilova, O.A.; Sevastyanov, V.G.; et al. Microstructural, Electrophysical and Gas-Sensing Properties of CeO2–Y2O3 Thin Films Obtained by the Sol-Gel Process. Ceram. Int. 2020, 46, 121–131. [Google Scholar] [CrossRef]
- Eriksson, J.; Khranovskyy, V.; Söderlind, F.; Käll, P.-O.; Yakimova, R.; Spetz, A.L. ZnO Nanoparticles or ZnO Films: A Comparison of the Gas Sensing Capabilities. Sens. Actuators B Chem. 2009, 137, 94–102. [Google Scholar] [CrossRef]
- Bektas, M.; Schönauer-Kamin, D.; Hagen, G.; Mergner, A.; Bojer, C.; Lippert, S.; Milius, W.; Breu, J.; Moos, R. BaFe1−XTaxO3−δ—A Material for Temperature Independent Resistive Oxygen Sensors. Sens. Actuators B Chem. 2014, 190, 208–213. [Google Scholar] [CrossRef]
- Lu, C.-C.; Huang, Y.-S.; Huang, J.-W.; Chang, C.-K.; Wu, S.-P. A Macroporous TiO2 Oxygen Sensor Fabricated Using Anodic Aluminium Oxide as an Etching Mask. Sensors 2010, 10, 670–683. [Google Scholar] [CrossRef]
- Llobet, E.; Espinosa, E.H.; Sotter, E.; Ionescu, R.; Vilanova, X.; Torres, J.; Felten, A.; Pireaux, J.J.; Ke, X.; Van Tendeloo, G.; et al. Carbon Nanotube–TiO2 Hybrid Films for Detecting Traces of O2. Nanotechnology 2008, 19, 375501. [Google Scholar] [CrossRef]
- IZU, N.; SHIN, W.; MATSUBARA, I.; MURAYAMA, N. Resistive Oxygen Sensor Using Hafnium-Doped Cerium Oxide. Electrochemistry 2005, 73, 478–480. [Google Scholar] [CrossRef]
- Izu, N.; Murayama, N.; Shin, W.; Matsubara, I.; Kanzaki, S. Resistive Oxygen Sensors Using Cerium Oxide Thin Films Prepared by Metal Organic Chemical Vapor Deposition and Sputtering. Jpn. J. Appl. Phys. 2004, 43, 6920–6924. [Google Scholar] [CrossRef]
- Izu, N.; Itoh, T.; Shin, W.; Matsubara, I.; Murayama, N. The Effect of Hafnia Doping on the Resistance of Ceria for Use in Resistive Oxygen Sensors. Sens. Actuators B Chem. 2007, 123, 407–412. [Google Scholar] [CrossRef]
- Sahner, K.; Moos, R.; Izu, N.; Shin, W.; Murayama, N. Response Kinetics of Temperature-Independent Resistive Oxygen Sensor Formulations: A Comparative Study. Sens. Actuators B Chem. 2006, 113, 112–119. [Google Scholar] [CrossRef]
- Li, N.; Tan, T.-C. A High-Temperature Metallic Oxide Resistive Oxygen Sensor. Sens. Actuators B Chem. 1992, 9, 91–96. [Google Scholar] [CrossRef]
- Herrmann, J.; Hagen, G.; Kita, J.; Noack, F.; Bleicker, D.; Moos, R. Multi-Gas Sensor to Detect Simultaneously Nitrogen Oxides and Oxygen. J. Sens. Sens. Syst. 2020, 9, 327–335. [Google Scholar] [CrossRef]
- Sanson, A.; Mercadelli, E.; Roncari, E.; Licheri, R.; Orrù, R.; Cao, G.; Merlone-Borla, E.; Marzorati, D.; Bonavita, A.; Micali, G.; et al. Influence of Processing Parameters on the Electrical Response of Screen Printed SrFe0.6Ti0.4O3−δ Thick Films. Ceram. Int. 2010, 36, 521–527. [Google Scholar] [CrossRef]
- Stratulat, A.; Serban, B.-C.; de Luca, A.; Avramescu, V.; Cobianu, C.; Brezeanu, M.; Buiu, O.; Diamandescu, L.; Feder, M.; Ali, S.; et al. Low Power Resistive Oxygen Sensor Based on Sonochemical SrTi0.6Fe0.4O2.8 (STFO40). Sensors 2015, 15, 17495–17506. [Google Scholar] [CrossRef]
- Neri, G.; Micali, G.; Bonavita, A.; Licheri, R.; Orrù, R.; Cao, G.; Marzorati, D.; Merlone Borla, E.; Roncari, E.; Sanson, A. FeSrTiO3-Based Resistive Oxygen Sensors for Application in Diesel Engines. Sens. Actuators B Chem. 2008, 134, 647–653. [Google Scholar] [CrossRef]
- Izu, N.; Shin, W.; Matsubara, I.; Murayama, N. The Effects of the Particle Size and Crystallite Size on the Response Time for Resistive Oxygen Gas Sensor Using Cerium Oxide Thick Film. Sens. Actuators B Chem. 2003, 94, 222–227. [Google Scholar] [CrossRef]
- Almaev, A.V.; Chernikov, E.V.; Davletkildeev, N.A.; Sokolov, D.V. Oxygen Sensors Based on Gallium Oxide Thin Films with Addition of Chromium. Superlattices Microstruct. 2020, 139, 106392. [Google Scholar] [CrossRef]
- Jin, G.; Choi, G.; Lee, W.; Park, J. Gas Sensing Property of Perovskite SrTi1−xFexO3−δ Fabricated by Thick Film Planar Technology. J. Nanosci. Nanotechnol. 2011, 11, 1738–1741. [Google Scholar] [CrossRef]
- Steiner, C.; Püls, S.; Bektas, M.; Müller, A.; Hagen, G.; Moos, R. Resistive, Temperature-Independent Metal Oxide Gas Sensor for Detecting the Oxygen Stoichiometry (Air-Fuel Ratio) of Lean Engine Exhaust Gases. Sensors 2023, 23, 3914. [Google Scholar] [CrossRef]
- Sahner, K.; Straub, J.; Moos, R. Cuprate-Ferrate Compositions for Temperature Independent Resistive Oxygen Sensors. J. Electroceram. 2006, 16, 179–186. [Google Scholar] [CrossRef]
- Murayama, N.; Izu, N.; Shin, W.; Matsubara, I. Application of Nano-Sized CeO2 Powder for Fast Response Oxygen Sensors. Key Eng. Mater. 2009, 421–422, 323–327. [Google Scholar] [CrossRef]
- Izu, N.; Oh-hori, N.; Itou, M.; Shin, W.; Matsubara, I.; Murayama, N. Resistive Oxygen Gas Sensors Based on Ce1−xZrxO2 Nano Powder Prepared Using New Precipitation Method. Sens. Actuators B Chem. 2005, 108, 238–243. [Google Scholar] [CrossRef]
- Beie, H.-J.; Gnörich, A. Oxygen Gas Sensors Based on CeO2 Thick and Thin Films. Sens. Actuators B Chem. 1991, 4, 393–399. [Google Scholar] [CrossRef]
- Exner, J.; Schubert, M.; Hanft, D.; Stöcker, T.; Fuierer, P.; Moos, R. Tuning of the Electrical Conductivity of Sr(Ti,Fe)O3 Oxygen Sensing Films by Aerosol Co-Deposition with Al2O3. Sens. Actuators B Chem. 2016, 230, 427–433. [Google Scholar] [CrossRef]
- Menesklou, W.; Schreiner, H.-J.; Moos, R.; Härdtl, K.H.; Ivers-Tiffée, E. Sr(Ti,Fe)O3: Materials for a Temperature Independent Resistive Oxygen Sensor. MRS Proc. 1999, 604, 305. [Google Scholar] [CrossRef]
- Chen, C.-Y.; Chang, K.-H. Temperature Independent Resistive Oxygen Sensor Prepared Using Zirconia-Doped Ceria Powders. Sens. Actuators B Chem. 2012, 162, 68–75. [Google Scholar] [CrossRef]
- Chen, C.-Y.; Chang, K.-H.; Chiang, H.-Y.; Shih, S.-J. Preparation of a Porous Ceria Coating for a Resistive Oxygen Sensor. Sens. Actuators B Chem. 2014, 204, 31–41. [Google Scholar] [CrossRef]
- Izu, N.; Itoh, T.; Nishibori, M.; Shin, W.; Matsubara, I. Resistive Type Sensor Using Ceria Thick Film with Nano Particles. Adv. Mat. Res. 2008, 47–50, 1522–1525. [Google Scholar] [CrossRef]
- Murayama, N.; Izu, N.; Shin, W.; Matsubara, I. Resistive Oxygen Gas Sensors Using Cerium Oxide Nanosized Powder. MRS Proc. 2004, 828, A3.2/K4.2. [Google Scholar] [CrossRef]
- Chang, K.H.; Liu, C.L.; Chan, Y.T.; Tsai, W.C.; Chen, C.Y. Resistive Oxygen Gas Sensing Behavior of Zirconia-Doped Ceria. Key Eng. Mater. 2011, 479, 143–148. [Google Scholar] [CrossRef]
- Bartic, M.; Baban, C.; Suzuki, H.; Ogita, M.; Isai, M. Β-Gallium Oxide as Oxygen Gas Sensors at a High Temperature. J. Am. Ceram. Soc. 2007, 90, 2879–2884. [Google Scholar] [CrossRef]
- American Elements. Available online: https://www.Americanelements.Com/Cerium-Oxide-1306-38-3 (accessed on 20 April 2024).
- Xavier, R.; Sivaperuman, K. Review on the of Physical Vapor Deposition on Imminent Chemiresistive Metal Oxide Gas Sensors and Their Future Scope. Mater. Today Commun. 2024, 38, 107831. [Google Scholar] [CrossRef]
- Wree, J.L.; Rogalla, D.; Ostendorf, A.; Schierbaum, K.D.; Devi, A. Plasma-Enhanced Atomic Layer Deposition of Molybdenum Oxide Thin Films at Low Temperatures for Hydrogen Gas Sensing. ACS Appl. Mater. Interfaces 2022, 15, 14502–14512. [Google Scholar] [CrossRef]




| Sector | Special Requirements |
|---|---|
| Healthcare |
|
| Food |
|
| Automotive |
|
| Environmental monitoring |
|
| HI-TECH |
|
| IoT |
|
| Working Temperature | Analyte | ||||
|---|---|---|---|---|---|
| H2 | CO | NH3 | H2S | O2 | |
| 250 °C | 1.4 | 1.4 | 1.4 | 6.6 | 4.4 |
| 300 °C | 1.3 | 1.3 | 1.3 | 4.8 | 3.7 |
| 350 °C | ~1 | <1 | 1.9 | 3.5 | 4.3 |
| 400 °C | ~1 | <1 | 2.9 | 1.9 | 3.7 |
| 450 °C | ~1 | <1 | 1.8 | <1 | 2.6 |
| Type of Oxygen Sensor | Mechanism of Operation | Key Materials | Applications | Ref. |
|---|---|---|---|---|
| Optical | This type of sensor uses dyes emitting light (luminescence) when exposed. The emitted light intensity is proportional to the amount of oxygen in the environment. | Luminescent dyes, polymers | Medical diagnostics, environmental monitoring, industrial process control | [18,54,55,56] |
| Schottky diode | It acts as a gas sensor due to the change in the electron work function resulting from the adsorption of gas molecules on the metal surface, which influences the conductivity of the metal–semiconductor junction. | Gold, nickel, platinum, SiC, GaAs | Industrial monitoring processes, environmental sensing, healthcare diagnostics | [57,58] |
| Electrochemical | Operation by the reaction of the electrolyte with the target gas, which generates an electrical signal proportional to the gas concentration. | Zirconia, Yttria-stabilized zirconia | Industrial, automotive, medical diagnostics | [6,59,60] |
| Magnetic | They are based on the paramagnetic properties of oxygen that cause the attraction of its molecules to a strong magnetic field, enabling accurate measurements of oxygen concentration. | Paramagnetic materials | Industrial process control, medical diagnostics | [36,37] |
| Chemiresistive (resistive) | They work based on the adsorption of oxygen molecules on the surface of the semiconductor material, which changes its resistance—the changes in this parameter are proportional to the concentration of oxygen. | SMO, carbon materials | Combustion engines sensors, harsh environment sensors | [61,62,63,64] |
| Magnetron Sputtering | Chemical Vapor Deposition | Atomic Layer Deposition |
|---|---|---|
| + Fast + Low cost + No harmful byproducts + Selection of the sputtered material independent of its melting point or chemical reactivity | + Very efficient + Very fast + Deposition possible on a complex surfaces + Simple process + Easy doping process | + Very precise + Deposition possible on very complex surfaces + Very high control of final product + Allows for repetitively obtaining extremely thin layers + Allows for simultaneous deposition on large quantities of samples + Very easy and precise doping or mixing of materials + Produces very homogeneous films |
| - Unable to conformally coat complex surfaces - Size of batches limited by the 2D size of chamber | - Many harmful byproducts - Aggressive precursors may damage substrates - Often needs high temperature, plasma, or lasers to activate precursors | - Slow process - Expensive - Harmful byproducts |
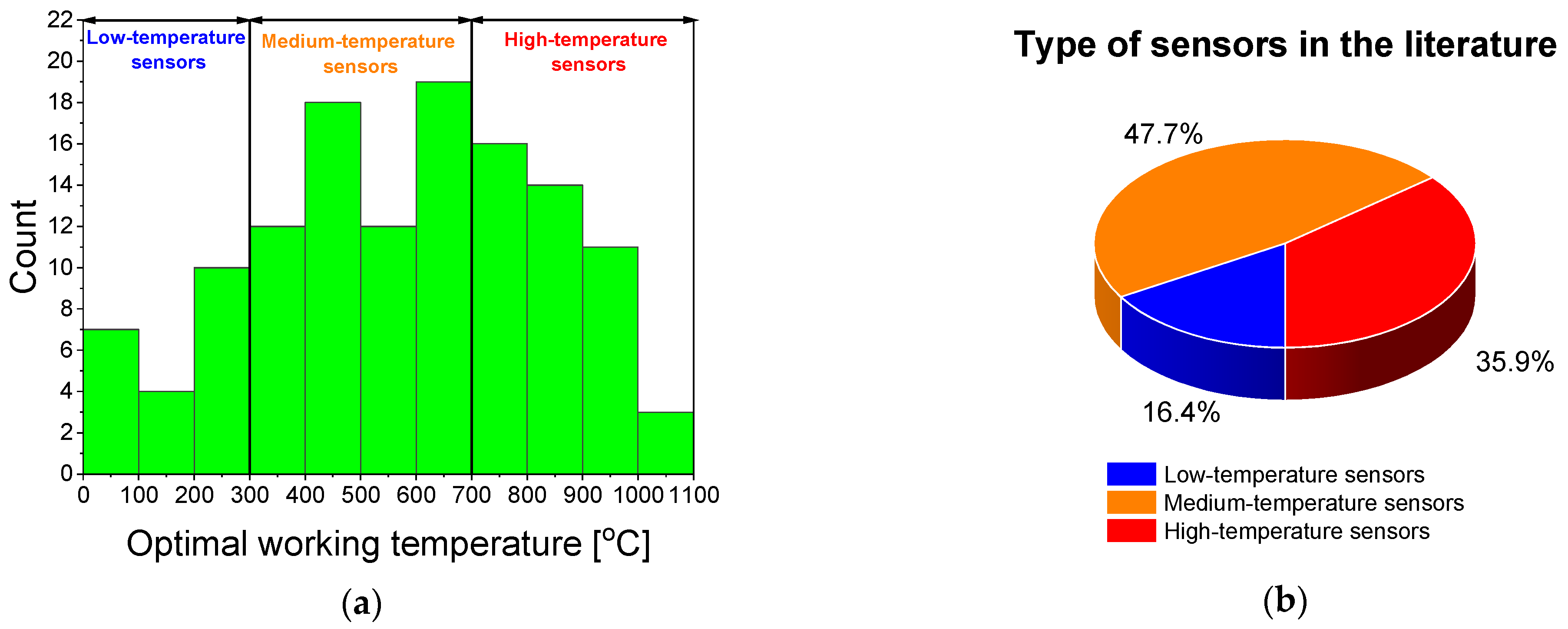
| Ref. | Material | Optimal Working Temperature [°C] | Thickness [nm] | Deposition Method | Min Range [%] | Max Range [%] | T90 [s] | Trec [s] |
|---|---|---|---|---|---|---|---|---|
| Low Temperature Sensors | ||||||||
| [89] | CNT | 20 | - | Dip casting | 0.3 | 100 | 60 | 180 |
| [90] | Bi2O2Se | 20 | 8.4 | CVD | 2.5 × 10−5 | 0.004 | - | - |
| [91] | SWCN:SrTiO3 | 20 | - | CVD, PLD | 10−5 | 10−3 | 21 | 170 |
| [92] | ZnO | 20 | - | Thermal evaporating | - | - | - | - |
| [93] | ZnO | 20 | - | Microwave–hydrothermal | 0 | 15 | 120 | 60 |
| [94] | CdO:ZnO (1:3) | 32 | 200 | Spin coating | - | - | 18 | 10 |
| [94] | ZnO | 32 | 200 | Spin coating | - | - | 38 | 30 |
| [94] | CdO | 100 | 200 | Spin coating | - | - | 14 | 38 |
| [52] | Ti2CTx | 125 | 50,000 | Micro-plotter printing | 1 | 20 | - | - |
| [94] | CdO:ZnO (3:1) | 150 | 200 | Spin coating | - | - | 20 | 35 |
| [95] | MWCNT | 160 | - | Drop casting | 0.3 | 50 | - | 0.9 |
| [96] | CdO:ZnO | 200 | - | RF magnetron sputtering | - | - | 25 | 45 |
| [50] | Nb2O5 | 200 | - | Screen printing | 0.4 | 20 | 71 | 57 |
| [97] | SnO2 | 225 | 120 | Electron bean evaporation | 0.05 | 0.4 | - | - |
| [97] | SnO2:2.72%Pt | 225 | 120 | Electron bean evaporation | 0.05 | 0.4 | - | - |
| [97] | SnO2:2.88%Pt | 225 | 120 | Electron bean evaporation | 0.05 | 0.4 | - | - |
| [97] | SnO2:3.74%Pt | 225 | 120 | Electron bean evaporation | 0.05 | 0.4 | - | - |
| [98] | CNT | 250 | PVD | - | - | - | - | |
| [99] | SnO2 | 270 | - | RGTO | 1 | 4 | - | - |
| [99] | SnO2 | 277 | 100 | Magnetron sputtering | 1 | 4 | - | - |
| [100] | ZnO NW: C MF | 280 | - | Chemical electrodeposition | 2 × 10−4 | 0.06 | 11 | 9 |
| [101] | CaCu3Ti4O12 | 300 | 450 | Spin coating | 0.05 | 12 | - | - |
| [102] | MoS2 | 300 | - | Dip casting | 2 | 100 | - | - |
| [103] | SnO2 | 300 | - | Screen printing | 0.1 | 100 | - | - |
| [104] | TiO2 | 300 | 30 | Molecular layering | 0.2 | 10 | 42 | 70 |
| [105] | Zn1−xAlxO (x = 0.2–10) | 300 | - | Spray pyrolysis | - | - | 170 | 270 |
| Medium Temperature Sensors | ||||||||
| [106] | TiO2 | 320 | 200 | Dip coating | - | - | - | - |
| [107] | ZrO2 | 320 | - | Screen printing | 0.003 | 100 | 15 | - |
| [108] | ZrO2:FeO2 (4:1) | 320 | - | Screen printing | 20 | - | 15 | - |
| [109] | Nb2O5 | 350 | - | Screen printing | 1 | 20 | - | - |
| [109] | Nb2O5—10% TiO2 | 350 | - | Screen printing | 1 | 20 | - | - |
| [109] | Nb2O5—5%TiO2 | 350 | - | Screen printing | 1 | 20 | - | - |
| [110] | Ce0.771Hf0.229O2 | 400 | 220 | RF magnetron sputtering | 20 | 100 | 37 | 48 |
| [110] | Ce0.801Hf0.199O2 | 400 | 220 | RF magnetron sputtering | 20 | 100 | 30 | 33 |
| [110] | Ce0.875Hf0.125O2 | 400 | 220 | RF magnetron sputtering | 20 | 100 | 15 | 20 |
| [110] | Ce0.888Hf0.112O2 | 400 | 220 | RF magnetron sputtering | 20 | 100 | 8 | 10 |
| [110] | Ce0.894Hf0.106O2 | 400 | 220 | RF magnetron sputtering | 20 | 100 | 8 | 10 |
| [110] | Ce0.91Hf0.09O2 | 400 | 220 | RF magnetron sputtering | 20 | 100 | 12 | 15 |
| [110] | Ce0.939Hf0.061O2 | 400 | 220 | RF magnetron sputtering | 20 | 100 | 15 | 17 |
| [111] | CeO2 | 400 | 55 | Sol–gel | 0.4 | 20 | 15 | 28 |
| [111] | CeO2—10% ZrO2 | 400 | 55 | Sol–gel | 0.4 | 20 | 15 | 28 |
| [111] | CeO2—20% ZrO2 | 400 | 55 | Sol–gel | 0.4 | 20 | 15 | 28 |
| [111] | CeO2—30% ZrO2 | 400 | 55 | Sol–gel | 0.4 | 20 | 15 | 28 |
| [111] | CeO2—5% ZrO2 | 400 | 55 | Sol–gel | 0.4 | 20 | 15 | 28 |
| [112] | Ga2O3+Si | 400 | 170 | RF magnetron sputtering | 9 | 100 | 11 | 70 |
| [113] | ZnO | 400 | 80 | Spin coating | 15 | 33 | - | - |
| [114] | CeO2 + 10%Y2O3 | 450 | 70 | Dip coating | 1 | 20 | 8 | - |
| [114] | CeO2 + 15% Y2O3 | 450 | 70 | Dip coating | 1 | 20 | 8 | - |
| [115] | ZnO (nano particles) | 450 | - | EDOC | 1 | 80 | 60 | 150 |
| [115] | ZnO (thin layer) | 450 | 250 | PE-MOCVD | 1 | 10 | - | - |
| [116] | BaFe0.3Ta0.7O3 | 500 | 3300 | Cold press | 1 | 100 | - | - |
| [116] | BaFe0.4Ta0.6O3 | 500 | 3300 | Cold press | 1 | 100 | - | - |
| [116] | BaFe0.5Ta0.5O3 | 500 | 3300 | Cold press | 1 | 100 | - | - |
| [116] | BaFe0.6Ta0.4O3 | 500 | 3300 | Cold press | 1 | 100 | - | - |
| [116] | BaFe0.7Ta0.3O3 | 500 | 3300 | Cold press | 1 | 100 | - | - |
| [116] | BaFe0.8Ta0.2O3 | 500 | 3300 | Cold press | 1 | 100 | - | - |
| [117] | TiO2 | 500 | 50 | RF magnetron sputtering | 0.4 | 0.6 | - | - |
| [118] | TiO2:CNT | 500 | - | Drop casting | 10−3 | - | 5 | - |
| [118] | TiO2:Nb | 500 | - | Drop casting | 10−3 | - | 1.5 | - |
| [118] | TiO2:Nb, CNT | 500 | - | Drop casting | 10−3 | - | 5 | - |
| [113] | ZnO:Cu (10:1) | 500 | 120 | Spin coating | 15 | 33 | - | - |
| [118] | TiO2 | 550 | - | Drop casting | 10−3 | - | 1.5 | - |
| [119] | Ce0.9Hf0.1O2 | 600 | 20,000 | Screen printing | 9 | 100 | - | - |
| [119] | Ce0.9Zr0.1O2 | 600 | 20,000 | Screen printing | 9 | 100 | - | - |
| [119] | CeO2 | 600 | 20,000 | Screen printing | 9 | 100 | - | - |
| [120] | CeO2 | 600 | 500 | (MO)CVD | - | - | 9 | - |
| [120] | CeO2 | 600 | 500 | Magnetron sputtering | - | - | 9 | - |
| [121] | CeO2:Hf | 600 | 27,000 | Screen printing | 10−15 | 100 | - | - |
| [122] | LaCu0.3Fe0.7O3 | 600 | 20,000 | Screen printing | 0.1 | 100 | 0.001 | 0.01 |
| [123] | YBa2Cu3O3 | 600 | 150,000 | Screen printing | 0.009 | 100 | 60 | 180 |
| [113] | ZnO:Al (10:1) | 600 | 40 | Spin coating | 15 | 33 | - | - |
| [124] | BaFe0.74Al0.01Ta0.25O3 | 650 | - | Screen printing | 0.1 | 100 | - | - |
| [61] | LaFeO3 (fibers) | 650 | - | Screen printing | 0.7 | 50 | 20 | 18 |
| [61] | LaFeO3 (powder + fibers) | 650 | - | Screen printing | 0.7 | 50 | 10 | 19 |
| [61] | LaFeO3 (powder) | 650 | - | Screen printing | 0.7 | 50 | 16 | 23 |
| [125] | SrFe0.6Ti0.4O3 | 650 | 30,000 | screen printing | 2.5 | 20 | - | - |
| [125] | SrFe0.6Ti0.4O3 | 650 | 10,000 | Screen printing | 2.5 | 20 | - | - |
| [126] | SrTi0.4Fe0.6O2.8 | 650 | - | Dipping | 1 | 16 | 25 | - |
| [126] | SrTi0.6Fe0.4O2.8 | 650 | - | Dipping | 1 | 16 | 25 | - |
| [127] | SrTi0.6Fe0.4O2.8 | 650 | 30,000 | Screen printing | 2.5 | 15 | 5 | 350 |
| [128] | CeO2 | 663 | 30,000 | Screen printing | 1 | 100 | 6 | - |
| High Temperature Sensors | ||||||||
| [129] | Ga2O3 | 700 | 200 | RF magnetron sputtering | 9 | 100 | 20 | 50 |
| [122] | La0.05Sr0.95Ti0.65Fe0.35O3 | 700 | 20,000 | Screen printing | 0.1 | 100 | 0.01 | 0.02 |
| [130] | Sr(Ti0.4Fe0.6)O3 | 700 | - | Screen printing | 1 | 20 | - | - |
| [130] | Sr(Ti0.6Fe0.4)O3 | 700 | - | Screen printing | 1 | 20 | - | - |
| [3] | Sr0.95La0.05(Ti0.7Fe0.3)0.95Ga0.05O3 | 700 | 677,000 | Screen printing | - | - | - | - |
| [130] | SrFeO3 | 700 | - | Screen printing | 1 | 20 | - | - |
| [3] | SrTi0.5Fe0.5O2.8 | 700 | 677,000 | Screen printing | - | - | - | - |
| [3] | SrTi0.7Fe0.3O2.8 | 700 | 677,000 | Screen printing | - | - | - | - |
| [3] | SrTi0.8Fe0.2O2.8 | 700 | 677,000 | Screen printing | - | - | - | - |
| [130] | SrTiO3 | 700 | - | Screen printing | 1 | 20 | - | - |
| [3] | SrTiO3 | 700 | 677,000 | Screen printing | - | - | - | - |
| [131] | BaFe0.74Al0.01Ta0.25O3 | 750 | 5000 | Powder aerosol deposition | 1 | 100 | - | - |
| [132] | LaFe0.7Cu0.3O3 | 750 | 20,000 | Screen printing | 0.1 | 100 | - | - |
| [132] | LaFe0.8Cu0.2O3 | 750 | 20,000 | Screen printing | 0.1 | 100 | - | - |
| [132] | LaFe0.9Cu0.1O3 | 750 | 20,000 | Screen printing | 0.1 | 100 | - | - |
| [132] | LaFeO3 | 750 | 20,000 | Screen printing | 0.1 | 100 | - | - |
| [133] | Ce0.8Zr0.2O2 | 800 | - | Screen printing | - | - | 9 | - |
| [133] | Ce0.95Zr0.05O2 | 800 | - | Screen printing | - | - | - | - |
| [133] | Ce0.9Zr0.1O2 | 800 | - | Screen printing | - | - | - | - |
| [133] | CeO2 | 800 | - | Screen printing | - | - | 12 | - |
| [134] | CeO2 | 800 | 8000 | Screen printing | 10−20 | 100 | - | - |
| [87] | CeO2 | 800 | - | Screen printing | - | - | 0.05 | - |
| [44] | CeO2 | 800 | 25,000 | Screen printing | 1 | 100 | 10 | - |
| [135] | CeO2 | 800 | 1000 | Magnetron sputtering | 10−8 | 100 | 0.005 | - |
| [135] | CeO2 | 800 | 2500 | Magnetron sputtering | 10−8 | 100 | 0.01 | - |
| [134] | CeO2—10% ZrO2 | 800 | 8000 | Screen printing | 10−20 | 100 | 0.016 | - |
| [134] | CeO2—20% ZrO2 | 800 | 8000 | Screen printing | 10−20 | 100 | 0.009 | - |
| [134] | CeO2—5% ZrO2 | 800 | 8000 | Screen printing | 10−20 | 100 | - | - |
| [136] | Sr(Ti,Fe)O3:Al2O3 (4:1) | 800 | 7500 | Aerosol deposition | 0.001 | 100 | - | - |
| [137] | Sr(Ti0.65Fe0.35)O3 | 800 | 15,000 | Screen printing | - | - | 0.003 | - |
| [138] | Ce0.85Zr0.15O2 | 900 | - | Screen printing | - | - | - | - |
| [138] | Ce0.95Zr0.05O2 | 900 | - | Screen printing | - | - | - | - |
| [138] | Ce0.9Zr0.1O2 | 900 | - | Screen printing | - | - | 16 | 49 |
| [139] | CeO2 | 900 | 10,800 | Screen printing | 10−4 | 100 | 11 | 38 |
| [138] | CeO2 | 900 | - | Screen printing | - | - | - | - |
| [53] | CeO2 | 900 | 10,000 | Screen printing | - | - | 0.012 | 0.001 |
| [140] | CeO2 | 900 | - | Screen printing | 2.5 | 6.5 | 0.012 | 0.001 |
| [141] | CeO2 | 900 | 10,000 | Screen printing | 2.5 | 6.5 | 0.012 | 0.001 |
| [88] | CeO2 | 900 | - | Screen printing | 1 | 100 | - | 11 |
| [142] | Ce0.9Zr0.1O2 | 950 | - | Screen printing | 1 | 100 | 13 | 29 |
| [142] | CeO2 | 950 | - | Screen printing | 1 | 100 | 21 | 59 |
| [143] | Ga2O3 | 1000 | 1000 | RF magnetron sputtering | 0.001 | 100 | 10 | 24 |
| [143] | Ga2O3 | 1000 | 106 | Single-crystal growth | 0.001 | 100 | 11 | 17 |
| [143] | Ga2O3 | 1000 | 700 | Spin coating | 0.001 | 100 | 12 | 25 |
| Thickness | Area | Density | Temperature | Heat Capacity |
|---|---|---|---|---|
| [cm] | [cm2] | [g/cm3] | [K] | |
| 0.002 | 1 | 7.6 | 1173 | 390 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bulowski, W.; Knura, R.; Socha, R.P.; Basiura, M.; Skibińska, K.; Wojnicki, M. Thin Film Semiconductor Metal Oxide Oxygen Sensors: Limitations, Challenges, and Future Progress. Electronics 2024, 13, 3409. https://doi.org/10.3390/electronics13173409
Bulowski W, Knura R, Socha RP, Basiura M, Skibińska K, Wojnicki M. Thin Film Semiconductor Metal Oxide Oxygen Sensors: Limitations, Challenges, and Future Progress. Electronics. 2024; 13(17):3409. https://doi.org/10.3390/electronics13173409
Chicago/Turabian StyleBulowski, Wojciech, Rafał Knura, Robert P. Socha, Maciej Basiura, Katarzyna Skibińska, and Marek Wojnicki. 2024. "Thin Film Semiconductor Metal Oxide Oxygen Sensors: Limitations, Challenges, and Future Progress" Electronics 13, no. 17: 3409. https://doi.org/10.3390/electronics13173409
APA StyleBulowski, W., Knura, R., Socha, R. P., Basiura, M., Skibińska, K., & Wojnicki, M. (2024). Thin Film Semiconductor Metal Oxide Oxygen Sensors: Limitations, Challenges, and Future Progress. Electronics, 13(17), 3409. https://doi.org/10.3390/electronics13173409









