Functional Mapping of the Brain for Brain–Computer Interfacing: A Review
Abstract
1. Introduction
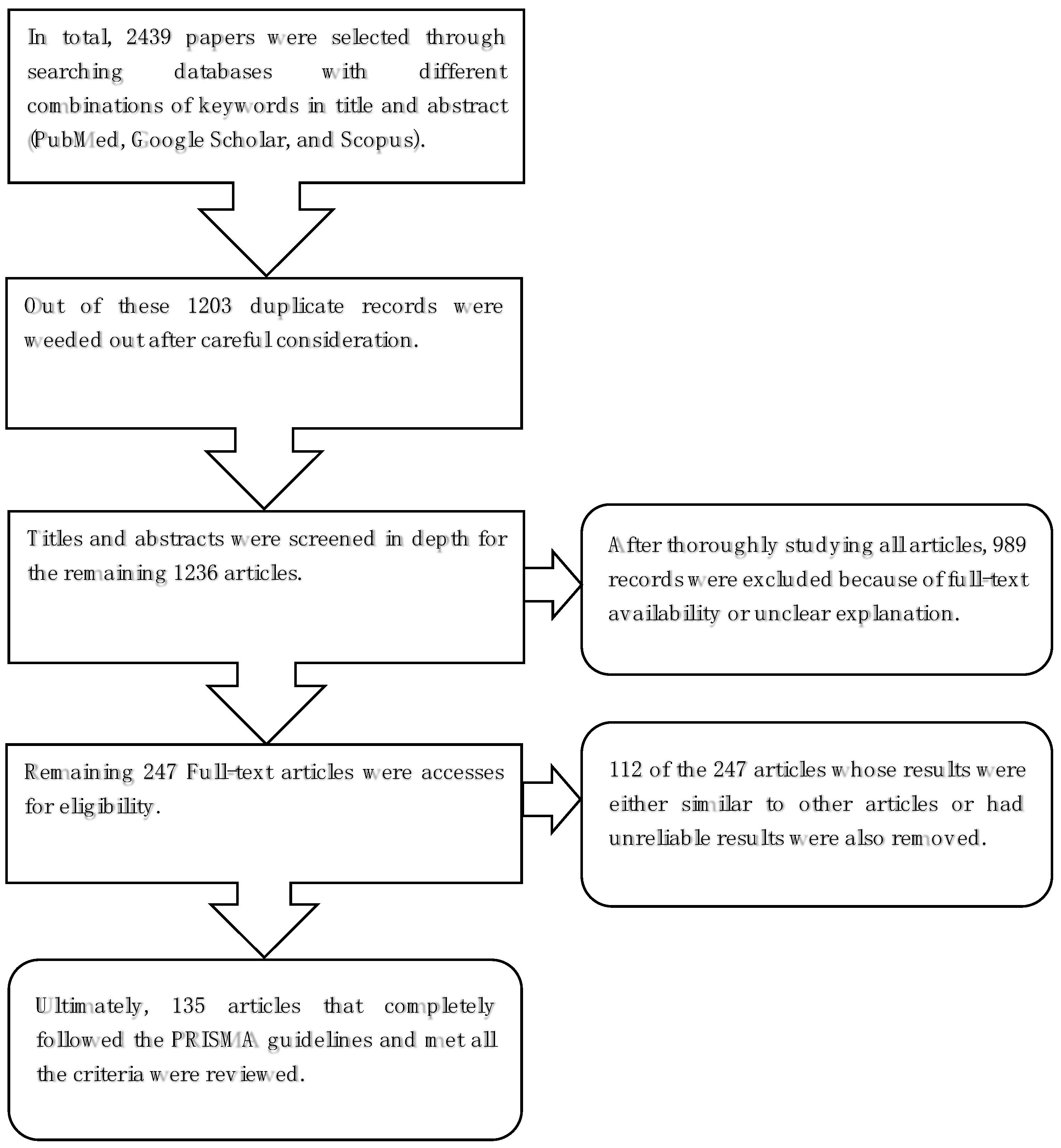
2. Materials and Methods
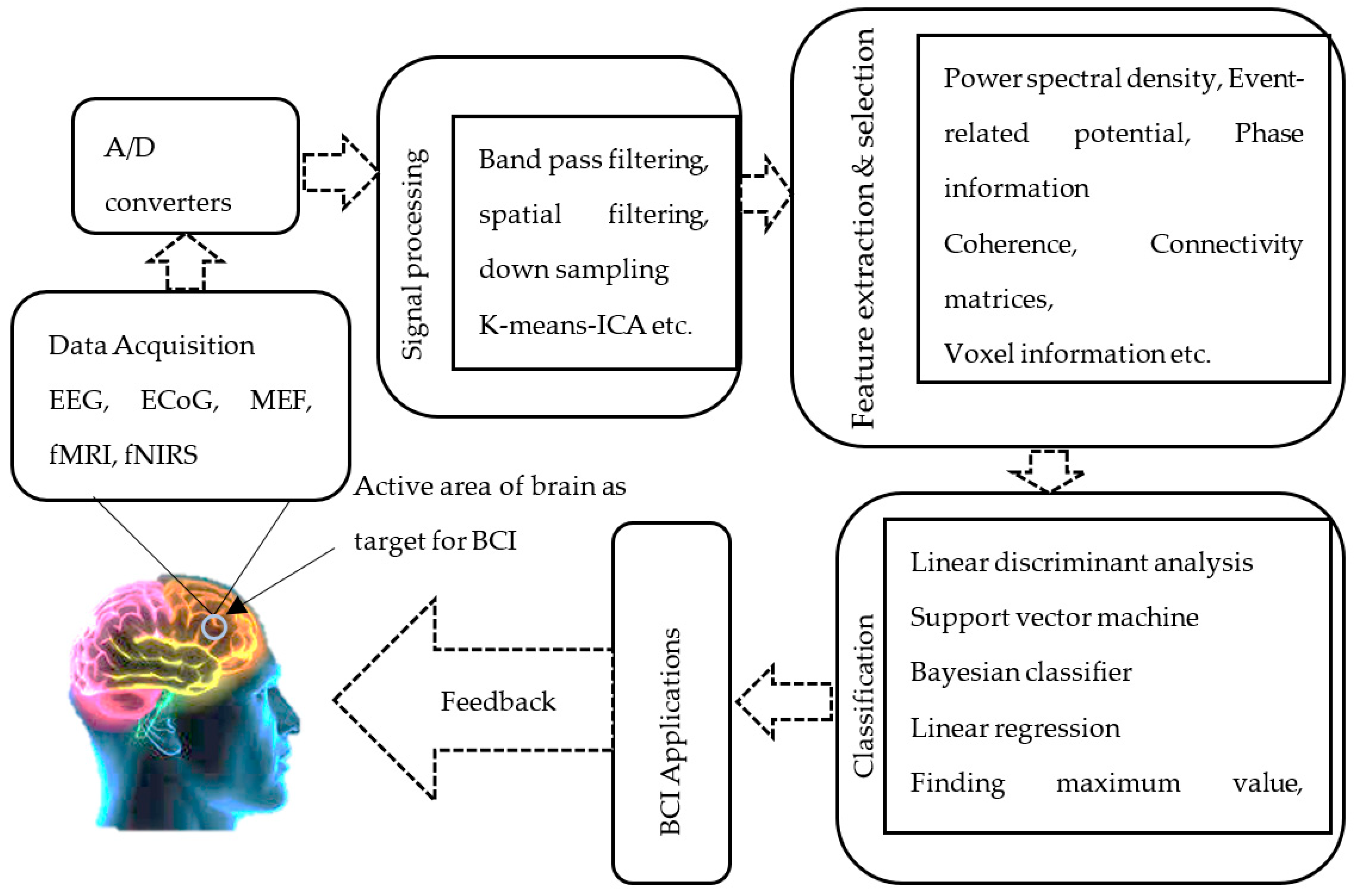
3. Basic Components in a Typical BCI System
3.1. Data Acquisition and Pre-Processing
3.2. Feature Extraction
3.3. Classification
4. Active Areas of the Brain as a Target for BCI
5. Functional Map of Brain for BCI with Different Neuroimaging Modalities
5.1. Hemodynamic Methods Based BCI (fMRI and fNIRS)
5.1.1. fMRI Based BCI
5.1.2. fNIRS-Based BCI
5.2. Electrophysiological-Based BCI (EEG and MEG)
5.2.1. EEG-Based BCI
5.2.2. MEG-Based BCI
6. Limitations and Future Scope
6.1. Hardware/Software
6.2. Knowledge of Biological Signals and Their Variability
6.3. Signal Processing
6.4. Limitations of Specific Task Measurement
6.5. Need for Calibration
6.6. Security Issues
7. Discussion
8. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wolpaw, J.R.; McFarland, D.J.; Vaughan, T.M. Brain-computer interface research at the Wadsworth Center. IEEE Trans. Rehabil. Eng. 2000, 8, 222–226. [Google Scholar] [CrossRef] [PubMed]
- Shih, J.J.; Krusienski, D.J.; Wolpaw, J.R. Brain-computer interfaces in medicine. Mayo Clin. Proc. 2012, 87, 268–279. [Google Scholar] [CrossRef] [PubMed]
- Synnott, J.; Dietzel, D.; Ioannou, M. A review of the polygraph: History, methodology and current status. Crime Psychol. Rev. 2015, 1, 59–83. [Google Scholar] [CrossRef]
- Machado, S.; Araújo, F.; Paes, F.; Velasques, B.; Cunha, M.; Budde, H.; Basile, L.F.; Anghinah, R.; Arias-Carrión, O.; Cagy, M.; et al. EEG-based brain-computer interfaces: An overview of basic concepts and clinical applications in neurorehabilitation. Rev. Neurosci. 2010, 21, 451–468. [Google Scholar] [CrossRef] [PubMed]
- Ramadan, R.A.; Vasilakos, A.V. Brain computer interface: Control signals review. Neurocomputing 2017, 223, 26–44. [Google Scholar] [CrossRef]
- Bogue, R. Brain-computer interfaces: Control by thought. Ind. Rob. 2010, 37, 126–132. [Google Scholar] [CrossRef]
- Leuthardt, E.C.; Schalk, G.; Wolpaw, J.R.; Ojemann, J.G.; Moran, D.W. A brain-computer interface using electrocorticographic signals in humans. J. Neural Eng. 2004, 1, 63–71. [Google Scholar] [CrossRef]
- Maynard, E.M.; Nordhausen, C.T.; Normann, R.A. The Utah Intracortical Electrode Array: A recording structure for potential brain-computer interfaces. Electroencephalogr. Clin. Neurophysiol. 1997, 102, 228–239. [Google Scholar] [CrossRef]
- Graimann, B.; Allison, B.; Pfurtscheller, G. Brain–Computer Interfaces: A Gentle Introduction. In Brain-Computer Interfaces. The Frontiers Collection; Springer: Berlin/Heidelberg, Germany, 2009; pp. 1–27. [Google Scholar]
- Looned, R.; Webb, J.; Xiao, Z.G.; Menon, C. Assisting drinking with an affordable BCI-controlled wearable robot and electrical stimulation: A preliminary investigation. J. Neuroeng. Rehabil. 2014, 11, 51. [Google Scholar] [CrossRef]
- Galán, F.; Nuttin, M.; Lew, E.; Ferrez, P.W.; Vanacker, G.; Philips, J.; Millán, J.d.R. A brain-actuated wheelchair: Asynchronous and non-invasive Brain-computer interfaces for continuous control of robots. Clin. Neurophysiol. 2008, 119, 2159–2169. [Google Scholar] [CrossRef]
- Sellers, E.W.; Vaughan, T.M.; Wolpaw, J.R. A brain-computer interface for long-term independent home use. Amyotroph. Lateral Scler. 2010, 11, 449–455. [Google Scholar] [CrossRef] [PubMed]
- Holz, E.M.; Botrel, L.; Kaufmann, T.; Kübler, A. Long-term independent brain-computer interface home use improves quality of life of a patient in the locked-in state: A case study. Arch. Phys. Med. Rehabil. 2015, 96, S16–S26. [Google Scholar] [CrossRef] [PubMed]
- Kaufmann, T.; Herweg, A.; Kübler, A. Toward brain-computer interface based wheelchair control utilizing tactually-evoked event-related potentials. J. Neuroeng. Rehabil. 2014, 11, 7. [Google Scholar] [CrossRef] [PubMed]
- Martel, A.; Daḧne, S.; Blankertz, B. EEG predictors of covert vigilant attention. J. Neural Eng. 2014, 11, 035009. [Google Scholar] [CrossRef] [PubMed]
- Haque, S.; Eberhart, Z.; Bansal, A.; McMillan, C. Semantic Similarity Metrics for Evaluating Source Code Summarization. In Proceedings of the 30th IEEE/ACM International Conference on Program Comprehension, Virtual Event, 16–17 May 2022. [Google Scholar] [CrossRef]
- Langs, G.; Wang, D.; Golland, P.; Mueller, S.; Pan, R.; Sabuncu, M.R.; Sun, W.; Li, K.; Liu, H. Identifying Shared Brain Networks in Individuals by Decoupling Functional and Anatomical Variability. Cereb. Cortex 2016, 26, 4004–4014. [Google Scholar] [CrossRef] [PubMed]
- Hramov, A.E.; Grubov, V.; Badarin, A.; Maksimenko, V.A.; Pisarchik, A.N. Functional near-infrared spectroscopy for the classification of motor-related brain activity on the sensor-level. Sensors 2020, 20, 2362. [Google Scholar] [CrossRef]
- Zander, T.O.; Kothe, C. Towards passive brain-computer interfaces: Applying brain-computer interface technology to human-machine systems in general. J. Neural Eng. 2011, 8, 025005. [Google Scholar] [CrossRef]
- Shi, T.W.; Chang, G.M.; Qiang, J.F.; Ren, L.; Cui, W.H. Brain computer interface system based on monocular vision and motor imagery for UAV indoor space target searching. Biomed. Signal Process. Control 2022, 79, 104114. [Google Scholar] [CrossRef]
- Enzinger, C.; Ropele, S.; Fazekas, F.; Loitfelder, M.; Gorani, F.; Seifert, T.; Reiter, G.; Neuper, C.; Pfurtscheller, G.; Müller-Putz, G. Brain motor system function in a patient with complete spinal cord injury following extensive brain-computer interface training. Exp. Brain Res. 2008, 190, 215–223. [Google Scholar] [CrossRef]
- Min, B.K.; Marzelli, M.J.; Yoo, S.S. Neuroimaging-based approaches in the brain-computer interface. Trends Biotechnol. 2010, 28, 552–560. [Google Scholar] [CrossRef]
- McFarland, D.J.; Wolpaw, J.R. Brain–computer interface use is a skill that user and system acquire together. PLoS Biol. 2018, 16, e2006719. [Google Scholar] [CrossRef] [PubMed]
- Nurse, E.S.; Karoly, P.J.; Grayden, D.B.; Freestone, D.R. A generalizable brain-computer interface (BCI) using machine learning for feature discovery. PLoS ONE 2015, 10, e0131328. [Google Scholar] [CrossRef] [PubMed]
- Krusienski, D.J.; Grosse-Wentrup, M.; Galán, F.; Coyle, D.; Miller, K.J.; Forney, E.; Anderson, C.W. Critical issues in state-of-the-art brain-computer interface signal processing. J. Neural Eng. 2011, 8, 025002. [Google Scholar] [CrossRef] [PubMed]
- McFarland, D.J.; Todd Lefkowicz, A.; Wolpaw, J.R. Design and operation of an EEG-based brain-computer interface with digital signal processing technology. Behav. Res. Methods Instrum. Comput. 1997, 29, 337–345. [Google Scholar] [CrossRef]
- Katona, J.; Kovari, A. A Brain-Computer Interface Project Applied in Computer Engineering. IEEE Trans. Educ. 2016, 59, 319–326. [Google Scholar] [CrossRef]
- Lotte, F. Signal processing approaches to minimize or suppress calibration time in oscillatory activity-based brain-computer interfaces. Proc. IEEE 2015, 103, 871–890. [Google Scholar] [CrossRef]
- Rao, R.P. Towards neural co-processors for the brain: Combining decoding and encoding in brain–computer interfaces. Curr. Opin. Neurobiol. 2019, 55, 142–151. [Google Scholar] [CrossRef]
- Hosseini-Nejad, H.; Jannesari, A.; Sodagar, A.M. Data compression in brain-machine/computer interfaces based on the walsh-hadamard transform. IEEE Trans. Biomed. Circuits Syst. 2014, 8, 129–137. [Google Scholar] [CrossRef]
- Obeid, I.; Wolf, P.D. Evaluation of spike-detection algorithms for a brain-machine interface application. IEEE Trans. Biomed. Eng. 2004, 51, 905–911. [Google Scholar] [CrossRef]
- Khalaf, A.; Sybeldon, M.; Sejdic, E.; Akcakaya, M. A brain-computer interface based on functional transcranial doppler ultrasound using wavelet transform and support vector machines. J. Neurosci. Methods 2018, 293, 174–182. [Google Scholar] [CrossRef]
- Gaur, P.; Pachori, R.B.; Wang, H.; Prasad, G. An Automatic Subject Specific Intrinsic Mode Function Selection for Enhancing Two-Class EEG-Based Motor Imagery-Brain Computer Interface. IEEE Sens. J. 2019, 19, 6938–6947. [Google Scholar] [CrossRef]
- Yang, B.; Li, H.; Wang, Q.; Zhang, Y. Subject-based feature extraction by using fisher WPD-CSP in brain-computer interfaces. Comput. Methods Programs Biomed. 2016, 129, 21–28. [Google Scholar] [CrossRef]
- Mousa, F.A.; El-Khoribi, R.A.; Shoman, M.E. A Novel Brain Computer Interface Based on Principle Component Analysis. In Procedia Computer Science; Elsevier, B.V.: Amsterdam, The Netherlands, 2016; Volume 82, pp. 49–56. [Google Scholar]
- Ting, W.; Guo-zheng, Y.; Bang-hua, Y.; Hong, S. EEG feature extraction based on wavelet packet decomposition for brain computer interface. Meas. J. Int. Meas. Confed. 2008, 41, 618–625. [Google Scholar] [CrossRef]
- Belwafi, K.; Romain, O.; Gannouni, S.; Ghaffari, F.; Djemal, R.; Ouni, B. An embedded implementation based on adaptive filter bank for brain–computer interface systems. J. Neurosci. Methods 2018, 305, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Rathee, D.; Raza, H.; Prasad, G.; Cecotti, H. Current source density estimation enhances the performance of motor-imagery-related brain–computer interface. IEEE Trans. Neural Syst. Rehabil. Eng. 2017, 25, 2461–2471. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; King, J.T.; Chuang, C.H.; Lin, C.T.; Jung, T.P. Spatial Filtering for EEG-Based Regression Problems in Brain-Computer Interface (BCI). IEEE Trans. Fuzzy Syst. 2018, 26, 771–781. [Google Scholar] [CrossRef]
- McFarland, D.J. The advantages of the surface Laplacian in brain-computer interface research. Int. J. Psychophysiol. 2015, 97, 271–276. [Google Scholar] [CrossRef]
- Ang, K.K.; Chin, Z.Y.; Zhang, H.; Guan, C. Filter Bank Common Spatial Pattern (FBCSP) in brain-computer interface. In Proceedings of the International Joint Conference on Neural Networks, Hong Kong, China, 1–8 June 2008; pp. 2390–2397. [Google Scholar]
- Brumberg, J.S.; Pitt, K.M.; Burnison, J.D. A Noninvasive Brain-Computer Interface for Real-Time Speech Synthesis: The Importance of Multimodal Feedback. IEEE Trans. Neural Syst. Rehabil. Eng. 2018, 26, 874–881. [Google Scholar] [CrossRef]
- Yao, L.; Brown, P.; Shoaran, M. Resting Tremor Detection in Parkinson’s Disease with Machine Learning and Kalman Filtering. In Proceedings of the 2018 IEEE Biomedical Circuits and Systems Conference, BioCAS 2018, Cleveland, OH, USA, 17–19 October 2018—Proceedings; Institute of Electrical and Electronics Engineers Inc.: Piscataway, NJ, USA, 2018. [Google Scholar]
- Brandman, D.M.; Hosman, T.; Saab, J.; Burkhart, M.C.; Shanahan, B.E.; Ciancibello, J.G.; Sarma, A.A.; Milstein, D.J.; Vargas-Irwin, C.E.; Franco, B.; et al. Rapid calibration of an intracortical brain-computer interface for people with tetraplegia. J. Neural Eng. 2018, 15, 026007. [Google Scholar] [CrossRef]
- Tsui, C.S.L.; Gan, J.Q.; Roberts, S.J. A self-paced brain - Computer interface for controlling a robot simulator: An online event labelling paradigm and an extended Kalman filter based algorithm for online training. Med. Biol. Eng. Comput. 2009, 47, 257–265. [Google Scholar] [CrossRef]
- Wang, J.; Xu, G.; Wang, L.; Zhang, H. Feature extraction of brain-computer interface based on improved multivariate adaptive autoregressive models. In Proceedings of the 2010 3rd International Conference on Biomedical Engineering and Informatics, BMEI, Yantai, China, 16–18 October 2010; Volume 2, pp. 895–898. [Google Scholar]
- Polak, M.; Kostov, A. Feature extraction in development of brain-computer interface: A case study. In Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology, Hong Kong, China, 1 November 1998; IEEE: Piscataway, NJ, USA, 1998; Volume 4, pp. 2058–2061. [Google Scholar]
- Fang, Y.; Chen, M.; Zheng, X. Extracting features from phase space of EEG signals in brain-computer interfaces. Neurocomputing 2015, 151, 1477–1485. [Google Scholar] [CrossRef]
- Wang, Z.; Cao, L.; Zhang, Z.; Gong, X.; Sun, Y.; Wang, H. Short time Fourier transformation and deep neural networks for motor imagery brain computer interface recognition. In Proceedings of the Concurrency Computation; John Wiley and Sons Ltd.: Hoboken, NJ, USA, 2018; Volume 30. [Google Scholar]
- Ilyas, M.Z.; Saad, P.; Ahmad, M.I.; Ghani, A.R.I. Classification of EEG signals for brain-computer interface applications: Performance comparison. In Proceedings of the 2016 International Conference on Robotics, Automation and Sciences, ICORAS 2016, Melaka, Malaysia, 5–6 November 2016; Institute of Electrical and Electronics Engineers Inc.: Piscataway, NJ, USA, 2017. [Google Scholar]
- Bostanov, V. BCI competition 2003-Data sets Ib and IIb: Feature extraction from event-related brain potentials with the continuous wavelet transform and the t-value scalogram. IEEE Trans. Biomed. Eng. 2004, 51, 1057–1061. [Google Scholar] [CrossRef] [PubMed]
- Krusienski, D.J.; Schalk, G.; McFarland, D.J.; Wolpaw, J.R. A μ-rhythm matched filter for continuous control of a brain-computer interface. IEEE Trans. Biomed. Eng. 2007, 54, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Ker, J.; Singh, S.P.; Bai, Y.; Rao, J.; Lim, T.; Wang, L. Image thresholding improves 3-dimensional convolutional neural network diagnosis of different acute brain hemorrhages on computed tomography scans. Sensors 2019, 19, 2167. [Google Scholar] [CrossRef]
- Nahata, H.; Singh, S.P. Deep Learning Solutions for Skin Cancer Detection and Diagnosis. In Machine Learning with Health Care Perspective. Learning and Analytics in Intelligent Systems; Springer: Cham, Switzerland, 2020; Volume 13, pp. 159–182. [Google Scholar]
- Singh, S.P.; Sharma, M.K.; Lay-Ekuakille, A.; Gangwar, D.; Gupta, S. Deep ConvLSTM with Self-Attention for Human Activity Decoding Using Wearable Sensors. IEEE Sens. J. 2021, 21, 8575–8582. [Google Scholar] [CrossRef]
- Singh, S.P.; Wang, L.; Gupta, S.; Gulyas, B.; Padmanabhan, P. Shallow 3D CNN for Detecting Acute Brain Hemorrhage from Medical Imaging Sensors. IEEE Sens. J. 2021, 21, 14290–14299. [Google Scholar] [CrossRef]
- Singh, S.P.; Wang, L.; Gupta, S.; Goli, H.; Padmanabhan, P.; Gulyás, B. 3d deep learning on medical images: A review. Sensors 2020, 20, 5097. [Google Scholar] [CrossRef]
- Sun, B.; Wu, Z.; Hu, Y.; Li, T. Golden subject is everyone: A subject transfer neural network for motor imagery-based brain computer interfaces. Neural Networks 2022, 151, 111–120. [Google Scholar] [CrossRef]
- Zhong, M.Y.; Yang, Q.Y.; Liu, Y.; Zhen, B.Y.; Zhao, F.D.; Xie, B.B. EEG emotion recognition based on TQWT-features and hybrid convolutional recurrent neural network. Biomed. Signal Process. Control 2023, 79, 104211. [Google Scholar] [CrossRef]
- Zhang, X.; Yao, L.; Wang, X.; Monaghan, J.; Mcalpine, D.; Zhang, Y. A survey on deep learning based brain computer interface: Recent advances and new frontiers. arXiv 2019, arXiv:1905.04149. [Google Scholar]
- Sharma, R.; Kim, M.; Gupta, A. Motor imagery classification in brain-machine interface with machine learning algorithms: Classical approach to multi-layer perceptron model. Biomed. Signal Process. Control 2022, 71, 103101. [Google Scholar] [CrossRef]
- Toma, F.M. A hybrid neuro-experimental decision support system to classify overconfidence and performance in a simulated bubble using a passive BCI. Expert Syst. Appl. 2023, 212, 118722. [Google Scholar] [CrossRef]
- Kosmyna, N.; Tarpin-Bernard, F.; Bonnefond, N.; Rivet, B. Feasibility of BCI control in a realistic smart home environment. Front. Hum. Neurosci. 2016, 10, 416. [Google Scholar] [CrossRef] [PubMed]
- Kothe, C.A.; Makeig, S. BCILAB: A platform for brain-computer interface development. J. Neural Eng. 2013, 10, 056014. [Google Scholar] [CrossRef]
- Ramadan, R.A.; Refat, S.; Elshahed, M.A.; Ali, R.A. Basics of brain computer interface. Intell. Syst. Ref. Libr. 2015, 74, 31–50. [Google Scholar] [CrossRef]
- Shah, S.; Haghi, B.; Kellis, S.; Bashford, L.; Kramer, D.; Lee, B.; Liu, C.; Andersen, R.; Emami, A. Decoding Kinematics from Human Parietal Cortex using Neural Networks. In Proceedings of the International IEEE/EMBS Conference on Neural Engineering, NER, San Francisco, CA, USA, 20–23 March 2019; IEEE Computer Society: Washington, DC, USA, 2019; Volume 2019, pp. 1138–1141. [Google Scholar]
- Swaminathan, R.; Prasad, S. Brain computer interface used in health care technologies. SpringerBriefs Appl. Sci. Technol. 2016, 7, 49–58. [Google Scholar] [CrossRef]
- Zhu, Y.; Gao, H.; Tong, L.; Li, Z.; Wang, L.; Zhang, C.; Yang, Q.; Yan, B. Emotion Regulation of Hippocampus Using Real-Time fMRI Neurofeedback in Healthy Human. Front. Hum. Neurosci. 2019, 13, 242. [Google Scholar] [CrossRef]
- Pfurtscheller, G.; Brunner, C.; Schlögl, A.; Lopes da Silva, F.H. Mu rhythm (de)synchronization and EEG single-trial classification of different motor imagery tasks. Neuroimage 2006, 31, 153–159. [Google Scholar] [CrossRef]
- Yin, G.; Gong, L. Direction control and speed control combined model of motor-imagery based brain-actuated vehicle. In Proceedings of the Chinese Control Conference, CCC, Dalian, China, 26–28 July 2017; pp. 2210–2214. [Google Scholar]
- Royer, A.S.; Doud, A.J.; Rose, M.L.; He, B. EEG control of a virtual helicopter in 3-dimensional space using intelligent control strategies. IEEE Trans. Neural Syst. Rehabil. Eng. 2010, 18, 581–589. [Google Scholar] [CrossRef]
- Lafleur, K.; Cassady, K.; Doud, A.; Shades, K.; Rogin, E.; He, B. Quadcopter control in three-dimensional space using a noninvasive motor imagery-based brain-computer interface. J. Neural Eng. 2013, 10, 046003. [Google Scholar] [CrossRef]
- Zhang, Z.; Duan, F.; Sole-Casals, J.; Dinares-Ferran, J.; Cichocki, A.; Yang, Z.; Sun, Z. A Novel Deep Learning Approach with Data Augmentation to Classify Motor Imagery Signals. IEEE Access 2019, 7, 15945–15954. [Google Scholar] [CrossRef]
- Lin, B.S.; Lin, B.S.; Yen, T.H.; Hsu, C.C.; Wang, Y.C. Design of wearable headset with steady state visually evoked potential-based brain computer interface. Micromachines 2019, 10, 681. [Google Scholar] [CrossRef]
- Yan, N.; Wang, C.; Tao, Y.; Li, J.; Zhang, K.; Chen, T.; Yuan, Z.; Yan, X.; Wang, G. Quadcopter Control System Using a Hybrid BCI Based on Off-Line Optimization and Enhanced Human-Machine Interaction. IEEE Access 2020, 8, 1160–1172. [Google Scholar] [CrossRef]
- Wang, M.; Li, R.; Zhang, R.; Li, G.; Zhang, D. A Wearable SSVEP-Based BCI System for Quadcopter Control Using Head-Mounted Device. IEEE Access 2018, 6, 26789–26798. [Google Scholar] [CrossRef]
- Chen, Y.J.; Chen, S.C.; Zaeni, I.A.E.; Wu, C.M. Fuzzy tracking and control algorithm for an SSVEP-based BCI system. Appl. Sci. 2016, 6, 270. [Google Scholar] [CrossRef]
- Duan, X.; Xie, S.; Xie, X.; Meng, Y.; Xu, Z. Quadcopter Flight Control Using a Non-invasive Multi-Modal Brain Computer Interface. Front. Neurorobot. 2019, 13, 23. [Google Scholar] [CrossRef]
- Emmert, K.; Breimhorst, M.; Bauermann, T.; Birklein, F.; Rebhorn, C.; Van De Ville, D.; Haller, S. Active pain coping is associated with the response in real-time fMRI neurofeedback during pain. Brain Imaging Behav. 2017, 11, 712–721. [Google Scholar] [CrossRef]
- Young, K.D.; Zotev, V.; Phillips, R.; Misaki, M.; Drevets, W.C.; Bodurka, J. Amygdala real-time functional magnetic resonance imaging neurofeedback for major depressive disorder: A review. Psychiatry Clin. Neurosci. 2018, 72, 466–481. [Google Scholar] [CrossRef]
- Sarkheil, P.; Zilverstand, A.; Kilian-Hütten, N.; Schneider, F.; Goebel, R.; Mathiak, K. fMRI feedback enhances emotion regulation as evidenced by a reduced amygdala response. Behav. Brain Res. 2015, 281, 326–332. [Google Scholar] [CrossRef]
- Koush, Y.; Meskaldji, D.E.; Pichon, S.; Rey, G.; Rieger, S.W.; Linden, D.E.J.; Van De Ville, D.; Vuilleumier, P.; Scharnowski, F. Learning Control Over Emotion Networks Through Connectivity-Based Neurofeedback. Cereb. Cortex 2017, 27, 1193–1202. [Google Scholar] [CrossRef]
- Nicholson, A.A.; Rabellino, D.; Densmore, M.; Frewen, P.A.; Paret, C.; Kluetsch, R.; Schmahl, C.; Théberge, J.; Neufeld, R.W.J.; McKinnon, M.C.; et al. The neurobiology of emotion regulation in posttraumatic stress disorder: Amygdala downregulation via real-time fMRI neurofeedback. Hum. Brain Mapp. 2017, 38, 541–560. [Google Scholar] [CrossRef] [PubMed]
- Buyukturkoglu, K.; Roettgers, H.; Sommer, J.; Rana, M.; Dietzsch, L.; Arikan, E.B.; Veit, R.; Malekshahi, R.; Kircher, T.; Birbaumer, N.; et al. Self-regulation of anterior insula with real-time fMRI and its behavioral effects in obsessive-compulsive disorder: A feasibility study. PLoS ONE 2015, 10, e0135872. [Google Scholar] [CrossRef]
- Emmert, K.; Kopel, R.; Koush, Y.; Maire, R.; Senn, P.; Van De Ville, D.; Haller, S. Continuous vs. intermittent neurofeedback to regulate auditory cortex activity of tinnitus patients using real-time fMRI—A pilot study. NeuroImage Clin. 2017, 14, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Zhang, G.; Yao, L.; Zhao, X. Impact of real-time fMRI working memory feedback training on the interactions between three core brain networks. Front. Behav. Neurosci. 2015, 9, 244. [Google Scholar] [CrossRef]
- Paret, C.; Zaehringer, J.; Ruf, M.; Ende, G.; Schmahl, C. The orbitofrontal cortex processes neurofeedback failure signals. Behav. Brain Res. 2019, 369, 111938. [Google Scholar] [CrossRef]
- Rubia, K.; Criaud, M.; Wulff, M.; Alegria, A.; Brinson, H.; Barker, G.; Stahl, D.; Giampietro, V. Functional connectivity changes associated with fMRI neurofeedback of right inferior frontal cortex in adolescents with ADHD. Neuroimage 2019, 188, 43–58. [Google Scholar] [CrossRef]
- Rota, G.; Sitaram, R.; Veit, R.; Erb, M.; Weiskopf, N.; Dogil, G.; Birbaumer, N. Self-regulation of regional cortical activity using real-time fmri: The right inferior frontal gyrus and linguistic processing. Hum. Brain Mapp. 2009, 30, 1605–1614. [Google Scholar] [CrossRef]
- Letra, L.; Pereira, D.; Castelo-Branco, M. Functional neuroimaging in obesity research. In Advances in Neurobiology; Springer: New York, NY, USA, 2017; Volume 19, pp. 239–248. [Google Scholar]
- Sokunbi, M.O. Using real-time fMRI brain-computer interfacing to treat eating disorders. J. Neurol. Sci. 2018, 388, 109–114. [Google Scholar] [CrossRef]
- Subramanian, L.; Morris, M.B.; Brosnan, M.; Turner, D.L.; Morris, H.R.; Linden, D.E.J. Functional magnetic resonance imaging neurofeedback-guided motor imagery training and motor training for parkinson’s disease: Randomized trial. Front. Behav. Neurosci. 2016, 10, 111. [Google Scholar] [CrossRef] [PubMed]
- Birbaumer, N.; Weber, C.; Neuper, C.; Buch, E.; Haapen, K.; Cohen, L. Chapter 24 Physiological regulation of thinking: Brain-computer interface (BCI) research. Prog. Brain Res. 2006, 159, 369–391. [Google Scholar]
- Zahn, R.; Weingartner, J.H.; Basilio, R.; Bado, P.; Mattos, P.; Sato, J.R.; de Oliveira-Souza, R.; Fontenelle, L.F.; Young, A.H.; Moll, J. Blame-rebalance fMRI neurofeedback in major depressive disorder: A randomised proof-of-concept trial. NeuroImage Clin. 2019, 24, 101992. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, Z.; Zou, Z.; Wu, X.; Gao, H.; Wang, C.; Zhou, J.; Qi, F.; Zhang, M.; He, J.; et al. Real-Time fMRI Neurofeedback Training Changes Brain Degree Centrality and Improves Sleep in Chronic Insomnia Disorder: A Resting-State fMRI Study. Front. Mol. Neurosci. 2022, 15, 825286. [Google Scholar] [CrossRef] [PubMed]
- Faress, A.; Chau, T. Towards a multimodal brain-computer interface: Combining fNIRS and fTCD measurements to enable higher classification accuracy. NeuroImage 2013, 77, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.; Nijholt, A. Brain-Computer Interfaces and Human-Computer Interaction. In Brain-Computer Interfaces; Human-Computer Interaction Series; Springer: London, UK, 2010; pp. 3–19. [Google Scholar]
- Kaiser, V.; Bauernfeind, G.; Kreilinger, A.; Kaufmann, T.; Kübler, A.; Neuper, C.; Müller-Putz, G.R. Cortical effects of user training in a motor imagery based brain-computer interface measured by fNIRS and EEG. NeuroImage 2014, 85, 432–444. [Google Scholar] [CrossRef] [PubMed]
- Naseer, N.; Hong, K.S. fNIRS-based brain-computer interfaces: A review. Front. Hum. Neurosci. 2015, 9, 3. [Google Scholar] [CrossRef]
- Noori, F.M.; Naseer, N.; Qureshi, N.K.; Nazeer, H.; Khan, R.A. Optimal feature selection from fNIRS signals using genetic algorithms for BCI. Neurosci. Lett. 2017, 647, 61–66. [Google Scholar] [CrossRef]
- Hong, K.S.; Naseer, N.; Kim, Y.H. Classification of prefrontal and motor cortex signals for three-class fNIRS-BCI. Neurosci. Lett. 2015, 587, 87–92. [Google Scholar] [CrossRef]
- Cui, X.; Bray, S.; Reiss, A.L. Functional near infrared spectroscopy (NIRS) signal improvement based on negative correlation between oxygenated and deoxygenated hemoglobin dynamics. NeuroImage 2010, 49, 3039–3046. [Google Scholar] [CrossRef]
- Zhang, Z.; Jiao, X.; Xu, F.; Jiang, J.; Yang, H.; Cao, Y.; Fu, J. The investigation of brain-computer interface for motor imagery and execution using functional near-infrared spectroscopy. In Proceedings of the International Conference on Innovative Optical Health Science; SPIE: Bellingham, WA, USA, 2017; Volume 10245, p. 102450I. [Google Scholar]
- Shin, J.; Jeong, J. Multiclass classification of hemodynamic responses for performance improvement of functional near-infrared spectroscopy-based brain–computer interface. J. Biomed. Opt. 2014, 19, 067009. [Google Scholar] [CrossRef]
- Chiarelli, A.M.; Croce, P.; Merla, A.; Zappasodi, F. Deep learning for hybrid EEG-fNIRS brain-computer interface: Application to motor imagery classification. J. Neural Eng. 2018, 15, 036028. [Google Scholar] [CrossRef]
- Erdoĝan, S.B.; Özsarfati, E.; Dilek, B.; Kadak, K.S.; Hanoĝlu, L.; Akin, A. Classification of motor imagery and execution signals with population-level feature sets: Implications for probe design in fNIRS based BCI. J. Neural Eng. 2019, 16, 026029. [Google Scholar] [CrossRef] [PubMed]
- Busch, M.A.; Schienkiewitz, A.; Nowossadeck, E.; Gößwald, A. Prävalenz des Schlaganfalls bei Erwachsenen im Alter von 40 bis 79 Jahren in Deutschland: Ergebnisse der Studie zur Gesundheit Erwachsener in Deutschland (DEGS1). Bundesgesundheitsblatt-Gesundheitsforsch.-Gesundheitsschutz 2013, 56, 656–660. [Google Scholar] [CrossRef] [PubMed]
- Reuter-Lorenz, P.A.; Park, D.C. How Does it STAC Up? Revisiting the Scaffolding Theory of Aging and Cognition. Neuropsychol. Rev. 2014, 24, 355–370. [Google Scholar] [CrossRef]
- Personnier, P.; Kubicki, A.; Laroche, D.; Papaxanthis, C. Temporal features of imagined locomotion in normal aging. Neurosci. Lett. 2010, 476, 146–149. [Google Scholar] [CrossRef] [PubMed]
- Skoura, X.; Personnier, P.; Vinter, A.; Pozzo, T.; Papaxanthis, C. Decline in motor prediction in elderly subjects: Right versus left arm differences in mentally simulated motor actions. Cortex 2008, 44, 1271–1278. [Google Scholar] [CrossRef]
- Jin, Z.; Zhou, G.; Gao, D.; Zhang, Y. EEG classification using sparse Bayesian extreme learning machine for brain–computer interface. Neural Comput. Appl. 2018, 32, 6601–6609. [Google Scholar] [CrossRef]
- Corsi, M.C.; Chavez, M.; Schwartz, D.; Hugueville, L.; Khambhati, A.N.; Bassett, D.S.; De Vico Fallani, F. Integrating EEG and MEG Signals to Improve Motor Imagery Classification in Brain-Computer Interface. Int. J. Neural Syst. 2019, 29, 1850014. [Google Scholar] [CrossRef]
- Blankertz, B.; Dornhege, G.; Krauledat, M.; Müller, K.R.; Kunzmann, V.; Losch, F.; Curio, G. The Berlin brain-computer interface: EEG-based communication without subject training. IEEE Trans. Neural Syst. Rehabil. Eng. 2006, 14, 147–152. [Google Scholar]
- Tomita, Y.; Vialatte, F.B.; Dreyfus, G.; Mitsukura, Y.; Bakardjian, H.; Cichocki, A. Bimodal BCI using simultaneously NIRS and EEG. IEEE Trans. Biomed. Eng. 2014, 61, 1274–1284. [Google Scholar] [CrossRef]
- Shin, J.; Kwon, J.; Choi, J.; Im, C.H. Ternary near-infrared spectroscopy brain-computer interface with increased information transfer rate using prefrontal hemodynamic changes during mental arithmetic, breath-holding, and idle state. IEEE Access 2018, 6, 19491–19498. [Google Scholar] [CrossRef]
- Bin, G.; Gao, X.; Wang, Y.; Hong, B.; Gao, S. VEP-based brain-computer interfaces: Time, frequency, and code modulations. IEEE Comput. Intell. Mag. 2009, 4, 22–26. [Google Scholar] [CrossRef]
- McFarland, D.J.; Wolpaw, J.R. EEG-based brain–computer interfaces. Curr. Opin. Biomed. Eng. 2017, 4, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Han, C.; Xu, G.; Xie, J.; Chen, C.; Zhang, S. Highly Interactive Brain-Computer Interface Based on Flicker-Free Steady-State Motion Visual Evoked Potential. Sci. Rep. 2018, 8, 5835. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.H.; Cho, J.H.; Lee, Y.E.; Lee, S.H.; Shin, G.H.; Kweon, Y.S.; Millán, J.d.R.; Müller, K.R.; Lee, S.W. 2020 International brain–computer interface competition: A review. Front. Hum. Neurosci. 2022, 16, 495. [Google Scholar] [CrossRef] [PubMed]
- Rashid, M.; Bari, B.S.; Hasan, M.J.; Razman, M.A.M.; Musa, R.M.; Nasir, A.F.A.; Majeed, A.P.P.A. The classification of motor imagery response: An accuracy enhancement through the ensemble of random subspace k-NN. PeerJ Comput. Sci. 2021, 7, e374. [Google Scholar] [CrossRef]
- Zhu, H.; Forenzo, D.; He, B. On the Deep Learning Models for EEG-Based Brain-Computer Interface Using Motor Imagery. IEEE Trans. Neural Syst. Rehabil. Eng. 2022, 30, 2283–2291. [Google Scholar] [CrossRef]
- Liu, X.; Shi, R.; Hui, Q.; Xu, S.; Wang, S.; Na, R.; Sun, Y.; Ding, W.; Zheng, D.; Chen, X. TCACNet: Temporal and channel attention convolutional network for motor imagery classification of EEG-based BCI. Inf. Process. Manag. 2022, 59, 103001. [Google Scholar] [CrossRef]
- Buch, E.; Weber, C.; Cohen, L.G.; Braun, C.; Dimyan, M.A.; Ard, T.; Mellinger, J.; Caria, A.; Soekadar, S.; Fourkas, A.; et al. Think to move: A neuromagnetic brain-computer interface (BCI) system for chronic stroke. Stroke 2008, 39, 910–917. [Google Scholar] [CrossRef]
- Kauhanen, L.; Nykopp, T.; Lehtonen, J.; Jylänki, P.; Heikkonen, J.; Rantanen, P.; Alaranta, H.; Sams, M. EEG and MEG brain-computer interface for tetraplegic patients. IEEE Trans. Neural Syst. Rehabil. Eng. 2006, 14, 190–193. [Google Scholar] [CrossRef]
- Mellinger, J.; Schalk, G.; Braun, C.; Preissl, H.; Rosenstiel, W.; Birbaumer, N.; Kübler, A. An MEG-based brain–computer interface (BCI). NeuroImage 2007, 36, 581–593. [Google Scholar] [CrossRef]
- Bianchi, L.; Sami, S.; Hillebrand, A.; Fawcett, I.P.; Quitadamo, L.R.; Seri, S. Which physiological components are more suitable for visual ERP based brain-computer interface? A preliminary MEG/EEG study. Brain Topogr. 2010, 23, 180–185. [Google Scholar] [CrossRef]
- Wittevrongel, B.; Holmes, N.; Boto, E.; Hill, R.; Rea, M.; Libert, A.; Khachatryan, E.; Van Hulle, M.M.; Bowtell, R.; Brookes, M.J. Practical real-time MEG-based neural interfacing with optically pumped magnetometers. BMC Biol. 2021, 19, 158. [Google Scholar] [CrossRef] [PubMed]
- Ovchinnikova, A.O.; Vasilyev, A.N.; Zubarev, I.P.; Kozyrskiy, B.L.; Shishkin, S.L. MEG-Based Detection of Voluntary Eye Fixations Used to Control a Computer. Front. Neurosci. 2021, 15, 619591. [Google Scholar] [CrossRef] [PubMed]
- Kwak, Y.; Song, W.J.; Kim, S.E. FGANet: FNIRS-Guided Attention Network for Hybrid EEG-fNIRS Brain-Computer Interfaces. IEEE Trans. Neural Syst. Rehabil. Eng. 2022, 30, 329–339. [Google Scholar] [CrossRef] [PubMed]
- Schalk, G.; McFarland, D.J.; Hinterberger, T.; Birbaumer, N.; Wolpaw, J.R. BCI2000: A general-purpose brain-computer interface (BCI) system. IEEE Trans. Biomed. Eng. 2004, 51, 1034–1043. [Google Scholar] [CrossRef] [PubMed]
- Renard, Y.; Lotte, F.; Gibert, G.; Congedo, M.; Maby, E.; Delannoy, V.; Bertrand, O.; Lécuyer, A. OpenViBE: An open-source software platform to design, test, and use brain-computer interfaces in real and virtual environments. Presence Teleoperators Virtual Environ. 2010, 19, 35–53. [Google Scholar] [CrossRef]
- Milsap, G.; Collard, M.; Coogan, C.; Crone, N.E. BCI2000Web and WebFM: Browser-based tools for brain computer interfaces and functional brain mapping. Front. Neurosci. 2019, 13, 1030. [Google Scholar] [CrossRef]
- Bowen, P.; Johnson, A.; Hash, J.; Smith, C.D.; Steinberg, D.I. An introductory resource guide for implementing the Health Insurance Portability and Accountability Act (HIPAA) security rule. NIST Spec. Publ. 2008, 800, 800–866. [Google Scholar]
- Fairclough, S.H. Designing human-computer interaction with neuroadaptive technology. In Current Research in Neuroadaptive Technology; Academic Press: Cambridge, MA, USA, 2022; pp. 1–15. ISBN 9780128214138. [Google Scholar]
- Bernal, S.L.; Celdrán, A.H.; Pérez, G.M.; Barros, M.T.; Balasubramaniam, S. Security in Brain-Computer Interfaces: State-of-the-Art, Opportunities, and Future Challenges. ACM Comput. Surv. 2021, 54, 1–35. [Google Scholar] [CrossRef]


| Reference | Functionality | Objective | Active Brain Areas | Methods | Outcome |
|---|---|---|---|---|---|
| [81] | fMRI-BCI | Emotion regulation | Lateral prefrontal cortex | Deficits in emotion regulation | The brain activity can be modified by fMRI feedback during the given task |
| [81] | -do- | Learning control over emotion network | Dorsal medial frontal cortex | Connectivity-based neurofeedback approach | Controlling specific behavioral changes |
| [84] | -do- | Self-regulation of the anterior insula | Anterior insula | Amygdala-based rtfMRI-neurofeedback | Achieved insula down-regulation and symptom alleviation in OCD disorder |
| [95] | -do- | Sleep improvement during Chronic insomnia disorder | Gyrus, rolandic operculum, insula | Amygdala-based rtfMRI-neurofeedback, voxel-based degree centrality | Sleep improvement in chronic insomnia-disoriented patients by altering intrinsic functional hubs |
| [100] | fNIRS-BCI | Classification of fNIRS signals for motor cortex signals | Motor cortex | Support vector machine on statistical features from the oxygenated hemoglobin | Constructed GA-optimized SVM and trained on statistical features. The classification was done on motor imagery vs. rest. |
| [18] | -do- | Classification of motor-related brain activities | Motor cortex | Motor execution proprioceptive feedback | The spatial dynamics in the motor cortex were classified with an accuracy of 100% for real movements and an accuracy of 90% for motor imagery |
| [105] | -do- | Motor imagery classification | Sensorimotor regions (C3 and C4) | Brain hemodynamics activities were recorded and classified using DNN, LDA, and SVM. | A combined EEG-fNIRS DNN framework was proposed and showed 83.28% accuracy on motor imagery classification |
| [129] | -do- | Motor arithmetic and motor imagery classification | Frontal, motor, and visual areas | fNIRS-guided attention network | The classification was done on motor arithmetic and motor imagery tasks with a mean classification accuracy of 91.96% and 78.59%, respectively |
| [120] | EEG-BCI | Classification of motor imagery response | Motor cortex | Training KNN on common spatial pattern features | Binary and multi-class motor imagery classification was performed and claimed 93.8% accuracy and compared with the state-of-the-art methods |
| [121] | -do- | Classification of motor imagery tasks | Mixed brain regions | Deep neural networks | Comparing state-of-the-art deep learning models for motor imagery tasks. The highest accuracy for EEGNet was reported, i.e., 75.5%. |
| [122] | -do- | Motor imagery tasks of body moves | Mixed brain regions | Attention-based CNN | BCI-based intelligent health care system for assisting disabled patients with an accuracy of 86.8% on the BCIC IV 2a dataset from the TCACNet and 96.2% accuracy on the HGD dataset |
| [125] | MEG-BCI | Source localization of the amplitude-modulated signal to the motor cortex | Motor cortex | Voluntary amplitude modulation of sensorimotor Mu rhythms | Analysis of communicating intentions by brain activities without involving muscles |
| [127] | -do- | Investigating the neural responses | Event-related potentials (ERPs) | OPM-MEG | Real-time MEG-BCI for “mind spelling” using optically pumped magnetometers |
| [128] | -do- | Single-trial eye fixation classification | 306 sensors at 102 positions around the head | LF-CNN and VR-CNN on MEG segments | Voluntary eye fixations ROC AUC of 0.66 for LF-CNN, and 0.67 for VAR-CNN (M ± SD) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singh, S.P.; Mishra, S.; Gupta, S.; Padmanabhan, P.; Jia, L.; Colin, T.K.A.; Tsai, Y.T.; Kejia, T.; Sankarapillai, P.; Mohan, A.; et al. Functional Mapping of the Brain for Brain–Computer Interfacing: A Review. Electronics 2023, 12, 604. https://doi.org/10.3390/electronics12030604
Singh SP, Mishra S, Gupta S, Padmanabhan P, Jia L, Colin TKA, Tsai YT, Kejia T, Sankarapillai P, Mohan A, et al. Functional Mapping of the Brain for Brain–Computer Interfacing: A Review. Electronics. 2023; 12(3):604. https://doi.org/10.3390/electronics12030604
Chicago/Turabian StyleSingh, Satya P., Sachin Mishra, Sukrit Gupta, Parasuraman Padmanabhan, Lu Jia, Teo Kok Ann Colin, Yeo Tseng Tsai, Teo Kejia, Pramod Sankarapillai, Anand Mohan, and et al. 2023. "Functional Mapping of the Brain for Brain–Computer Interfacing: A Review" Electronics 12, no. 3: 604. https://doi.org/10.3390/electronics12030604
APA StyleSingh, S. P., Mishra, S., Gupta, S., Padmanabhan, P., Jia, L., Colin, T. K. A., Tsai, Y. T., Kejia, T., Sankarapillai, P., Mohan, A., & Gulyás, B. (2023). Functional Mapping of the Brain for Brain–Computer Interfacing: A Review. Electronics, 12(3), 604. https://doi.org/10.3390/electronics12030604







