A Novel Framework for Classification of Different Alzheimer’s Disease Stages Using CNN Model
Abstract
1. Introduction
- Early-symptomatic aliment;
- Prevenient aliments, i.e., those with apparent early dementia AD;
- AD or common variants of AD [16].
2. Related Work
3. Problem Description and Solution Strategy
4. Methods and Materials
5. The Proposed Framework
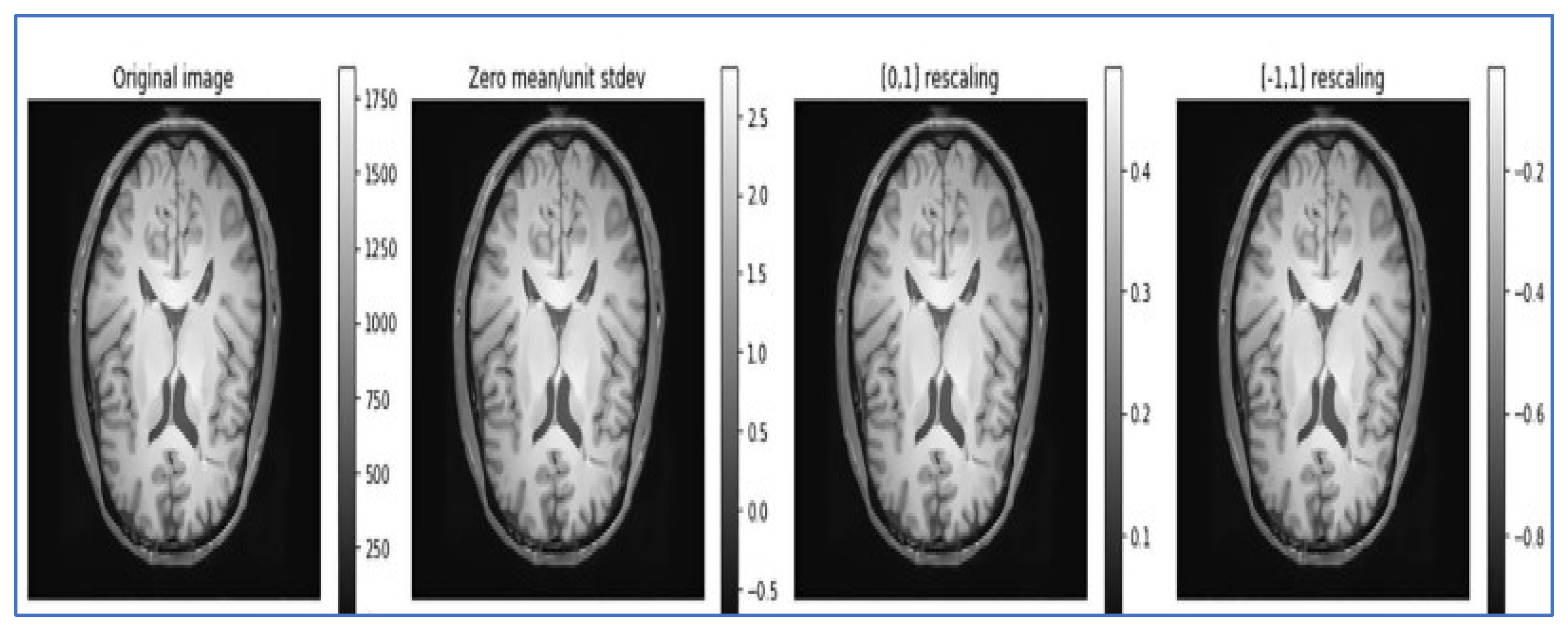
- Data normalization: Data normalization is beneficial for removing different redundancies from the datasets, such as varied contrasts and varied subject poses, to simplify subtle difference detection. It rescales the attributes with a mean value of 0 and a standard deviation of 1. Different types of normalization techniques, such as Z normalization, called standardization; min–max normalization; and unit vector normalization, are applied to the dataset. We applied unit vector normalization to our dataset.
- Unit vector normalization: It shrinks/stretches a vector and scales it to a unit length. We applied it to the whole dataset, and the transformed data are viewed as a cluster of vectors with distant trajectories on the d-dimensional unit sphere. The general formulae for unit vector normalization are , where = normalized vector, = non-Zero vector, and = length of U.
6. Proposed Classification Methods and Techniques
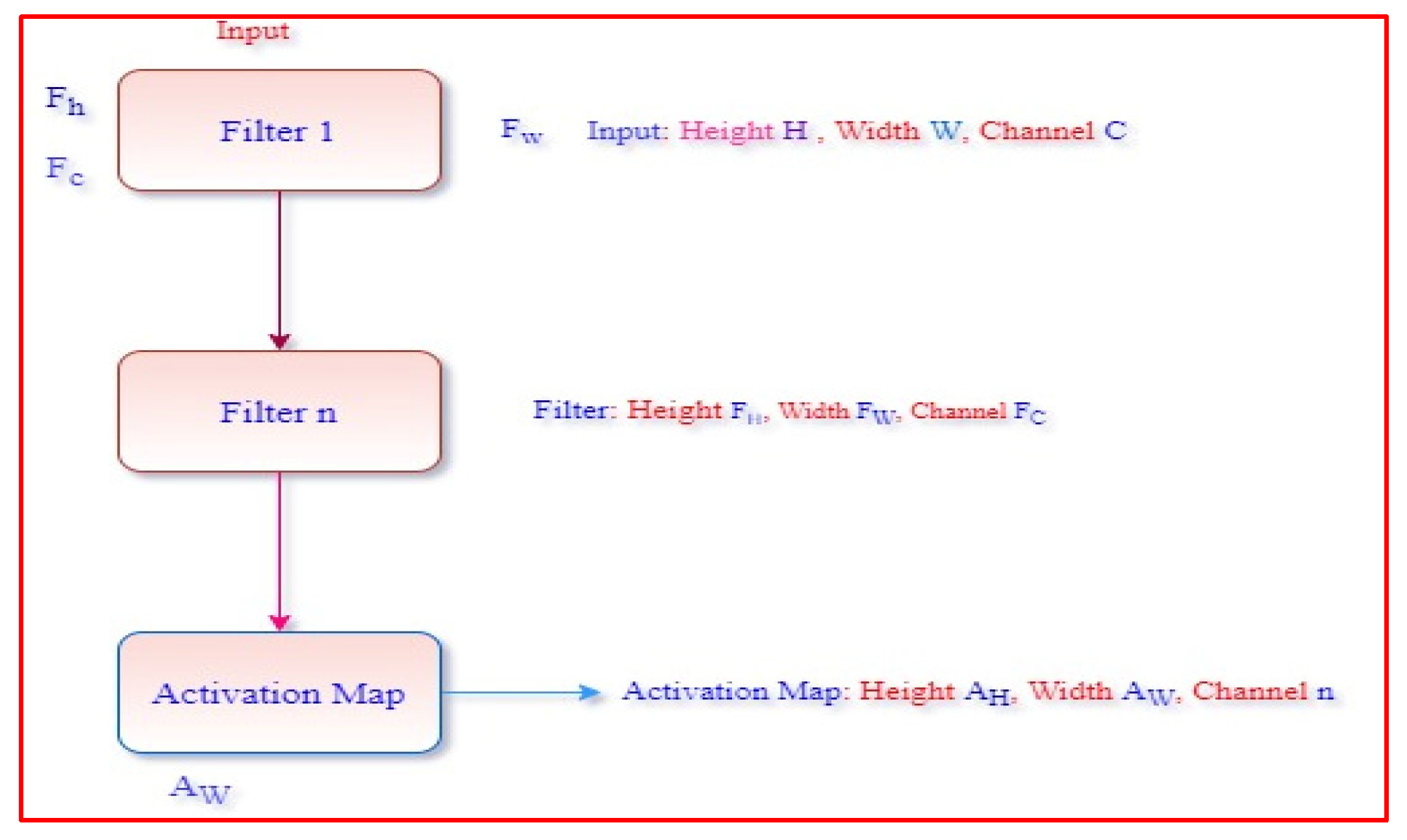
6.1. Convolution Layer
6.2. Polling Layer
7. Experimental Findings and Model Evaluation
Model Evaluation
8. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Prince, M.J.; Comas-Herrera, A.; Knapp, M.; Guerchet, M.M.; Karagiannidou, M. World Alzheimer Report 2016—Improving Healthcare for People Living with Dementia: Coverage, Quality and Costs Now and in the Future; Alzheimer’s Disease International (ADI): London, UK, 2016. [Google Scholar]
- Prince, M.; Wimo, A.; Guerchet, M.; Ali, G.; Wu, Y.; Prina, M. World Alzheimer Report 2015; Alzheimer’s Disease International(ADI): London, UK, 2015; pp. 1–92. Available online: https://www.alz.co.uk/research/WorldAlzheimerReport2015.pdf (accessed on 14 March 2022).
- Armstrong, R.A. The molecular biology of senile plaques and neurofibrillary tangles in Alzheimer’s disease. Folia Neuropathol. 2009, 47, 289–299. [Google Scholar] [PubMed]
- Bin Tufail, A.; Ullah, K.; Khan, R.A.; Shakir, M.; Khan, M.A.; Ullah, I.; Ma, Y.-K.; Ali, S. On Improved 3D-CNN-Based Binary and Multiclass Classification of Alzheimer’s Disease Using Neuroimaging Modalities and Data Augmentation Methods. J. Healthc. Eng. 2022, 2022, 1302170. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.; Ullah, T.; Ahmad, I.; Al-Sharabi, A.; Ullah, K.; Khan, R.A.; Rasheed, S.; Ullah, I.; Uddin, N.; Ali, S. A Novel Hybrid Deep Learning Model for Metastatic Cancer Detection. Comput. Intell. Neurosci. 2022, 2022, 8141530. [Google Scholar] [CrossRef] [PubMed]
- Bin Tufail, A.; Ullah, I.; Khan, W.U.; Asif, M.; Ahmad, I.; Ma, Y.-K.; Khan, R.; Kalimullah; Ali, S. Diagnosis of Diabetic Retinopathy through Retinal Fundus Images and 3D Convolutional Neural Networks with Limited Number of Samples. Wirel. Commun. Mob. Comput. 2021, 2021, 6013448. [Google Scholar] [CrossRef]
- Ahmad, I.; Ullah, I.; Khan, W.U.; Rehman, A.U.; Adrees, M.S.; Saleem, M.Q.; Cheikhrouhou, O.; Hamam, H.; Shafiq, M. Efficient algorithms for E-healthcare to solve multiobject fuse detection problem. J. Healthc. Eng. 2021, 2021, 9500304. [Google Scholar] [CrossRef]
- Porter, J.H.; Prus, A.J. The Discriminative Stimulus Properties of Drugs Used to Treat Depression and Anxiety. Brain Imag. Behav. Neurosci. 2012, 5, 289–320. [Google Scholar]
- Noble, W.; Europe PMC Funders Group. Advances in tau-based drug discovery. Expert. Opin. Drug. Discov. 2011, 6, 797–810. [Google Scholar] [CrossRef] [PubMed]
- Ferrera, P.; Arias, C. Differential effects of COX inhibitors against b -amyloid-induced neurotoxicity in human neuroblastoma cells. Neurochem. Int. 2005, 47, 589–596. [Google Scholar] [CrossRef]
- Gasparini, L.; Ongoing, E.; Wenk, G. Non-steroidal anti-inflammatory drugs (NSAIDs) in Alzheimer’s disease: Old and new mechanisms of action. J. Neurochem. 2004, 91, 521–536. [Google Scholar] [CrossRef]
- Reitz, C. Alzheimer’s disease and the amyloid cascade hypothesis: A critical review. Int. J. Alzheimer’s Dis. 2012, 2012, 369808. [Google Scholar] [CrossRef]
- Gustafson, D.R.; Morris, M.C.; Scarmeas, N.; Shah, R.C.; Sijben, J.; Yaffe, K.; Zhu, X. New Perspectives on Alzheimer’s Disease and Nutrition. J. Alzheimer’s Dis. 2015, 46, 1111–1127. [Google Scholar] [CrossRef] [PubMed]
- Shah, R.C. Medical foods for Alzheimer’s disease. Drugs Aging 2011, 28, 421–428. [Google Scholar] [CrossRef] [PubMed]
- Cummings, J. Alzheimer’s disease diagnostic criteria: Practical applications. Alzheimer’s Res. Ther. 2012, 4, 35. [Google Scholar] [CrossRef] [PubMed]
- Dubois, B.; Feldman, H.; Jacova, C.; Dekosky, S.; Barberger-Gateau, P.; Cummings, J.; Delacourte, A.; Galasko, D.; Gauthier, S.; Jicha, G.; et al. Research criteria for the diagnosis of Alzheimer’s disease: Revising the NINCDS-ADRDA criteria. Lancet Neurol. 2007, 6, 734–746. [Google Scholar] [CrossRef]
- Viola, K.L.; Sbarboro, J.; Sureka, R.; De, M.; Bicca, M.A.; Wang, J.; Vasavada, S.; Satpathy, S.; Wu, S.; Joshi, H.; et al. Towards non-invasive diagnostic imaging of early-stage Alzheimer’s disease. Nat. Nanotechnol. 2015, 10, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Grundman, M.; Petersen, R.; Ferris, S.; Thomas, R.; Aisen, P.; Bennett, D.; Foster, N.; Galasko, D.; Doody, R.; Kaye, J.; et al. Mild Cognitive Impairment Can Be Distinguished from Alzheimer Disease and Normal Aging for Clinical Trials. Arch. Neurol. 2004, 61, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Ito, H.; Shimada, H.; Shinotoh, H.; Takano, H.; Sasaki, T.; Nogami, T.; Suzuki, M.; Nagashima, T.; Takahata, K.; Seki, C.; et al. Quantitative analysis of amyloid deposition in Alzheimer’s disease using PET and the radiotracer 11 C-AZD2184. J. Nucl. Med. 2014, 55, 932–938. [Google Scholar] [CrossRef]
- Bron, E.E.; Smits, M.; van der Flier, W.M.; Vrenken, H.; Barkhof, F.; Scheltens, P.; Papma, J.M.; Steketee, R.M.; Orellana, C.M.; Meijboom, R.; et al. Standardized evaluation of algorithms for computer-aided diagnosis of dementia based on structural MRI: The CADDementia challenge. NeuroImage 2015, 111, 562–579. [Google Scholar] [CrossRef]
- Janousova, E.; Vounou, M.; Wolz, R.; Gray, K.R.; Rueckert, D.; Montana, G.; the Alzheimer’s Disease Neuroimaging Initiative. Biomarker discovery for sparse classification of brain images in Alzheimer’s disease. Ann. BMVA 2012, 2012, 1–11. [Google Scholar]
- Payan, A.; Montana, G. Predicting Alzheimer’s disease a neuroimaging study with 3D convolutional neural networks. In Proceedings of the ICPRAM 2015—4th International Conference on Pattern Recognition Applications and Methods, Lisbon, Portugal, 10–12 January 2015; Volume 2, pp. 355–362. [Google Scholar]
- Bengio, Y.; Courville, A.; Vincent, P. Representation learning: A review and new perspectives. IEEE Trans. Pattern Anal. Mach. Intell. 2013, 35, 1798–1828. [Google Scholar] [CrossRef]
- Ebrahimighahnavieh, A.; Luo, S.; Chiong, R. Deep learning to detect Alzheimer’s disease from neuroimaging: A systematic literature review. Comput. Methods Programs Biomed. 2020, 187, 105242. [Google Scholar] [CrossRef] [PubMed]
- Pan, D.; Huang, Y.; Zeng, A.; Jia, L.; Song, X. Early Diagnosis of Alzheimer’s Disease Based on Deep Learning and Was. In Human Brain and Artificial Intelligence; Zeng, A., Pan, D., Hao, T., Zhang, D., Shi, Y., Song, X., Eds.; Springer: Singapore, 2019; pp. 52–68. [Google Scholar]
- Zhang, F.; Li, Z.; Zhang, B.; Du, H.; Wang, B.; Zhang, X. Multimodal deep learning model for auxiliary diagnosis of Alzheimer’s disease. Neurocomputing 2019, 361, 185–195. Available online: http://www.sciencedirect.com/science/article/pii/S0169260719310946 (accessed on 6 January 2023). [CrossRef]
- Spasov, S.; Passamonti, L.; Duggento, A.; Liò, P.; Toschi, N. A parameter-efficient deep learning approach to predict conversion from mild cognitive impairment to Alzheimer’s disease. Neuroimage 2019, 189, 276–287. [Google Scholar] [CrossRef] [PubMed]
- Park, C.; Ha, J.; Park, S. Prediction of Alzheimer’s disease based on the deep neural network by integrating gene expression and DNA methylation dataset. Expert Syst. Appl. 2020, 140, 112873. [Google Scholar] [CrossRef]
- Sarraf, S.; Tofghi, G. Classification of Alzheimer’s disease structural MRI data by deep learning convolutional neural networks. arXiv 2016, arXiv:1607.06583. [Google Scholar]
- Hosseini-asl, E.; Kenton, R.; El-baz, A. Alzheimer’s Disease Diagnostics by Adaptation of 3d Convolutional Network. Electrical and Computer Engineering Department. University of Louisville: Louisville. In Proceedings of the 2016 IEEE International Conference on Image Processing (ICIP), Phoenix, AZ, USA, 25–28 September 2016; Volume 502. [Google Scholar]
- Gupta, A.; Ayhan, M.; Maida, A. Natural Image Bases to Represent Neuroimaging Data. In Proceedings of the 30th International Conference on International Conference on Machine Learning, Atlanta, GA, USA, 16–21 June 2013; pp. 987–994. [Google Scholar]
- Brosch, T.; Tam, R. Manifold learning of brain MRIs by deep learning. In Lecture Notes in Computer Science, Proceedings of the International Conference on Medical Image Computing and Computer-Assisted Intervention, Nagoya, Japan, 22–26 September 2013; Springer Nature Switzerland AG.: Cham, Switzerlands, 2013; pp. 633–640. [Google Scholar]
- Tufail, A.B.; Ma, Y.-K.; Zhang, Q.-N.; Khan, A.; Zhao, L.; Yang, Q.; Adeel, M.; Khan, R.; Ullah, I. 3D convolutional neural networks-based multiclass classification of Alzheimer’s and Parkinson’s diseases using PET and SPECT neuroimaging modalities. Brain Inform. 2021, 8, 23. [Google Scholar] [CrossRef]
- Bilal, A.; Shafiq, M.; Fang, F.; Waqar, M.; Ullah, I.; Ghadi, Y.Y.; Long, H.; Zeng, R. IGWO-IVNet3: DL-Based Automatic Diagnosis of Lung Nodules Using an Improved Gray Wolf Optimization and InceptionNet-V3. Sensors 2022, 22, 9603. [Google Scholar] [CrossRef]
- Mazhar, T.; Nasir, Q.; Haq, I.; Kamal, M.M.; Ullah, I.; Kim, T.; Mohamed, H.G.; Alwadai, N. A Novel Expert System for the Diagnosis and Treatment of Heart Disease. Electronics 2022, 11, 3989. [Google Scholar]
- Tufail, A.B.; Ullah, I.; Rehman, A.U.; Khan, R.A.; Khan, M.A.; Ma, Y.K.; Khokhar, N.H.; Sadiq, M.T.; Khan, R.; Shafiq, M.; et al. On Disharmony in Batch Normalization and Dropout Methods for Early Categorization of Alzheimer’s Disease. Sustainability 2022, 14, 14695. [Google Scholar] [CrossRef]
- Liu, F.; Shen, C. Learning deep convolutional features for MRI based Alzheimer’s disease classification. arXiv 2014, arXiv:1404.3366. [Google Scholar]
- Korolev, S.; Safullin, A.; Belyaev, M.; Dodonova, Y. Residual, and plain convolutional neural networks for 3d brain MRI classification. In Proceedings of the 2017 IEEE 14th International Symposium on Biomedical Imaging (ISBI 2017), Melbourne, VIC, Australia, 18–21 April 2017; pp. 835–838. [Google Scholar]
- Sarraf, S.; Tofghi, G. Classification of Alzheimer’s disease using fMRI data and deep learning convolutional neural networks. arXiv 2016, arXiv:1603.08631. [Google Scholar]
- Suk, H.I.; Lee, S.W.; Shen, D.; the Alzheimers Disease Neuroimaging Initiative. Hierarchical feature representation and multimodal fusion with deep learning for AD/MCI diagnosis. Neuroimage 2014, 101, 569–582. [Google Scholar] [CrossRef] [PubMed]
- Suk, H.I.; Shen, D. Deep learning-based feature representation for ad/MCI classification. Med Image Comput. Comput. Assist. Interv. 2013, 16, 583–590. [Google Scholar] [PubMed]
- Suk, H.I.; Lee, S.-W.; Shen, D.; the Alzheimer’s Disease Neuroimaging Initiative. Latent feature representation with stacked auto-encoder for AD/MCI diagnosis. Brain Struct Funct. 2015, 220, 841–859. [Google Scholar] [CrossRef]
- Suk, H.I.; Shen, D.; the Alzheimer’s Disease Neuroimaging Initiative. Deep Learning in the Diagnosis of Brain Disorders. In Recent Progress in Brain and Cognitive Engineering; Springer Nature Switzerland AG.: Chem, Switzerland, 2015; pp. 203–213. [Google Scholar]
- Wang, Y.; Yang, Y.; Guo, X.; Ye, C.; Gao, N.; Fang, Y.; Ma, H.T. A novel multimodal MRI analysis for Alzheimer’s disease based on convolutional neural network. In Proceedings of the 2018 40th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Honolulu, HI, USA, 18–21 July 2018; pp. 754–757. [Google Scholar]
- Song, T.-A.; Chowdhury, S.R.; Yang, F.; Jacobs, H.; El Fakhri, G.; Li, Q.; Johnson, K.; Dutta, J. Graph convolutional neural networks for Alzheimer’s disease. In Proceedings of the 2019 IEEE 16th International Symposium on Biomedical Imaging (ISBI 2019), Venice, Italy, 8–11 April 2019; pp. 414–417. [Google Scholar]
- Jain, R.; Jain, N.; Aggarwal, A.; Hemanth, D.J. ScienceDirect Convolutional neural network-based Alzheimer’s disease classification from magnetic resonance brain images. Cogn. Syst. Res. 2019, 57, 147–159. [Google Scholar] [CrossRef]
- Spasov, S.E.; Passamonti, L.; Duggento, A.; Lio, P.; Toschi, N. A Multi-modal Convolutional Neural Network Framework for the Prediction of Alzheimer’s Disease. In Proceedings of the 2018 40th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Honolulu, HI, USA, 18–21 July 2018; pp. 1271–1274. [Google Scholar] [CrossRef]
- Bin Tufail, A.; Anwar, N.; Ben Othman, M.T.; Ullah, I.; Khan, R.A.; Ma, Y.-K.; Adhikari, D.; Rehman, A.U.; Shafiq, M.; Hamam, H. Early-Stage Alzheimer’s Disease Categorization Using PET Neuroimaging Modality and Convolutional Neural Networks in the 2D and 3D Domains. Sensors 2022, 22, 4609. [Google Scholar] [CrossRef]
- Haq, I.; Mazhar, T.; Malik, M.A.; Kamal, M.M.; Ullah, I.; Kim, T.; Hamdi, M.; Hamam, H. Lung Nodules Localization and Report Analysis from Computerized Tomography (CT) Scan Using a Novel Machine Learning Approach. Appl. Sci. 2022, 12, 12614. [Google Scholar] [CrossRef]
- Bin Tufail, A.; Ullah, I.; Khan, R.; Ali, L.; Yousaf, A.; Rehman, A.U.; Alhakami, W.; Hamam, H.; Cheikhrouhou, O.; Ma, Y.-K. Recognition of Ziziphus lotus through Aerial Imaging and Deep Transfer Learning Approach. Mob. Inf. Syst. 2021, 2021, 4310321. [Google Scholar] [CrossRef]
- Khan, R.; Yang, Q.; Ullah, I.; Rehman, A.U.; Bin Tufail, A.; Noor, A.; Rehman, A.; Cengiz, K. 3D convolutional neural networks based automatic modulation classification in the presence of channel noise. IET Commun. 2021, 16, 497–509. [Google Scholar] [CrossRef]
- Bin Tufail, A.; Ma, Y.-K.; Kaabar, M.K.A.; Martínez, F.; Junejo, A.R.; Ullah, I.; Khan, R. Deep Learning in Cancer Diagnosis and Prognosis Prediction: A Minireview on Challenges, Recent Trends, and Future Directions. Comput. Math. Methods Med. 2021, 2021, 9025470. [Google Scholar] [CrossRef]
- Sahumbaiev, I.; Popov, A.; Ramirez, J.; Gorriz, J.M.; Ortiz, A. 3D-CNN HadNet classification of MRI for Alzheimer’s Disease diagnosis. In Proceedings of the 2018 IEEE Nuclear Science Symposium and Medical Imaging Conference Proceedings (NSS/MIC), Sydney, NSW, Australia, 10–17 November 2018; pp. 3–6. [Google Scholar]
- Ansarullah, S.I.; Saif, S.M.; Andrabi, S.A.B.; Kumhar, S.H.; Kirmani, M.M.; Kumar, P. An Intelligent and Reliable Hyperparameter Optimization Machine Learning Model for Early Heart Disease Assessment Using Imperative Risk Attributes. J. Healthc. Eng. 2022, 2022, 9882288. [Google Scholar] [CrossRef] [PubMed]
- Ansarullah, S.I.; Saif, S.M.; Kumar, P.; Kirmani, M.M. Significance of Visible Non-Invasive Risk Attributes for the Initial Prediction of Heart Disease Using Different Machine Learning Techniques. Comput. Intell. Neurosci. 2022, 2022, 9580896. [Google Scholar] [CrossRef] [PubMed]
- Ansarullah, S.I.; Kumar, P. A systematic literature review on cardiovascular disorder identification using knowledge mining and machine learning method. Int. J. Recent Technol. Eng. 2019, 7, 1009–1015. [Google Scholar]
- Saif, S.M.; Ansarullah, S.I.; Ben Othman, M.T.; Alshmrany, S.; Shafiq, M.; Hamam, H. Impact of ICT in Modernizing the Global Education Industry to Yield Better Academic Outreach. Sustainability 2022, 14, 6884. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Z.; Yin, L.; Zhu, Z.; Qi, G.; Liu, Y. X-Net: A dual encoding–Decoding method in medical image segmentation. Vis. Comput. 2021, 1–11. [Google Scholar] [CrossRef]
- Xu, Y.; He, X.; Xu, G.; Qi, G.; Yu, K.; Yin, L.; Yang, P.; Yin, Y.; Chen, H. A medical image segmentation method based on multi-dimensional statistical features. Front. Neurosci. 2022, 16, 1009581. [Google Scholar] [CrossRef]
- Sharma, A.; Singh, P.; Dar, G. Artificial Intelligence and Machine Learning for Healthcare Solutions. In Data Analytics in Bioinformatics: A Machine Learning Perspective; Scrivener Publishing LLC: Beverly, MA, USA, 2021; pp. 281–291. [Google Scholar]
- Mohiuddin, G.; Sharma, A.; Singh, P. Deep Learning Models for Detection and Diagnosis of Alzheimer’s Disease. In Machine Learning and Data Analytics for Predicting, Managing, and Monitoring Disease; IGI Global: Hershey, PA, USA, 2021; pp. 140–149. [Google Scholar]
- Zhu, Z.; He, X.; Qi, G.; Li, Y.; Cong, B.; Liu, Y. Brain tumor segmentation based on the fusion of deep semantics and edge information in multimodal MRI. Inf. Fusion 2023, 91, 376–387. [Google Scholar] [CrossRef]




| Class | Training Data | Validation Data | Testing Data | Total |
|---|---|---|---|---|
| CN | 400 | 90 | 90 | 580 |
| MCI | 400 | 90 | 90 | 580 |
| EMCI | 400 | 90 | 90 | 580 |
| LMCI | 400 | 90 | 90 | 580 |
| AD | 400 | 90 | 90 | 580 |
| TOTAL | 2000 | 450 | 450 | 2900 |
| Layer | Output Shape | Param |
|---|---|---|
| input1 (Input Layer) | (None, 224, 224, 3) | 0 |
| convu1 (Conv2D) | (None, 112, 112, 32) | 864 |
| convolution1 (Batch Normalization) | (None, 112, 112, 32) | 128 |
| convolution1 (ReLU) | (None, 112, 112, 32) | 0 |
| convolution1_dw (DepthwiseConv2D) | (None, 112, 112, 32) | 288 |
| convolution1_ bn (Batch Normalization) | (None, 112, 112, 32) | 128 |
| convolution1_dw_relu (ReLU) | (None, 112, 112, 32) | 0 |
| convolution1_pw (Conv2D) | (None, 112, 112, 64) | 2048 |
| convolution1_pw_1_bn (Batch Normalization) | (None, 112, 112, 64) | 256 |
| convolution1_pw_relu (ReLU) | (None, 112, 112, 64) | 0 |
| convolution2_pad (ZeroPadding2D) | (None, 113, 113, 64) | 0 |
| convolution2_dw (DepthwiseConv2D) | (None, 56, 56, 64) | 576 |
| convolution2_dw_bn (Batch Normalization) | (None, 56, 56, 64) | 256 |
| convolution2_dw_relu (ReLU) | (None, 56, 56, 64) | 0 |
| convolution2_pw (Conv2D) | (None, 56, 56, 128) | 8192 |
| convolution2_pw_bn (Batch Normalization) | (None, 56, 56, 128) | 512 |
| convolution2_pw_relu (ReLU) | (None, 56, 56, 128) | 0 |
| convolution3_dw (DepthwiseConv2D) | (None, 56, 56, 128) | 1152 |
| flatten (Flatten) | (None, 50176) | 0 |
| dense (Dense) | (None, 512) | 25,690,624 |
| batch_normalization_1 (normalization) | (Batch (None, 512)) | 2048 |
| dense1 (Dense) | (None, 512) | 262,656 |
| dropout (Dropout) | (None, 512) | 0 |
| dense2 (Dense) | (None, 5) | 2565 |
| Total params: 29,190,853 Trainable params: 25,958,917 Non-trainable params: 3,231,936 |
| Metrics | Precision | Recall | F1-Score | Support |
|---|---|---|---|---|
| Final AD JPEG | 0.98 | 0.98 | 0.98 | 90 |
| Final CN JPEG | 0.95 | 0.90 | 0.93 | 90 |
| Final EMCI JPEG | 0.96 | 0.96 | 0.96 | 90 |
| Final LMCI JPEG | 1.00 | 1.00 | 1.00 | 90 |
| Final MCI JPEG | 0.93 | 0.98 | 0.95 | 90 |
| Accuracy | 0.96 | 450 | ||
| Macro Avg. | 0.96 | 0.96 | 0.96 | 450 |
| Weighted Avg. | 0.96 | 0.96 | 0.96 | 450 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohi ud din dar, G.; Bhagat, A.; Ansarullah, S.I.; Othman, M.T.B.; Hamid, Y.; Alkahtani, H.K.; Ullah, I.; Hamam, H. A Novel Framework for Classification of Different Alzheimer’s Disease Stages Using CNN Model. Electronics 2023, 12, 469. https://doi.org/10.3390/electronics12020469
Mohi ud din dar G, Bhagat A, Ansarullah SI, Othman MTB, Hamid Y, Alkahtani HK, Ullah I, Hamam H. A Novel Framework for Classification of Different Alzheimer’s Disease Stages Using CNN Model. Electronics. 2023; 12(2):469. https://doi.org/10.3390/electronics12020469
Chicago/Turabian StyleMohi ud din dar, Gowhar, Avinash Bhagat, Syed Immamul Ansarullah, Mohamed Tahar Ben Othman, Yasir Hamid, Hend Khalid Alkahtani, Inam Ullah, and Habib Hamam. 2023. "A Novel Framework for Classification of Different Alzheimer’s Disease Stages Using CNN Model" Electronics 12, no. 2: 469. https://doi.org/10.3390/electronics12020469
APA StyleMohi ud din dar, G., Bhagat, A., Ansarullah, S. I., Othman, M. T. B., Hamid, Y., Alkahtani, H. K., Ullah, I., & Hamam, H. (2023). A Novel Framework for Classification of Different Alzheimer’s Disease Stages Using CNN Model. Electronics, 12(2), 469. https://doi.org/10.3390/electronics12020469