Low Leakage Current Metal–Insulator–Metal Device Based on a Beryllium Oxide Insulator Created by a Two-Step Spin-Coating Method as a Novel Type of Modified Pechini Synthesis
Abstract
1. Introduction
2. Materials and Methods
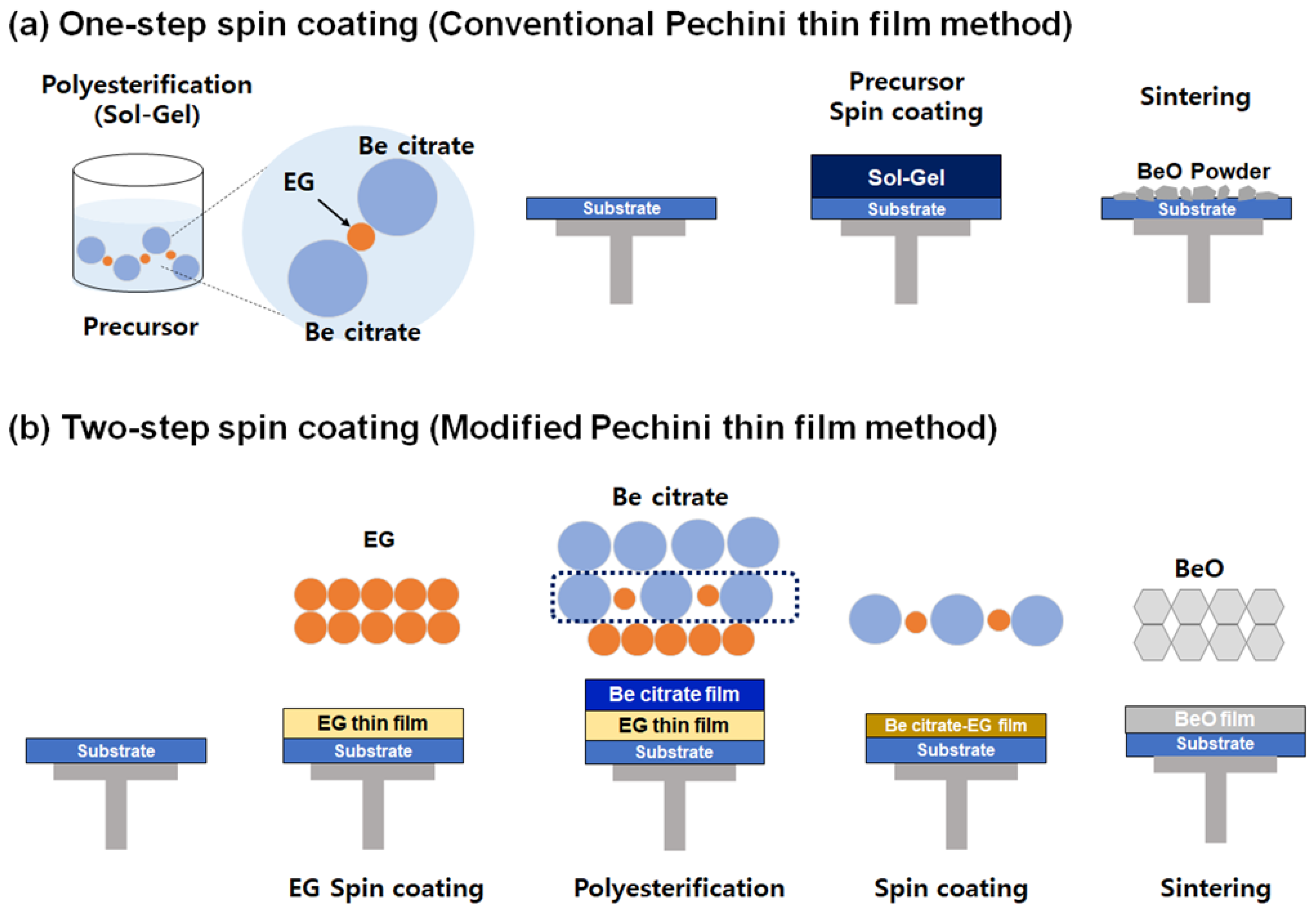
2.1. Modified Pechini Synthesis by Spin-Coating [19]
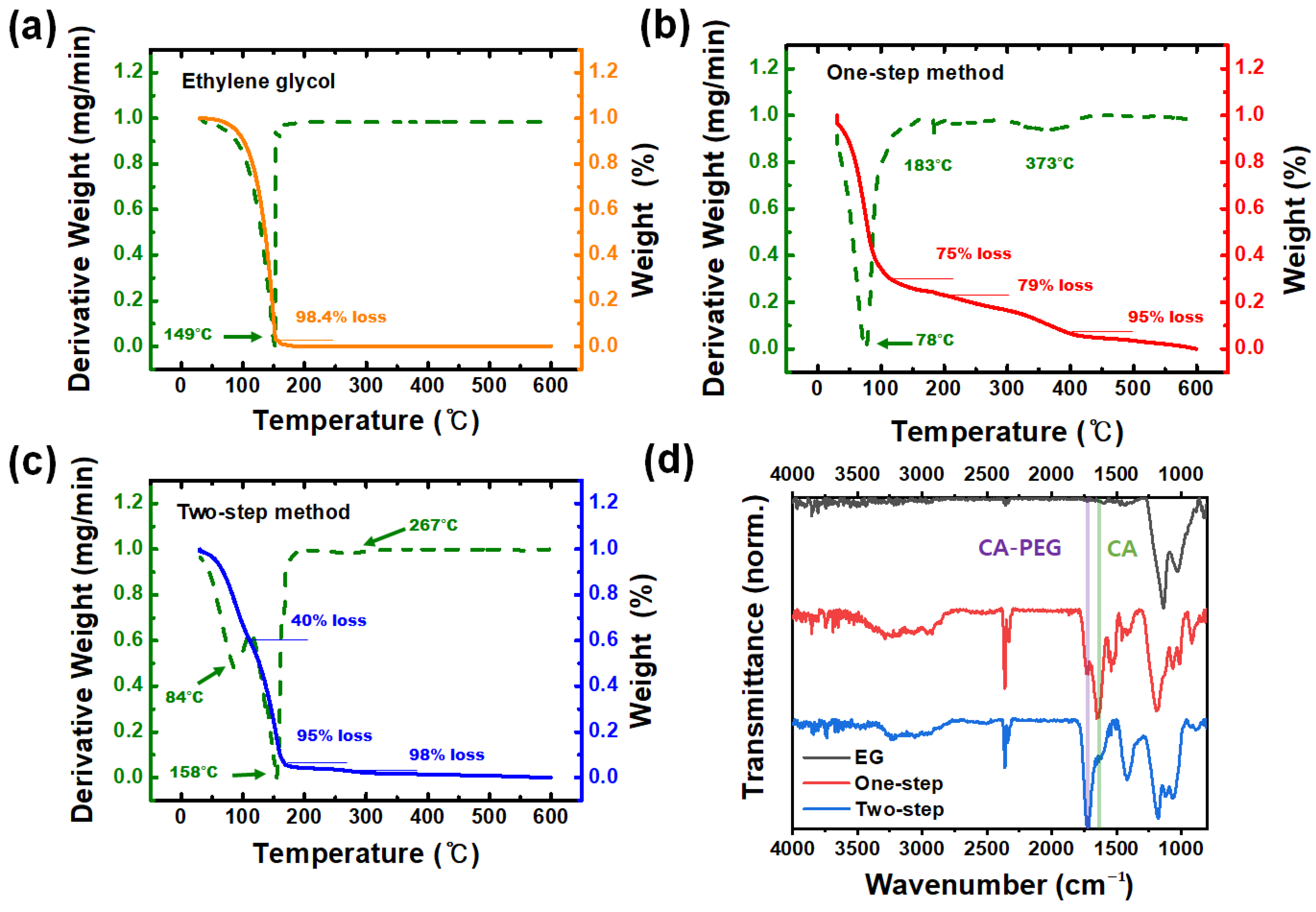
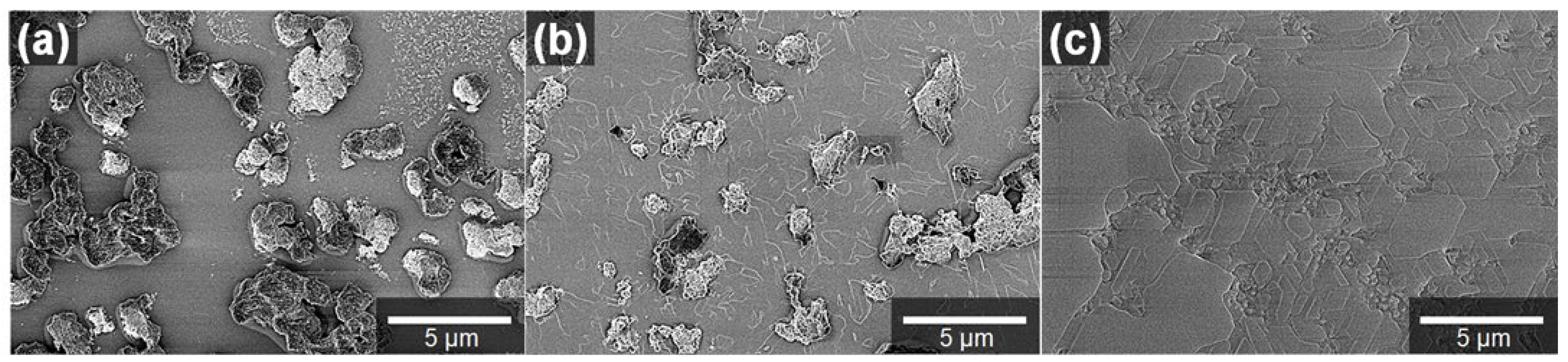
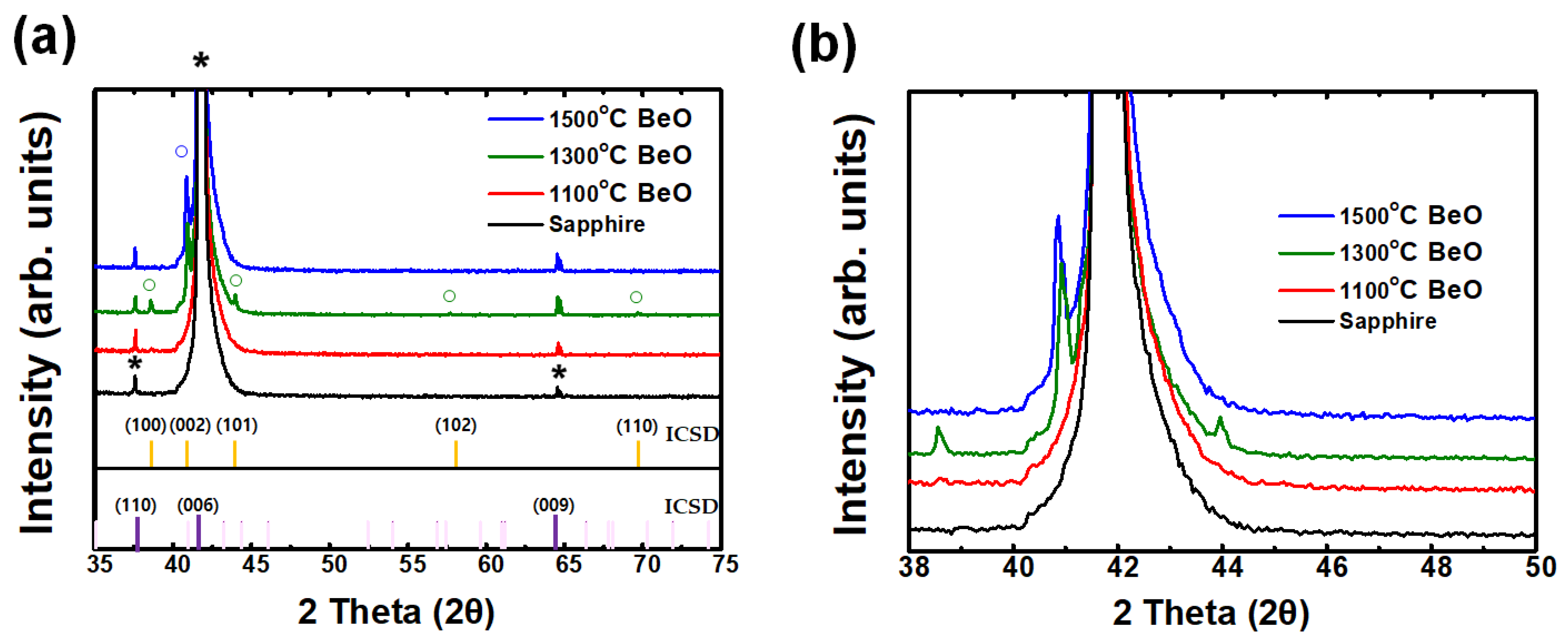
2.2. Characterization of BeO Thin Films
2.3. Fabrication and Leakage Current Measurement of Metal–Insulator–Metal Devices
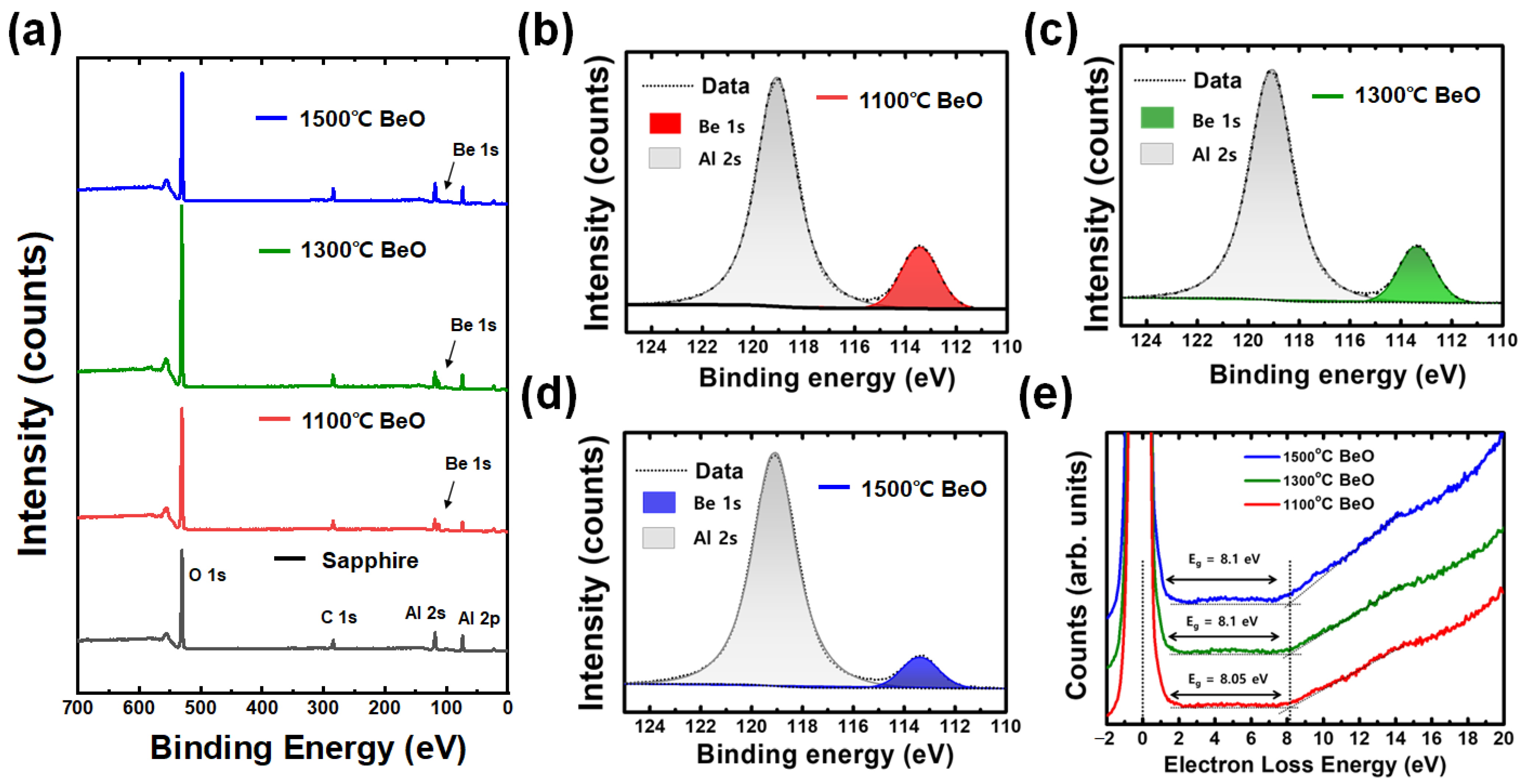
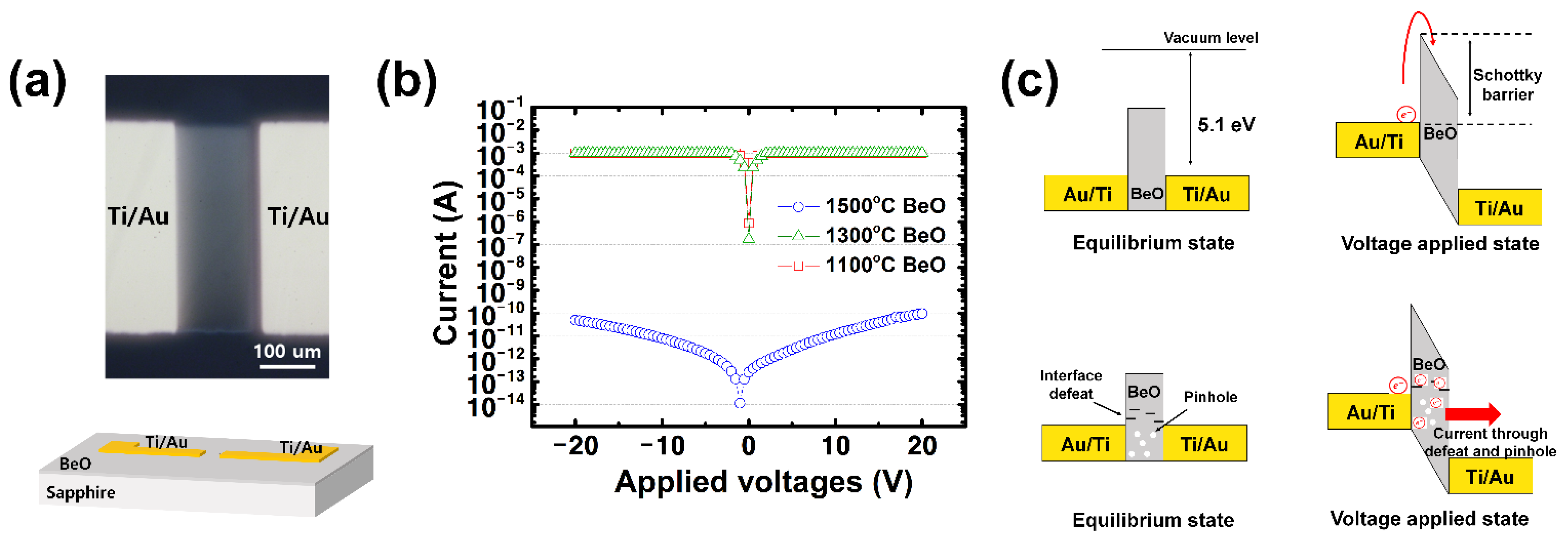
3. Results
4. Conclusions
5. Patents
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, H.; Holbrook, M.; Cheng, F.; Nam, H.; Liu, M.; Pan, C.-R.; West, D.; Zhang, S.; Chou, M.Y.; Shih, C.-K. Epitaxial growth of two-dimensional insulator monolayer honeycomb BeO. ACS Nano 2021, 15, 2497–2505. [Google Scholar] [CrossRef] [PubMed]
- Gaskins, J.T.; Hopkins, P.E.; Merrill, D.R.; Bauers, S.R.; Hadland, E.; Johnson, D.C.; Koh, D.; Yum, J.H.; Banerjee, S.; Nordell, B.J.; et al. Investigation and review of the thermal, mechanical, electrical, optical, and structural properties of atomic layer deposited high-k dielectrics: Beryllium oxide, aluminum oxide, hafnium oxide, and aluminum nitride. ECS J. Solid State Sci. Technol. 2017, 6, N189. [Google Scholar] [CrossRef]
- Li, W.; Mingo, N. Thermal conductivity of bulk and nanowire InAs, AlN, and BeO polymorphs from first principles. J. Appl. Phys. 2013, 114, 183505. [Google Scholar] [CrossRef]
- Mortazavi, B.; Shojaei, F.; Rabczuk, T.; Zhuang, X. High tensile strength and thermal conductivity in BeO monolayer: A first-principles study. FlatChem 2021, 28, 100257. [Google Scholar] [CrossRef]
- Slack, G.A.; Austerman, S.B. Thermal conductivity of BeO single crystals. J. Appl. Phys. 1971, 42, 4713–4717. [Google Scholar] [CrossRef]
- Malakkal, L.; Szpunar, B.; Siripurapu, R.K.; Zuniga, J.C.; Szpunar, J.A. Thermal conductivity of wurtzite and zinc blende cubic phases of BeO from ab initio calculations. Solid State Sci. 2017, 65, 79–87. [Google Scholar] [CrossRef]
- Yim, K.H.; Yong, Y.; Lee, J.H.; Lee, K.H.; Nahm, H.-H.; Yoo, J.H.; Lee, C.H.; Hwang, C.S.; Han, S.W. Novel high-κ dielectrics for next-generation electronic devices screened by automated ab initio calculations. NPG Asia Mater. 2015, 7, e190. [Google Scholar] [CrossRef]
- Roessler, D.M.; Walker, W.C.; Loh, E. Electronic spectrum of crystalline beryllium oxide. J. Phys. Chem. Solids 1969, 30, 157–167. [Google Scholar] [CrossRef]
- Hazen, R.M.; Finger, L.W. High-pressure and high-temperature crystal chemistry of beryllium oxide. J. Appl. Phys. 1986, 59, 3728–3733. [Google Scholar] [CrossRef]
- Schirmer, O.F. The structure of the paramagnetic lithium center in zinc oxide and beryllium oxide. J. Phys. Chem. Solids 1968, 29, 1407–1429. [Google Scholar] [CrossRef]
- Smith, D.K.; NewKirk, H.W.; Kahn, J.S. The crystal structure and polarity of beryllium oxide. J. Electrochem. Soc. 1964, 111, 78. [Google Scholar] [CrossRef]
- Felten, E.J. Sintering behavior of beryllium oxide. J. Am. Ceram. Soc. 1961, 44, 251–255. [Google Scholar] [CrossRef]
- Emsley, J. Nature’s building blocks: An A-Z guide to the elements; Oxford University Press: Oxford, UK, 2003; p. 57. [Google Scholar]
- Luckey, T.D.; Venugopal, B. Physiologic and Chemical Basis for Metal Toxicity; Springer: Berlin/Heidelberg, Germany, 1977; pp. 167–168. [Google Scholar]
- Pechini, M.P. Method of Preparing Lead and Alkaline Earth Titanates and Niobates and Coating Method Using the Same to form a Capacitor. U.S. Patent US3330697A, 11 July 1967. [Google Scholar]
- Wang, X.; Wang, R.; Peng, C.; Li, T.; Liu, B. Polyacrylamide gel method: Synthesis and property of BeO nanopowders. J. Sol-Gel Sci. Technol. 2011, 57, 115–127. [Google Scholar] [CrossRef]
- Dimesso, L. Pechini Processes: An Alternate Approach of the Sol–Gel Method, Preparation, Properties, and Applications. In Handbook of Sol-Gel Science and Technology; Springer: Cham, Switzerland, 2016. [Google Scholar] [CrossRef]
- Lu, X.; Wang, T.; Zhang, Y.; Shahzad, M.B.; Qi, Y. An enhancement in structural and superconducting properties of Bi2212 epitaxial thin films grown by the Pechini sol–gel method. J. Appl. Crystallogr. 2013, 46, 379–386. [Google Scholar] [CrossRef]
- Im, J.-H.; Kim, H.-S.; Park, N.-G. Morphology-photovoltaic property correlation in perovskite solar cells: One-step versus two-step deposition of CH3NH3PbI3. APL Mater. 2014, 2, 081510. [Google Scholar] [CrossRef]
- Mohassel, R.; Sobhani, A.; Salavati-Niasari, M.; Goudarzi, M. Pechini synthesis and characteristics of Gd2CoMnO6 nanostructures and its structural, optical and photocatalytic properties. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 204, 232–240. [Google Scholar] [CrossRef]
- Rudisill, S.G.; Hein, N.M.; Terzic, D.; Stein, A. Controlling microstructural evolution in pechini gels through the interplay between precursor complexation, step-growth polymerization, and template confinement. Chem. Mater. 2013, 25, 745–753. [Google Scholar] [CrossRef]
- Sarakinos, K. A review on morphological evolution of thin metal films on weakly-interacting substrates. Thin Solid Film. 2019, 688, 137312. [Google Scholar] [CrossRef]
- Teixeira, M.S.M.; Santos, L.M.N.B.F.; Costa, J.C.S. Nucleation, coalescence, and thin-film growth of triflate-based ionic liquids on ITO, Ag, and Au surfaces. Coll. Interfaces 2022, 6, 46. [Google Scholar] [CrossRef]
- Legg, B.A.; Voïtchovsky, K.; De Yireo, J.J. Hydroxide films on mica form charge-stabilized microphases that circumvent nucleation barriers. Sci. Adv. 2022, 8, eabn7087. [Google Scholar] [CrossRef]
- Guo, X.; Tan, Y.; Hu, Y.; Zafar, Z.; Liu, J.; Zou, J. High quality VO2 thin films synthesized from V2O5 powder for sensitive near-infrared detection. Sci. Rep. 2021, 11, 21749. [Google Scholar] [CrossRef] [PubMed]
- LaFollette, D.K.; Canova, K.L.; Zhang, Z.V.; Abelson, J.R. Coalescence of ultrathin films by atomic layer deposition or chemical vapor deposition: Models of the minimum thickness based on nucleation and growth rates. J. Vac. Sci. Technol. A Vac. Surf. Film. 2022, 40, 023403. [Google Scholar] [CrossRef]
- Jang, Y.S.; Jung, D.H.; Sultane, P.R.; Larsen, E.S.; Bielawski, C.W.; Oh, J.W. Low temperature growth of beryllium oxide thin films prepared via plasma enhanced atomic layer deposition. Appl. Surf. Sci. 2022, 572, 151405. [Google Scholar] [CrossRef]
- Kim, H.-S.; Park, N.-G. Importance of tailoring lattice strain in halide perovskite crystals. NPG Asia Mater. 2020, 12, 78. [Google Scholar] [CrossRef]
- Mallinson, C.F.; Castle, J.E. Beryllium and beryllium oxide by XPS. Surf. Sci. Spectra 2013, 20, 86–96. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Prakash, R.; Kumar, S.; Kumar, V.; Choudhary, R.J.; Phase, D.M. Optical and x-ray photoelectron spectroscopy studies of α-Al2O3. AIP Conf. Proc. 2016, 1731, 050097. [Google Scholar]
- Tago, T.; Kataoka, N.; Tanaka, H.; Kinoshita, K.; Kishida, S. XPS study from a clean surface of Al2O3 single crystals. Procedia Eng. 2017, 216, 175–181. [Google Scholar] [CrossRef]
- Jung, D.H.; Jang, Y.S.; Sultane, P.R.; Bielawski, C.W.; Oh, J.W. Energy band offsets of BeO dielectrics grown via atomic-layer deposition on β-Ga2O3 substrates. J. Alloy. Compd. 2022, 922, 166197. [Google Scholar] [CrossRef]
- Yum, J.H.; Bersuker, G.; Ferrer, D.A.; Akyol, T.; Lei, M.; Park, K.W.; Hudnall, T.W.; Downer, M.C.; Bielawski, C.W.; Yu, E.T.; et al. ALD beryllium oxide: Novel barrier layer for high performance gate stacks on Si and high mobility substrates. In Proceedings of the 2011 International Electron Devices Meeting, Washington, DC, USA, 5–7 December 2011; IEEE: New York, NY, USA, 2011. [Google Scholar]
- Koh, D.; Banerjee, S.K.; Brockman, J.; Kuhn, M.; King, S.W. X-ray photoelectron spectroscopy investigation of the valence band offset at beryllium oxide-diamond interfaces. Diam. Relat. Mater. 2020, 101, 107647. [Google Scholar] [CrossRef]
- Valedbagi, S.; Jalilian, J.; Elahi, S.M.; Majidi, S.; Fathalian, A.; Dalouji, V. Ab initio calculations of electronic and optical properties of BeO nanosheet. Electron. Mater. Lett. 2014, 10, 5–11. [Google Scholar] [CrossRef]
- Austerman, S.B.; Newkirk, J.B.; Smith, D.K.; Newkirk, H.W. Etching studies of beryllium oxide crystals. J. Mater. Sci. 1967, 2, 378–387. [Google Scholar] [CrossRef]
- Chia, C.; Schulaker, M.M.; Provine, J.; Jeffrey, S.S.; Howe, R.T. ALD HfO2 films for defining microelectrodes for electrochemical sensing and other applications. ACS Appl. Mater. Interfaces 2019, 11, 26082–26092. [Google Scholar] [CrossRef] [PubMed]
- Dongquoc, V.; Seo, D.-B.; Anh, C.V.; Lee, J.-H.; Park, J.-H.; Kim, E.-T. Controlled surface morphology and electrical properties of sputtered titanium nitride thin film for metal–insulator–metal structures. Appl. Sci. 2022, 12, 10415. [Google Scholar] [CrossRef]
- Sun, S.; Lawandi, S.R.; Sarangan, A.M.; Banerjee, P.P. Dark electron tunneling current in metal–insulator–metal structures: Modeling, fabrication, and measurement. Opt. Eng. 2022, 61, 027101. [Google Scholar] [CrossRef]
- Qaid, M.M.; AlSalhi, M.S. Leakage current and charge transport mechanism in Poly(phenylene oxdie)-conductor Schottly bilayers. Mater. Chem. Phys. 2021, 259, 124054. [Google Scholar] [CrossRef]
- Ma, J.; Matioli, E. 2 kV slanted tri-gate GaN-on-Si Schottky barrier diodes with ultra-low leakage current. Appl. Phys. Lett. 2018, 112, 052101. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual 165 author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to 166 people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeon, Y.P.; Hong, D.; Lee, S.-h.; Lee, E.J.; Cho, T.W.; Kim, D.Y.; Kim, C.Y.; Park, J.; Kim, Y.J.; Yoo, Y.J.; et al. Low Leakage Current Metal–Insulator–Metal Device Based on a Beryllium Oxide Insulator Created by a Two-Step Spin-Coating Method as a Novel Type of Modified Pechini Synthesis. Electronics 2023, 12, 47. https://doi.org/10.3390/electronics12010047
Jeon YP, Hong D, Lee S-h, Lee EJ, Cho TW, Kim DY, Kim CY, Park J, Kim YJ, Yoo YJ, et al. Low Leakage Current Metal–Insulator–Metal Device Based on a Beryllium Oxide Insulator Created by a Two-Step Spin-Coating Method as a Novel Type of Modified Pechini Synthesis. Electronics. 2023; 12(1):47. https://doi.org/10.3390/electronics12010047
Chicago/Turabian StyleJeon, Young Pyo, Dongpyo Hong, Sang-hwa Lee, Eun Jung Lee, Tae Woong Cho, Do Yeon Kim, Chae Yeon Kim, JuSang Park, Young Jun Kim, Young Joon Yoo, and et al. 2023. "Low Leakage Current Metal–Insulator–Metal Device Based on a Beryllium Oxide Insulator Created by a Two-Step Spin-Coating Method as a Novel Type of Modified Pechini Synthesis" Electronics 12, no. 1: 47. https://doi.org/10.3390/electronics12010047
APA StyleJeon, Y. P., Hong, D., Lee, S.-h., Lee, E. J., Cho, T. W., Kim, D. Y., Kim, C. Y., Park, J., Kim, Y. J., Yoo, Y. J., & Park, S. Y. (2023). Low Leakage Current Metal–Insulator–Metal Device Based on a Beryllium Oxide Insulator Created by a Two-Step Spin-Coating Method as a Novel Type of Modified Pechini Synthesis. Electronics, 12(1), 47. https://doi.org/10.3390/electronics12010047






