Sensitivity Characterization of Multi-Band THz Metamaterial Sensor for Possible Virus Detection
Abstract
:1. Introduction
2. Materials and Methods
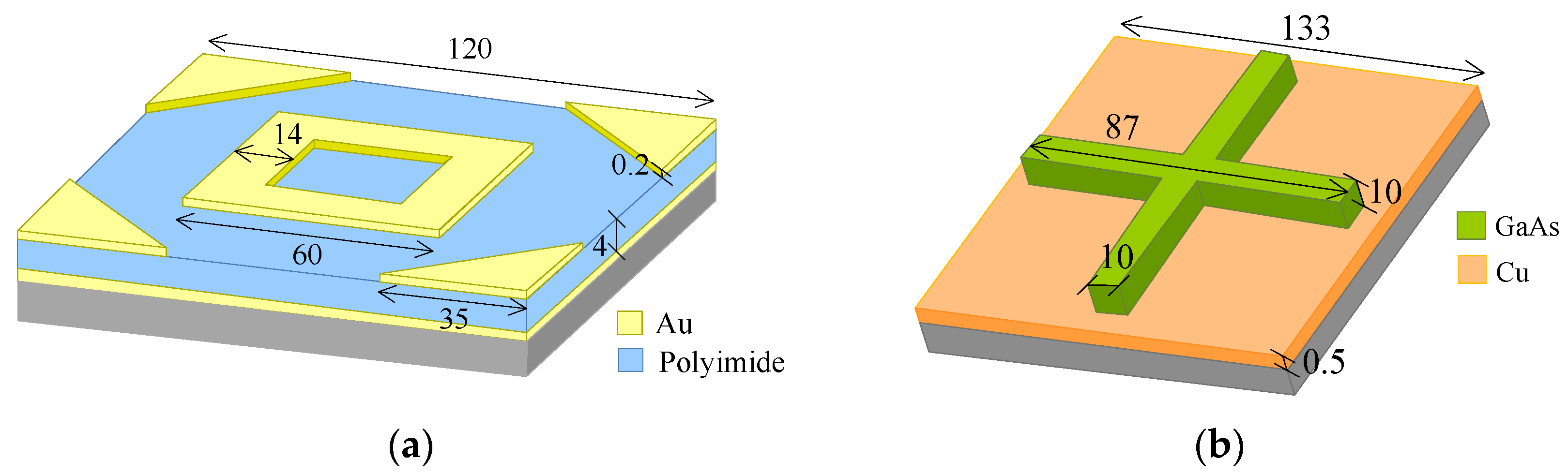
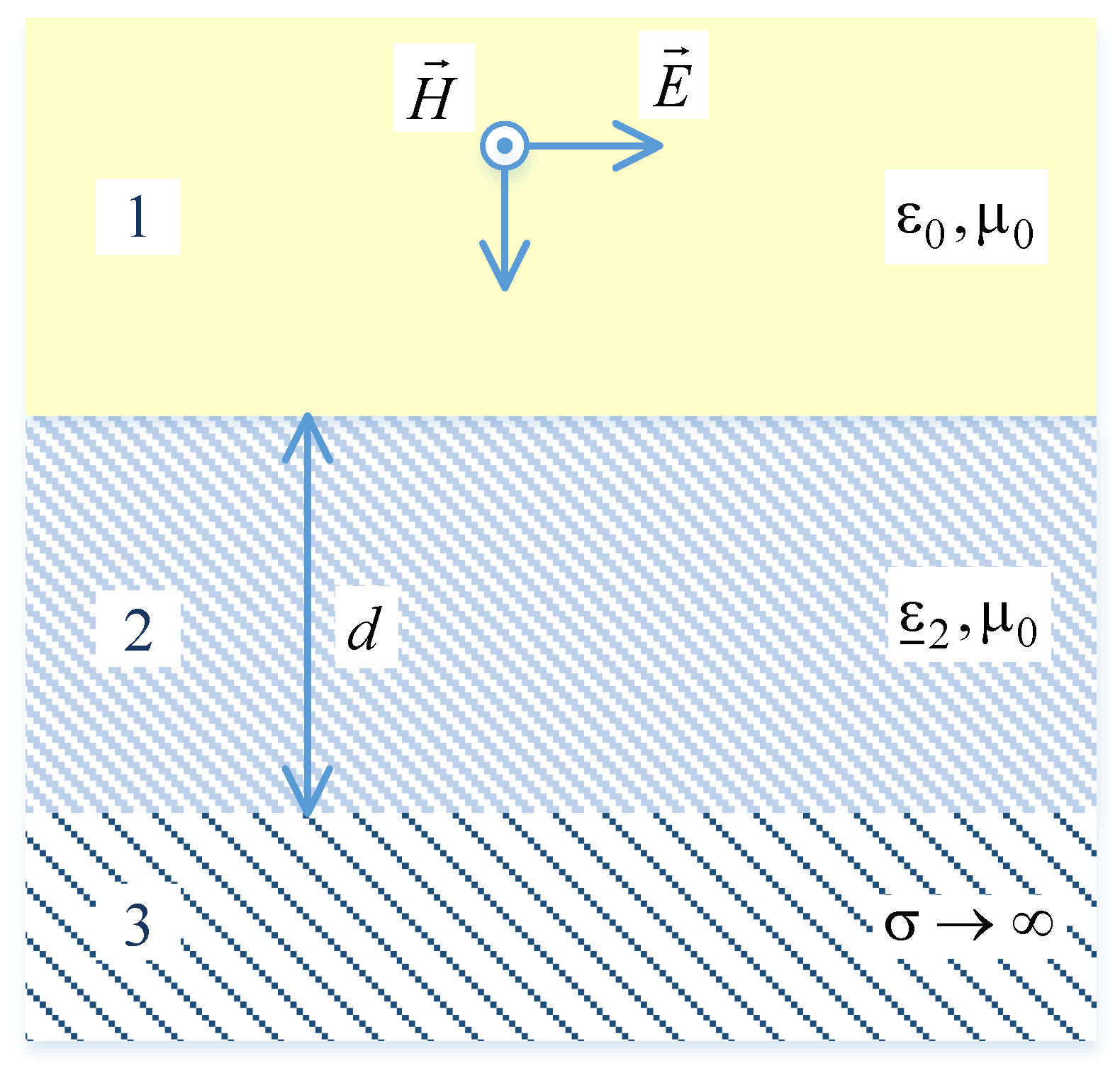
2.1. Unit Cells Design and Modelling
2.2. Virus Samples
3. Results and Discussion
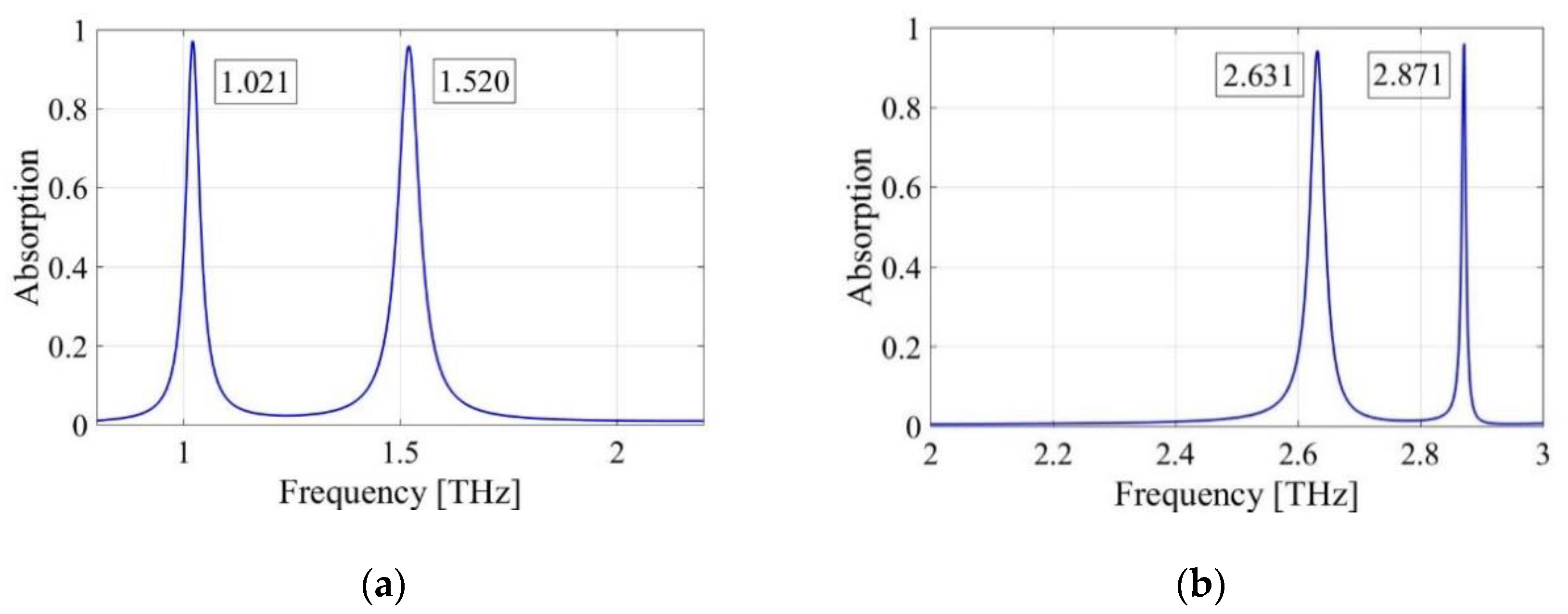
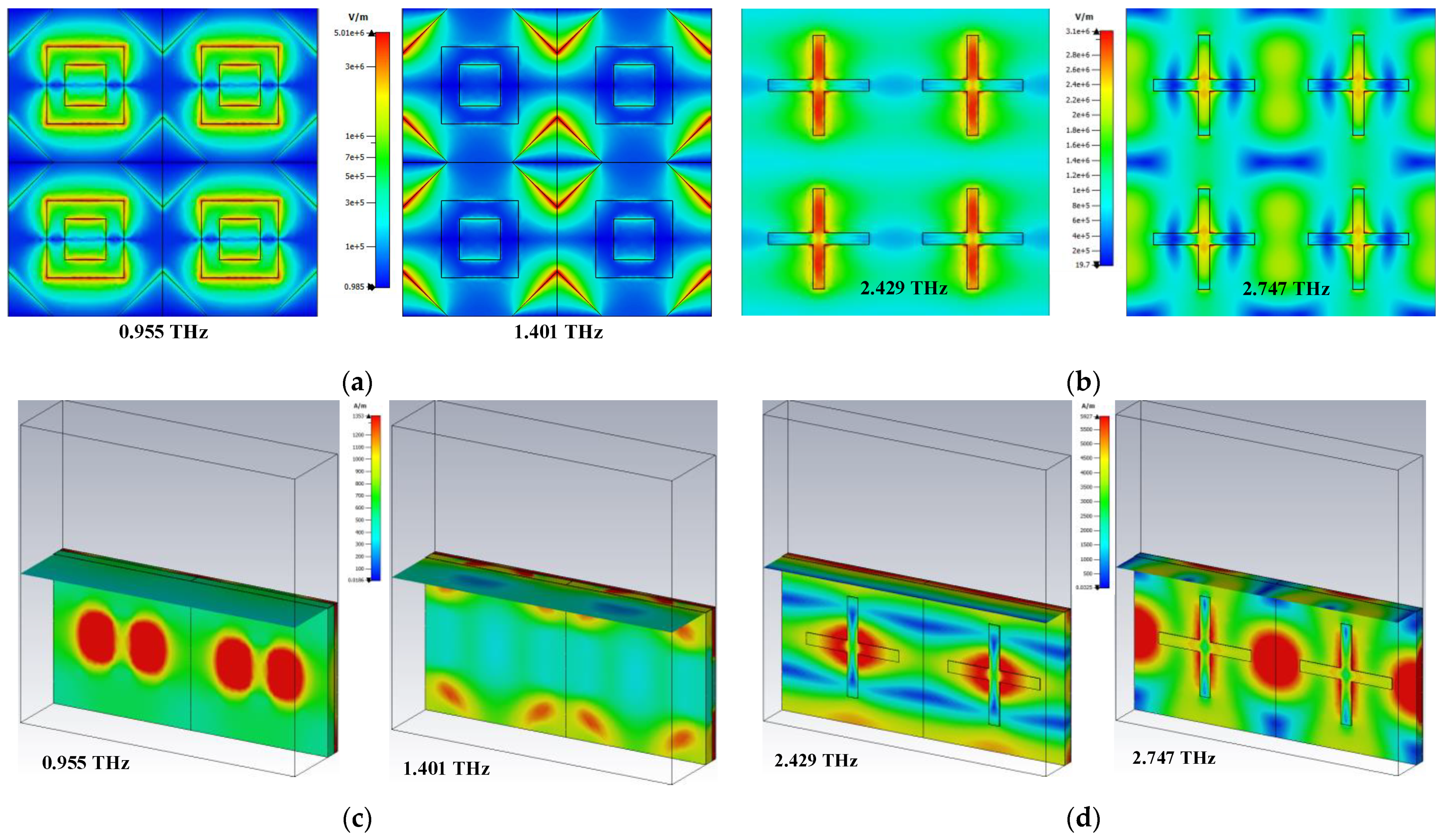
3.1. Absorption Responses of Sensor
3.2. Principles of Detection
3.3. Introducing Quantities of Interest for Sensitivity Analysis
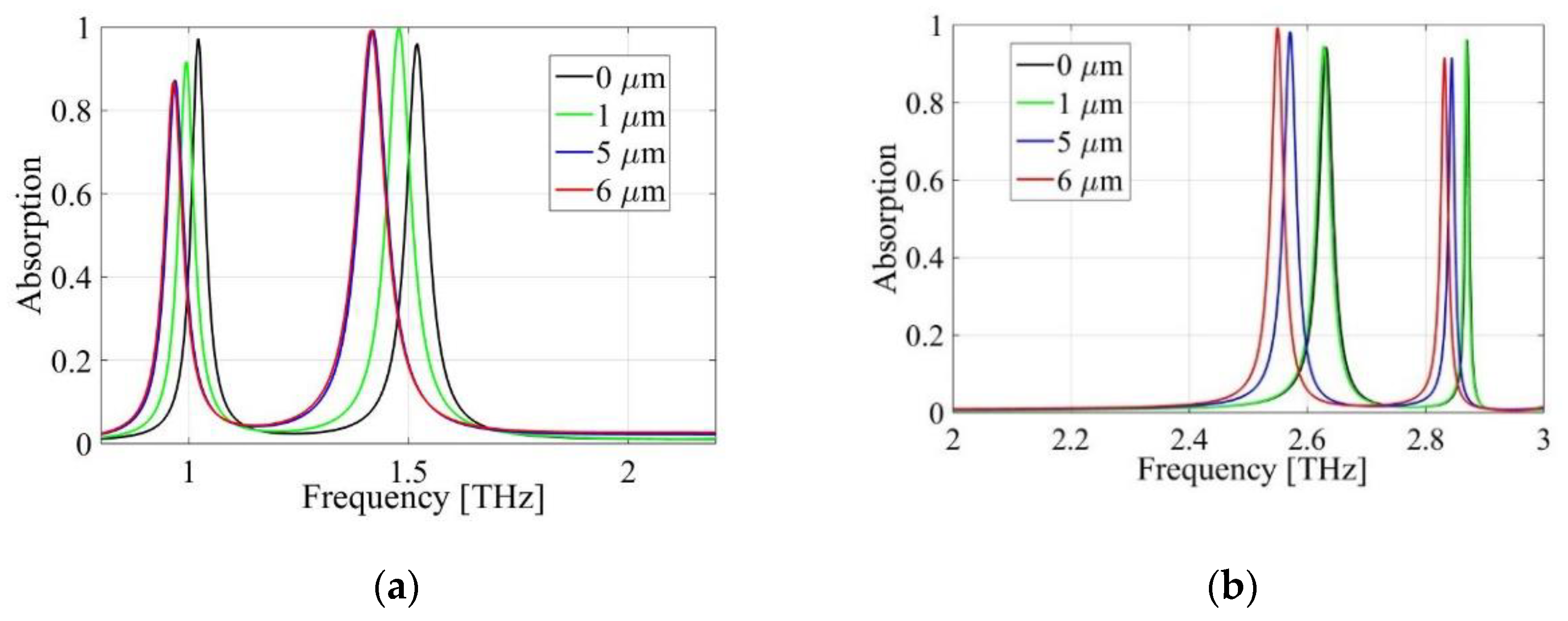
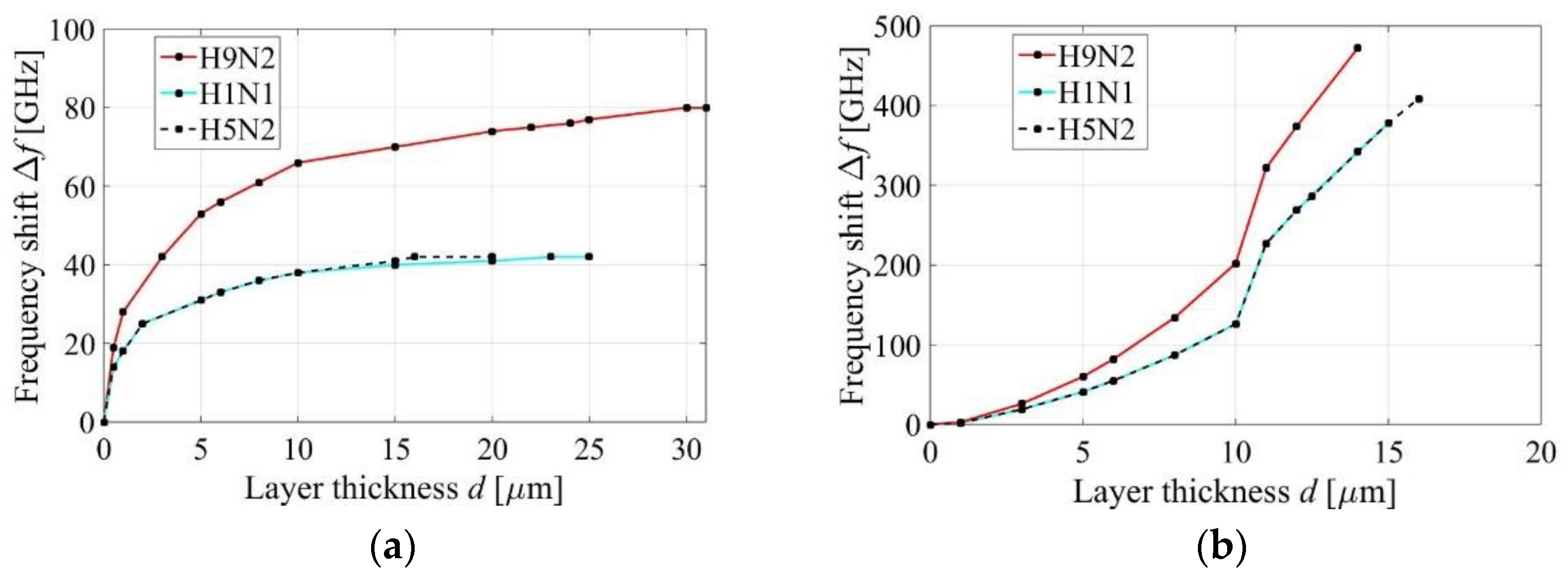
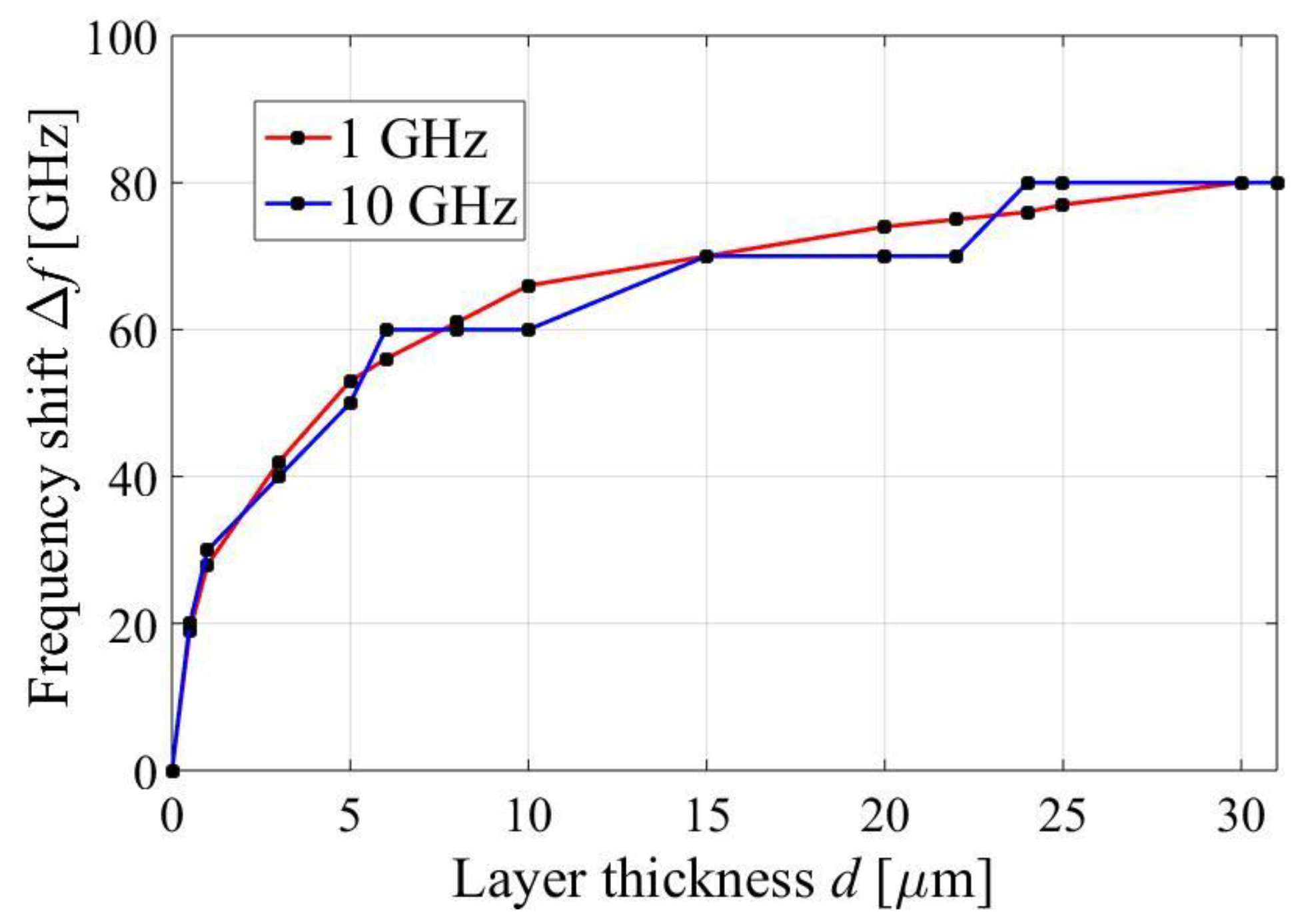
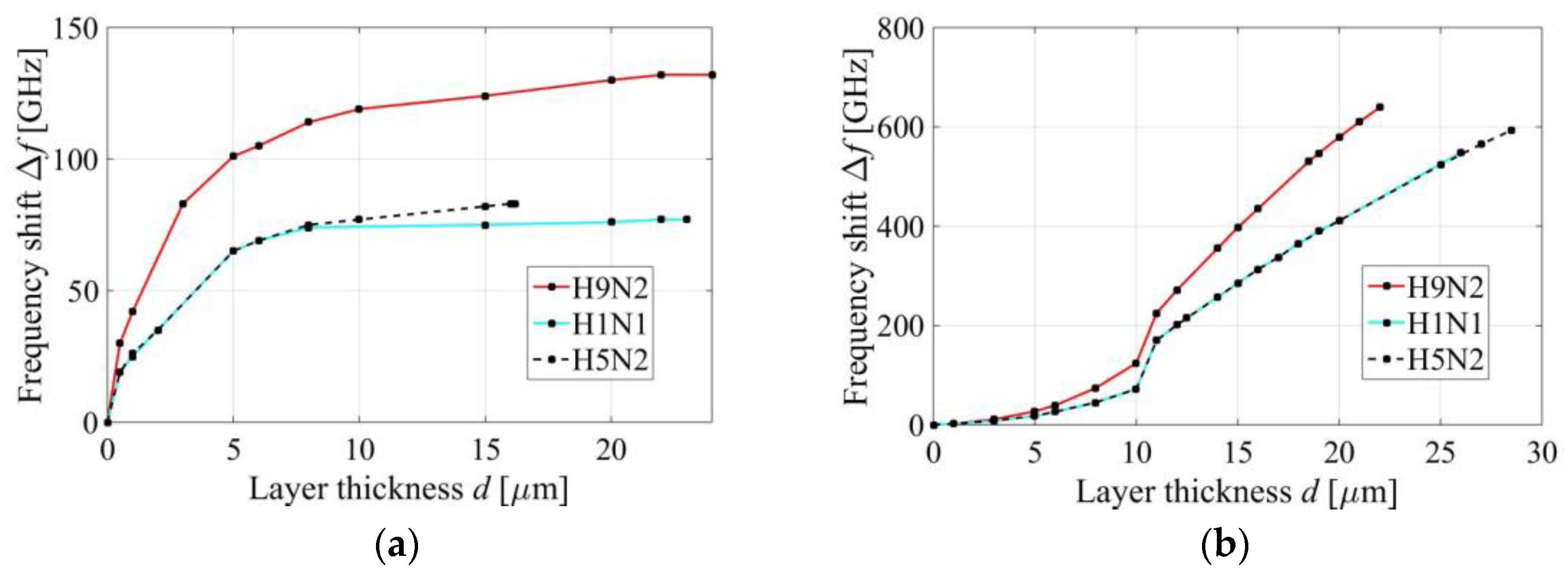
3.4. First Peak of the Absorption Response
3.5. Second Peak of the Absorption Response
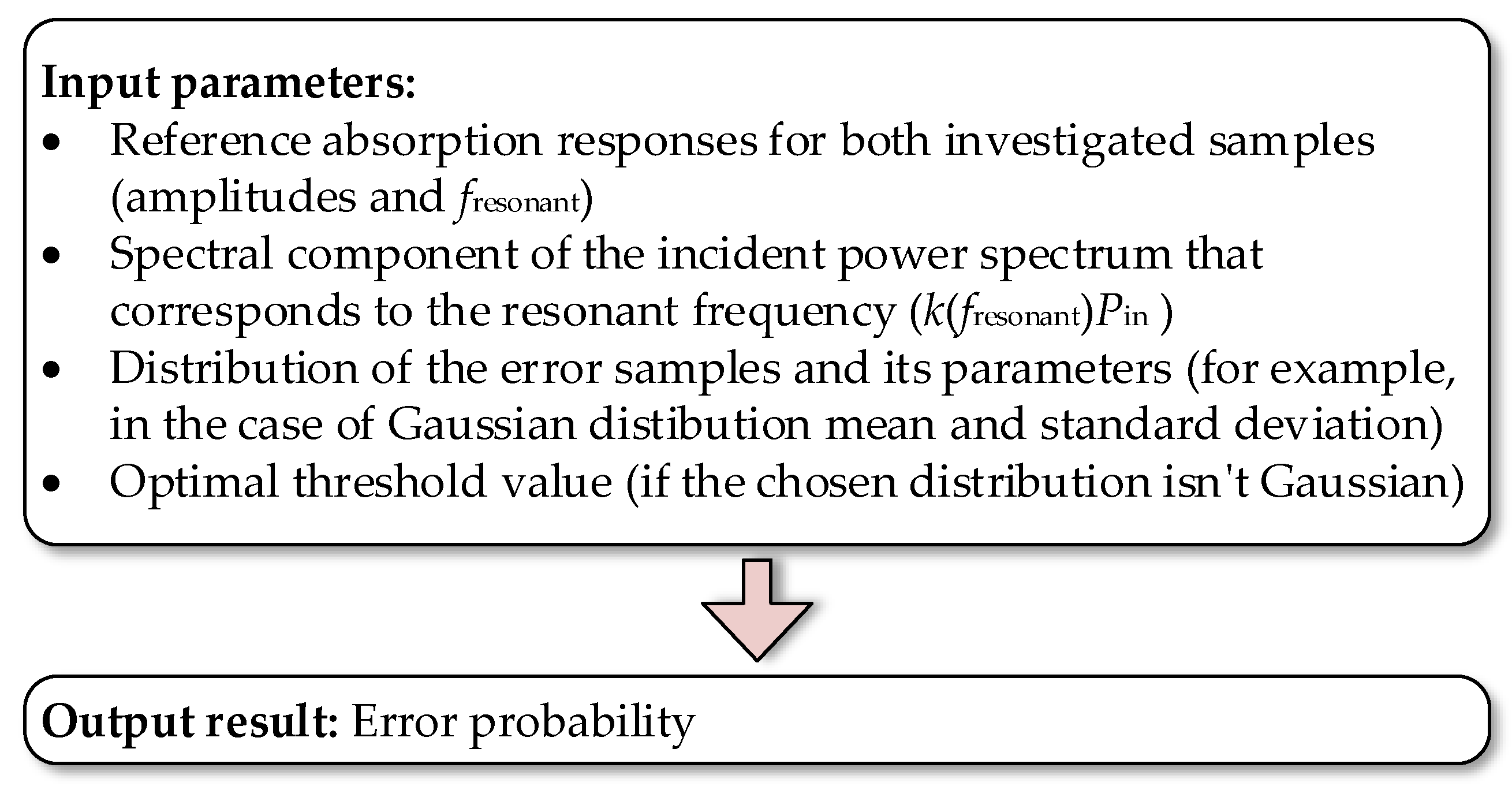
3.6. Qualitative Analysis of Sensor Detection
- (1)
- Decreasing the resolution frequency if possible,
- (2)
- Adding the peak amplitude as another reference parameter.
- Since the difference between reference absorption amplitudes for samples is rather small, if the incident power spectral component for is relatively low and simultaneously the standard deviation of the error sample distribution is relatively high, the estimated error probability is extremely high as it is approaching 50%. It should be noted that the error probability of 50% can be achieved without any measurement with a random guess of the sample type.
- The estimated error probability decreases significantly with a decrease in the standard deviation of the error sample distribution.
- If we increase the input power, we can decrease the error probability to some extent, but not significantly since the values of error samples have the predominant effect on the error probability.
- With the increase in virus layer thickness, the difference between the reference amplitudes increases. As a result, there is an improvement in the error probability.
3.7. Comparison of Sensors
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kutter, J.S.; Spronken, M.I.; Fraaij, P.L.; Fouchier, R.A.; Herfst, S. Transmission routes of respiratory viruses among humans. Curr. Opin. Virol. 2018, 28, 142–151. [Google Scholar] [CrossRef]
- Influenza-Vaccine-Preventable Diseases. Available online: https://www.who.int/immunization/monitoring_surveillance/burden/vpd/WHO_SurveillanceVaccinePreventable_09_Influenza_R2.pdf?ua=1 (accessed on 29 December 2021).
- Krokhine, S.; Torabi, H.; Doostmohammadi, A.; Rezai, P. Conventional and microfluidic methods for airborne virus isolation and detection. Colloid Surf. B 2021, 206, 111962. [Google Scholar] [CrossRef] [PubMed]
- Kovačević, A.; Potrebić, M.; Tošić, D. Sensitivity Analysis of Possible THz Virus Detection Using Quad-Band Metamaterial Sensor. In Proceedings of the IEEE 32nd International Conference on Microelectronics (MIEL), Nis, Serbia, 12–14 September 2021; pp. 107–110. [Google Scholar]
- Xu, W.; Xie, L.; Ying, Y. Mechanisms and applications of terahertz metamaterial sensing: A review. Nanoscale 2017, 37, 13864–13878. [Google Scholar] [CrossRef]
- Akter, N.; Hasan, M.M.; Pala, N. A Review of THz Technologies for Rapid Sensing and Detection of Viruses including SARS-CoV-2. Biosensors 2021, 11, 349. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, B.; Menon, A.; Jha, R.M. Active Terahertz Metamaterial for Biomedical Applications; Springer: Singapore, 2016. [Google Scholar]
- Kodama, C.H.; Coutu, R.A., Jr. THz Metamaterial Characterization Using THz-TDS. In Terahertz Spectroscopy—A Cutting Edge Technology; Uddin, J., Ed.; IntechOpen: London, UK, 2017; Chapter 6. [Google Scholar]
- Xie, Q.; Dong, G.; Wang, B.; Huang, W. Design of Quad-Band Terahertz Metamaterial Absorber Using a Perforated Rectangular Resonator for Sensing Applications. Nanoscale Res. Lett. 2018, 13, 137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niknam, S.; Yazdi, M.; Amlashi, S.B. Enhanced Ultra-Sensitive Metamaterial Resonance Sensor based on Double Corrugated Metal stripe for Terahertz Sensing. Sci. Rep. 2019, 9, 7516. [Google Scholar] [CrossRef] [Green Version]
- Ma, A.; Zhong, R.; Wu, Z.; Wang, Y.; Yang, L.; Liang, Z.; Fang, Z.; Liu, S. Ultrasensitive THz Sensor Based on Centrosymmetric F-Shaped Metamaterial Resonators. Front. Phys. 2020, 8, 584639. [Google Scholar] [CrossRef]
- Cheng, D.; He, X.; Huang, X.; Zhang, B.; Liu, G.; Shu, G.; Fang, C.; Wang, J.; Luo, Y. Terahertz Biosensing Metamaterial Absorber for Virus Detection Based on Spoof Surface Plasmon Polaritons. Int. J. RF Microw. Comput.-Aided Eng. 2018, 28, e21448. [Google Scholar] [CrossRef]
- Keshavarz, A.; Vafapour, Z. Sensing Avian Influenza Viruses Using Terahertz Metamaterial Reflector. IEEE Sens. J. 2019, 19, 5161–5166. [Google Scholar] [CrossRef]
- Hong, J.T.; Jun, S.W.; Cha, S.H.; Park, J.Y.; Lee, S.; Shin, G.A.; Ahn, Y.H. Enhanced Sensitivity in THz Plasmonic Sensors with Silver Nanowires. Sci. Rep. 2018, 8, 15536. [Google Scholar] [CrossRef] [Green Version]
- Ahmadivand, A.; Gerislioglu, B.; Tomitaka, A.; Manickam, P.; Kaushik, A.; Bhansali, S.; Nair, M.; Pala, N. Extreme Sensitive Metasensor for Targeted Biomarkers Identification Using Colloidal Nanoparticles-Integrated Plasmonic Unit Cells. Biomed. Opt. Express 2018, 9, 373–386. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.-K.; Kang, J.-H.; Kwon, J.; Lee, J.-S.; Lee, S.; Woo, D.H.; Kim, J.H.; Song, C.-S.; Park, Q.-H.; Seo, M. Nano Metamaterials for Ultrasensitive Terahertz Biosensing. Sci. Rep. 2017, 7, 8146. [Google Scholar] [CrossRef]
- Park, S.J.; Cha, S.H.; Shin, G.A.; Ahn, Y.H. Sensing Viruses Using Terahertz Nano-Gap Metamaterials. Biomed. Opt. Express 2017, 8, 3551–3558. [Google Scholar] [CrossRef] [Green Version]
- Wang, B.X.; Huang, W.Q.; Wang, L.L. Ultra-narrow terahertz perfect light absorber based on surface lattice resonance of a sandwich resonator for sensing applications. RSC Adv. 2017, 7, 42956–42963. [Google Scholar] [CrossRef] [Green Version]
- Yan, F.; Li, Q.; Tian, H.; Wang, Z.; Li, L. Ultrahigh Q-factor dual-band terahertz perfect absorber with dielectric grating slit waveguide for sensing. J. Phys. D Appl. Phys. 2020, 53, 235103. [Google Scholar] [CrossRef]
- Hu, D.; Meng, T.; Wang, H.; Ma, Y.; Zhu, Q. Ultra-narrow-band terahertz perfect metamaterial absorber for refractive index sensing application. Results Phys. 2020, 19, 103567. [Google Scholar] [CrossRef]
- Yin, W.; Shen, Z.; Li, S.; Zhang, L.; Chen, X. A Three-Dimensional Dual-Band Terahertz Perfect Absorber as a Highly Sensitive Sensor. Front. Phys. 2021, 9, 665280. [Google Scholar] [CrossRef]
- Stebunov, Y.V.; Yakubovsky, D.I.; Fedyanin, D.Y.; Arsenin, A.V.; Volkov, V.S. Superior sensitivity of copper-based plasmonic biosensors. Langmuir 2018, 34, 4681–4687. [Google Scholar] [CrossRef] [Green Version]
- Cong, L.; Tan, S.; Yahiaoui, R.; Yan, F.; Zhang, W.; Singh, R. Experimental demonstration of ultrasensitive sensing with terahertz metamaterial absorbers: A comparison with the metasurfaces. Appl. Phys. Lett. 2015, 106, 031107. [Google Scholar] [CrossRef]
- WIPL-D Pro 17, 3D Electromagnetic Solver, WIPL-D d.o.o., Belgrade, Serbia. 2021. Available online: http://www.wipl-d.com (accessed on 14 February 2022).
- Summary of Influenza Risk Assessment Tool (IRAT) Results. Available online: https://www.cdc.gov/flu/pandemic-resources/monitoring/irat-virus-summaries.htm (accessed on 29 December 2021).
- Amin, M.; Siddiqui, O.; Abutarboush, H.; Farhat, M.; Ramzan, R. A THz graphene metasurface for polarization selective virus sensing. Carbon 2021, 176, 580–591. [Google Scholar] [CrossRef]
- Wang, B.; Sadeqi, A.; Ma, R.; Wang, P.; Tsujita, W.; Sadamoto, K.; Sawa, Y.; Nejad, H.R.; Sonkusale, S.; Wang, C.; et al. Metamaterial Absorber for THz Polarimetric Sensing. In Proceedings of the SPIE, Terahertz, RF, Millimeter, and Submillimeter-Wave Technology and Applications XI, San Francisco, CA, USA, 29 January–1 February 2018; Volume 10531, pp. 1–7. [Google Scholar]
- Vajda, J.; Weber, D.; Brekel, D.; Hundt, B.; Müller, E. Size distribution analysis of influenza virus particles using size exclusion chromatography. J. Chromatogr. A 2016, 1465, 117–125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naftaly, M. Metrology Issues and Solutions in THz Time-Domain Spectroscopy: Noise, Errors, Calibration. IEEE Sens. J. 2013, 13, 8–17. [Google Scholar] [CrossRef]
- Hayt, W.H., Jr.; Buck, J.A. Engineering Electromagnetics; McGraw-Hill Education: New York, NY, USA, 2019. [Google Scholar]
- Duvillaret, L.; Garet, F.; Coutaz, J.-L. Influence of noise on the characterization of materials by terahertz time-domain spectroscopy. J. Opt. Soc. Am. B 2000, 17, 452–461. [Google Scholar] [CrossRef] [Green Version]
- Withayachumnankul, W.; Fischer, B.M.; Lin, H.; Abbott, D. Uncertainty in terahertz time-domain spectroscopy measurement. J. Opt. Soc. Am. B 2008, 25, 1059–1072. [Google Scholar] [CrossRef] [Green Version]
- Naftaly, M.; Miles, R.E. Terahertz Time-Domain Spectroscopy for Material Characterization. Proc. IEEE 2007, 95, 1658–1665. [Google Scholar] [CrossRef]
- Zaytsev, K.I.; Gavdush, A.A.; Chernomyrdin, N.V.; Yurchenko, S.O. Highly Accurate in Vivo Terahertz Spectroscopy of Healthy Skin: Variation of Refractive Index and Absorption Coefficient Along the Human Body. IEEE Trans. Terahertz Sci. Technol. 2015, 5, 817–827. [Google Scholar] [CrossRef]
- Hu, X.; Xu, G.; Wen, L.; Wang, H.; Zhao, Y.; Zhang, Y.; Cumming, D.R.S.; Chen, Q. Metamaterial absorber integrated microfluidic terahertz sensors. Laser Photonics Rev. 2016, 10, 962–969. [Google Scholar] [CrossRef] [Green Version]
- Lan, F.; Luo, F.; Mazumder, P.; Yang, Z.; Meng, L.; Bao, Z.; Zhou, J.; Zhang, Y.; Liang, S.; Shi, Z.; et al. Dual-band refractometric terahertz biosensing with intense wave-matter-overlap microfluidic channel. Biomed. Opt. Express 2019, 10, 3790. [Google Scholar] [CrossRef]
- Cong, L.; Singh, R. Sensing with THz metamaterial absorbers. arXiv 2014, arXiv:1408.3711. [Google Scholar]
- Wang, B.X.; Zhai, X.; Wang, G.Z.; Huang, W.Q.; Wang, L.L. A novel dual-band terahertz metamaterial absorber for a sensor application. J. Appl. Phys. 2015, 117, 014504. [Google Scholar] [CrossRef]

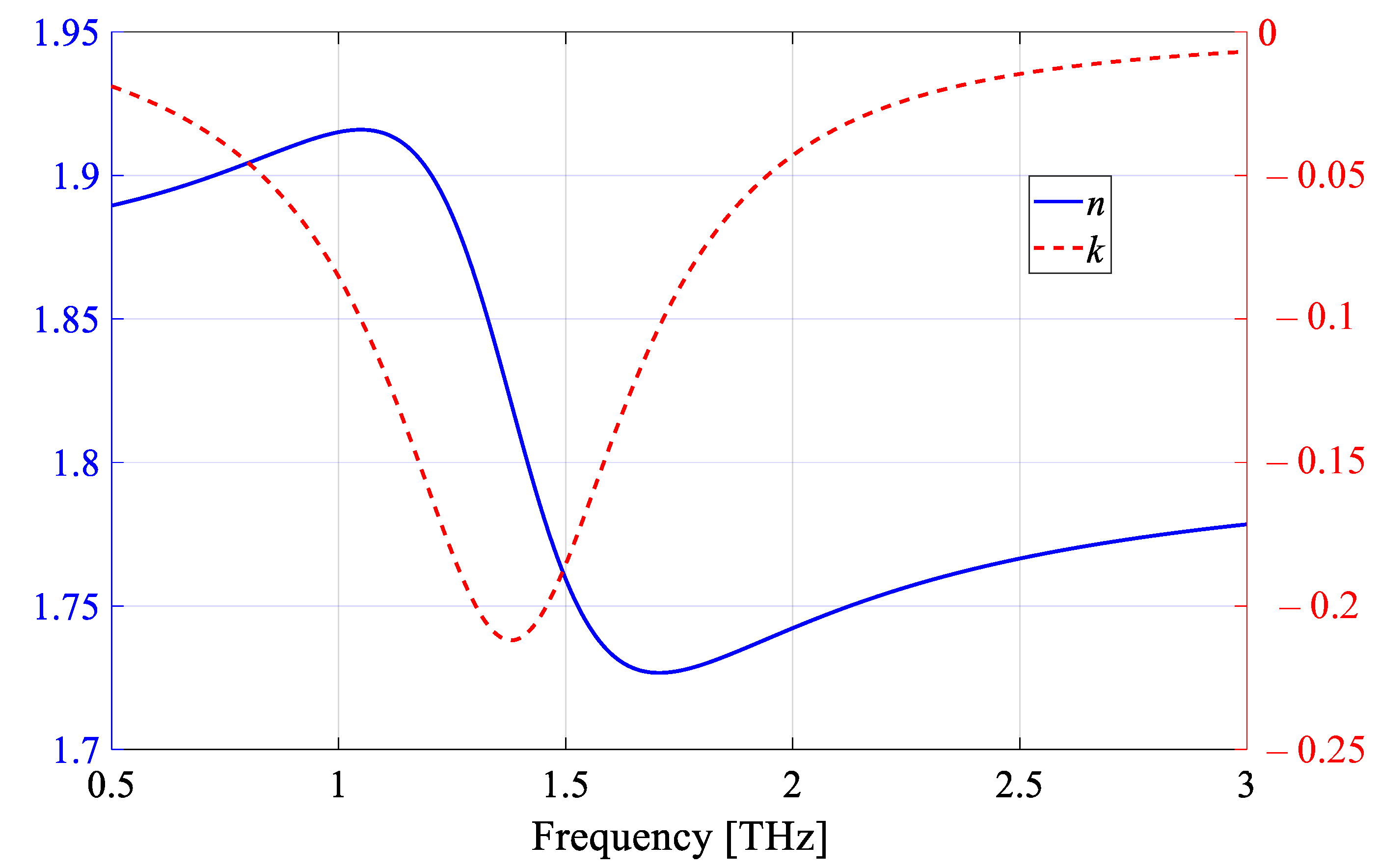


| Strain Name | Protein Concentration [mg/mL] | Refractive Index: A n + B j k | |
|---|---|---|---|
| A | B | ||
| H1N1 | 0.54 | 1 | 1.4 |
| H5N2 | 0.2 | 1 | 1 |
| H9N2 | 0.28 | 1.2 | 1.4 |
| Structure | Resonant Frequency [THz] | Q-Factor | S [GHz/RIU] | FOM [RIU−1] |
|---|---|---|---|---|
| SquarePMA | 1.021 | 26.2 | 100 | 2.56 |
| 1.520 | 25.8 | 168 | 2.85 | |
| CrossPMA | 2.631 | 94 | 1900 | 67.86 |
| 2.871 | 410 | 1050 | 150 |
| Virus Subtypes | H9N2 | H1N1 | H5N2 | ||||
|---|---|---|---|---|---|---|---|
| Resolution | [GHz] | 1 | 10 | 1 | 10 | 1 | 10 |
| dmin | [μm] | 0.1 | 0.25 | 0.1 | 0.5 | 0.1 | 0.5 |
| dmax | 28.33 | 22 | 20 | 8 | 15 | 8 | |
| dmax − dmin | 28.23 | 21.75 | 19.9 | 7.5 | 14.9 | 7.5 | |
| Sensitivity [GHz/nm] | General (×10−3) | 2.6744 | 2.7586 | 1.9347 | 2.6667 | 2.5839 | 2.6667 |
| d ≤ 1 μm | 0.0272 | 0.0267 | 0.0172 | 0.02 | 0.0172 | 0.02 | |
| d ≥ 15 μm (×10−4) | 6.7517 | 0 | 2 | 0 | 0 | 0 | |
| sssVirus Subtypes | H9N2 | H1N1 | H5N2 | |
|---|---|---|---|---|
| dmin | [μm] | 0.333 | 0.5 | 0.5 |
| dmax | 14 | 15 | 16 | |
| dmax − dmin | 13.667 | 14.5 | 15.5 | |
| Sensitivity [GHz/nm] | General (×10−3) | 34.46 | 26 | 26.32 |
| d ≤ 1 μm | 0.003 | 0.002 | 0.002 | |
| d ≥ 15 μm (×10−4) | 0 | 0 | 310 | |
| Virus Subtypes | H9N2 | H1N1 | H5N2 | |
|---|---|---|---|---|
| dmin | [μm] | 0.1 | 0.1 | 0.1 |
| dmax | 21 | 20 | 15 | |
| dmax − dmin | 20.9 | 19.9 | 14.9 | |
| Sensitivity [GHz/nm] | General (×10−3) | 5.9809 | 3.628 | 5.248 |
| d ≤ 1 μm | 0.04 | 0.0236 | 0.0247 | |
| d ≥ 15 μm (×10−4) | 11.667 | 2 | 0 | |
| Virus Subtypes | H9N2 | H1N1 | H5N2 | |
|---|---|---|---|---|
| dmin | [μm] | 0.5 | 0.5 | 0.5 |
| dmax | 22 | 26 | 28.5 | |
| dmax − dmin | 21.5 | 25.5 | 28 | |
| Sensitivity [GHz/nm] | General (×10−3) | 29.67 | 21.45 | 21.14 |
| d ≤ 1 μm | 0.002 | 0.002 | 0.002 | |
| d ≥ 15 μm (×10−4) | 345.7 | 239.1 | 228.1 | |
| Input | Output | |||||
|---|---|---|---|---|---|---|
| Samples | fresonant [THz] | εr (fresonant) | A (fresonant) | Pin [W] | SD | Pe |
| 1 µm: H1N1, H5N2 | 2.629 | H1N1: 2.176 + j 0.035 H5N2: 2.176 + j 0.025 | H1N1: 0.94535 H5N2: 0.94476 | 0.2 | 0.05 | 50% |
| 0.2 | 0.00001 | 0% | ||||
| 1.2 | 0.05 | 49.4% | ||||
| 1.2 | 0.0005 | 27.2% | ||||
| 6 µm: H1N1, H5N2 | 2.576 | H1N1: 2.173 + j 0.038 H5N2: 2.173 + j 0.027 | H1N1: 0.99192 H5N2: 0.98562 | 1.2 | 0.0005 | 0% |
| Structure | Resonant Frequency [THz] | Q-Factor | S [GHz/RIU] | FOM [RIU−1] |
|---|---|---|---|---|
| Graphene H-shaped reflector [13] | 1.7164 | 9.18 | 540 | 2.86 |
| Cross-shaped absorber [37] | 0.637 | 7.036 | 152 | 2.67 |
| Complementary cross-shaped absorber [37] | 0.792 | 7.189 | 163 | 2.05 |
| Metallic strip absorber [38] | 1.420 | 7.1 | 300 | 1.5 |
| 2.990 | 59.8 | 2200 | 34 | |
| SquarePMA | 1.021 | 26.2 | 100 | 2.56 |
| 1.520 | 25.8 | 168 | 2.85 | |
| CrossPMA | 2.631 | 94 | 1900 | 67.86 |
| 2.871 | 410.1 | 1050 | 150 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kovačević, A.; Potrebić, M.; Tošić, D. Sensitivity Characterization of Multi-Band THz Metamaterial Sensor for Possible Virus Detection. Electronics 2022, 11, 699. https://doi.org/10.3390/electronics11050699
Kovačević A, Potrebić M, Tošić D. Sensitivity Characterization of Multi-Band THz Metamaterial Sensor for Possible Virus Detection. Electronics. 2022; 11(5):699. https://doi.org/10.3390/electronics11050699
Chicago/Turabian StyleKovačević, Anja, Milka Potrebić, and Dejan Tošić. 2022. "Sensitivity Characterization of Multi-Band THz Metamaterial Sensor for Possible Virus Detection" Electronics 11, no. 5: 699. https://doi.org/10.3390/electronics11050699
APA StyleKovačević, A., Potrebić, M., & Tošić, D. (2022). Sensitivity Characterization of Multi-Band THz Metamaterial Sensor for Possible Virus Detection. Electronics, 11(5), 699. https://doi.org/10.3390/electronics11050699








