From Transparent Cranial Windows to Multifunctional Smart Cranial Platforms
Abstract
:1. Role of Cranial Window in Brain Research
2. Development of Cranial Windows
2.1. Natural Cranial Windows
2.2. Implanted Optical Cranial Windows
2.3. Multifunctional Cranial Windows
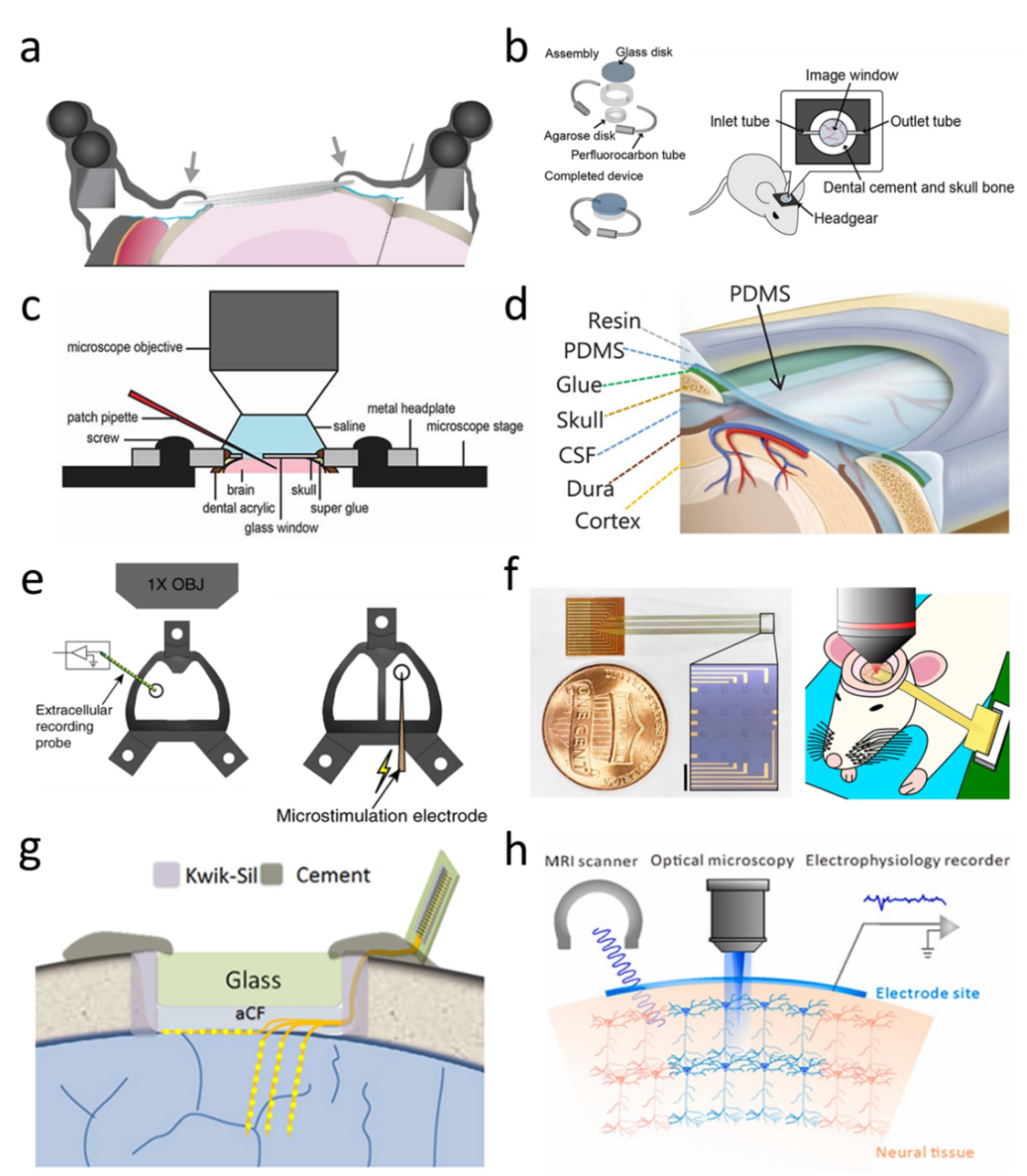
3. A Hybrid Ti-PDMS Structure for Large-Area Transparent Cranial Window
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Kennedy, D.; Norman, C. What don’t we know? Science 2005, 309, 75. [Google Scholar] [CrossRef] [PubMed]
- McGaugh, J.L. Memory—A century of consolidation. Science 2000, 287, 248–251. [Google Scholar] [CrossRef] [PubMed]
- Todd, J.J.; Marois, R. Capacity limit of visual short-term memory in human posterior parietal cortex. Nature 2004, 428, 751–754. [Google Scholar] [CrossRef] [PubMed]
- Park, H.J.; Friston, K. Structural and functional brain networks: From connections to cognition. Science 2013, 342, 1238411. [Google Scholar] [CrossRef]
- Mizusaki, B.E.P.; Stepanyants, A.; Chklovskii, D.B.; Sjöström, P.J. Neocortex: A lean mean memory storage machine. Nat. Neurosci. 2016, 19, 643–644. [Google Scholar] [CrossRef]
- Lodato, S.; Arlotta, P. Generating neuronal diversity in the mammalian cerebral cortex. Annu. Rev. Cell Dev. Biol. 2015, 31, 699–720. [Google Scholar] [CrossRef]
- Fields, R. Neuroscience: Map the other brain. Nature 2013, 501, 25–27. [Google Scholar] [CrossRef]
- Sunwoo, J.; Cornelius, N.R.; Doerschuk, P.C.; Schaffer, C.B. Estimating Brain Microvascular Blood Flows from Partial Two-Photon Microscopy Data by Computation with a Circuit Model. In Proceedings of the 2011 Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Boston, MA, USA, 30 August–3 September 2011; pp. 174–177. [Google Scholar]
- Shih, A.Y.; Driscoll, J.D.; Drew, P.J.; Nishimura, N.; Schaffer, C.B.; Kleinfeld, D. Two-photon microscopy as a tool to study blood flow and neurovascular coupling in the rodent brain. J. Cereb. Blood Flow Metab. 2012, 32, 1277–1309. [Google Scholar] [CrossRef]
- Jessen, K. Glial cells. Int. J. Biochem. Cell Biol. 2004, 36, 1861–1867. [Google Scholar] [CrossRef]
- Allen, N.J.; Lyons, D.A. Glia as architects of central nervous system formation and function. Science 2018, 362, 181–185. [Google Scholar] [CrossRef]
- De Silva, T.M.; Faraci, F.M. Microvascular dysfunction and cognitive impairment. Cell. Mol. Neurobiol. 2016, 36, 241–258. [Google Scholar] [CrossRef] [PubMed]
- Hosford, P.S.; Gourine, A.V. What is the key mediator of the neurovascular coupling response? Neurosci. Biobehav. Rev. 2019, 96, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Iadecola, C. The neurovascular unit coming of age: A journey through neurovascular coupling in health and disease. Neuron 2017, 96, 17–42. [Google Scholar] [CrossRef] [PubMed]
- Miles, K.A. Brain perfusion: Computed tomography applications. Neuroradiology 2004, 46, S194–S200. [Google Scholar] [CrossRef] [PubMed]
- Cook, C.K.L. Computed tomography of the brain: A pictorial review. Hosp. Med. 2004, 65, 8–12. [Google Scholar] [CrossRef]
- Boto, E.; Holmes, N.; Leggett, J.; Roberts, G.; Shah, V.; Meyer, S.S.; Muñoz, L.D.; Mullinger, K.J.; Tierney, T.M.; Bestmann, S.; et al. Moving magnetoencephalography towards real-world applications with a wearable system. Nature 2018, 555, 657–661. [Google Scholar] [CrossRef] [PubMed]
- Baillet, S. Magnetoencephalography for brain electrophysiology and imaging. Nat. Neurosci. 2017, 20, 327–339. [Google Scholar] [CrossRef] [PubMed]
- Montagne, A.; Nation, D.A.; Sagare, A.P.; Barisano, G.; Sweeney, M.D.; Chakhoyan, A.; Pachicano, M.; Joe, E.; Nelson, A.R.; D’Orazio, L.M.; et al. APOE4 leads to blood–brain barrier dysfunction predicting cognitive decline. Nature 2020, 581, 71–76. [Google Scholar] [CrossRef]
- Townsend, D.W. Positron emission tomography/computed tomography. Semin. Nucl. Med. 2008, 38, 152–166. [Google Scholar] [CrossRef]
- Buckner, R.L.; Krienen, F.M.; Yeo, B.T.T. Opportunities and limitations of intrinsic functional connectivity MRI. Nat. Neurosci. 2013, 16, 832–837. [Google Scholar] [CrossRef]
- Zuo, X.N.; Xing, X.X. Test-retest reliabilities of resting-state FMRI measurements in human brain functional connectomics: A systems neuroscience perspective. Neurosci. Biobehav. Rev. 2014, 45, 100–118. [Google Scholar] [CrossRef] [PubMed]
- Ganesh, G.; Nakamura, K.; Saetia, S.; Tobar, A.M.; Yoshida, E.; Ando, H.; Yoshimura, N.; Koike, Y. Utilizing sensory prediction errors for movement intention decoding: A new methodology. Sci. Adv. 2018, 4, eaaq0183. [Google Scholar] [CrossRef] [PubMed]
- Obidin, N.; Tasnim, F.; Dagdeviren, C. The future of neuroimplantable devices: A materials science and regulatory perspective. Adv. Mater. 2020, 32, 1901482. [Google Scholar] [CrossRef] [PubMed]
- Luan, L.; Robinson, J.T.; Aazhang, B.; Chi, T.; Yang, K.; Li, X.; Rathore, H.; Singer, A.; Yellapantula, S.; Fan, Y.; et al. Recent advances in electrical neural interface engineering: Minimal invasiveness, longevity, and scalability. Neuron 2020, 108, 302–321. [Google Scholar] [CrossRef]
- Hong, G.; Lieber, C. Novel electrode technologies for neural recordings. Nat. Rev. Neurosci. 2019, 20, 330–345. [Google Scholar] [CrossRef] [PubMed]
- Hochberg, L.R.; Bacher, D.; Jarosiewicz, B.; Masse, N.Y.; Simeral, J.D.; Vogel, J.; Haddadin, S.; Liu, J.; Cash, S.S.; van der Smagt, P.; et al. Reach and grasp by people with tetraplegia using a neurally controlled robotic arm. Nature 2012, 485, 372–375. [Google Scholar] [CrossRef]
- Ajiboye, A.B.; Willett, F.R.; Young, D.R.; Memberg, W.D.; Murphy, B.A.; Miller, J.P.; Walter, B.L.; Sweet, J.A.; Hoyen, H.A.; Keith, M.W.; et al. Restoration of reaching and grasping movements through brain-controlled muscle stimulation in a person with tetraplegia: A proof-of-concept demonstration. Lancet 2017, 389, 1821–1830. [Google Scholar] [CrossRef]
- Brochier, T.; Zehl, L.; Hao, Y.; Duret, M.; Sprenger, J.; Denker, M.; Grün, S.; Riehle, A. Massively parallel recordings in macaque motor cortex during an instructed delayed reach-to-grasp task. Sci. Data 2018, 5, 180055. [Google Scholar] [CrossRef]
- Chen, X.; Wang, F.; Fernandez, E.; Roelfsema, P.R. Shape perception via a high-channel-count neuroprosthesis in monkey visual cortex. Science 2020, 370, 1191–1196. [Google Scholar] [CrossRef]
- Moses, D.A.; Leonard, M.K.; Makin, J.G.; Chang, E.F. Real-time decoding of question-and-answer speech dialogue using human cortical activity. Nat. Commun. 2019, 10, 3096–3110. [Google Scholar] [CrossRef]
- Beauchamp, M.S.; Oswalt, D.; Sun, P.; Foster, B.L.; Magnotti, J.F.; Niketeghad, S.; Pouratian, N.; Bosking, W.H.; Yoshor, D. Dynamic stimulation of visual cortex produces form vision in sighted and blind humans. Cell 2020, 181, 774–783.e775. [Google Scholar] [CrossRef] [PubMed]
- Fekete, Z. Recent advances in silicon-based neural microelectrodes and microsystems: A review. Sens. Actuators B Chem. 2015, 215, 300–315. [Google Scholar] [CrossRef]
- Vetter, R.J.; Williams, J.C.; Hetke, J.F.; Nunamaker, E.A.; Kipke, D.R. Chronic neural recording using silicon-substrate microelectrode arrays implanted in cerebral cortex. IEEE Trans. Biomed. Eng. 2004, 51, 896–904. [Google Scholar] [CrossRef] [PubMed]
- Krauss, J.K.; Lipsman, N.; Aziz, T.; Boutet, A.; Brown, P.; Chang, J.W.; Davidson, B.; Grill, W.M.; Hariz, M.I.; Horn, A.; et al. Technology of deep brain stimulation: Current status and future directions. Nat. Rev. Neurol. 2021, 17, 75–87. [Google Scholar] [CrossRef] [PubMed]
- Cramer, S.W.; Carter, R.E.; Aronson, J.D.; Kodandaramaiah, S.B.; Ebner, T.J.; Chen, C.C. Through the looking glass: A review of cranial window technology for optical access to the brain. J. Neurosci. Methods 2021, 354, 109100. [Google Scholar] [CrossRef] [PubMed]
- Dunn, A.K.; Devor, A.; Bolay, H.; Andermann, M.L.; Moskowitz, M.A.; Dale, A.M.; Boas, D.A. Simultaneous imaging of total cerebral hemoglobin concentration, oxygenation, and blood flow during functional activation. Opt. Lett. 2003, 28, 28–30. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Wang, Y.; Chen, S.; Luo, W.; Li, P.; Luo, Q. Simultaneous monitoring of intracellular pH changes and hemodynamic response during cortical spreading depression by fluorescence-corrected multimodal optical imaging. Neuroimage 2011, 57, 873–884. [Google Scholar] [CrossRef]
- Farkas, E.; Bari, F.; Obrenovitch, T.P. Multi-modal imaging of anoxic depolarization and hemodynamic changes induced by cardiac arrest in the rat cerebral cortex. Neuroimage 2010, 51, 734–742. [Google Scholar] [CrossRef]
- Rickgauer, J.P.; Deisseroth, K.; Tank, D.W. Simultaneous cellular-resolution optical perturbation and imaging of place cell firing fields. Nat. Neurosci. 2014, 17, 1816–1824. [Google Scholar] [CrossRef]
- Packer, A.M.; Russell, L.E.; Dalgleish, H.W.P.; Häusser, M. Simultaneous all-optical manipulation and recording of neural circuit activity with cellular resolution in vivo. Nat. Meth. 2015, 12, 140–146. [Google Scholar] [CrossRef]
- Ju, N.; Jiang, R.; Macknik, S.L.; Martinez-Conde, S.; Tang, S. Long-term all-optical interrogation of cortical neurons in awake-behaving nonhuman primates. PLoS Biol. 2018, 16, e2005839. [Google Scholar] [CrossRef] [PubMed]
- Yang, W. Manipulating neuronal circuits, in concert. Science 2021, 373, 635. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.V.; Li, N.; Huber, D.; Ophir, E.; Gutnisky, D.; Ting, J.T.; Feng, G.; Svoboda, K. Flow of cortical activity underlying a tactile decision in mice. Neuron 2014, 81, 179–194. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.H.; Zhang, Y.; Lecoq, J.; Jung, J.C.; Li, J.; Zeng, H.; Niell, C.M.; Schnitzer, M.J. Long-term optical access to an estimated one million neurons in the live mouse cortex. Cell Rep. 2016, 17, 3385–3394. [Google Scholar] [CrossRef] [PubMed]
- Helmchen, F.; Denk, W. Deep tissue two-photon microscopy. Nat. Meth. 2005, 2, 932–940. [Google Scholar] [CrossRef]
- Stosiek, C.; Garaschuk, O.; Holthoff, K.; Konnerth, A. In vivo two-photon calcium imaging of neuronal networks. Proc. Natl. Acad. Sci. USA 2003, 100, 7319. [Google Scholar] [CrossRef]
- Baker, W.B.; Sun, Z.; Hiraki, T.; Putt, M.E.; Durduran, T.; Reivich, M.; Yodh, A.G.; Greenberg, J.H. Neurovascular coupling varies with level of global cerebral ischemia in a rat model. J. Cereb. Blood Flow Metab. 2012, 33, 97–105. [Google Scholar] [CrossRef]
- Liu, Y.H.; Liao, L.D.; Tan, S.S.H.; Kwon, K.Y.; Ling, J.M.; Bandla, A.; Shih, Y.-Y.I.; Tan, E.T.W.; Li, W.; Ng, W.H.; et al. Assessment of neurovascular dynamics during transient ischemic attack by the novel integration of micro-electrocorticography electrode array with functional photoacoustic microscopy. Neurobiol. Dis. 2015, 82, 455–465. [Google Scholar] [CrossRef]
- Luan, L.; Sullender, C.T.; Li, X.; Zhao, Z.; Zhu, H.; Wei, X.; Xie, C.; Dunn, A.K. Nanoelectronics enabled chronic multimodal neural platform in a mouse ischemic model. J. Neurosci. Methods 2018, 295, 68–76. [Google Scholar] [CrossRef]
- Park, D.W.; Schendel, A.A.; Mikael, S.; Brodnick, S.K.; Richner, T.J.; Ness, J.P.; Hayat, M.R.; Atry, F.; Frye, S.T.; Pashaie, R.; et al. Graphene-based carbon-layered electrode array technology for neural imaging and optogenetic applications. Nat. Commun. 2014, 5, 5258–5269. [Google Scholar] [CrossRef]
- Wang, B.; Zhou, J.; Carney, P.; Jiang, H. A novel detachable head-mounted device for simultaneous EEG and photoacoustic monitoring of epilepsy in freely moving rats. Neurosci. Res. 2015, 91, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Liao, L.D.; Liu, Y.H.; Lai, H.Y.; Bandla, A.; Shih, Y.Y.I.; Chen, Y.Y.; Thakor, N.V. Rescue of cortical neurovascular functions during the hyperacute phase of ischemia by peripheral sensory stimulation. Neurobiol. Dis. 2015, 75, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Li, D.; Guo, J.; Li, B.; Kong, Y.; Hu, Y.; Du, B.; Ding, Y.; Li, X.; Liu, H.; et al. The neurovascular couplings between electrophysiological and hemodynamic activities in anticipatory selective attention. Cereb. Cortex 2022, bhab525, Online ahead of print. [Google Scholar] [CrossRef]
- Kuzum, D.; Takano, H.; Shim, E.; Reed, J.C.; Juul, H.; Richardson, A.G.; de Vries, J.; Bink, H.; Dichter, M.A.; Lucas, T.H.; et al. Transparent and flexible low noise graphene electrodes for simultaneous electrophysiology and neuroimaging. Nat. Commun. 2014, 5, 5259. [Google Scholar] [CrossRef] [PubMed]
- Luan, L.; Wei, X.; Zhao, Z.; Siegel, J.J.; Potnis, O.; Tuppen, C.A.; Lin, S.; Kazmi, S.; Fowler, R.A.; Holloway, S.; et al. Ultraflexible nanoelectronic probes form reliable, glial scar–free neural integration. Sci. Adv. 2017, 3, e1601966. [Google Scholar] [CrossRef] [PubMed]
- Richner, T.J.; Thongpang, S.; Brodnick, S.K.; Schendel, A.A.; Falk, R.W.; Krugner-Higby, L.A.; Pashaie, R.; Williams, J.C. Optogenetic micro-electrocorticography for modulating and localizing cerebral cortex activity. J. Neural Eng. 2014, 11, 016010. [Google Scholar] [CrossRef]
- Park, K.; You, J.; Du, C.; Pan, Y. Cranial window implantation on mouse cortex to study microvascular change induced by cocaine. Quant. Imaging Med. Surg. 2014, 5, 97–107. [Google Scholar] [CrossRef]
- Park, D.W.; Brodnick, S.K.; Ness, J.P.; Atry, F.; Krugner-Higby, L.; Sandberg, A.; Mikael, S.; Richner, T.J.; Novello, J.; Kim, H.; et al. Fabrication and utility of a transparent graphene neural electrode array for electrophysiology, in vivo imaging, and optogenetics. Nat. Protoc. 2016, 11, 2201–2222. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, X.; Xu, W.; Luo, W.; Li, M.; Chu, F.; Xu, L.; Cao, A.; Guan, J.; Tang, S.; et al. Stretchable transparent electrode arrays for simultaneous electrical and optical interrogation of neural circuits in vivo. Nano Lett. 2018, 18, 2903–2911. [Google Scholar] [CrossRef]
- Ghanbari, L.; Carter, R.E.; Rynes, M.L.; Dominguez, J.; Chen, G.; Naik, A.; Hu, J.; Sagar, M.A.K.; Haltom, L.; Mossazghi, N.; et al. Cortex-wide neural interfacing via transparent polymer skulls. Nat. Commun. 2019, 10, 1500–1513. [Google Scholar] [CrossRef]
- Wang, X.; Wang, M.; Sheng, H.; Zhu, L.; Zhu, J.; Zhang, H.; Liu, Y.; Zhan, L.; Wang, X.; Zhang, J.; et al. Subdural neural interfaces for long-term electrical recording, optical microscopy and magnetic resonance imaging. Biomaterials 2022, 281, 121352. [Google Scholar] [CrossRef] [PubMed]
- Yang, N.; Liu, F.; Zhang, X.; Chen, C.; Xia, Z.; Fu, S.; Wang, J.; Xu, J.; Cui, S.; Zhang, Y.; et al. A hybrid titanium-softmaterial, high-strength, transparent cranial window for transcranial injection and neuroimaging. Biosensors 2022, 12, 129. [Google Scholar] [CrossRef] [PubMed]
- Roome, C.J.; Kuhn, B. Chronic cranial window with access port for repeated cellular manipulations, drug application, and electrophysiology. Front. Cell. Neurosci. 2014, 8, 379. [Google Scholar] [CrossRef] [PubMed]
- Goldey, G.J.; Roumis, D.K.; Glickfeld, L.L.; Kerlin, A.M.; Reid, R.C.; Bonin, V.; Schafer, D.P.; Andermann, M.L. Removable cranial windows for long-term imaging in awake mice. Nat. Protoc. 2014, 9, 2515–2538. [Google Scholar] [CrossRef]
- Tokuno, H.; Hatanaka, N.; Chiken, S.; Ishizuka, N. An improved method with a long-shanked glass micropipette and ultrasonography for drug injection into deep brain structure of the monkey. Brain Res. Protoc. 2002, 10, 16–22. [Google Scholar] [CrossRef]
- Takehara, H.; Nagaoka, A.; Noguchi, J.; Akagi, T.; Kasai, H.; Ichiki, T. Lab-on-a-brain: Implantable micro-optical fluidic devices for neural cell analysis in vivo. Sci. Rep. 2014, 4, 6721. [Google Scholar] [CrossRef] [PubMed]
- Vieira, D.B.; Gamarra, L.F. Getting into the brain: Liposome-based strategies for effective drug delivery across the blood-brain barrier. Int. J. Nanomed. 2016, 11, 5381–5414. [Google Scholar] [CrossRef]
- Chen, W.; Zuo, H.; Zhang, E.; Li, L.; Henrich-Noack, P.; Cooper, H.; Qian, Y.; Xu, Z.P. Brain targeting delivery facilitated by ligand-functionalized layered double hydroxide nanoparticles. ACS Appl. Mater. Interfaces 2018, 10, 20326–20333. [Google Scholar] [CrossRef]
- Liang, J.; Gao, C.; Zhu, Y.; Ling, C.; Wang, Q.; Huang, Y.; Qin, J.; Wang, J.; Lu, W.; Wang, J. Natural brain penetration enhancer-modified albumin nanoparticles for glioma targeting delivery. ACS Appl. Mater. Interfaces 2018, 10, 30201–30213. [Google Scholar] [CrossRef]
- Gernert, M.; Feja, M. Bypassing the blood-brain barrier: Direct intracranial drug delivery in epilepsies. Pharmaceutics 2020, 12, 1134. [Google Scholar] [CrossRef]
- Han, H.; Xia, Z.; Chen, H.; Hou, C.; Li, W. Simple diffusion delivery via brain interstitial route for the treatment of cerebral ischemia. Sci. China Life Sci. 2011, 54, 235–239. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Jin, K.; Sahasrabudhe, A.; Chiang, P.-H.; Maalouf, J.H.; Koehler, F.; Rosenfeld, D.; Rao, S.; Tanaka, T.; Khudiyev, T.; et al. In situ electrochemical generation of nitric oxide for neuronal modulation. Nat. Nanotechnol. 2020, 15, 690–697. [Google Scholar] [CrossRef] [PubMed]
- Heo, C.; Park, H.; Kim, Y.T.; Baeg, E.; Kim, Y.H.; Kim, S.G.; Suh, M. A soft, transparent, freely accessible cranial window for chronic imaging and electrophysiology. Sci. Rep. 2016, 6, 27818. [Google Scholar] [CrossRef] [PubMed]
- Kunori, N.; Takashima, I. An implantable cranial window using a collagen membrane for chronic voltage-sensitive dye imaging. Micromachines 2019, 10, 789. [Google Scholar] [CrossRef]
- Wang, Y.; Liang, G.; Liu, F.; Chen, Q.; Xi, L. A long-term cranial window for high-resolution photoacoustic imaging. IEEE Trans. Biomed. Eng. 2021, 68, 706–711. [Google Scholar] [CrossRef]
- Donders, F. De bewegingen der hersenen en de veranderingen der vaatvulling van de pia mater, ook bij gesloten onuitzetbaren schedel regtstreeks onderzocht. Ned. Lancet 1850, 5, 521–553. [Google Scholar]
- Donders, F.C. Physiologie des Menschen; S. Hirzel Verlag: Hirzel, Germany, 1859. [Google Scholar]
- Forbes, H.S. The cerebral circulation: Observation and measurement of pial vessels. Arch. Neurol. Psychiatry 1928, 19, 751–761. [Google Scholar] [CrossRef]
- Leyden, E. Beiträge und untersuchungen zur physiologie und pathologie des gehirns. Arch. Pathol. Anat. Physiol. Klin. Med. 1866, 37, 519–559. [Google Scholar] [CrossRef]
- Cushing, H. Some experimental and clinical observations concerning states of increased intracranial tension. Am. J. Med. Sci. 1902, 124, 375. [Google Scholar] [CrossRef]
- Lee, F.C. The effect of histamine on cerebrospinal fluid pressure. Am. J. Physiol.-Leg. Content 1925, 74, 317–325. [Google Scholar] [CrossRef]
- Yang, G.; Pan, F.; Parkhurst, C.N.; Grutzendler, J.; Gan, W.B. Thinned-skull cranial window technique for long-term imaging of the cortex in live mice. Nat. Protoc. 2010, 5, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Christie, R.H.; Bacskai, B.J.; Zipfel, W.R.; Williams, R.M.; Kajdasz, S.T.; Webb, W.W.; Hyman, B.T. Growth arrest of individual senile plaques in a model of Alzheimer’s disease observed by in vivo multiphoton microscopy. J. Neurosci. 2001, 21, 858–864. [Google Scholar] [CrossRef] [PubMed]
- Jeong, D.C.; Tsai, P.S.; Kleinfeld, D. All-optical osteotomy to create windows for transcranial imaging in mice. Opt. Express 2013, 21, 23160–23168. [Google Scholar] [CrossRef]
- Nimmerjahn, A.; Kirchhoff, F.; Helmchen, F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science 2005, 308, 1314–1318. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.V.; Kang, S.S.; Dustin, M.L.; McGavern, D.B. Myelomonocytic cell recruitment causes fatal CNS vascular injury during acute viral meningitis. Nature 2009, 457, 191–195. [Google Scholar] [CrossRef] [PubMed]
- Tsai, J.; Grutzendler, J.; Duff, K.; Gan, W.B. Fibrillar amyloid deposition leads to local synaptic abnormalities and breakage of neuronal branches. Nat. Neurosci. 2004, 7, 1181–1183. [Google Scholar] [CrossRef] [PubMed]
- Zuo, Y.; Lin, A.; Chang, P.; Gan, W.B. Development of long-term dendritic spine stability in diverse regions of cerebral cortex. Neuron 2005, 46, 181–189. [Google Scholar] [CrossRef]
- Zuo, Y.; Yang, G.; Kwon, E.; Gan, W.B. Long-term sensory deprivation prevents dendritic spine loss in primary somatosensory cortex. Nature 2005, 436, 261–265. [Google Scholar] [CrossRef]
- Grutzendler, J.; Kasthuri, N.; Gan, W.B. Long-term dendritic spine stability in the adult cortex. Nature 2002, 420, 812–816. [Google Scholar] [CrossRef]
- Holtmaat, A.; Bonhoeffer, T.; Chow, D.K.; Chuckowree, J.; De Paola, V.; Hofer, S.B.; Hübener, M.; Keck, T.; Knott, G.; Lee, W.-C.A.; et al. Long-term, high-resolution imaging in the mouse neocortex through a chronic cranial window. Nat. Protoc. 2009, 4, 1128–1144. [Google Scholar] [CrossRef]
- Drew, P.J.; Shih, A.Y.; Driscoll, J.D.; Knutsen, P.M.; Blinder, P.; Davalos, D.; Akassoglou, K.; Tsai, P.S.; Kleinfeld, D. Chronic optical access through a polished and reinforced thinned skull. Nat. Meth. 2010, 7, 981–984. [Google Scholar] [CrossRef] [PubMed]
- Helm, P.J.; Ottersen, O.P.; Nase, G. Analysis of optical properties of the mouse cranium-Implications for in vivo multi photon laser scanning microscopy. J. Neurosci. Methods 2009, 178, 316–322. [Google Scholar] [CrossRef] [PubMed]
- Tohmi, M.; Kitaura, H.; Komagata, S.; Kudoh, M.; Shibuki, K. Enduring critical period plasticity visualized by transcranial flavoprotein imaging in mouse primary visual cortex. J. Neurosci. 2006, 26, 11775–11785. [Google Scholar] [CrossRef] [PubMed]
- Hira, R.; Honkura, N.; Noguchi, J.; Maruyama, Y.; Augustine, G.J.; Kasai, H.; Matsuzaki, M. Transcranial optogenetic stimulation for functional mapping of the motor cortex. J. Neurosci. Methods 2009, 179, 258–263. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, Y.; Xu, T.H.; Luo, Q.M.; Zhu, D. An innovative transparent cranial window based on skull optical clearing. Laser Phys. Lett. 2012, 9, 469–473. [Google Scholar] [CrossRef]
- Zhang, C.; Zhao, Y.; Shi, R.; Zhu, D. A Rapid and Reversible Skull Optical Clearing Method for Monitoring Cortical Blood Flow. In Dynamics and Fluctuations in Biomedical Photonics XIII; SPIE BiOS: San Francisco, CA, USA, 2016; Volume 9707, p. 970717. [Google Scholar]
- Yang, X.; Zhang, Y.; Zhao, K.; Zhao, Y.; Liu, Y.; Gong, H.; Luo, Q.; Zhu, D. Skull optical clearing solution for enhancing ultrasonic and photoacoustic imaging. IEEE Trans. Med. Imaging 2016, 35, 1903–1906. [Google Scholar] [CrossRef]
- Zhao, Y.; Yu, T.; Zhang, C.; Li, Z.; Luo, Q.; Xu, T.H.; Zhu, D. Skull optical clearing window for in vivo imaging of the mouse cortex at synaptic resolution. Light Sci. Appl. 2018, 7, 17153. [Google Scholar] [CrossRef]
- Zhang, C.; Feng, W.; Zhao, Y.; Yu, T.; Li, P.; Xu, T.; Luo, Q.; Zhu, D. A large, switchable optical clearing skull window for cerebrovascular imaging. Theranostics 2018, 8, 2696–2708. [Google Scholar] [CrossRef]
- Li, D.Y.; Zheng, Z.; Yu, T.T.; Tang, B.Z.; Fei, P.; Qian, J.; Zhu, D. Visible-near infrared-II skull optical clearing window for in vivo cortical vasculature imaging and targeted manipulation. J. Biophotonics 2020, 13, e202000142. [Google Scholar] [CrossRef]
- Zhang, C.; Feng, W. Assessment of tissue-specific changes in structure and function induced by in vivo skin/skull optical clearing techniques. Lasers Surg. Med. 2021, 54, 447–458. [Google Scholar] [CrossRef]
- Svoboda, K.; Denk, W.; Kleinfeld, D.; Tank, D.W. In vivo dendritic calcium dynamics in neocortical pyramidal neurons. Nature 1997, 385, 161–165. [Google Scholar] [CrossRef] [PubMed]
- Lendvai, B.; Stern, E.A.; Chen, B.; Svoboda, K. Experience-dependent plasticity of dendritic spines in the developing rat barrel cortex in vivo. Nature 2000, 404, 876–881. [Google Scholar] [CrossRef] [PubMed]
- Trachtenberg, J.T.; Chen, B.E.; Knott, G.W.; Feng, G.; Sanes, J.R.; Welker, E.; Svoboda, K. Long-term in vivo imaging of experience-dependent synaptic plasticity in adult cortex. Nature 2002, 420, 788–794. [Google Scholar] [CrossRef] [PubMed]
- Portera-Cailliau, C.; Weimer, R.M.; De Paola, V.; Caroni, P.; Svoboda, K. Diverse modes of axon elaboration in the developing neocortex. PLoS Biol. 2005, 3, e272. [Google Scholar] [CrossRef] [PubMed]
- De Paola, V.; Holtmaat, A.; Knott, G.; Song, S.; Wilbrecht, L.; Caroni, P.; Svoboda, K. Cell type-specific structural plasticity of axonal branches and boutons in the adult neocortex. Neuron 2006, 49, 861–875. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.C.; Huang, H.; Feng, G.; Sanes, J.R.; Brown, E.N.; So, P.T.; Nedivi, E. Dynamic remodeling of dendritic arbors in GABAergic interneurons of adult visual cortex. PLoS Biol. 2006, 4, e29. [Google Scholar] [CrossRef]
- Holtmaat, A.; de Paola, V.; Wilbrecht, L.; Trachtenberg, J.T.; Svoboda, K.; Portera-Cailliau, C. Imaging neocortical neurons through a chronic cranial window. Cold Spring Harb. Protoc. 2012, 2012, 694–701. [Google Scholar] [CrossRef]
- Brown, C.E.; Aminoltejari, K.; Erb, H.; Winship, I.R.; Murphy, T.H. In vivo voltage-sensitive dye imaging in adult mice reveals that somatosensory maps lost to stroke are replaced over weeks by new structural and functional circuits with prolonged modes of activation within both the peri-infarct zone and distant sites. J. Neurosci. 2009, 29, 1719–1734. [Google Scholar] [CrossRef]
- Keck, T.; Mrsic-Flogel, T.D.; Vaz Afonso, M.; Eysel, U.T.; Bonhoeffer, T.; Hübener, M. Massive restructuring of neuronal circuits during functional reorganization of adult visual cortex. Nat. Neurosci. 2008, 11, 1162–1167. [Google Scholar] [CrossRef]
- O’Connor, D.H.; Peron, S.P.; Huber, D.; Svoboda, K. Neural activity in barrel cortex underlying vibrissa-based object localization in mice. Neuron 2010, 67, 1048–1061. [Google Scholar] [CrossRef]
- Kobat, D.; Horton, N.; Xu, C. In vivo two-photon microscopy to 1.6-mm depth in mouse cortex. J. Biomed. Opt. 2011, 16, 106014. [Google Scholar] [CrossRef] [PubMed]
- Horton, N.G.; Wang, K.; Kobat, D.; Clark, C.G.; Wise, F.W.; Schaffer, C.B.; Xu, C. In vivo three-photon microscopy of subcortical structures within an intact mouse brain. Nat. Photonics 2013, 7, 205–209. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.F.; Hübener, M. Chronic calcium imaging of neurons in the mouse visual cortex using a troponin C-based indicator. Cold Spring Harb. Protoc. 2014, 2014, 544–551. [Google Scholar] [CrossRef] [PubMed]
- Choi, W.J.; Wang, R.K. Swept-source optical coherence tomography powered by a 1.3-µm vertical cavity surface emitting laser enables 2.3-mm-deep brain imaging in mice in vivo. J. Biomed. Opt. 2015, 20, 106004. [Google Scholar] [CrossRef]
- Dombeck, D.; Tank, D. Two-photon imaging of neural activity in awake mobile mice. Cold Spring Harb. Protoc. 2014, 2014, 726–736. [Google Scholar] [CrossRef]
- Koletar, M.M.; Dorr, A.; Brown, M.E.; McLaurin, J.; Stefanovic, B. Refinement of a chronic cranial window implant in the rat for longitudinal in vivo two–photon fluorescence microscopy of neurovascular function. Sci. Rep. 2019, 9, 5499. [Google Scholar] [CrossRef]
- Scott, B.B.; Thiberge, S.Y.; Guo, C.; Tervo, D.G.R.; Brody, C.D.; Karpova, A.Y.; Tank, D.W. Imaging cortical dynamics in GCaMP transgenic rats with a head-mounted widefield macroscope. Neuron 2018, 100, 1045–1058. [Google Scholar] [CrossRef]
- Klein, S.P.; De Sloovere, V.; Meyfroidt, G.; Depreitere, B. Autoregulation assessment by direct visualisation of pial arterial blood flow in the piglet brain. Sci. Rep. 2019, 9, 13333. [Google Scholar] [CrossRef]
- Smith, G.B.; Hein, B.; Whitney, D.E.; Fitzpatrick, D.; Kaschube, M. Distributed network interactions and their emergence in developing neocortex. Nat. Neurosci. 2018, 21, 1600–1608. [Google Scholar] [CrossRef]
- Stettler, D.D.; Yamahachi, H.; Li, W.; Denk, W.; Gilbert, C.D. Axons and synaptic boutons are highly dynamic in adult visual cortex. Neuron 2006, 49, 877–887. [Google Scholar] [CrossRef]
- Orringer, D.A.; Chen, T.; Huang, D.L.; Armstead, W.M.; Hoff, B.A.; Koo, Y.E.; Keep, R.F.; Philbert, M.A.; Kopelman, R.; Sagher, O. The brain tumor window model: A combined cranial window and implanted glioma model for evaluating intraoperative contrast agents. Neurosurgery 2010, 66, 736–743. [Google Scholar] [CrossRef] [PubMed]
- Sunil, S.; Erdener, S.E.; Lee, B.S.; Postnov, D.; Tang, J.; Kura, S.; Cheng, X.; Chen, I.A.; Boas, D.A.; Kılıç, K. Awake chronic mouse model of targeted pial vessel occlusion via photothrombosis. Neurophotonics 2020, 7, 015005. [Google Scholar] [CrossRef] [PubMed]
- Kauvar, I.V.; Machado, T.A.; Yuen, E.; Kochalka, J.; Choi, M.; Allen, W.E.; Wetzstein, G.; Deisseroth, K. Cortical observation by synchronous multifocal optical sampling reveals widespread population encoding of actions. Neuron 2020, 107, 351–367. [Google Scholar] [CrossRef] [PubMed]
- Andermann, M.L.; Gilfoy, N.B.; Goldey, G.J.; Sachdev, R.N.; Wölfel, M.; McCormick, D.A.; Reid, R.C.; Levene, M.J. Chronic cellular imaging of entire cortical columns in awake mice using microprisms. Neuron 2013, 80, 900–913. [Google Scholar] [CrossRef]
- Low, R.J.; Gu, Y.; Tank, D.W. Cellular resolution optical access to brain regions in fissures: Imaging medial prefrontal cortex and grid cells in entorhinal cortex. Proc. Natl. Acad. Sci. USA 2014, 111, 18739–18744. [Google Scholar] [CrossRef]
- Beckmann, L.; Zhang, X.; Nadkarni, N.A.; Cai, Z.; Batra, A.; Sullivan, D.P.; Muller, W.A.; Sun, C.; Kuranov, R.; Zhang, H.F. Longitudinal deep-brain imaging in mouse using visible-light optical coherence tomography through chronic microprism cranial window. Biomed. Opt. Express 2019, 10, 5235–5250. [Google Scholar] [CrossRef]
- Levene, M.J.; Dombeck, D.A.; Kasischke, K.A.; Molloy, R.P.; Webb, W.W. In vivo multiphoton microscopy of deep brain tissue. J. Neurophysiol. 2004, 91, 1908–1912. [Google Scholar] [CrossRef]
- Bocarsly, M.E.; Jiang, W.C.; Wang, C.; Dudman, J.T.; Ji, N.; Aponte, Y. Minimally invasive microendoscopy system for in vivo functional imaging of deep nuclei in the mouse brain. Biomed. Opt. Express 2015, 6, 4546–4556. [Google Scholar] [CrossRef]
- Pernici, C.D.; Rowe, R.K.; Doughty, P.T.; Madadi, M.; Lifshitz, J.; Murray, T.A. Longitudinal optical imaging technique to visualize progressive axonal damage after brain injury in mice reveals responses to different minocycline treatments. Sci. Rep. 2020, 10, 7815. [Google Scholar] [CrossRef]
- Sato, M.; Sano, S.; Watanabe, H.; Kudo, Y.; Nakai, J. An aspherical microlens assembly for deep brain fluorescence microendoscopy. Biochem. Biophys. Res. Commun. 2020, 527, 447–452. [Google Scholar] [CrossRef]
- Park, H.; You, N.; Lee, J.; Suh, M. Longitudinal study of hemodynamics and dendritic membrane potential changes in the mouse cortex following a soft cranial window installation. Neurophotonics 2019, 6, 015006. [Google Scholar] [CrossRef] [PubMed]
- Rosenthal, Z.P.; Raut, R.V.; Yan, P.; Koko, D.; Kraft, A.W.; Czerniewski, L.; Acland, B.; Mitra, A.; Snyder, L.H.; Bauer, A.Q.; et al. Local perturbations of cortical excitability propagate differentially through large-scale functional networks. Cereb. Cortex 2020, 30, 3352–3369. [Google Scholar] [CrossRef] [PubMed]
- Miao, P.; Zhang, L.; Li, M.; Zhang, Y.; Feng, S.; Wang, Q.; Thakor, N.V. Chronic wide-field imaging of brain hemodynamics in behaving animals. Biomed. Opt. Express 2017, 8, 436–445. [Google Scholar] [CrossRef] [PubMed]
- Thunemann, M.; Lu, Y.; Liu, X.; Kılıç, K.; Desjardins, M.; Vandenberghe, M.; Sadegh, S.; Saisan, P.A.; Cheng, Q.; Weldy, K.L.; et al. Deep 2-photon imaging and artifact-free optogenetics through transparent graphene microelectrode arrays. Nat. Commun. 2018, 9, 2035–2047. [Google Scholar] [CrossRef]
- Kozai, T.D.Y.; Vazquez, A.L. Photoelectric artefact from optogenetics and imaging on microelectrodes and bioelectronics: New challenges and opportunities. J. Mater. Chem. B 2015, 3, 4965–4978. [Google Scholar] [CrossRef] [PubMed]
- Benfenati, V.; Toffanin, S.; Bonetti, S.; Turatti, G.; Pistone, A.; Chiappalone, M.; Sagnella, A.; Stefani, A.; Generali, G.; Ruani, G.; et al. A transparent organic transistor structure for bidirectional stimulation and recording of primary neurons. Nat. Mater. 2013, 12, 672–680. [Google Scholar] [CrossRef]
- Lee, W.; Kim, D.; Matsuhisa, N.; Nagase, M.; Sekino, M.; Malliaras, G.G.; Yokota, T.; Someya, T. Transparent, conformable, active multielectrode array using organic electrochemical transistors. Proc. Natl. Acad. Sci. USA 2017, 114, 10554–10559. [Google Scholar] [CrossRef]
- Rengier, F.; Mehndiratta, A.; von Tengg-Kobligk, H.; Zechmann, C.M.; Unterhinninghofen, R.; Kauczor, H.U.; Giesel, F.L. 3D printing based on imaging data: Review of medical applications. Int. J. Comput. Assist. Radiol. Surg. 2010, 5, 335–341. [Google Scholar] [CrossRef]
- Naftulin, J.S.; Kimchi, E.Y.; Cash, S.S. Streamlined, inexpensive 3D printing of the brain and skull. PLoS ONE 2015, 10, e0136198. [Google Scholar] [CrossRef]
- Zhou, L.Y.; Fu, J.; He, Y. A review of 3D printing technologies for soft polymer materials. Adv. Funct. Mater. 2020, 30, 2000187. [Google Scholar] [CrossRef]
- Li, J.; Pumera, M. 3D printing of functional microrobots. Chem. Soc. Rev. 2021, 50, 2794–2838. [Google Scholar] [CrossRef] [PubMed]
- Ma, I.T.; Symon, M.R.; Bristol, R.E.; Beals, S.P.; Joganic, E.F.; Adelson, P.D.; Shafron, D.H.; Singh, D.J. Outcomes of titanium mesh cranioplasty in pediatric patients. J. Craniofac. Surg. 2018, 29, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Liu, B.; Xie, Z.; Ding, S.; Zhuang, Z.; Lin, L.; Guo, Y.; Chen, H.; Yu, X. Comparison of manually shaped and computer-shaped titanium mesh for repairing large frontotemporoparietal skull defects after traumatic brain injury. Neurosurg. Focus 2012, 33, e13. [Google Scholar] [CrossRef]
- Lam, S.; Kuether, J.; Fong, A.; Reid, R. Cranioplasty for large-sized calvarial defects in the pediatric population: A review. Craniomaxillofac. Trauma Reconstr. 2015, 8, 159–170. [Google Scholar] [CrossRef]
- Goldstein, J.A.; Paliga, J.T.; Bartlett, S.P. Cranioplasty: Indications and advances. Curr. Opin. Otolaryngol. Head Neck Surg. 2013, 21, 400–409. [Google Scholar] [CrossRef]
- Zhu, S.; Chen, Y.; Lin, F.; Chen, Z.; Jiang, X.; Zhang, J.; Wang, J. Complications following titanium cranioplasty compared with nontitanium implants cranioplasty: A systematic review and meta-analysis. J. Clin. Neurosci. 2021, 84, 66–74. [Google Scholar] [CrossRef]
- Lewitz, M.; Salma, A.; Welzel Saravia, H.; Sakellaropoulou, I.; Sarkis, H.M.; Ewelt, C.; Fortmann, T.; Wilbers, E.; Schipmann, S.; Suero Molina, E.; et al. Load-bearing capacity and design advantages of a custom-made, thin pure-titanium cranioplasty (craniotop). J. Craniofac. Surg. 2021, 32, 1291–1296. [Google Scholar] [CrossRef]
- Maher, S.; Kaur, G.; Lima-Marques, L.; Evdokiou, A.; Losic, D. Engineering of micro- to nanostructured 3D-printed drug-releasing titanium implants for enhanced osseointegration and localized delivery of anticancer drugs. ACS Appl. Mater. Interfaces 2017, 9, 29562–29570. [Google Scholar] [CrossRef] [PubMed]
- Qi, C.; Jiang, F.; Yang, S. Advanced honeycomb designs for improving mechanical properties: A review. Compos. B. Eng. 2021, 227, 109393. [Google Scholar] [CrossRef]
- Zhang, J.; Lu, G.; You, Z. Large deformation and energy absorption of additively manufactured auxetic materials and structures: A review. Compos. B. Eng. 2020, 201, 108340. [Google Scholar] [CrossRef]
- Wang, Q.; Li, Z.; Zhang, Y.; Cui, S.; Yang, Z.; Lu, Z. Ultra-low density architectured metamaterial with superior mechanical properties and energy absorption capability. Compos. B. Eng. 2020, 202, 108379. [Google Scholar] [CrossRef]
- Chen, S.; Tan, X.; Hu, J.; Zhu, S.; Wang, B.; Wang, L.; Jin, Y.; Wu, L. A novel gradient negative stiffness honeycomb for recoverable energy absorption. Compos. B. Eng. 2021, 215, 108745. [Google Scholar] [CrossRef]
- Hales, T.C. The honeycomb conjecture. Discret. Comput. Geom. 2001, 25, 1–22. [Google Scholar] [CrossRef]
- Zhang, Q.; Yang, X.; Li, P.; Huang, G.; Feng, S.; Shen, C.; Han, B.; Zhang, X.; Jin, F.; Xu, F.; et al. Bioinspired engineering of honeycomb structure -using nature to inspire human innovation. Prog. Mater. Sci. 2015, 74, 332–400. [Google Scholar] [CrossRef]
- Torimitsu, S.; Nishida, Y.; Takano, T.; Yajima, D.; Inokuchi, G.; Makino, Y.; Motomura, A.; Chiba, F.; Yamaguchi, R.; Hashimoto, M.; et al. Differences in biomechanical properties and thickness among frontal and parietal bones in a Japanese sample. Forensic Sci. Int. 2015, 252, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.M.; Heider, B.; Williams, G.V.; Healy, F.L.; Ramsden, B.M.; Roe, A.W. A chamber and artificial dura method for long-term optical imaging in the monkey. J. Neurosci. Methods 2002, 113, 41–49. [Google Scholar] [CrossRef]
- Feng, P.; Wang, X.; Lu, B.; Pan, G.; Leng, X.; Ma, X.; Zhang, J.; Zhao, W. Ionic liquids-filled patterned cavities improve transmittance of transparent and stretchable electronic polydimethylsiloxane films. J. Mater. Sci. 2019, 54, 11134–11144. [Google Scholar] [CrossRef]
- Guan, F.; Song, Z.; Xin, F.; Wang, H.; Yu, D.; Li, G.; Liu, W. Preparation of hydrophobic transparent paper via using polydimethylsiloxane as transparent agent. J. Bioresour. Bioprod. 2020, 5, 37–43. [Google Scholar] [CrossRef]
- Świerczek-Lasek, B.; Keremidarska-Markova, M.; Hristova-Panusheva, K.; Vladkova, T.; Ciemerych, M.A.; Archacka, K.; Krasteva, N. Polydimethylsiloxane materials with supraphysiological elasticity enable differentiation of myogenic cells. J. Biomed. Mater. Res. A 2019, 107, 2619–2628. [Google Scholar] [CrossRef]
- Shtoyerman, E.; Arieli, A.; Slovin, H.; Vanzetta, I.; Grinvald, A. Long-term optical imaging and spectroscopy reveal mechanisms underlying the intrinsic signal and stability of cortical maps in V1 of behaving monkeys. J. Neurosci. Methods 2000, 20, 8111–8121. [Google Scholar] [CrossRef]
- Ryu, M.; Kim, J.; Lee, S.; Kim, J.; Song, T. Stretchable and transparent paper based on PDMS–CNC composite for direct printing. Adv. Mater. Technol. 2021, 6, 2100156. [Google Scholar] [CrossRef]
- Musk, E. An integrated brain-machine interface platform with thousands of channels. J. Med. Internet Res. 2019, 21, e16194. [Google Scholar] [CrossRef] [PubMed]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, N.; Li, Q.; Zhang, X.; Xu, J.; Xu, S.; Liu, F. From Transparent Cranial Windows to Multifunctional Smart Cranial Platforms. Electronics 2022, 11, 2559. https://doi.org/10.3390/electronics11162559
Yang N, Li Q, Zhang X, Xu J, Xu S, Liu F. From Transparent Cranial Windows to Multifunctional Smart Cranial Platforms. Electronics. 2022; 11(16):2559. https://doi.org/10.3390/electronics11162559
Chicago/Turabian StyleYang, Nana, Qing Li, Xinyue Zhang, Jingjing Xu, Shengyong Xu, and Fengyu Liu. 2022. "From Transparent Cranial Windows to Multifunctional Smart Cranial Platforms" Electronics 11, no. 16: 2559. https://doi.org/10.3390/electronics11162559
APA StyleYang, N., Li, Q., Zhang, X., Xu, J., Xu, S., & Liu, F. (2022). From Transparent Cranial Windows to Multifunctional Smart Cranial Platforms. Electronics, 11(16), 2559. https://doi.org/10.3390/electronics11162559







