Abstract
Technological innovation in the cosmetic field must necessarily consider not only the safety and efficacy requirements but also the growing attention to environmental issues and the need for sensory pleasure during application to satisfy the consumers’ expectations. This work aimed to formulate an oil-in-water fluid emulsion stabilized by the combination of organic and inorganic powders, namely Zea mays starch and zinc oxide associated at different ratios in presence of a polysaccharidic rheological modifier. After verifying the physical stability of the prototypes with a mechanical stress test in a centrifuge, the rheological analyses were conducted in continuous and oscillatory flow conditions, and the immersion/de-immersion test, conducted using a texture analyzer, allowed identifying the system with the viscoelastic characteristics and the parameters of textures, such as firmness, consistency, and adhesiveness, more suitable for the formulation of skin-care products with low viscosity and high spreadability. Finally, we studied the compatibility of the powders with sunscreen filters and pigments, to optimize the systems and assess their potential use in finished products.
1. Introduction
The widespread use of emulsions in the cosmetic industry is due to their ability to guarantee a simultaneous and synergistic action of the components and to be versatile systems, that can be applied in different areas such as skin care, hair care, personal care, or colour cosmetics [1]. Cosmetic emulsions are colloidal dispersions of two immiscible liquids, generally, oil and water, usually enriched with additives that can contribute to enhancing the functionality, sensorial properties, or quality of the final product. In these complex systems, the presence of the emulsifier is necessary to prevent separation phenomena caused by the high surface energy of the interface [2]. The most common stabilizers are those belonging to the category of surfactants, amphiphilic molecules that stabilize the system by reducing the potential energy at the oil-water interface. To achieve kinetic stability, surfactants are often used in mixed systems or in combination with polymeric emulsifiers; the latter are usually hydrophilic molecules able to swell in water so that the steric hindrance of the chains increases the viscosity of the water phase and prevent droplets’ coalescence thus stabilizing the emulsions [3].
It’s easy to understand that emulsion formulation with kinetic stability is not such a trivial task that became even more challenging in the last years in which environmental issues and social media influence led customers to be more informed and demanding. Consequently, cosmetic market demand is shifting to simple formulations able to ensure not only efficacy and safety but also environmental sustainability through biodegradable components [4]. In this scenario, a particular type of emulsions, the so-called Pickering emulsions, already known since the early twentieth century in literature but still little used, began to arouse interest. Their popularity is a consequence of their “surfactant-free” nature since stabilization is due to solid particle placement at the oil-water interface [5]. Thanks to their partial wettability for both phases, solid particles can adsorb permanently to create a physical shell around dispersed phase droplets; this shield promotes droplet repulsion and minimizes irreversible instability phenomena such as Ostwald ripening or coalescence, leading to the formation of a system subjected only to reversible instability such as creaming or sedimentation [6]. In addition, the absence of surfactants embraces the demand for products intended for sensitive skin, since they may alter the skin pH and cause skin irritation due to the alteration of the multilamellar lipid structure of the stratum corneum [7].
Formulating Pickering emulsions also promotes the use of multifunctional natural cosmetic powders as it is possible to combine their main function such as texturizing, absorbing, viscosifying, and optic properties, with their stabilizing activity. Despite all the advantages provided by Pickering emulsions, it must be noted that the strongest stabilization occurs only when a great quantity of solid particles is used, to build an efficient stabilizing network around droplets [8,9]. Systems formulated with a high concentration of internal phase or solid particles may appear greasy on the skin. Unpleasant sensory feelings must be avoided in a cosmetic product, in order not to affect the consumer’s acceptability and guarantee a continuous use of the product and repeated purchases. Consequently, it is important to know the contribution that each selected raw material, i.e., powders, polymers, emollients, and active substances, will give to the product in terms of texture, application, and sensorial properties, to combine chemical-physical stability with pleasantness during application. For this purpose, rheological and texture analyses are useful instrumental techniques that can help the cosmetic product design: rheological measurements are used to study the flow behaviour and the structure viscoelastic properties, and to forecast the stability issues of products [10,11]. Texture analysis, on the other hand, allows studying the mechanical properties of products during pick-up and application through parameters that can be related to usability and sensoriality, such as firmness, consistency, adhesiveness, and stringiness [12,13,14,15].
Starting from the concept of Pickering emulsions, this work aims to demonstrate the possibility of formulating a surfactant-free, stable, fluid oil in water emulsion, stabilized by the association of mays starch and zinc oxide, adaptable as a versatile basis for the development of cosmetic products. Chemically, starch consists of units of β-D-glucopyranose linked by α-glycosidic bonds arranged in helical structures. Being the storage form of glucose, its components, amylose, and amylopectin, are contained in grains held in many plants’ seeds and stems. Thanks to its great availability, it is widely used in different industrial fields, such as food, pharmaceutics, and cosmetics [16]. Starch is a water-dispersible powder and thanks to its capability to bind water it can swell leading to solvent jellification [17]: for these properties, it can be used as an emulsifier or thickener in cosmetic products, but it can also act as a texturizer that modulates structural characteristics linked to spreadability, playtime, and after-feel. Zinc oxide is an inorganic compound that can be found in nature in the form of a yellow mineral, Zincite, usually together with manganese. In its natural form, zinc oxide crystallizes forming hexagonal structures; in each crystalline unit, the zinc ion is surrounded by four oxygens arranged in a tetrahedral structure, and similarly, oxygen is enveloped by four zinc ions arranged in the same geometry [18]. In the cosmetic industry, it occurs in the form of white powder, and it is used as physic sunscreen which protects the skin from acute and chronic damage of UVB and UVA rays thanks to the absorption, reflection, and diffusion of the radiations [19].
This project was carried out through the preparation of low viscosity o/w emulsions stabilized by binary associations of Zea mays starch and zinc oxide powders used at different ratios and concentrations, on which chemical-physical stability studies, rheological, and texture analyses were performed. Since industrial development requires that the innovation must satisfy market requirements, the formulation must be compatible with the introduction of both technical and active ingredients that can confer the specific cosmetic efficacy properties claimed, also guaranteeing stability, safety, and pleasantness of use. With this concept in mind, we further studied the systems obtained evaluating the structural and applicative characteristics in relation to the introduction of different types of polysaccharidic rheological modifiers and the variation of the composition of the oily phase, considering the compatibility and stability issues. In the second phase of the work, more complex systems were created by introducing specific ingredients such as skin-friendly preservative systems to ensure safety and microbiological quality, a mixture of colored pigments to evaluate the potential use of the system in the development of make-up products, different blends of organic sunscreens to evaluate the potential use of the system in the development of sun protection products.
2. Materials and Methods
2.1. Materials
Organic texturizer: A—CELUS BI FEEL® 10 (INCI name: Zea mays starch, Polyvinyl alcohol, Glycerin) by Roelmi HPC (Origgio (VA), Milan, Italy). It consists of a polymeric blend with predominantly physical interactions, where composition is: Zea mays (corn) starch 50–75%, Polyvinyl alcohol 25–50%, Glycerin 5–10%. Particle size is 5–15 µm with an apparent density of 0.458 g/cm3.
Inorganic texturizer: Z-ZINCO OSSIDO SIG. ORO USP® (INCI name: Zinc oxide) by Res Pharma (Trezzo sull’Adda, Milano, Italy). This inorganic white powder has a relative density of 5.61 g/cm3 and a particle size of 45 µm.
Rheology modifiers: D-KELCO CARE DIUTAN GUM® (INCI name: Sphingomonas ferment extract) by CP Kelco (Atlanta, GA, USA); S- RHEOZAN® SH (INCI name: Succinoglycan) by Solvay (Bruxelles, Belgium); X-XANTHAN GUM® FNCSP-PC (INCI name: Xanthan gum) by Jungbunzlauer (Basel, Switzerland).
Emollients: O1—CETIOL® CC (INCI name: Dicaprylyl carbonate) and O2—CETIOL® OE (INCI name: Dicaprylyl ether, Tocopherol, Glycine soja oil) by BASF (Ludwigshafen am Rhein, Germany); O3—DUB® MCT (INCI name: Caprylic/capryc triglyceride) and O7—DUB® DIPA (INCI name: Dibutyl adipate) by Stearinerie Dubois (Boulogne-Billancourt, France); O4—XIAMETER® PMX-200 SILICONE FLUID (INCI name: Dimethicone) by Dow Corning (Midland, MI, USA); O5—SILKFLO® 366 (INCI name: Hydrogenated polydecene) and O6—DW JOJOBA COLORLESS ORGANIC® (INCI name: Simmondsia chinensis seed oil) by Vantage (Chicago, IL, USA).
Preservatives: P1—DERMOSOFT OCTIOL® (INCI name: Caprylyl glycol) by Evonik (Essenn, Germany); P3—EUXIL PE® 9010 (INCI name: Phenoxyethanol, Ethylhexylglycerin) by Ashland (Wilmington, NC, USA); P2—HYDROLITE® 5 (INCI name: Pentylene glycol) by Symrise (Holzminden, Germany); P4—LIPACIDE® C8G (INCI name: Capryloyl glycine), and LIPACIDE® UG (INCI name: Undecylenoyl glycine) by SEPPIC (Courbevoie, France).
Pigments: Y—TIMICA TERRA® BROWN MN4509 (INCI name: Mica, Iron oxides, Titanium dioxide), TIMICA TERRA® WHITE MN4501 (INCI name: Mica, Titanium dioxide), and TIMICA TERRA® YELLOW MN4502 (INCI name: Mica, Iron oxide, Titanium dioxide) by BASF (Ludwigshafen am Rhein, Germany).
Sun filters: U—PARSOL® 1798 (INCI name: Butylmethoxydibenzoylmethane) by DSM (Kaiseraugst, Switzerland); TINOSORB® S (INCI name: Bis-Ethylhexyloxyphenolmethoxyphenyl Triazine), UVINUL® A PLUS (INCI name: Diethylaminohydroxybenzoylhexyl Benzoate), and UVINUL® T150 (INCI name: Ethylhexyltriazon) all by BASF (Ludwigshafen am Rhein, Germany). The sunscreens filters mixtures required to obtain an SPF of 15, 30, and 50 have been determined in silico using the “Sunscreen simulator” software by BASF that performs calculations according to ISO 24444 of the International Organization for Standardization. This software allows the estimation of several parameters of a mixture of filters, namely the effective protection factor and the absorption values throughout the UV-Vis spectrum. All the materials taken into consideration are last-generation filters authorized by the European Union. All the mixtures are described in Table S1 and they have a ratio between the UVA protection factor (UVA-PF) and the overall protection factor (SPF) higher than 3.
2.2. Methods
The listed materials were used to formulate o/w emulsions and the detailed formulation procedures and percentage concentrations are reported in the “Supplementary Materials” file. Independently on the composition of the emulsion, water (1) and oil (2) phases were heated under stirring at 40–45 °C and emulsification was performed thanks to an L5T laboratory mixer by Silverson (Waterside, Chesham, UK); phase 2 was carefully introduced into phase 1 and then emulsified at 4500 rpm for 5 min.
Zea mays starch (A) and Zinc Oxide (Z) were used both separately and in association; as shown in Table S2, A was dispersed in phase 1 under stirring at 40 °C, while Z was dispersed in phase 2 at 45 °C. The polysaccharides used in the ternary association’s Xanthan gum (X), Succinoglycan (S), and Diutan gum (D) were dispersed in phase 1 at 40 °C (Table S3). The emollients used in the oily phase and the relative concentrations of use are listed in Table S4. Broad spectrum preservatives have been introduced according to their chemical properties (Table S5): Caprylyl glycol (P1) and Pentylen glycol (P2) have been introduced into phase 1 before emulsification; Capryloyl glycine and Undecylenoyl glycine (P4) have been introduced into phase 2 before emulsification; Phenoxyethanol and Ethylhexylglycerin (P3) were introduced after emulsification at a temperature lower than 40 °C. Pigments (Y) and sunscreen filters (U) mixtures were added to the oil phase before emulsification as shown in Tables S6 and S7. All the described emulsions had a pH value of 7.5 ± 0.2.
To predict physical instability phenomena, samples were subjected to strong mechanical stress conditions by using the centrifuge MPW-56 of MED, at 3000 rpm (835 RCF) and 4800 rpm (2138 RCF) for 30 min. To investigate long-term stability, the samples were subjected to the accelerated aging test at 40 °C in a thermostatic oven for 90 days; samples were placed in pyrex glass jars and parameters such as organoleptic aspect, viscosity, and pH were monitored monthly. The morphological analysis has been performed using a LEICA DM1000 optical microscope and images were taken with a 40× objective.
The rheological tests have been performed using an Anton Paar MCR 302e controlled rate rheometer (Anton Paar GmbH, Graz, Austria) equipped with a PP50-P2 sensor (50 mm parallel plates with serrated surfaces, gap 1 mm) and with a Peltier system for temperature control. The rheological tests have been performed at 23 ± 0.05 °C. Each sample was subjected to both rotational and oscillatory tests. The controlled share rate test (CSR) allowed obtaining a flow curve representing the variation of the viscosity depending on the applied shear rate (from 0.001 s−1 to 1000 s−1). Amplitude sweep tests (AS) were performed increasing the strain (γ) from 0.01% to 1000%, at a fixed oscillation frequency of 1 Hz, to identify the samples’ linear viscoelastic region (LVER) and the critical strain (γ G′ = G″). Frequency sweep tests were performed by decreasing the oscillation frequency from 10 Hz to 0.01 Hz, at a fixed strain inside the LVER, to analyze the emulsions’ inner structure and the trend of storage (G′) and loss (G″) moduli.
Texture analyses were performed with TEXTURE ANALYZER TMS-PRO of Food Technology Corporation (Sterling, VA, USA), equipped with a load cell of 10 Newtons and a spherical nylon probe with a diameter of 2 cm. The analysis speed was set to 80 mm/min and the displacement, the depth of penetration of the probe into the sample, to 10 mm: the probe moved vertically inside the sample loaded in specific jars with a volume of 50 mL, and then returned to initial position. Texture Lab Pro software was used to collect and elaborate the data, showing a curve plotted as load (N) vs. cumulative displacement (mm) where we identified five texture parameters: firmness (N) is the maximum value of force; consistency (N.mm) is the area under the positive curve; cohesiveness (N) is the negative peak; adhesiveness (N.mm) is the area under the negative portion of the curve; stringiness (mm) is the extension of the filament the sample formed during the de-immersion phase.
3. Results and Discussions
In the first phase of this work, the stabilizing properties of the selected raw materials, i.e., Zea mays starch (A) and zinc oxide (Z) used alone and in association with each other, were investigated. Starches are known in the cosmetic field for their viscosifying and texturizing capacity. Thanks to their ability to swell in water and to position themselves at the interface of the emulsion, the starch granules can be useful for improving the stability of the system, giving the product marked sensory performances during application and reducing its greasiness [20]. In addition to being commonly used as a physical filter in solar products, zinc oxide is a material of considerable interest for the formulation of Pickering emulsions, as evidenced by several scientific articles on the use of zinc oxide nanoparticles for the stabilization of the emulsions dispersed droplets [21,22]. The selected powders A and Z were inserted individually at 5% w/w in oil-in-water emulsions with increasing oil phase (O) content from 20% to 40% w/w composed by a mixture of Dicaprylyl carbonate and Caprylic/capric triglyceride in equal amounts (ratio 1:1). While the emulsions containing starch (A) were all stable after 24 h at room temperature, among the emulsions with zinc oxide (Z) only the one containing 40% w/w of oily phase was stable. Lower percentages of the oily phase determined a reduction of the viscosity of the system leading to phase separation and flocculation of zinc oxide particles. None of these emulsions containing 5% w/w of powders was stable at the centrifuge test conducted at 3000 rpm for 30 min, showing phase separation. To obtain stable systems even when subjected to mechanical stress tests, emulsions were prepared by keeping the oily phase constant at 40% w/w and varying the solid phase concentration (5–7.5–10–15% w/w). The emulsions thus formulated were all stable at room temperature after 24 h; however, only the one containing 15% of A (A15 O40) and the one containing 15% of Z (Z15 O40) passed the centrifuge test, showing no phase separation (Table 1).

Table 1.
Stability test results of systems emulsified with Zea mays starch (A) and zinc oxide (Z) used alone at different concentrations, varying the percentages of oily phase (O).
This preliminary screening made it possible to highlight that stable emulsions are formed only in the presence of high percentages of powders and emollients, respectively at 15% w/w and 40% w/w. However, these systems were characterized by a pronounced sticky and greasy after-feel due to the high concentration of lipids, which had a negative impact on the sensory profile and consumers’ acceptability.
Considering the importance of the sensory aspects in addition to the functional ones to obtain a successful cosmetic product, since the aim of the work was to formulate low-viscosity emulsions with a light texture, we used the powders A and Z in binary associations, evaluating their stabilizing properties (Table 2). A formulation systematic was set up keeping the number of oils at 40% w/w constant and increasing the total concentration of powders (5–10–15% w/w), keeping the ratio between A and Z fixed at 1:1. These samples were stable at room temperature 24 h after formulation. The mechanical stability test in the centrifuge, conducted at 3000 rpm for 30 min, confirmed what was observed previously with the emulsions containing a single powder: the only emulsion stable was AZ15 O40, with high concentrations of powders (15% w/w), which determined the same sensorial critical issues encountered in the previous section. In fact, the comparison between the parameters registered during the de-immersion phase of the texture analysis of the emulsions prepared by increasing the amount of total solid phase at fixed oil amount (40% w/w), revealed that AZ15 O40 with 7.5% w/w of Zea mays starch and 7.5% w/w of zinc oxide was characterized by the highest values of adhesiveness (Figure 1a) and stringiness (Figure 1b). These parameters are often related to the adhesive and film-forming properties of the formulation, but if they are too unbalanced, they can describe systems that are not pleasant during the application phase because they have poor spreadability and are judged sticky and stringy.

Table 2.
Stability test results of systems emulsified with binary associations of Zea mays starch (A) and zinc oxide (Z) (ratio 1:1) varying the percentages of the total solid phase and of the oily phase (O).
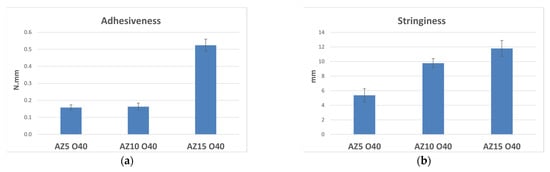
Figure 1.
Textural parameters and standard deviations of adhesiveness (a) and stringiness (b) measured by an immersion/de-immersion test for the emulsions prepared with A-Z associations (1:1) increasing the amount of the total solid phase (5–10–15% w/w) at fixed oil amount (40% w/w).
We have therefore formulated emulsions containing an intermediate concentration of powders (10% w/w) keeping the ratio between A and Z fixed and reducing the oily phase (40–30–20% w/w). By decreasing the oily phase there was a worsening of stability, even at room temperature since the sample AZ10 O20 formulated with 20% w/w of emollients showed phase separation within 24 h. The consequent increase in the percentage of aqueous phase leads to a decrease in the viscosity of the system, accelerating the onset of instability phenomena.
3.1. Ternary Systems: Zea mays Starch (A), Zinc Oxide (Z), and a Polysaccharide
To obtain stable and sensorial pleasant emulsions even with a low percentage of emollients, we have added a polysaccharide raw material to the systems containing binary combinations of A and Z. The multifunctionality of polysaccharides is well known in the cosmetic field, since they are the first-choice ingredients when formulating a green product, especially for their biodegradability and versatility. In fact, they are widely used as moisturizers, suspenders, and rheological modifiers, as they can interact with the water molecules and swell, giving high viscosity and stability to the system. We selected three polysaccharides, namely xanthan gum (X), succinoglycan (S), and diutan gum (D) whose rheological behaviour has been extensively studied in the scientific literature [23] and which are used in the cosmetic formulation for their efficient viscosifying and texture characteristics, showing high stability at low-stress conditions and pronounced shear thinning properties. These polysaccharides have been inserted at increasing concentrations in emulsions containing an A-Z combination at 10% w/w (ratio 1:1) with an oily phase of 10% w/w. The stability of the obtained systems was studied, and the results are summarized in Table 3. All the emulsions were stable after 24 h from the formulation at room temperature. As revealed by the results of the mechanical stress test in centrifuge conducted at 3000 and 4800 rpm, emulsions with xanthan gum were not stable, whereas succinoglycan was able to stabilize emulsions only when used in concentrations equal to or greater than 0.3% w/w. Only diutan gum was able to stabilize the systems even at a concentration of 0.1% w/w.

Table 3.
Stability test results of systems with 10% w/w of oil phase emulsified with the ternary associations of Zea mays starch (A), zinc oxide (Z), and a polysaccharide at increasing concentrations.
In Figure 2 a rheological characterization of the systems formulated with the same concentration of polysaccharides (0.3% w/w) is shown. All the emulsions subjected to a Controlled Shear Rate test showed a pseudoplastic shear-thinning behaviour since, after the so-called Newtonian plateau region in which the viscosity remained almost constant, the viscosity progressively decreased by increasing the values of shear rate (Figure 2a). The emulsion with xanthan gum (AZ10 X03) showed up to two decades lower values of viscosity than the emulsion with succinoglycan (AZ10 S03) and diutan gum (AZ10 D03). The mechanical spectrum obtained through a Frequency Sweep test described a liquid-like behaviour of the emulsion containing X, since the loss modulus G″ was greater than the storage modulus G′ (Figure 2b). This is an indication of a system that is not stable over time, in accordance with the results of the mechanical centrifuge test. On the contrary, the emulsions containing S and D showed a typical weak-gel trend in which G′ is always greater than G″ in the whole range of frequencies investigated. The moduli were located between the first and the second decades and their course was dependent on the frequency applied. This rheological profile indicates that the systems are held together by weak physical interactions between the polymer chains and the vehicle.
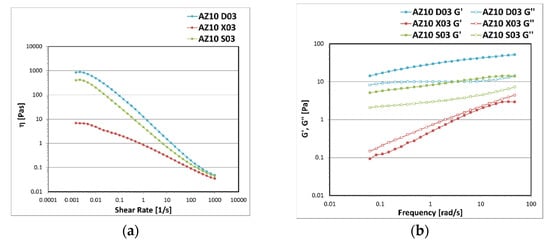
Figure 2.
Viscosity trend as a function of shear rate (a) and storage G′ and loss G″ moduli trend as a function of frequency (b) measured for the emulsions prepared with A-Z associations (1:1) and the polysaccharides D, X, and S at 0.3% w/w.
In addition to having higher values of viscoelasticity, the AZ10 D03 emulsion showed higher values of the texture parameters’ firmness and adhesiveness than AZ10 X03 and AZ10 S03 (Figure 3). Diutan Gum is a microbial-origin anionic polymer formed by a main chain of D-glucose, D-glucuronic acid, D-glucose, and L-rhamnose, and a side chain of two L-rhamnoses. This chemical structure allows the formation of a more regular and orderly double helix structure than Xanthan gum and Succinoglycan: the carboxylate groups placed inside the double helix structure together with the formation of hydrogen bridges and intra-chain interactions help to stabilize the association of the two backbones, forming stiffer and resistant networks. Moreover, a large number of water molecules can be efficiently internalized and retained in the polymeric core.
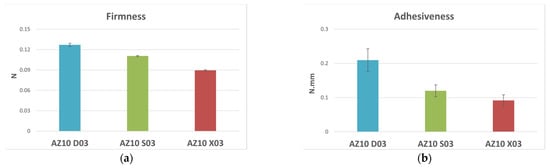
Figure 3.
Textural parameters and standard deviations of firmness (a) and adhesiveness (b) measured by an immersion/de-immersion test for the emulsions prepared with A-Z associations (1:1) and the polysaccharides D, S, and X at 0.3% w/w.
Images obtained with optical microscopy of the samples AZ D03 and AZ S03 highlighted the great efficiency of diutan gum polysaccharide in the formation of a more structured network, where the internal phase is more homogeneously dispersed (Figure 4a) than in the sample with succinoglycan. Moreover, in sample AZ10 S03 (Figure 4b) reddish areas are visible which correspond to agglomerates of zinc oxide, that are not present in sample AZ10 D03, indicating a greater suspending ability of polysaccharide D.
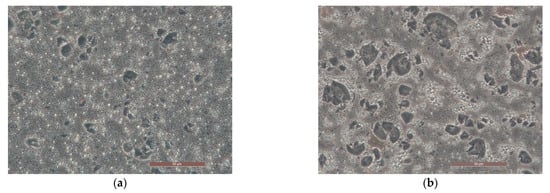
Figure 4.
Optical microscopies with 40× magnification of emulsion AZ10 D03 (a) and AZ10 S03 (b) prepared with A-Z (ratio 1:1), 10% w/w of oily phase O and 0.3% w/w of polysaccharides D (Diutan gum) and S (Succinoglycan).
We evaluated the influence of increasing the concentration of diutan gum (from 0.1 to 0.5% w/w) on the morphological, rheological, and texture properties of the emulsions formulated with 10% of powders (A-Z ratio 1:1) in presence of 10 or 40% w/w of the oily phase. All the systems were stable at the centrifuge test conducted both at 3000 and 4800 rpm. Morphological analyses performed through optical microscopy on the samples prepared with 40% w/w of oils confirmed that diutan gum improved the stability of the systems: internal phase droplets in emulsions AZ D01 O40 and AZ D05 O40 (Figure 5b,c) are smaller and more homogeneous when compared to the emulsion AZ10 O40 (Figure 5a), with no polysaccharide. In addition, the increment of both the polymer and the internal phase concentrations led to the formation of very structured and stiff systems where zinc oxide was not efficiently dispersed and tended to pack in reddish agglomerates.
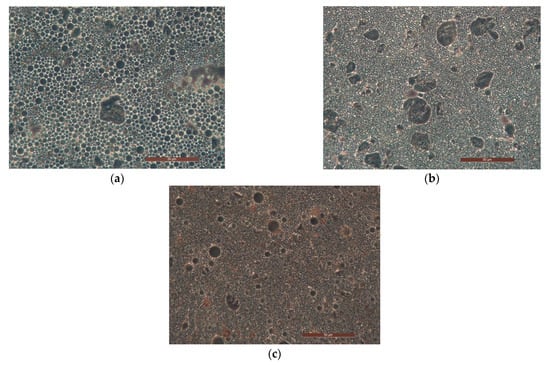
Figure 5.
Optical microscopies with 40× magnification of emulsions prepared with A-Z association (ratio 1:1), 40% w/w of oil phase O without (a), and with the polysaccharide D at 0.1% w/w (b), and D at 0.5 % w/w (c).
The rheological curves in Figure 4 obtained from the AS analyses show that the viscoelastic structure increased by increasing the percentage of the polysaccharide, as the polymer chains were closely packed and in intimate contact forming a three-dimensional network that trapped the water. Inside the LVER, which is characterized by constant and stress−amplitude independent moduli, since the material does not change irreversibly, G′ was larger than G″ indicating a predominantly elastic behaviour of the sample. After this region, G′ progressively decreased with the increase of the strain until a critical strain point was located, in which it intercepts G″ with consequent inversion of the moduli, indicating the irreversible breakage of the structure. By increasing the amount of D an extension of the LVER and a shift of the critical strain at higher percentage values can be noted, indicating the ability of the polysaccharide to stabilize the system making it more resistant to the applied deformation. As expected, the emulsions formulated with 10% w/w of the oily phase (Figure 6a) showed lower values of moduli than the emulsions formulated with 40% w/w of the oily phase (Figure 6b), since the increase of the inner phase volume and the consequent decrease of the aqueous outer phase determine an increase of the viscosity of the system.
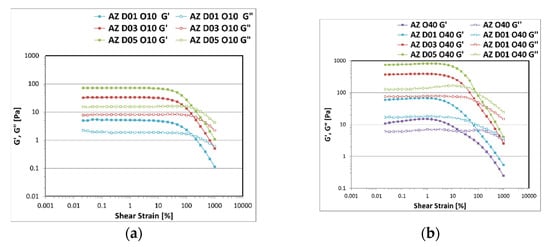
Figure 6.
Storage G′ and loss G″ moduli trend as a function of the oscillation strain measured for the emulsions prepared with A-Z associations (1:1) and increasing concentration of D (0.1–0.3–0.5% w/w) with 10% w/w (a) and 40% w/w of oily phase (b).
Consistently with the rheological data, in the histograms shown in Figure 7, it is possible to note that the increase in the concentration of D led to an increase in the texture parameters of firmness (Figure 7a) and adhesiveness (Figure 7b), while the stringiness decreased since the more the system became structured, the less filament was formed during the de-immersion phase of the probe, indicating a greater resistance of the system (Figure 7c). These variations were more evident in the emulsions formulated with 40% w/w of oily phase compared to those with 10% w/w of oily phase in which the texture parameters of firmness and adhesiveness were significantly lower.
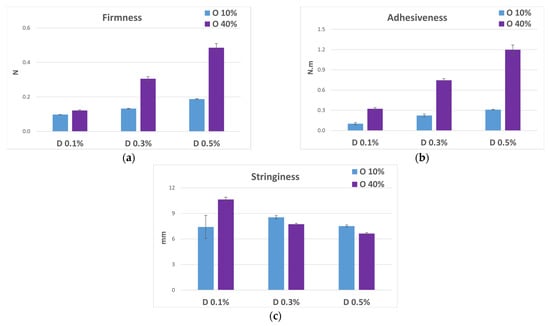
Figure 7.
Textural parameters and standard deviations of firmness (a), adhesiveness (b), and stringiness (c) measured by an immersion/de-immersion test for the emulsions prepared with A-Z associations (1:1), D at increasing concentrations (0.1–0.3–0.5% w/w) with 10% w/w (blue columns) and 40% w/w (purple columns) of oily phase O.
By reducing the amount of the solid phase composed by the association between A and Z, and keeping the concentration of D fixed at 0.1% w/w with an oily phase of 10% w/w, there was a loss of the physical stability of the system, since a phase separation and zinc oxide precipitation was observed after the mechanical stress tests conducted in the centrifuge at 4800 rpm for 30 min (Table 4). The quantitative reduction of the solid phase influences the thickness of the mechanical barrier created around the droplets of the dispersed phase, which are prone to coalescence, determining a reduction of the interfacial areas available for zinc oxide particles, which precipitate. The rheological AS curves obtained for the emulsions in the function of the total amount of the solid phase AZ are reported in Figure 8. The reduction of the powders determined a quantitative decrease in the viscoaelastic moduli of the system.

Table 4.
Stability test results of emulsions formulated with 10% w/w of oil phase O, 0.1% w/w of diutan gum D and the different total amounts of solid phase composed by Zea mays starch A and zinc oxide Z (ratio 1:1).
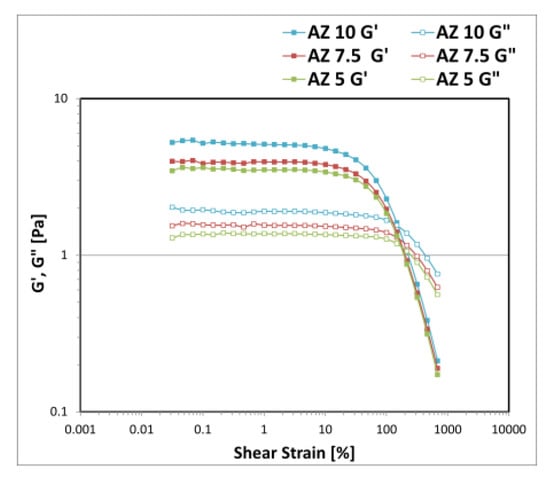
Figure 8.
Storage G′ and loss G″ moduli trend as a function of the oscillation strain measured for the emulsions prepared with A-Z associations (1:1) decreasing the total solid-phase amount (10–7.5–5% w/w) with D at 0.1% w/w and 10% of oily phase.
3.2. Variation of the A-Z Ratios
The physical-mechanical behavior of the emulsions was studied as the ratio between the stabilizing powders A and Z changed, which until now had been kept fixed at 1:1. While keeping the content of polysaccharide D fixed at 0.1% w/w, oily phase O fixed at 10% w/w and the total percentage of solid phase AZ fixed at 10% w/w, emulsions were formulated by varying the ratios between A and Z and the stability of the systems obtained was investigated by centrifuge tests (Table 5).

Table 5.
Stability test results of emulsions formulated with 10% w/w of oil phase O, 0.1% w/w of diutan gum D and different ratios between Zea mays starch A and zinc oxide Z (total solid phase at 10% w/w).
Only the samples formulated with an A:Z ratio equal to 1:1, 1:2, 2:1 were stable after centrifugation at 4800 rpm for 30 min, confirming that a balance between the powders is mandatory to homogeneously coat the surface of the dispersed phase droplets and obtain stable formulas. The structural properties of the emulsions that passed the stability tests were evaluated by rheological tests in oscillatory flow conditions (Figure 9). The trends of G′ and G″ as a function of frequency, measured by keeping constant the strain value within the LVER, represent the emulsion’s “mechanical spectra” and describe its structural properties. By varying the ratios between the powders, with the same concentration of polysaccharide D, the emulsions do not show significant differences in the viscoelastic properties (Figure 9a). G′ was always greater than G″ in the whole frequency range investigated and the moduli trend was dependent on the frequencies applied. By increasing the concentration of D at 0.2% w/w (Figure 9b) and at 0.3% w/w (Figure 9c), a quantitative increase of both moduli was obtained, since the aqueous external dispersing phase was retained and bound by the polysaccharide chains.
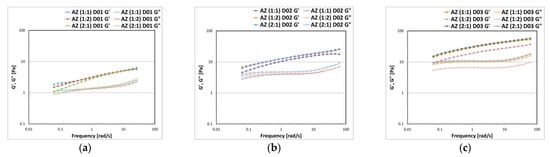
Figure 9.
Storage G′ and loss G″ moduli trend as a function of the oscillation frequency measured for the emulsions prepared with 10% w/w of oily phase O, A-Z associations at different ratios (1:1–1:2–2:1) at the same total solid phase amount (10% w/w) and D at 0.1% w/w (a), 0.2% w/w (b), and 0.3% w/w (c).
The texture properties of the emulsions were analyzed through an immersion de-immersion test and the results in terms of firmness and adhesiveness are reported in Figure 10. In accordance with the rheology results, there were no significant differences between the emulsions prepared by varying the ratios between A and Z, keeping the concentration of D fixed. It was possible to note only a consistent increase in the values of the texture parameters as the percentage of polysaccharide rheological modifier increased (Figure 10a,b).
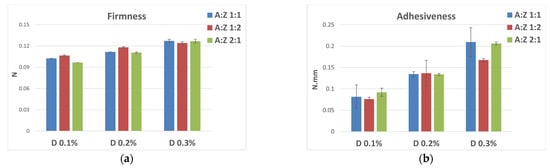
Figure 10.
Textural parameters and standard deviations of firmness (a) and adhesiveness (b) measured by an immersion/de-immersion test for the emulsions prepared with A-Z associations (1:1) and the polysaccharides D, S, and X at 0.3% w/w.
For the next phases of the work, we have chosen to continue the compatibility and stability studies on emulsions formulated with an A:Z ratio of 1:1 (total content of powders 10% w/w), with the polysaccharide rheological modifier D at 0.1% w/w and the oily phase fixed at 10% w/w. This combination allowed us to obtain fluid, stable, sensorily pleasing emulsified systems with balanced texture properties.
3.3. Variation of the Composition of the Oily Phase
The emulsions formulated up to now had an oily phase O composed of a mixture of Dicaprylyl carbonate and Caprylic/capric triglyceride in equal amounts (ratio 1:1). To study how the qualitative composition of the oily phase can affect the application characteristics of the system, this binary mixture has been replaced by emollients belonging to different chemical classes and which differ in polarity, density, and solvent capacity. The emulsions thus obtained, and the relative results of the stability studies are summarized in Table 6.

Table 6.
Stability test results of emulsions formulated with Zea mays starch A and zinc oxide Z (total solid phase at 10% w/w), diutan gum D at 0.1% w/w, and different oil phases O at 10% w/w.
The system was stable when formulated with esters of medium-chain saturated fatty acids, as in the sample AZD O1 with Dicaprylic carbonate, and triglycerides, as in the sample AZD O3 with Caprylic/capric triglyceride, or silicone oils, as in the sample AZD O4 with dimethicone. On the contrary, the systems AZD O6 formulated with the natural origin oil with a high content of unsaturated fatty acids and AZD O7 formulated with the diester Dibutyl adipate showed physical instability phenomena in the centrifuge test conducted at 3000 rpm. The emulsions that resulted stable at the test conducted at 4800 rpm underwent rheological and texture analyses (Figure 11). The mechanical spectra did not show significant differences between the samples formulated with different oily phases (Figure 11a). Their behaviour was typical of low viscosity emulsions, since the moduli were in the first decade, indicating stability over time, having the elastic component represented by the G′ modulus always dominant in the whole frequency range. The textural curves reported in Figure 11b were almost overlapped since no significative differences could be found in terms of texture parameters. All emulsions showed low values of firmness, consistency, and adhesiveness, indicating a fluid and spreadable system with a light and non-greasy texture.
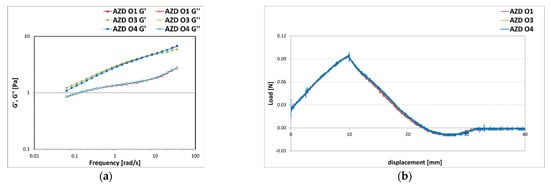
Figure 11.
Storage G′ and loss G″ moduli trend as a function of the oscillation frequency (a) and texture curves obtained from an immersion/de-immersion test (b) for the emulsions prepared with A-Z associations (ratio 1:1) at a total solid amount of 10% w/w, D at 0.1% w/w, and different oily phase O1, O3, and O4 at 10% w/w.
3.4. Inclusion of a Preservative System
Using the starting oily phase composed of Dicaprylyl carbonate and Caprylic/capric triglyceride, we decided to introduce preservative molecules into the system, evaluating the compatibility and the impact on the stability and mechanical properties. Cosmetic products, in fact, require adequate protection against microbial contamination to ensure consumer safety and increase the shelf-life. The preservative molecules (P) selected in this study are characterized by a broad-spectrum activity and compatibility with a wide range of pH. The emulsions obtained have been submitted to stability tests in the centrifuge and the results are summarized in Table 7.

Table 7.
Stability test results of emulsions formulated with Zea mays starch A and zinc oxide Z (total solid phase at 10% w/w), diutan gum D at 0.1% w/w, oily phase at 10% w/w and different preservative molecules P.
Except for AZD P4, all formulations were stable in centrifuge and analyzed by rheological and texture analyses (Figure 12). The results showed negligible differences, which do not impact the application properties. The AZD P2 sample had slightly more elastic properties than the others, especially at lower oscillation frequencies (Figure 12a). In addition, it also showed higher firmness than the other samples, while maintaining the same values of adhesiveness, cohesiveness, and stringiness (Figure 12b).
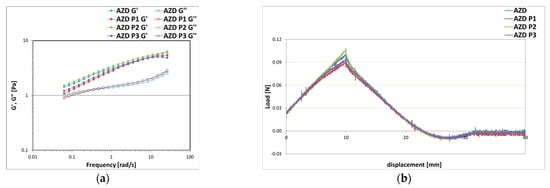
Figure 12.
Storage G′ and loss G″ moduli trend as a function of the oscillation frequency (a) and texture curves obtained from an immersion/de-immersion test (b) for the emulsions prepared with A-Z associations (ratio 1:1) at a total solid amount of 10% w/w, D at 0.1% w/w, oily phase at 10% w/w and different preservative molecules P1, P2, and P3.
Samples AZD P2 and AZD P3 were subjected to the accelerated aging test in a thermostatic oven at 40 °C and were compared with the same samples at room temperature. After three months no changes in the viscosity, organoleptic aspects, and pH values were registered neither for the samples at 40 °C and 23 °C.
3.5. Inclusion of Pigments
In this phase of the study, we evaluated the possibility of using the type of emulsion studied for a make-up product with a fluid texture. A mixture of three pigments (Y) was added to the formulated emulsion keeping the ratio between the powders A and Z fixed (1:1) at a total solid phase concentration of 10% w/w, in the presence of 0.1% w/w of polysaccharide D, using an oily phase composed of Dicaprylyl carbonate and Caprylic/capric triglyceride at 10% w/w and a preservative system composed of Phenoxyethanol and Ethylhexyl glycerine at 1% w/w. The emulsion thus formulated was not stable in the centrifuge stability tests since powder precipitation and phase separation was observed. The percentage of polysaccharide rheological modifier D was thus increased to stabilize the system. The results of the stability tests on the formulated emulsions are shown in Table 8.

Table 8.
Stability test results of emulsions formulated with Zea mays starch A and zinc oxide Z (total solid phase at 10% w/w), the oily phase at 10% w/w, a mixture of pigments Y at 3% w/w, and diutan gum D at increasing concentrations (0.1–0.2–0.3% w/w).
By increasing the concentration of the polysaccharide, we ensured physical stability to the system because the degree of structuring of the external aqueous phase had increased, creating a three-dimensional network able to efficiently keep the powders in suspension.
Figure 13 shows the comparison between the physical and mechanical properties of the formulation that resulted to be stable under mechanical stress and the pigment-free formulation with the same amount of powders A and Z (10% w/w) and polysaccharide D (0.3% w/w). The introduction of pigments did not alter the rheological structural properties of the emulsion (Figure 13a) and had no impact on the texture properties, as the curves obtained are superimposed (Figure 13b). Even if the D content has been increased, the emulsions were still fluid and flowing, having both G′ and G″ moduli between the first and second decade and firmness values between 0.10 and 0.15 N.
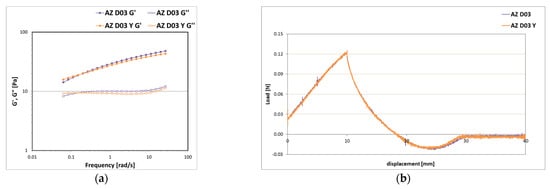
Figure 13.
Storage G′ and loss G″ moduli trend as a function of the oscillation frequency (a) and texture curves obtained from an immersion/de-immersion test (b) for the emulsions prepared with A-Z associations (ratio 1:1) at a total solid amount of 10% w/w, D at 0.3% w/w, an oily phase at 10% w/w with and without 3% w/w of a pigments mixture Y.
The inclusion of pigments in the formula did not change the long-term stability of the system: after three months at 40 °C in a thermostatic oven, the sample did not show any irreversible changes.
3.6. Inclusion of Sunscreen Filters
The emulsions object of this study, being stabilized thanks to zinc oxide, a compound widely known for its UV filter properties, could represent an interesting and innovative basis for the formulation of sun protection products. We evaluated the possibility of inserting a mixture of latest-generation high-spectrum sun filters in the emulsions containing the association between A and Z (ratio 1:1) at a total solid phase concentration of 10% w/w, in the presence of 0.1% w/w of polysaccharide D, using an oily phase composed of Dicaprylyl carbonate and Caprylic/capric triglyceride and a preservative system composed of Phenoxyethanol and Ethylhexyl glycerine at 1% w/w. The ratio between the filter mixture U and the oily phase was kept fixed at 1:1. The amount of filters and consequently of oils has been varied to guarantee an SPF protection factor of 15, 30, and 50 evaluated in silico using the “Sunscreen Simulator” software produced by BASF Care Creations® (see Materials and Methods sections). All the emulsions obtained were stable in the centrifuge test conducted at 4800 rpm for 30 min (Table 9).

Table 9.
Stability test results of emulsions formulated with Zea mays starch A and zinc oxide Z (total solid phase at 10% w/w), diutan gum D at 0.1% w/w, and increasing concentration of oily phase O and a mixture sunscreen filters U (9–15–23.5% w/w).
Comparing the formulation AZD without filters and the formulation AZD U1 with 9% w/w of sunscreens mixture (SPF 15) with the same amount of emollients, stabilizing powders A and Z, and diutan gum D, it is noted that the introduction of sunscreens did not significantly change the rheological and texture properties of the system that remain almost unchanged (Figure 14).
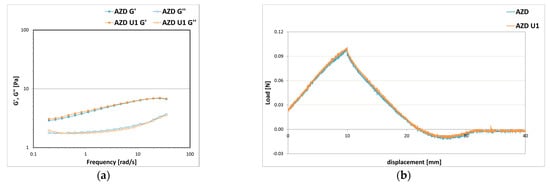
Figure 14.
Storage G′ and loss G″ moduli trend as a function of the oscillation frequency (a) and texture curves obtained from an immersion/de-immersion test (b) for the emulsions prepared with A-Z associations (ratio 1:1) at a total solid amount of 10% w/w, D at 0.3% w/w, an oily phase at 9% w/w with and without 9% w/w of sun filters mixture U.
In Figure 15 a comparison between the rheological profiles of the emulsions containing increasing concentrations of filters and oil phase (9–15–23.5% w/w), variation necessary to guarantee a sun protection factor of 15, 30, and 50 respectively, is shown. The increase in the volumetric fraction of the dispersed internal phase was reflected in the increase in the elastic properties of the system as shown by the increase in the complex modulus G* values and the decrease in the damping factor tan δ values. G* is defined as the ratio between the total stress and the strain to which a material is subjected at a given frequency and describes the entire viscoelastic behaviour of the sample. AZD U3 showed the highest G* values, indicating more structured and stiffer systems (Figure 15a) Tanδ is a rheological parameter that describes the ratio between the viscous and the elastic modulus; values of tan δ < 1 correspond to viscoelastic behaviour. AZD U3 showed the lowest tan δ values thus indicating a more elastic structure (Figure 15b).
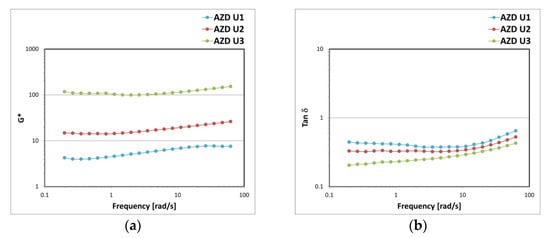
Figure 15.
Complex G* modulus (a) and damping factor tanδ (b) trend as a function of the oscillation frequency for the emulsions prepared with A-Z associations (ratio 1:1) at a total solid amount of 10% w/w, D at 0.1% w/w, and increasing amounts of a mixture of filters U (AZD U1 with 9% w/w of U; AZD U2 with 15-% w/w of U; AZD U3 with 23.5% w/w of U).
The results obtained with the texture analysis were in accordance with those obtained from the rheological tests, as the AZD U3 sample with increased internal phase showed greater values of firmness, consistency, and adhesiveness (Figure 16). If compared to the samples AZD U1 and AZD U2 it was characterized by lower spreadability, since more force was necessary to deform it, and could determine a more persistent after-feel.
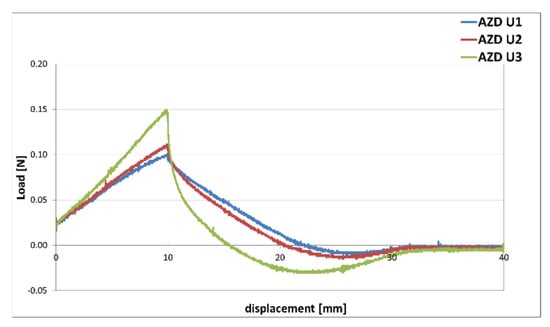
Figure 16.
Texture curves obtained from an immersion/de-immersion test for the emulsions prepared with A-Z associations (ratio 1:1) at a total solid amount of 10% w/w, D at 0.1% w/w, and increasing amounts of a mixture of filters U (AZD U1 with 9% w/w of U; AZD U2 with 15% w/w of U; AZD U3 with 23.5% w/w of U).
The inclusion of sunscreens did not affect the long-term stability of the system: organoleptic properties, viscosity, and pH values remained unchanged after three months at 40 °C in the thermostatic oven.
4. Conclusions
This work has confirmed how the characterization of the physical-mechanical properties of cosmetic emulsions allows the identification of the most suitable ratios between all the components of the formula and the optimization of the formulation process to obtain stable and sensorily pleasing systems. The preparation of formulation systematics and the instrumental approach utilizing the combination of rheology and texture analyses made it possible to evaluate the potential of the use of a base formulation for low viscosity surfactant-free emulsions stabilized by the association of zea starch A and zinc oxide Z, inspiring from the concept of Pickering emulsion. A versatile system was obtained that is adaptable to market needs, which include, in addition to stability and safety, also sensoriality and effectiveness, and, therefore, the ability to convey technical and active ingredients such as pigments and sunscreens. The more balanced A:Z ratios (1:2, 1:1, and 2:1), in the presence of a polysaccharide rheological modifier such as diutan gum D at low concentrations (0.1–0.3% w/w), guarantee the obtainment of stable samples, showing low to medium viscosity, pick-up, and adhesiveness parameters. The analytical techniques used made it possible to carefully compare the formulas, highlighting their different structural characteristics and application properties, and to evaluate their compatibility with emollients and preservative molecules belonging to different chemical classes. The powders stabilized emulsion model studied in this work proved to be versatile and modulable, suitable for use in various cosmetic applications, from make-up products to sunscreens, when low viscosities, easy application and sensorial characteristics are required.
5. Patents
This work led to the filing of a patent in Italy about an oil-in-water powder stabilized emulsified composition [24].
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cosmetics9060123/s1, Table S1: sunscreen filters and relative concentrations used to prepare the mixtures inserted in the samples with a protection factor SPF of 15, 30, and 50 calculated with the Sunscreen Simulator software by BASF. Table S2: formulation scheme of emulsions stabilized by Zea mays starch (A) and Zinc oxide (Z), and relative concentration ranges used. Table S3: formulation scheme of emulsions stabilized by the ternary association of Zea mays starch (A), Zinc oxide (Z) and Xanthan gum (X), Sclerotium gum (S), and Diutan gum (D), and relative concentration ranges used. Table S4: formulation scheme of emulsions stabilized by the ternary association of Zea mays starch (A), Zinc oxide (Z), and Diutan gum (D) with different emollients and relative concentrations used. Table S5: formulation scheme of emulsions stabilized by the ternary association of Zea mays starch (A), Zinc oxide (Z), and Diutan gum (D) with the introduction of different preservative molecules and relative concentrations used. Table S6: formulation scheme of emulsions stabilized by the ternary association of Zea mays starch (A), Zinc oxide (Z), and increasing concentrations of Diutan gum (D) with the introduction of 2.5% w/w of pigments. Table S7: formulation scheme of emulsions stabilized by the ternary association of Zea mays starch (A), Zinc oxide (Z) and and increasing concentrations of Diutan gum (D) and different amounts of sun filters mixture (U).
Author Contributions
Conceptualization, A.S. and A.C.; investigation, E.D.D. and G.T.; resources, L.B.; project administration A.C.; formal analyses, G.T.; writing—original draft preparation, G.T.; writing—review and editing, E.D.D.; supervision, G.B. and S.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors would like to acknowledge Giulia Galizia from Unired Srl. for writing assistance and language editing.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Venkataramani, D.; Tsulaia, A.; Amin, S. Fundamentals and applications of particle stabilized emulsions in cosmetic formulations. Adv. Colloid Interface Sci. 2020, 283, 102234. [Google Scholar] [CrossRef]
- Guzmán, E.; Ortega, F.; Rubio, R.G. Pickering Emulsions: A novel tool for cosmetic formulators. Cosmetics 2022, 9, 68. [Google Scholar] [CrossRef]
- Carlotti, M.E.; Pattarino, I.F.; Gasco, M.R.; Cavalli, R. Use of polymeric and non-polymeric surfactants in o/w emulsion formulation. Int. J. Cosmet. Sci. 1995, 17, 13–25. [Google Scholar] [CrossRef]
- Bordes, C.; Bolzinger, M.A.; El Achak, M.; Pirot, F.; Arquier, D.; Agusti, G.; Chevalier, Y. Formulation of Pickering emulsions for the development of surfactant-free sunscreen creams. Int. J. Cosmet. Sci. 2021, 43, 432–445. [Google Scholar] [CrossRef]
- Sun, Z.; Yan, X.; Xiao, Y.; Hu, L.; Eggersdorfer, M.; Chen, D.; Yang, Z.; Weitz, D.A. Pickering emulsions stabilized by colloidal surfactants: Role of solid particles. Particuology 2022, 64, 153–163. [Google Scholar] [CrossRef]
- Chevalier, Y.; Bolzigner, M.A. Emulsions stabilized with solid nanoparticles: Pickering emulsions. Colloids Surf. A Physiochem. Eng. Asp. 2013, 439, 23–34. [Google Scholar] [CrossRef]
- Sewerin, A. Interactions between surfactants and the skin—Theory and practice. Adv. Colloid Interface Sci. 2018, 256, 242–255. [Google Scholar] [CrossRef] [PubMed]
- Pang, B.; Huan, L.; Zhang, K. Recent progress on Pickering emulsions stabilized by polysaccharides-based micro/nanoparticles. Adv. Colloid Interface Sci. 2021, 296, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Jie, W.; Guang, H.M. Recent Studies of Pickering Emulsions: Particles Make the Difference. Nano Micro-Small J. 2016, 34, 4633–4648. [Google Scholar]
- Tadros, T. Application of rheology for assessment and prediction of the long-term physical stability of emulsions. Adv. Colloid Interface Sci. 2004, 108-109, 227–258. [Google Scholar] [CrossRef]
- Kulawik-Pióro, A.; Ptaszek, A.; Kruk, G. Effective tool for assessment of the quality of barrier creams—Relationships between rheological, textural and sensory properties. Regul. Toxicol. Pharmacol. 2019, 103, 113–123. [Google Scholar] [CrossRef]
- Gilbert, L.; Picard, C.; Savary, G.; Grisel, M. Rheological and textural characterization of cosmetic emulsions containing natural and synthetic polymers: Relationships between both data. Colloids Surf. A Physiochem. Eng. Asp. 2013, 421, 150–163. [Google Scholar] [CrossRef]
- Calixto, M.S.; Maia Campos, P.M.B.G. Physical–Mechanical characterization of cosmetic formulations and correlation between instrumental measurements and sensorial properties. Int. J. Cosmet. Sci. 2017, 39, 527–534. [Google Scholar] [CrossRef] [PubMed]
- Vieira, G.S.; Lavarde, M.; Fréville, V.; Rocha-Filho, P.A.; Pensé-Lhéritier, A. Combining sensory and texturometer parameters to characterize different type of cosmetic ingredients. Int. J. Cosmet. Sci. 2020, 42, 156–166. [Google Scholar] [CrossRef] [PubMed]
- Tai, A.; Bianchini, R.; Jachowicz, J. Texture analysis of cosmetic/pharmaceutical raw materials and formulations. Int. J. Cosmet. Sci. 2014, 36, 291–304. [Google Scholar] [CrossRef]
- Jenkins, P.J.; Donald, A.M. The influence of amylose on starch granule structure. Int. J. Biol. Macromol. 1995, 17, 315–321. [Google Scholar] [CrossRef]
- Marku, D.; Wahlgren, M.; Rayner, M.; Sjöö, M.; Timgren, A. Characterization of starch Pickering emulsions for potential applications in topical formulations. Int. J. Pharm. 2012, 428, 1–7. [Google Scholar] [CrossRef]
- Klingshirn, C. ZnO: From basics towards applications. Phys. Status Solidi B 2007, 244, 3027–3073. [Google Scholar] [CrossRef]
- Schneider, S.L.; Lim, H.W. A review of inorganic UV filters zinc oxide and titanium dioxide. Photoderm. Photoimm. Photomed. 2019, 35, 442–446. [Google Scholar] [CrossRef]
- Terescenco, D.; Hucher, N.; Picard, C.; Savary, G. Sensory perception of textural properties of cosmetic Pickering emulsions. Int. J. Cosmet. Sci. 2020, 42, 198–207. [Google Scholar] [CrossRef]
- Marto, J.; Ascenso, A.; Gonçalves, L.M.; Gouveia, L.F.; Manteigas, P.; Pinto, P.; Oliveira, E.; Almeida, A.J.; Ribeiro, H.M. Melatonin-based pickering emulsions for skin’s photoprotection. Drug Deliv. 2016, 23, 1594–1607. [Google Scholar] [CrossRef] [PubMed]
- Cionti, C.; Vavassori, G.; Pargoletti, E.; Meroni, D.; Cappelletti, G. One-step, highly stable Pickering emulsions stabilized by ZnO: Tuning emulsion stability by in situ functionalization. J. Colloid Interface Sci. 2022, 628, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Lapasin, R.; Pricl, S. Rheology of Industrial Polysaccarides, Theory and Application; Blackie Academic & Professional: London, UK, 1995. [Google Scholar]
- Baratto, G.; Semenzato, A.; Costantini, A.; Tafuro, G.; Unifarco, S.P.A. Powders Stabilized Emulsifying Composition. Italy Patent IT 102022000003131, 21 February 2022. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).