Current and Prospective Applications of 3D Printing in Cosmetics: A Literature Review
Abstract
1. Introduction
2. Types of 3D Printing Technologies
2.1. Inkjet Printing
2.2. Extrusion-Based Printing
2.3. Photopolymersation
- Stereolithography (SLA)
- Digital light processing (DLP)
- Continuous liquid interface production (CLIP)
- Two-photon polymerisation (TPP)
3. Types of 3D Printed Delivery Platforms
- Skin patches
- Microneedles
Fabrication Methods
4. Materials Used in 3DP Platforms
- Materials used in 3DP skin patches
- Materials used in 3DP microneedles
5. Characterisation of 3DP Platforms
6. Release and Skin Delivery of Actives Used in 3DP Platforms
7. Research in Borderline Areas
8. Conclusions and Future Prospects
Author Contributions
Funding
Conflicts of Interest
References
- Hadgraft, J.; Lane, M.E. Advanced topical formulations (ATF). Int. J. Pharm. 2016, 514, 52–57. [Google Scholar] [CrossRef]
- Luo, L.; Lane, M.E. Topical and transdermal delivery of caffeine. Int. J. Pharm. 2015, 490, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Economidou, S.N.; Lamprou, D.A.; Douroumis, D. 3D printing applications for transdermal drug delivery. Int. J. Pharm. 2018, 544, 415–424. [Google Scholar] [CrossRef] [PubMed]
- Menditto, E.; Orlando, V.; de Rosa, G.; Minghetti, P.; Musazzi, U.M.; Cahir, C.; Kurczewska-Michalak, M.; Kardas, P.; Costa, E.; Lobo, J.M.S.; et al. Patient centric pharmaceutical drug product design—The impact on medication adherence. Pharmaceutics 2020, 12, 44. [Google Scholar] [CrossRef]
- Martinez, P.R.; Goyanes, A.; Basit, A.W.; Gaisford, S. Fabrication of drug-loaded hydrogels with stereolithographic 3D printing. Int. J. Pharm. 2017, 532, 313–317. [Google Scholar] [CrossRef] [PubMed]
- Trenfield, S.J.; Awad, A.; Goyanes, A.; Gaisford, S.; Basit, A.W. 3D Printing Pharmaceuticals: Drug Development to Frontline Care. Trends Pharmacol. Sci. 2018, 39, 440–451. [Google Scholar] [CrossRef] [PubMed]
- Azad, M.A.; Olawuni, D.; Kimbell, G.; Badruddoza, A.Z.M.; Hossain, M.S.; Sultana, T. Polymers for extrusion-based 3D printing of pharmaceuticals: A holistic materials–process perspective. Pharmaceutics 2020, 12, 124. [Google Scholar] [CrossRef] [PubMed]
- Chinga-Carrasco, G.; Ehman, N.V.; Filgueira, D.; Johansson, J.; Vallejos, M.E.; Felissia, F.E.; Håkansson, J.; Area, M.C. Bagasse—A major agro-industrial residue as potential resource for nanocellulose inks for 3D printing of wound dressing devices. Addit. Manuf. 2019, 28, 267–274. [Google Scholar] [CrossRef]
- Varaprasad, K.; Jayaramudu, T.; Kanikireddy, V.; Toro, C.; Sadiku, E.R. Alginate-based composite materials for wound dressing application:A mini review. Carbohydr. Polym. 2020, 236, 116025. [Google Scholar] [CrossRef] [PubMed]
- Souto, E.B.; Campos, J.C.; Filho, S.C.; Teixeira, M.C.; Martins-Gomes, C.; Zielinska, A.; Carbone, C.; Silva, A.M. 3D printing in the design of pharmaceutical dosage forms. Pharm. Dev. Technol. 2019, 24, 1044–1053. [Google Scholar] [CrossRef]
- Bird, D.; Eker, E.; Ravindra, N.M. 3D printing of pharmaceuticals and transdermal drug delivery—An overview. In Proceedings of the TMS 2019 148th Annual Meeting & Exhibition Supplemental Proceedings, San Antonio, TX, USA, 10–14 March 2019; Springer International Publishing: Cham, Switzerland, 2019; pp. 1563–1573. [Google Scholar]
- Elahpour, N.; Pahlevanzadeh, F.; Kharaziha, M.; Bakhsheshi-Rad, H.R.; Ramakrishna, S.; Berto, F. 3D printed microneedles for transdermal drug delivery: A brief review of two decades. Int. J. Pharm. 2021, 597, 120301. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Yu, H.; Wang, L.; Shen, D.; Ni, Z.; Ren, S.; Lu, Y.; Chen, X.; Yang, J.; Hong, Y. Research progress on cosmetic microneedle systems: Preparation, property and application. Eur. Polym. J. 2022, 163, 110942. [Google Scholar] [CrossRef]
- Hu, X.; He, H. A review of cosmetic skin delivery. J. Cosmet. Dermatol. 2021, 20, 2020–2030. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, R.S.; Fantaus, S.S.; Guillot, A.J.; Melero, A.; Beck, R.C.R. 3D-Printed Products for Topical Skin Applications: From Personalized Dressings to Drug Delivery. Pharmaceutics 2021, 13, 1946. [Google Scholar] [CrossRef]
- Trenfield, S.J.; Awad, A.; Madla, C.M.; Hatton, G.B.; Firth, J.; Goyanes, A.; Gaisford, S.; Basit, A.W. Shaping the future: Recent advances of 3D printing in drug delivery and healthcare. Expert Opin. Drug Deliv. 2019, 16, 1081–1094. [Google Scholar] [CrossRef] [PubMed]
- Shahrubudin, N.; Lee, T.C.; Ramlan, R. An overview on 3D printing technology: Technological, materials, and applications. Procedia Manuf. 2019, 35, 1286–1296. [Google Scholar] [CrossRef]
- Yao, W.; Li, D.; Zhao, Y.; Zhan, Z.; Jin, G.; Liang, H.; Yang, R. 3D Printed Multi-Functional Hydrogel Microneedles Based on High-Precision Digital Light Processing. Micromachines 2019, 11, 17. [Google Scholar] [CrossRef] [PubMed]
- Cordeiro, A.S.; Tekko, I.A.; Jomaa, M.H.; Vora, L.; McAlister, E.; Volpe-Zanutto, F.; Nethery, M.; Baine, P.T.; Mitchell, N.; McNeill, D.W.; et al. Two-Photon Polymerisation 3D Printing of Microneedle Array Templates with Versatile Designs: Application in the Development of Polymeric Drug Delivery Systems. Pharm. Res. 2020, 37, 174. [Google Scholar] [CrossRef]
- Pere, C.P.P.; Economidou, S.N.; Lall, G.; Ziraud, C.; Boateng, J.S.; Alexander, B.D.; Lamprou, D.A.; Douroumis, D. 3D printed microneedles for insulin skin delivery. Int. J. Pharm. 2018, 544, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Economidou, S.N.; Pere, C.P.P.; Reid, A.; Uddin, M.J.; Windmill, J.F.C.; Lamprou, D.A.; Douroumis, D. 3D printed microneedle patches using stereolithography (SLA)for intradermal insulin delivery. Mater. Sci. Eng. C 2019, 102, 743–755. [Google Scholar] [CrossRef]
- Evans, S.E.; Harrington, T.; Rivero, M.C.R.; Rognin, E.; Tuladhar, T.; Daly, R. 2D and 3D inkjet printing of biopharmaceuticals—A review of trends and future perspectives in research and manufacturing. Int. J. Pharm. 2021, 599, 120443. [Google Scholar] [CrossRef] [PubMed]
- Jain, V.; Haider, N.; Jain, K. 3D Printing in Personalized Drug Delivery. Curr. Pharm. Des. 2019, 24, 5062–5071. [Google Scholar] [CrossRef] [PubMed]
- Vithani, K.; Goyanes, A.; Jannin, V.; Basit, A.W.; Gaisford, S.; Boyd, B.J. An Overview of 3D Printing Technologies for Soft Materials and Potential Opportunities for Lipid-based Drug Delivery Systems. Pharm. Res. 2019, 36, 4. [Google Scholar] [CrossRef]
- Stevic, M.; Hill, P.; Tamburic, S. Exploring the use of 3D Printing Technology in the Fabrication of Personalised Lipstick Applicators. J. Dermatol. Cosmetol. 2017, 1, 00024. [Google Scholar] [CrossRef][Green Version]
- Yang, Q.; Zhong, W.; Xu, L.; Li, H.; Yan, Q.; She, Y.; Yang, G. Recent progress of 3D-printed microneedles for transdermal drug delivery. Int. J. Pharm. 2021, 593, 120106. [Google Scholar] [CrossRef] [PubMed]
- Goyanes, A.; Det-Amornrat, U.; Wang, J.; Basit, A.W.; Gaisford, S. 3D scanning and 3D printing as innovative technologies for fabricating personalized topical drug delivery systems. J. Control Release 2016, 234, 41–48. [Google Scholar] [CrossRef]
- Muwaffak, Z.; Goyanes, A.; Clark, V.; Basit, A.W.; Hilton, S.T.; Gaisford, S. Patient-specific 3D scanned and 3D printed antimicrobial polycaprolactone wound dressings. Int. J. Pharm. 2017, 527, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Liu, L.; Xiang, S.; Jiang, C.; Wu, W.; Ruan, S.; Du, Q.; Chen, T.; Xue, Y.; Chen, H.; et al. Formulation and Characterization of a 3D-Printed Cryptotanshinone-Loaded Niosomal Hydrogel for Topical Therapy of Acne. AAPS PharmSciTech 2020, 21, 159. [Google Scholar] [CrossRef] [PubMed]
- Tang, T.O.; Simon, G.P. Biodegradation of 3D-printed polylactic acid milliprojections under physiological conditions. J. Appl. Polym. Sci. 2020, 137. [Google Scholar] [CrossRef]
- Luzuriaga, M.A.; Berry, D.R.; Reagan, J.C.; Smaldone, R.A.; Gassensmith, J.J. Biodegradable 3D printed polymer microneedles for transdermal drug delivery. Lab Chip 2018, 18, 1223–1230. [Google Scholar] [CrossRef]
- Camović, M.; Biščević, A.; Brčić, I.; Borčak, K.; Bušatlić, S.; Ćenanović, N.; Dedović, A.; Mulalić, A.; Osmanlić, M.; Sirbubalo, M.; et al. Coated 3D printed PLA microneedles as transdermal drug delivery systems. In Proceedings of the CMBEBIH 2019, Banja Luka, Bosnia and Herzegovina, 16–18 May 2019; Springer International Publishing: Cham, Switzerland, 2019; pp. 735–742. [Google Scholar]
- Wu, M.; Zhang, Y.; Huang, H.; Li, J.; Liu, H.; Guo, Z.; Xue, L.; Liu, S.; Lei, Y. Assisted 3D printing of microneedle patches for minimally invasive glucose control in diabetes. Mater. Sci. Eng. C 2020, 117, 111299. [Google Scholar] [CrossRef] [PubMed]
- Xenikakis, I.; Tzimtzimis, M.; Tsongas, K.; Andreadis, D.; Demiri, E.; Tzetzis, D.; Fatouros, D.G. Fabrication and finite element analysis of stereolithographic 3D printed microneedles for transdermal delivery of model dyes across human skin in vitro. Eur. J. Pharm. Sci. 2019, 137, 104976. [Google Scholar] [CrossRef] [PubMed]
- Amer, R.I.; El-Osaily, G.H.; Bakr, R.O.; el Dine, R.S.; Fayez, A.M. Characterization and Pharmacological Evaluation of Anti-Cellulite Herbal Product(s) Encapsulated in 3D-Fabricated Polymeric Microneedles. Sci. Rep. 2020, 10, 6316. [Google Scholar] [CrossRef]
- Gittard, S.D.; Miller, P.R.; Jin, C.; Martin, T.N.; Boehm, R.D.; Chisholm, B.J.; Stafslien, S.J.; Daniels, J.W.; Cilz, N.; Monteiro-Riviere, N.A.; et al. Deposition of antimicrobial coatings on microstereolithography-fabricated microneedles. JOM 2011, 63, 59–68. [Google Scholar] [CrossRef]
- Caudill, C.L.; Perry, J.L.; Tian, S.; Luft, J.C.; DeSimone, J.M. Spatially controlled coating of continuous liquid interface production microneedles for transdermal protein delivery. J. Control Release 2018, 284, 122–132. [Google Scholar] [CrossRef] [PubMed]
- Pazhamannil, R.V.; Govindan, P. Current state and future scope of additive manufacturing technologies via vat photopolymerization. Mater. Today Proc. 2021, 43, 130–136. [Google Scholar] [CrossRef]
- Ovsianikov, A.; Chichkov, B.; Mente, P.; Monteiro-Riviere, N.A.; Doraiswamy, A.; Narayan, R.J. Two Photon Polymerization of Polymer-Ceramic Hybrid Materials for Transdermal Drug Delivery. Int. J. Appl. Ceram. Technol. 2007, 4, 22–29. [Google Scholar] [CrossRef]
- Doraiswamy, A.; Ovsianikov, A.; Gittard, S.D.; Monteiro-Riviere, N.A.; Crombez, R.; Montalvo, E.; Shen, W.; Chichkov, B.N.; Narayan, R.J. Fabrication of Microneedles Using Two Photon Polymerization for Transdermal Delivery of Nanomaterials. J. Nanosci. Nanotechnol. 2010, 10, 6305–6312. [Google Scholar] [CrossRef] [PubMed]
- Gittard, S.D.; Ovsianikov, A.; Chichkov, B.N.; Doraiswamy, A.; Narayan, R.J. Two-photon polymerization of microneedles for transdermal drug delivery. Expert Opin. Drug Deliv. 2010, 7, 513–533. [Google Scholar] [CrossRef]
- Anantrao, J.H.; Nath, P.A.; Nivrutti, P.R. Drug Penetration Enhancement Techniques in Transdermal Drug Delivery System: A Review. J. Pharm. Res. Int. 2021, 33, 46–61. [Google Scholar] [CrossRef]
- Chandan, S.; Nishant, T.; Bhupinder, K.; Manish, G. Recent advancements in transdermal patches. Int. J. Health Sci. 2022, 6, 6443–6460. [Google Scholar] [CrossRef]
- Pastore, M.N.; Kalia, Y.N.; Horstmann, M.; Roberts, M.S. Transdermal patches: History, development and pharmacology. Br. J. Pharmacol. 2015, 172, 2179–2209. [Google Scholar] [CrossRef] [PubMed]
- Kadam, C.Y.; Muchandi, A.; Alabade, P.P.; Narwade, P.P.; Khandwe, S.R. Transdermal Drug Delivery System: A Painless Method for Healthy Skin—A Review. Int. J. Sci. Dev. Res. 2022, 7, 123–130. [Google Scholar]
- Brooks, Z.; Goswami, T.; Neidhard-Doll, A.; Goswami, T. Transdermal drug delivery systems: Analysis of adhesion failure. J. Pharm. Biopharm. Res. 2022, 4, 256–270. [Google Scholar] [CrossRef]
- Cilurzo, F.; Gennari, C.G.M.; Minghetti, P. Adhesive properties: A critical issue in transdermal patch development. Expert Opin. Drug Deliv. 2012, 9, 33–45. [Google Scholar] [CrossRef]
- Brown, M.B.; Traynor, M.J.; Martin, G.P.; Akomeah, F.K. Transdermal drug delivery systems: Skin perturbation devices. In Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2008; pp. 119–139. [Google Scholar]
- Patravale, V.B.; Mandawgade, S.D. Novel cosmetic delivery systems: An application update. Int. J. Cosmet. Sci. 2008, 30, 19–33. [Google Scholar] [CrossRef]
- Hadgraft, J.; Lane, M.E. Drug crystallization—Implications for topical and transdermal delivery. Expert Opin. Drug Deliv. 2016, 13, 817–830. [Google Scholar] [CrossRef]
- Bird, D.; Ravindra, N.M. Transdermal Drug Delivery and Patches—An Overview. Med. Devices Sens. 2020, 3, e10069. [Google Scholar] [CrossRef]
- Prodduturi, S.; Sadrieh, N.; Wokovich, A.M.; Doub, W.H.; Westenberger, B.J.; Buhse, L. Transdermal delivery of fentanyl from matrix and reservoir systems: Effect of heat and compromised skin. J. Pharm. Sci. 2010, 99, 2357–2366. [Google Scholar] [CrossRef]
- Pawar, R.; Mishra, D.N.; Pawar, N. An Updated Review on Global Pharmaceutical Formulation Developments and Future Potential of Non-invasive Transdermal Drug Delivery System. Int. J. Pharm. Sci. Res. 2022, 13, 1896–1907. [Google Scholar]
- Reddy, R.D.P.; Sharma, V. Additive manufacturing in drug delivery applications: A review. Int. J. Pharm. 2020, 589, 119820. [Google Scholar] [CrossRef]
- Sirbubalo, M.; Tucak, A.; Muhamedagic, K.; Hindija, L.; Rahić, O.; Hadžiabdić, J.; Cekic, A.; Begic-Hajdarevic, D.; Husic, M.C.; Dervišević, A.; et al. 3D Printing—A “Touch-Button” Approach to Manufacture Microneedles for Transdermal Drug Delivery. Pharmaceutics 2021, 13, 924. [Google Scholar] [CrossRef]
- Hirao, T. Structure and function of skin from a cosmetic aspect. In Cosmetic Science and Technology: Theoretical Principles and Applications; Sakamoto, K., Lochhead, R., Maibach, H., Yamashita, Y., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 673–683. [Google Scholar]
- Kochhar, J.S.; Tan, J.J.Y.; Kwang, Y.C.; Kang, L. Microneedles for Transdermal Drug Delivery; Springer International Publishing AG: Cham, Switzerland, 2019. [Google Scholar]
- Teymourian, H.; Tehrani, F.; Mahato, K.; Wang, J. Lab under the Skin: Microneedle Based Wearable Devices. Adv. Healthc. Mater. 2021, 10, e2002255. [Google Scholar] [CrossRef]
- Fonseca, D.F.S.; Vilela, C.; Silvestre, A.J.D.; Freire, C.S.R. A compendium of current developments on polysaccharide and protein-based microneedles. Int. J. Biol. Macromol. 2019, 136, 704–728. [Google Scholar] [CrossRef] [PubMed]
- Guillot, A.J.; Cordeiro, A.S.; Donnelly, R.F.; Montesinos, M.C.; Garrigues, T.M.; Melero, A. Microneedle-based delivery: An overview of current applications and trends. Pharmaceutics 2020, 12, 569. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.D.; Zhang, X.P.; Zhang, B.L.; Hao, Y.Y.; Guo, X.D. Safety Assessment of Microneedle Technology for Transdermal Drug Delivery: A Review. Adv. Ther. 2020, 3, 2000033. [Google Scholar] [CrossRef]
- Aldawood, F.K.; Andar, A.; Desai, S. A Comprehensive Review of Microneedles: Types, Materials, Processes, Characterizations and Applications. Polymers 2021, 13, 2815. [Google Scholar] [CrossRef]
- Markiewicz, A.; Zasada, M.; Erkiert-Polguj, A.; Wieckowska-Szakiel, M.; Budzisz, E. An evaluation of the antiaging properties of strawberry hydrolysate treatment enriched with L-ascorbic acid applied with microneedle mesotherapy. J. Cosmet. Dermatol. 2019, 18, 129–135. [Google Scholar] [CrossRef]
- Serrano-Castañeda, P.; Escobar-Chávez, J.J.; Rodríguez-Cruz, I.M.; Melgoza-Contreras, L.M.; Martínez-Hernández, J. Microneedles as enhancer of drug absorption through the skin and applications in medicine and cosmetology. J. Pharm. Pharm. Sci. 2018, 21, 73–93. [Google Scholar] [CrossRef]
- Cohen, I.D.; Bratescu, D.; Althea, K.E.; Thomas, M. Dissolvable Microneedles Comprising One or More Encapsulated Cosmetic Ingredients. U.S. Patent Application No. US20140200509A1, 17 July 2014. [Google Scholar]
- Choi, S.Y.; Kwon, H.J.; Ahn, G.R.; Ko, E.J.; Yoo, K.H.; Kim, B.J.; Lee, C.; Kim, D. Hyaluronic acid microneedle patch for the improvement of crow’s feet wrinkles. Dermatol. Ther. 2017, 30, e12546. [Google Scholar] [CrossRef]
- Bhatnagar, S.; Dave, K.; Venuganti, V.V.K. Microneedles in the clinic. J. Control. Release 2017, 260, 164–182. [Google Scholar] [CrossRef]
- McCrudden, M.T.; Alkilani, A.Z.; McCrudden, C.M.; McAlister, E.; McCarthy, H.O.; Woolfson, A.D.; Donnelly, R.F. Design and physicochemical characterisation of novel dissolving polymeric microneedle arrays for transdermal delivery of high dose, low molecular weight drugs. J. Control. Release 2014, 180, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Bhatnagar, S.; Gadeela, P.R.; Thathireddy, P.; Venuganti, V.V.K. Microneedle-based drug delivery: Materials of construction. J. Chem. Sci. 2019, 131, 90. [Google Scholar] [CrossRef]
- Kang, N.; Kim, S.; Lee, J.; Kim, K.; Choi, Y.; Oh, Y.; Kim, J.; Kim, D.; Park, J. Microneedles for drug delivery: Recent advances in materials and geometry for preclinical and clinical studies. Expert Opin. Drug Deliv. 2021, 18, 929–947. [Google Scholar] [CrossRef]
- Lee, C.; Eom, Y.A.; Yang, H.; Jang, M.; Jung, S.U.; Park, Y.O.; Lee, S.E.; Jung, H. Skin Barrier Restoration and Moisturization Using Horse Oil-Loaded Dissolving Microneedle Patches. Ski. Pharmacol. Physiol. 2018, 31, 163–171. [Google Scholar] [CrossRef]
- Koyani, R.D. Biopolymers for microneedle synthesis: From then to now. Biomanufacturing Rev. 2019, 4, 1. [Google Scholar] [CrossRef]
- Lee, K.; Lee, H.C.; Lee, D.; Jung, H. Drawing Lithography: Three-Dimensional Fabrication of an Ultrahigh-Aspect-Ratio Microneedle. Adv. Mater. 2010, 22, 483–486. [Google Scholar] [CrossRef]
- Ebrahiminejad, V.; Rad, Z.F.; Prewett, P.D.; Davies, G.J. Fabrication and testing of polymer microneedles for transdermal drug delivery. Beilstein J. Nanotechnol. 2022, 13, 629–640. [Google Scholar] [CrossRef]
- Sonetha, V.; Majumdar, S.; Shah, S. Step-wise micro-fabrication techniques of microneedle arrays with applications in transdermal drug delivery—A review. J. Drug Deliv. Sci. Technol. 2022, 68, 103119. [Google Scholar] [CrossRef]
- Kim, J.D.; Kim, M.; Yang, H.; Lee, K.; Jung, H. Droplet-born air blowing: Novel dissolving microneedle fabrication. J. Control. Release 2013, 170, 430–436. [Google Scholar] [CrossRef]
- Kim, M.; Yang, H.; Kim, H.; Jung, H.; Jung, H. Novel cosmetic patches for wrinkle improvement: Retinyl retinoate- and ascorbic acid-loaded dissolving microneedles. Int. J. Cosmet. Sci. 2014, 36, 207–212. [Google Scholar] [CrossRef]
- Kim, S.; Yang, H.; Kim, M.; Baek, J.H.; Kim, S.J.; An, S.M.; Koh, J.S.; Seo, R.; Jung, H. 4-n-butylresorcinol dissolving microneedle patch for skin depigmentation: A randomized, double-blind, placebo-controlled trial. J. Cosmet. Dermatol. 2016, 15, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Dangol, M.; Kang, G.; Lahiji, S.F.; Yang, H.; Jang, M.; Ma, Y.; Li, C.; Lee, S.G.; Kim, C.H.; et al. Enhanced Transdermal Delivery by Combined Application of Dissolving Microneedle Patch on Serum-Treated Skin. Mol. Pharm. 2017, 14, 2024–2031. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Yang, H.; Kim, S.; Kim, M.; Kang, H.; Kim, N.; An, S.; Koh, J.; Jung, H. Evaluation of the anti-wrinkle effect of an ascorbic acid-loaded dissolving microneedle patch via a double-blind, placebo-controlled clinical study. Int. J. Cosmet. Sci. 2016, 38, 375–381. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Kim, S.; Jang, M.; Kim, H.; Lee, S.; Kim, Y.; Eom, Y.A.; Kang, G.; Chiang, L.; Baek, J.H.; et al. Two-phase delivery using a horse oil and adenosine-loaded dissolving microneedle patch for skin barrier restoration, moisturization, and wrinkle improvement. J. Cosmet. Dermatol. 2019, 18, 936–943. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Song, C.; Baik, S.; Kim, D.; Hyeon, T.; Kim, D. Device-assisted transdermal drug delivery. Adv. Drug Deliv. Rev. 2018, 127, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.H.; Ng, J.Y.; Kang, L. Three-dimensional printing of a microneedle array on personalized curved surfaces for dual-pronged treatment of trigger finger. Biofabrication 2017, 9, 015010. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.H.; Tiew, W.J.; Zhang, J.; Ho, P.C.L.; Kachouie, N.N.; Kang, L. Geometrical optimisation of a personalised microneedle eye patch for transdermal delivery of anti-wrinkle small peptide. Biofabrication 2020, 12, 035003. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.H.; Kathuria, H.; Amir, M.H.B.; Zhang, X.; Duong, H.T.T.; Ho, P.C.L.; Kang, L. High resolution photopolymer for 3D printing of personalised microneedle for transdermal delivery of anti-wrinkle small peptide. J. Control. Release 2021, 329, 907–918. [Google Scholar] [CrossRef] [PubMed]
- Johnson, A.R.; Caudill, C.L.; Tumbleston, J.R.; Bloomquist, C.J.; Moga, K.A.; Ermoshkin, A.; Shirvanyants, D.; Mecham, S.J.; Luft, J.C.; DeSimone, J.M. Single-step fabrication of computationally designed microneedles by continuous liquid interface production. PLoS ONE 2016, 11, e0162518. [Google Scholar] [CrossRef] [PubMed]
- Zamboulis, A.; Michailidou, G.; Koumentakou, I.; Bikiaris, D.N. Polysaccharide 3D Printing for Drug Delivery Applications. Pharmaceutics 2022, 14, 145. [Google Scholar] [CrossRef] [PubMed]
- Diañez, I.; Gallegos, C.; la Fuente, E.B.; Martínez, I.; Valencia, C.; Sánchez, M.C.; Diaz, M.; Franco, J. 3D printing in situ gelification of κ-carrageenan solutions: Effect of printing variables on the rheological response. Food Hydrocoll. 2019, 87, 321–330. [Google Scholar] [CrossRef]
- Boonlai, W.; Tantishaiyakul, V.; Hirun, N. Characterization of κ-carrageenan/methylcellulose/cellulose nanocrystal hydrogels for 3D bioprinting. Polym. Int. 2021, 71, 181–191. [Google Scholar] [CrossRef]
- Sommer, M.R.; Alison, L.; Minas, C.; Tervoort, E.; Rühs, P.A.; Studart, A.R. 3D printing of concentrated emulsions into multiphase biocompatible soft materials. Soft Matter. 2017, 13, 1794–1803. [Google Scholar] [CrossRef] [PubMed]
- Aranaz, I.; Acosta, N.; Civera, C.; Elorza, B.; Mingo, J.; Castro, C.; de Los Llanos Gandía, M.; Caballero, A.H. Cosmetics and Cosmeceutical Applications of Chitin, Chitosan and Their Derivatives. Polymers 2018, 10, 213. [Google Scholar] [CrossRef] [PubMed]
- Rajabi, M.; McConnell, M.; Cabral, J.; Ali, M.A. Chitosan hydrogels in 3D printing for biomedical applications. Carbohydr. Polym. 2021, 260, 117768. [Google Scholar] [CrossRef]
- Camcı, Y.; Türk, S.; Gepek, E.; İyibilgin, O.; Özsoy, M.İ. Fabrication and characterization of innovative chitosan/doxorubicin coated 3D printed microneedle patch for prolonged drug delivery. J. Appl. Polym. Sci. 2022, 139. [Google Scholar] [CrossRef]
- Bukhari, S.N.A.; Roswandi, N.L.; Waqas, M.; Habib, H.; Hussain, F.; Khan, S.; Sohail, M.; Ramli, N.A.; Thu, H.E.; Hussain, Z. Hyaluronic acid, a promising skin rejuvenating biomedicine: A review of recent updates and pre-clinical and clinical investigations on cosmetic and nutricosmetic effects. Int. J. Biol. Macromol. 2018, 120, 1682–1695. [Google Scholar] [CrossRef]
- Graça, M.F.P.; Miguel, S.P.; Cabral, C.S.D.; Correia, I.J. Hyaluronic acid—Based wound dressings: A review. Carbohydr. Polym. 2020, 241, 116364. [Google Scholar] [CrossRef]
- Maiz-Fernández, S.; Barroso, N.; Pérez-Álvarez, L.; Silván, U.; Vilas-Vilela, J.L.; Lanceros-Mendez, S. 3D printable self-healing hyaluronic acid/chitosan polycomplex hydrogels with drug release capability. Int. J. Biol. Macromol. 2021, 188, 820–832. [Google Scholar] [CrossRef]
- Mbituyimana, B.; Liu, L.; Ye, W.; Boni, B.O.O.; Zhang, K.; Chen, J.; Thomas, S.; Vasilievich, R.V.; Shi, Z.; Yang, G. Bacterial cellulose-based composites for biomedical and cosmetic applications: Research progress and existing products. Carbohydr. Polym. 2021, 273, 118565. [Google Scholar] [CrossRef] [PubMed]
- Mendoza, D.J.; Maliha, M.; Raghuwanshi, V.S.; Browne, C.; Mouterde, L.M.M.; Simon, G.P.; Allais, F.; Garnier, G. Diethyl sinapate-grafted cellulose nanocrystals as nature-inspired UV filters in cosmetic formulations. Mater. Today Bio 2021, 12, 100126. [Google Scholar] [CrossRef] [PubMed]
- Mohan, D.; Teong, Z.K.; Bakir, A.N.; Sajab, M.S.; Kaco, H. Extending Cellulose-Based Polymers Application in Additive Manufacturing Technology: A Review of Recent Approaches. Polymers 2020, 12, 1876. [Google Scholar] [CrossRef]
- Li, V.C.; Dunn, C.K.; Zhang, Z.; Deng, Y.; Qi, H.J. Direct Ink Write (DIW) 3D Printed Cellulose Nanocrystal Aerogel Structures. Sci. Rep. 2017, 7, 8018. [Google Scholar] [CrossRef]
- Xiong, S.; Zhang, X.; Lu, P.; Wu, Y.; Wang, Q.; Sun, H.; Heng, B.C.; Bunpetch, V.; Zhang, S.; Ouyang, H. A Gelatin-sulfonated Silk Composite Scaffold based on 3D Printing Technology Enhances Skin Regeneration by Stimulating Epidermal Growth and Dermal Neovascularization. Sci. Rep. 2017, 7, 4288. [Google Scholar] [CrossRef] [PubMed]
- Al-Nimry, S.; Dayah, A.A.; Hasan, I.; Daghmash, R. Cosmetic, Biomedical and Pharmaceutical Applications of Fish Gelatin/Hydrolysates. Mar. Drugs 2021, 19, 145. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Kumar, H.; Tian, Z.; Jin, X.; Holzman, J.F.; Menard, F.; Kim, K. Visible Light Photoinitiation of Cell-Adhesive Gelatin Methacryloyl Hydrogels for Stereolithography 3D Bioprinting. ACS Appl. Mater. Interfaces 2018, 10, 26859–26869. [Google Scholar] [CrossRef]
- Liu, J.; Tagami, T.; Ozeki, T. Fabrication of 3D-Printed Fish-Gelatin-Based Polymer Hydrogel Patches for Local Delivery of PEGylated Liposomal Doxorubicin. Mar. Drugs 2020, 18, 325. [Google Scholar] [CrossRef]
- Karavasili, C.; Tsongas, K.; Andreadis, I.I.; Andriotis, E.G.; Papachristou, E.T.; Papi, R.M.; Tzetzis, D.; Fatouros, D.G. Physico-mechanical and finite element analysis evaluation of 3D printable alginate-methylcellulose inks for wound healing applications. Carbohydr. Polym. 2020, 247, 116666. [Google Scholar] [CrossRef]
- Hajleh, M.N.A.; Al-Samydai, A.A.; Al-Dujaili, E.A.S. Nano, micro particulate and cosmetic delivery systems of polylactic acid: A mini review. J. Cosmet. Dermatol. 2020, 19, 2805–2811. [Google Scholar] [CrossRef]
- Rydz, J.; Sikorska, W.; Musioł, M.; Janeczek, H.; Włodarczyk, J.; Misiurska-Marczak, M.; Łęczycka, J.; Kowalczuk, M. 3D-Printed Polyester-Based Prototypes for Cosmetic Applications—Future Directions at the Forensic Engineering of Advanced Polymeric Materials. Materials 2019, 12, 994. [Google Scholar] [CrossRef]
- Blanco, I. End-life Prediction of Commercial PLA Used for Food Packaging through Short Term TGA Experiments: Real Chance or Low Reliability? Chin. J. Polym. Sci. 2014, 32, 681–689. [Google Scholar] [CrossRef]
- Kesente, M.; Kavetsou, E.; Roussaki, M.; Blidi, S.; Loupassaki, S.; Chanioti, S.; Siamandoura, P.; Stamatogianni, C.; Philippou, E.; Papaspyrides, C.J.B.; et al. Encapsulation of Olive Leaves Extracts in Biodegradable PLA Nanoparticles for Use in Cosmetic Formulation. Bioengineering 2017, 4, 75. [Google Scholar] [CrossRef] [PubMed]
- Ausejo, J.G.; Rydz, J.; Musioł, M.; Sikorska, W.; Sobota, M.; Włodarczyk, J.; Adamus, G.; Janeczek, H.; Kwiecień, I.; Hercog, A.; et al. A comparative study of three-dimensional printing directions: The degradation and toxicological profile of a PLA/PHA blend. Polym. Degrad. Stab. 2018, 152, 191–207. [Google Scholar] [CrossRef]
- Asthana, N.; Pal, K.; Aljabali, A.A.A.; Tambuwala, M.M.; de Souza, F.G.; Pandey, K. Polyvinyl alcohol (PVA) mixed green–clay and aloe vera based polymeric membrane optimization: Peel-off mask formulation for skin care cosmeceuticals in green nanotechnology. J. Mol. Struct. 2021, 1229, 129592. [Google Scholar] [CrossRef]
- Badri, W.; Miladi, K.; Eddabra, R.; Fessi, H.; Elaissari, A. Elaboration of Nanoparticles Containing Indomethacin: Argan Oil for Transdermal Local and Cosmetic Application. J. Nanomater. 2015, 16, 113. [Google Scholar] [CrossRef]
- Yang, S.; Jeong, J.; Lim, Y.; Park, J. Synthesis and characterization of PVP microneedle patch using metal bioelectrodes for novel drug delivery system. Mater. Des. 2021, 201, 109485. [Google Scholar] [CrossRef]
- Park, Y.; Park, J.; Chu, G.S.; Kim, K.S.; Sung, J.H.; Kim, B. Transdermal delivery of cosmetic ingredients using dissolving polymer microneedle arrays. Biotechnol. Bioprocess Eng. 2015, 20, 543–549. [Google Scholar] [CrossRef]
- Adli, S.A.; Ali, F.; Azmi, A.S.; Anuar, H.; Nasir, N.A.M.; Hasham, R.; Idris, M.K.H. Development of biodegradable cosmetic patch using a polylactic acid/phycocyanin-alginate composite. Polymers 2020, 12, 1669. [Google Scholar] [CrossRef]
- Rees, A.; Powell, L.C.; Chinga-Carrasco, G.; Gethin, D.T.; Syverud, K.; Hill, K.E.; Thomas, D.W. 3D Bioprinting of Carboxymethylated-Periodate Oxidized Nanocellulose Constructs for Wound Dressing Applications. BioMed Res. Int. 2015, 2015, 925757. [Google Scholar] [CrossRef]
- Domínguez-Robles, J.; Martin, N.K.; Fong, M.L.; Stewart, S.A.; Irwin, N.J.; Rial-Hermida, M.I.; Donnelly, R.F.; Larrañeta, E. Antioxidant pla composites containing lignin for 3D printing applications: A potential material for healthcare applications. Pharmaceutics 2019, 11, 165. [Google Scholar] [CrossRef] [PubMed]
- Bom, S.; Santos, C.; Barros, R.; Martins, A.M.; Paradiso, P.; Cláudio, R.; Pinto, P.; Ribeiro, H.; Marto, J. Effects of starch incorporation on the physicochemical properties and release kinetics of alginate-based 3D hydrogel patches for topical delivery. Pharmaceutics 2020, 12, 719. [Google Scholar] [CrossRef] [PubMed]
- Nagarkar, R.; Singh, M.; Nguyen, H.X.; Jonnalagadda, S. A review of recent advances in microneedle technology for transdermal drug delivery. J. Drug Deliv. Sci. Technol. 2020, 59, 101923. [Google Scholar] [CrossRef]
- Loizidou, E.Z.; Williams, N.A.; Barrow, D.A.; Eaton, M.J.; McCrory, J.; Evans, S.L.; Allender, C.J. Structural characterisation and transdermal delivery studies on sugar microneedles: Experimental and finite element modelling analyses. Eur. J. Pharm. Biopharm. 2015, 89, 224–231. [Google Scholar] [CrossRef]
- Economidou, S.N.; Douroumis, D. 3D printing as a transformative tool for microneedle systems: Recent advances, manufacturing considerations and market potential. Adv. Drug Deliv. Rev. 2021, 173, 60–69. [Google Scholar] [CrossRef]
- Wallis, M.; Al-Dulimi, Z.; Tan, D.K.; Maniruzzaman, M.; Nokhodchi, A. 3D printing for enhanced drug delivery: Current state-of-the-art and challenges. Drug Dev. Ind. Pharm. 2020, 46, 1385–1401. [Google Scholar] [CrossRef]
- Cha, K.J.; Kim, T.; Park, S.J.; Kim, D.S. Simple and cost-effective fabrication of solid biodegradable polymer microneedle arrays with adjustable aspect ratio for transdermal drug delivery using acupuncture microneedles. J. Micromechanics Microengineering 2014, 24, 115015. [Google Scholar] [CrossRef]
- Tang, T.O.; Holmes, S.; Dean, K.; Simon, G.P. Design and fabrication of transdermal drug delivery patch with milliprojections using material extrusion 3D printing. J. Appl. Polym. Sci. 2020, 137, 48777. [Google Scholar] [CrossRef]
- Zvezdin, V.; Kasatkina, T.; Kasatkin, I.; Gavrilova, M.; Kazakova, O. Microneedle patch based on dissolving, detachable microneedle technology for improved skin quality of the periorbital region. Part 2: Clinical Evaluation. Int. J. Cosmet. Sci. 2020, 42, 429–435. [Google Scholar] [CrossRef]
- Zvezdin, V.; Peno-Mazzarino, L.; Radionov, N.; Kasatkina, T.; Kasatkin, I. Microneedle patch based on dissolving, detachable microneedle technology for improved skin quality—Part 1: Ex vivo safety evaluation. Int. J. Cosmet. Sci. 2020, 42, 369–376. [Google Scholar] [CrossRef]
- Jang, M.; Baek, S.; Kang, G.; Yang, H.; Kim, S.; Jung, H. Dissolving microneedle with high molecular weight hyaluronic acid to improve skin wrinkles, dermal density and elasticity. Int. J. Cosmet. Sci. 2020, 42, 302–309. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Fu, X.; Chen, G.; Wang, Y.; Zhao, Y. Versatile Ice Microneedles for Transdermal Delivery of Diverse Actives. Adv. Sci. 2021, 8, e2101210. [Google Scholar] [CrossRef] [PubMed]
- Shim, W.S.; Hwang, Y.M.; Park, S.G.; Lee, C.K.; Kang, N.G. Role of Polyvinylpyrrolidone in Dissolving Microneedle for Efficient Transdermal Drug Delivery: In vitro and Clinical Studies. Bull. Korean Chem. Soc. 2018, 39, 789–793. [Google Scholar] [CrossRef]
- Fang, J.; Liu, C.; Hsu, R.; Chen, Y.; Chiang, W.; Wang, H.D.; Hu, S.-H. Transdermal Composite Microneedle Composed of Mesoporous Iron Oxide Nanoraspberry and PVA for Androgenetic Alopecia Treatment. Polymers 2020, 12, 1392. [Google Scholar] [CrossRef]
- El-Sayed, N.; Vaut, L.; Schneider, M. Customized fast-separable microneedles prepared with the aid of 3D printing for nanoparticle delivery. Eur. J. Pharm. Biopharm. 2020, 154, 166–174. [Google Scholar] [CrossRef]
- Aung, N.N.; Ngawhirunpat, T.; Rojanarata, T.; Patrojanasophon, P.; Opanasopit, P.; Pamornpathomkul, B. HPMC/PVP Dissolving Microneedles: A Promising Delivery Platform to Promote Trans-Epidermal Delivery of Alpha-Arbutin for Skin Lightening. AAPS PharmSciTech 2020, 21, 25. [Google Scholar] [CrossRef]
- Wasti, S.; Adhikari, S. Use of Biomaterials for 3D Printing by Fused Deposition Modeling Technique: A Review. Front. Chem. 2020, 8, 315. [Google Scholar] [CrossRef]
- le Duigou, A.; Correa, D.; Ueda, M.; Matsuzaki, R.; Castro, M. A review of 3D and 4D printing of natural fibre biocomposites. Mater. Des. 2020, 194, 108911. [Google Scholar] [CrossRef]
- Chaudhari, V.S.; Malakar, T.K.; Murty, U.S.; Banerjee, S. Extruded filaments derived 3D printed medicated skin patch to mitigate destructive pulmonary tuberculosis: Design to delivery. Expert Opin. Drug Deliv. 2020, 18, 301–313. [Google Scholar] [CrossRef]
- Davis, S.P.; Landis, B.J.; Adams, Z.H.; Allen, M.G.; Prausnitz, M.R. Insertion of microneedles into skin: Measurement and prediction of insertion force and needle fracture force. J. Biomech. 2004, 37, 1155–1163. [Google Scholar] [CrossRef]
- Arany, P.; Róka, E.; Mollet, L.; Coleman, A.W.; Perret, F.; Kim, B.; Kovács, R.; Kazsoki, A.; Zelkó, R.; Gesztelyi, R.; et al. Fused depositionmodeling 3D printing: Test platforms for evaluating post-fabrication chemical modifications and in-vitro biological properties. Pharmaceutics 2019, 11, 277. [Google Scholar]
- Hao, Y.Y.; Yang, Y.; Li, Q.Y.; Zhang, X.P.; Shen, C.B.; Zhang, C.; Cui, Y.; Guo, X.D. Effect of polymer microneedle pre-treatment on drug distributions in the skin in vivo. J. Drug Target. 2020, 28, 811–817. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Lahiji, S.F.; Jang, J.; Jang, M.; Jung, H. Micro-pillar integrated dissolving microneedles for enhanced transdermal drug delivery. Pharmaceutics 2019, 11, 402. [Google Scholar] [CrossRef] [PubMed]
- Economidou, S.N.; Pere, C.P.P.; Okereke, M.; Douroumis, D. Optimisation of Design and Manufacturing Parameters of 3D Printed Solid Microneedles for Improved Strength, Sharpness, and Drug Delivery. Micromachines 2021, 12, 117. [Google Scholar] [CrossRef]
- Economidou, S.N.; Uddin, M.J.; Marques, M.J.; Douroumis, D.; Sow, W.T.; Li, H.; Reid, A.; Windmill, J.F.; Podoleanu, A. A Novel 3D Printed Hollow Microneedle Microelectromechanical System for Controlled, Personalised Transdermal Drug Delivery. Addit. Manuf. 2020, 38, 101815. [Google Scholar]
- Donnelly, R.F.; Singh, T.R.R.; Garland, M.J.; Migalska, K.; Majithiya, R.; McCrudden, C.M.; Kole, P.L.; Mahmood, T.M.T.; McCarthy, H.O.; Woolfson, A.D. Hydrogel-forming microneedle arrays for enhanced transdermal drug delivery. Adv. Funct. Mater. 2012, 22, 4879–4890. [Google Scholar] [CrossRef]
- Ratz-Łyko, A.; Arct, J. Resveratrol as an active ingredient for cosmetic and dermatological applications: A review. J. Cosmet. Laser Ther. 2019, 21, 84–90. [Google Scholar] [CrossRef]
- Siddique, S.; Parveen, Z.; Ali, Z.; Zaheer, M. Qualitative and Quantitative Estimation of Hydroquinone in Skin Whitening Cosmetics. J. Cosmet. Dermatol. Sci. Appl. 2012, 2, 224–228. [Google Scholar] [CrossRef]
- Tabriz, A.G.; Douroumis, D. Recent advances in 3D printing for wound healing: A systematic review. J. Drug Deliv. Sci. Technol. 2022, 74, 103564. [Google Scholar] [CrossRef]
- Waghule, T.; Singhvi, G.; Dubey, S.K.; Pandey, M.M.; Gupta, G.; Singh, M.; Dua, K. Microneedles: A smart approach and increasing potential for transdermal drug delivery system. Biomed. Pharmacother. 2019, 109, 1249–1258. [Google Scholar] [CrossRef]
- Iliopoulos, F.; Lane, M.E.; Caspers, P.; Puppels, G. Franz cell diffusion testing and quantitative confocal raman spectroscopy: In vitro-in vivo correlation. Pharmaceutics 2020, 12, 887. [Google Scholar] [CrossRef]
- Krombholz, R.; Lunter, D. A new method for in-situ skin penetration aAnalysis by confocal raman microscopy. Molecules 2020, 25, 4222. [Google Scholar] [CrossRef] [PubMed]
- Munem, M.; Djuphammar, A.; Sjölander, L.; Hagvall, L.; Malmberg, P. Animal- free skin permeation analysis using mass spectrometry imaging. Toxicol. Vitr. 2021, 71, 105062. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Tan, C.; Li, L. Review of 3D printable hydrogels and constructs. Mater. Des. 2018, 159, 20–38. [Google Scholar] [CrossRef]
- Olejnik, A.; Semba, J.A.; Kulpa, A.; Dańczak-Pazdrowska, A.; Rybka, J.D.; Gornowicz-Porowska, J. 3D Bioprinting in Skin Related Research: Recent Achievements and Application Perspectives. ACS Synth. Biol. 2022, 11, 26–38. [Google Scholar] [CrossRef] [PubMed]
- Coltelli, M.; Panariello, L.; Morganti, P.; Danti, S.; Baroni, A.; Lazzeri, A.; Fusco, A.; Donnarumma, G. Skin-Compatible Biobased Beauty Masks Prepared by Extrusion. J. Funct. Biomater. 2020, 11, 23. [Google Scholar] [CrossRef] [PubMed]
- Azizoğlu, E.; Özer, Ö. Fabrication of Montelukast sodium loaded filaments and 3D printing transdermal patches onto packaging material. Int. J. Pharm. 2020, 587, 119588. [Google Scholar] [CrossRef]
- Alkilani, A.; McCrudden, M.T.; Donnelly, R. Transdermal Drug Delivery: Innovative Pharmaceutical Developments Based on Disruption of the Barrier Properties of the Stratum Corneum. Pharmaceutics 2015, 7, 438–470. [Google Scholar] [CrossRef]

| 3DP | Schematic Diagram | Ink | Printing Method |
|---|---|---|---|
| Ink jet printing | 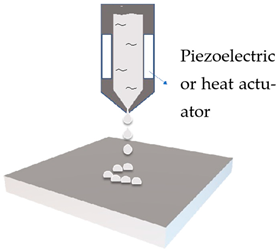 | Emulsion | Drop-on-demand controlled by the actuated printhead |
| Extrusion based printing | 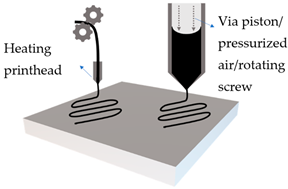 | Solid filament | Mechanical roller with heating, to extrude solid filament |
| Viscous emulsion | Pressure or mechanical extrusion of viscous emulsion | ||
| Photopolymerisation | 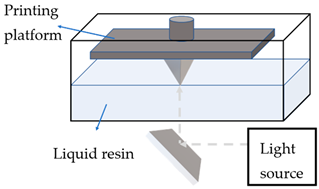 | Photopolymerisable liquid resin | Solidifying polymer via photopolymerisation, with either moving light source or moving printing platform |
| Class | Type of Delivery | Loading of Actives | Delivery Mechanism | Pros | Cons |
|---|---|---|---|---|---|
| Solid | Poke with patch | In separate topical formulations, before or after insertion of MNs | Creates transient pores, then passive diffusion | Excellent mechanical properties | Two-step-application |
| Coated | Coat and poke | Layer-by-layer, e.g., dip coating, spin coating | From coated layers on the surface of MNs | Efficient drug delivery with precise amount | Low drug loading |
| Dissolving/separable | Poke and release | Encapsulated within hydrophilic polymer matrix | Dissolve upon insertion after minutes | Safer, larger dose, no biohazardous waste, facilitate rapid delivery of macromolecules | Clogging, can be resolved by side opening on the tip |
| Hollow | Poke and flow | Within liquid reservoir | Pressure-driven delivery of liquid formulations | Large amount of formulation loading | Possibility of blocking by skin tissue, complex design |
| Swellable (hydrogel-forming) | Poke and release | Within voids of polymer matrix | Upon absorption of skin interstitial fluid, forming continuous unblockable microchannels for active delivery | Intact removal of MNs array after use, leaving no polymer residues | Limited drug loading, low ability to perforate skin, weak mechanical strength. |
| Types of MNs | Solid MNs | Coated MNs | Dissolving MNs | Hollow MNs | Swellable MNs |
|---|---|---|---|---|---|
| Just before insertion | 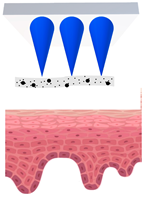 | 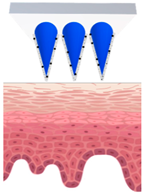 | 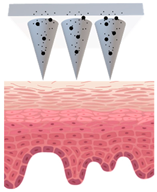 |  |  |
| After application | 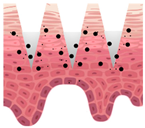 | 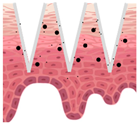 | 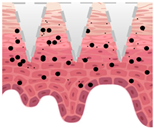 | 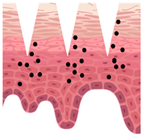 | 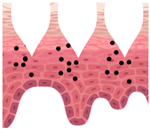 |
 —MN loaded with active ingredients;
—MN loaded with active ingredients; —solid needle;
—solid needle; —dissolving/swellable needle;
—dissolving/swellable needle; —stratum corneum.
—stratum corneum.| Material | Characteristics | Cosmetic Benefits | 3D Printability |
|---|---|---|---|
| Carrageenan (sulphated anionic polysaccharide) | Simple cold-setting gelation, biodegradable, renewable, safe, low cost, viscoelastic properties, so it can be modified easily. No addition of additives or initiators required. | As stabiliser and thickener for emulsions, to achieve desired product consistency, hydration. | Extrusion method: gel strength linearly increases by decreasing printing speed and layer height, at printing temperature below ~80 °C [88]. Addition of crosslinkers, methylcellulose and cellulose nanocrystal, can improve rheological behaviour and compressive mechanical strength [89]. The pore size of 3D printed structure is adjustable, produces soft and flexible structure [90]. |
| Chitosan (synthetised cationic polysaccharide from deacetylation of chitin) | Low-cost production, biodegradable, hydrogel can be produced by various ways (both physical and chemical crosslinking). Controlled release of actives is possible. Low water solubility at neutral pH and low mechanical integrity of 3D printed structure. | Absorbs UV, used in sunscreens; has intrinsic antimicrobial and antifungal properties, moisture absorbing properties, acts also as emulsion stabiliser [91]. | Extrusion method and photopolymerisation method, widely used for studies on 3D-printed wound dressing due to bioactivity, flexibility, and self-adhesion properties of 3D printed patches. The addition of other biomaterial could increase the printability [92]. Chitosan was also studied as a coating for MNs, where it increased drug loading capacity [93]. |
| Hyaluronic acid (linear, weak polyanion, non- sulphated glycosaminoglycan) | Hydrophilic, biocompatible, and biodegradable, viscoelastic. | It possesses skin regenerating and collagen stimulating efficacy, with hydrating, anti-wrinkle, and anti-aging effects [94] | Extrusion based: widely used in wound healing [95]. 3D printed hydrogel can achieve controlled release of actives [96] |
| Cellulose (nano- cellulose, bacterial cellulose, and other derivatives; polysaccharide) | Most abundant biopolymer, sustainable, biocompatible, high strength, high elasticity. | Produces facial masks for prolonged release of actives [97]. Used as UV filter [98]. | Extrusion-based [99]. Direct ink writing 3DP and freeze drying to produce versatile aerogels [100]. |
| Collagen (protein) | Biocompatible, low antigenic, biodegradable, highly soluble at neutral pH. | Derivatives are antioxidant, UV protective, anti-aging, moisturising. | Extrusion-based, studied for wound healing. Due to the porous nature of the 3D printed structure, actives could be easily coated [101]. |
| Gelatin (derived from collagen) | Low toughness, various modification methods available to improve its low melting point and poor stability. | Reduces the effect of photo aging and oxidative damage. UV protection [102]. | Photopolymerisation with the addition of photo initiator [103]; UV exposure time and shape affect the release; both can be controlled [104]. |
| Alginate (anionic linear polysaccharide) | Biocompatible, biodegradable. High strength. | Moisturising. Used for production of biodegradable cosmetic patches. | Extrusion based: studied for wound healing [105]. |
| Polylactic acid (PLA, thermoplastic polylactide) | Biocompatible, high elasticity, may cause inflammation. | As makeup products additive. For development of biodegradable novel cosmetic delivery platform [106] and for packaging [107,108]. For producing novel cosmetic emulsion [109]. | Extrusion method (FDM) to produce 3D printed specimen of cosmetic container [107,110], also used for coated microneedles [32]. |
| Polyvinyl alcohol (PVA, synthetic polymer) | Biocompatible, water soluble, stable to temperature variations, film forming. | Producing cosmetic delivery platforms and peel-off masks [111], also nanoparticles for cosmetic emulsions [112]. | Extrusion method and photopolymerisation method (DLP). |
| Poly(vinyl pyrrolidone) (PVP, linear polymer) | Low toxicity, inert and biocompatible, brittle, low reactivity towards photopolymerisation, can be adjusted by addition of another photopolymer. | Produce metal-coated [113] and dissolving [114] cosmetic MNs. | Photopolymerisation method (DLP) [85]. |
| Cosmetic Benefits | Active Ingredient | Characteristics | 3D Printed Delivery Platforms |
|---|---|---|---|
| Anti-wrinkle | Acetyl-hexapeptide 3 (AHP-3) | Peptide, hydrophilic, large MW. | DLP 3D printing of polyethylene glycol diacrylate (PEGDA) and vinyl pyrrolidone (VP) to produce personalised MN patch. AHP-3 was loaded by mixing in pre-polymer resin, but not incorporated in the polymer structure, aiming for easy release from the printed MNs [85]. |
| Anti-acne (anti-microbial) | Salicylic acid | Obtained from plant extract. Beta-hydroxyl acid, small MW, potentially good skin penetrant. | Salicylic acid was loaded to polylactic acid by hot melt extrusion. 3D printed nose patch made by FDM failed due to its complex structure. Flexible nose patch was successfully fabricated with PEGDA and PEG using SLA printer [27]. |
| Anti-aging and anti-acne (antioxidant and anti-inflammatory properties); skin-whitening | Resveratrol | Obtained from plant extract, polyphenol phytoalexin. Skin permeation from aqueous was better than from oily system [143]. | Extrusion based method followed by freeze-drying for the fabrication of 3DP edible oleogel from emulsion containing gelatin and gellan gum. The bioactivity of actives has improved. The method has potential to produce cosmetic soft patch with resveratrol. |
| Skin-whitening/lightening | Hydroquinone | Inhibits melanin synthesis, side effects related to long-term application [144] | It has been used an initiator for SLA 3D printing in producing wound dressings [145] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiao, Y.; Stevic, M.; Buanz, A.; Uddin, M.J.; Tamburic, S. Current and Prospective Applications of 3D Printing in Cosmetics: A Literature Review. Cosmetics 2022, 9, 115. https://doi.org/10.3390/cosmetics9060115
Jiao Y, Stevic M, Buanz A, Uddin MJ, Tamburic S. Current and Prospective Applications of 3D Printing in Cosmetics: A Literature Review. Cosmetics. 2022; 9(6):115. https://doi.org/10.3390/cosmetics9060115
Chicago/Turabian StyleJiao, Yimeng, Milica Stevic, Asma Buanz, Md Jasim Uddin, and Slobodanka Tamburic. 2022. "Current and Prospective Applications of 3D Printing in Cosmetics: A Literature Review" Cosmetics 9, no. 6: 115. https://doi.org/10.3390/cosmetics9060115
APA StyleJiao, Y., Stevic, M., Buanz, A., Uddin, M. J., & Tamburic, S. (2022). Current and Prospective Applications of 3D Printing in Cosmetics: A Literature Review. Cosmetics, 9(6), 115. https://doi.org/10.3390/cosmetics9060115







