Measurement of Stress Relief during Scented Cosmetic Product Application Using a Mood Questionnaire, Stress Hormone Levels and Brain Activation
Abstract
1. Introduction
2. Materials and Methods
2.1. Sensory Evaluation of the Active Ingredient
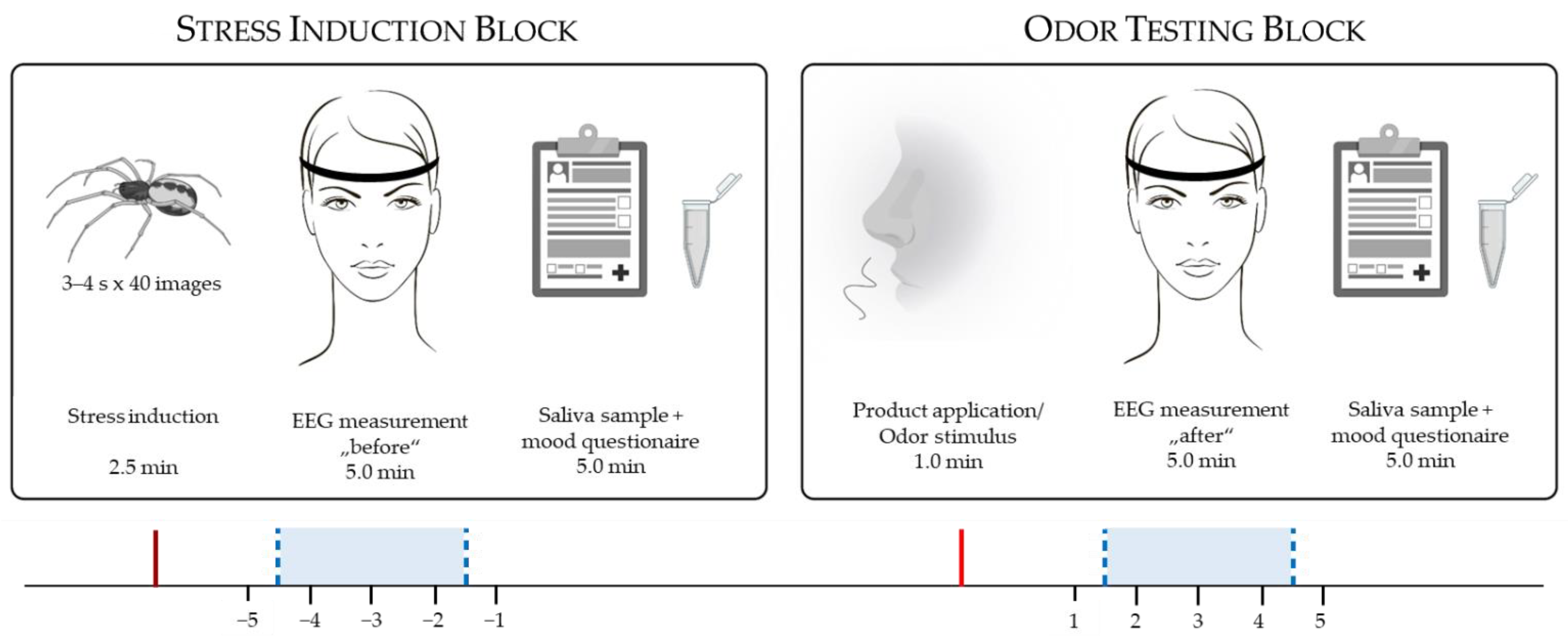
2.2. Method Development for Instrumental Measurement of Stress Relief
3. Results
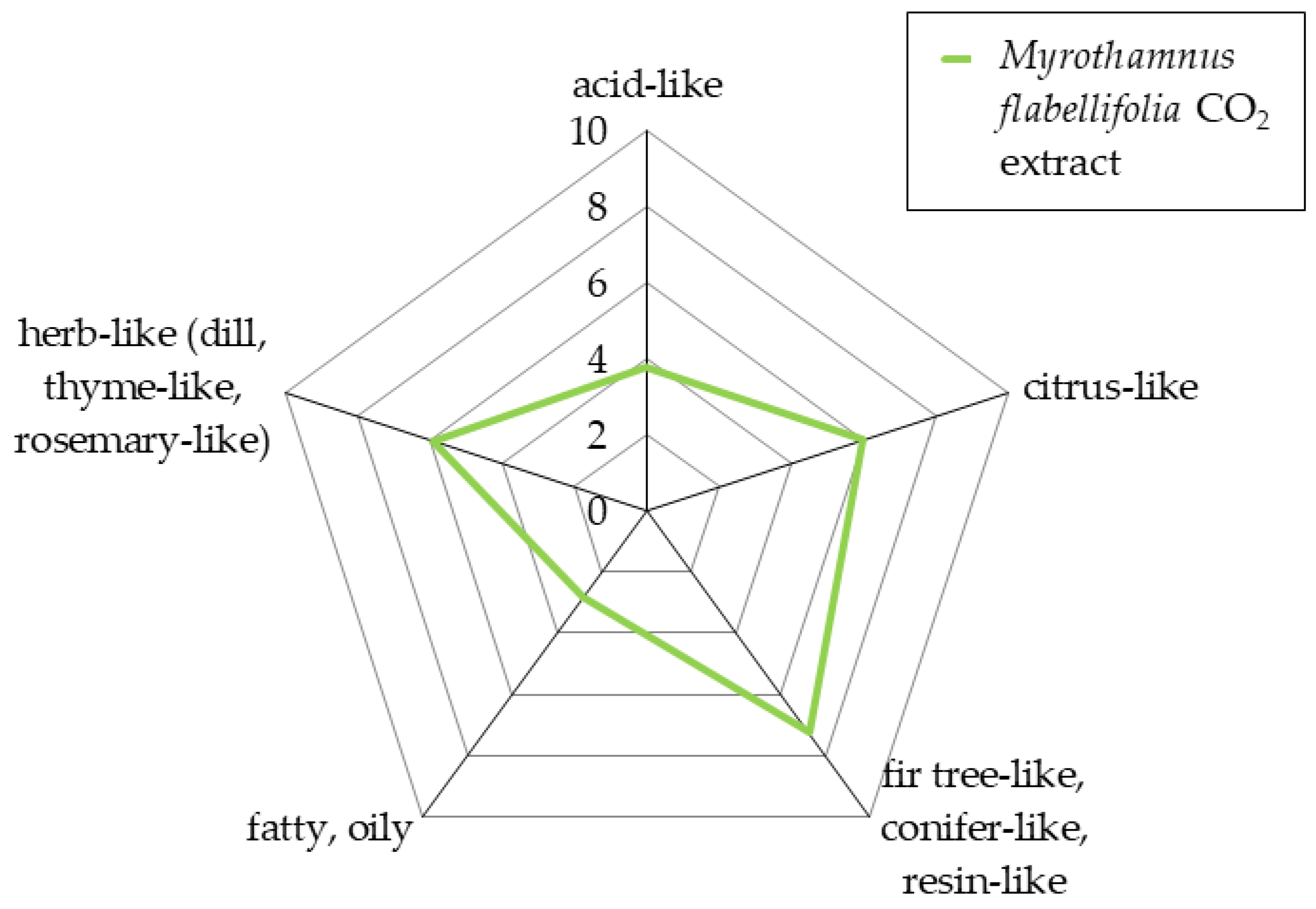
3.1. Sensory Aroma Evaluation
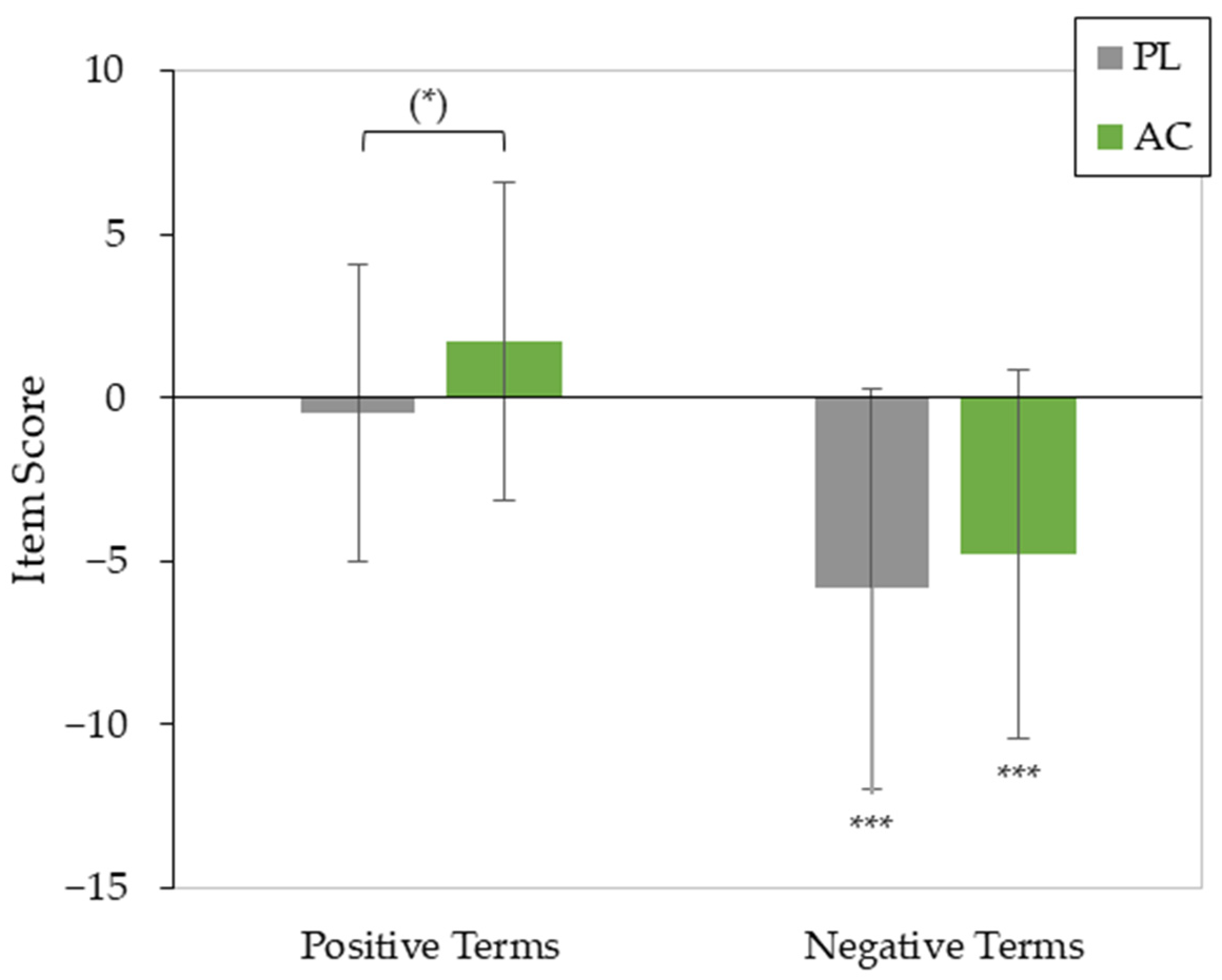
3.2. Effects of AC Application on Mood
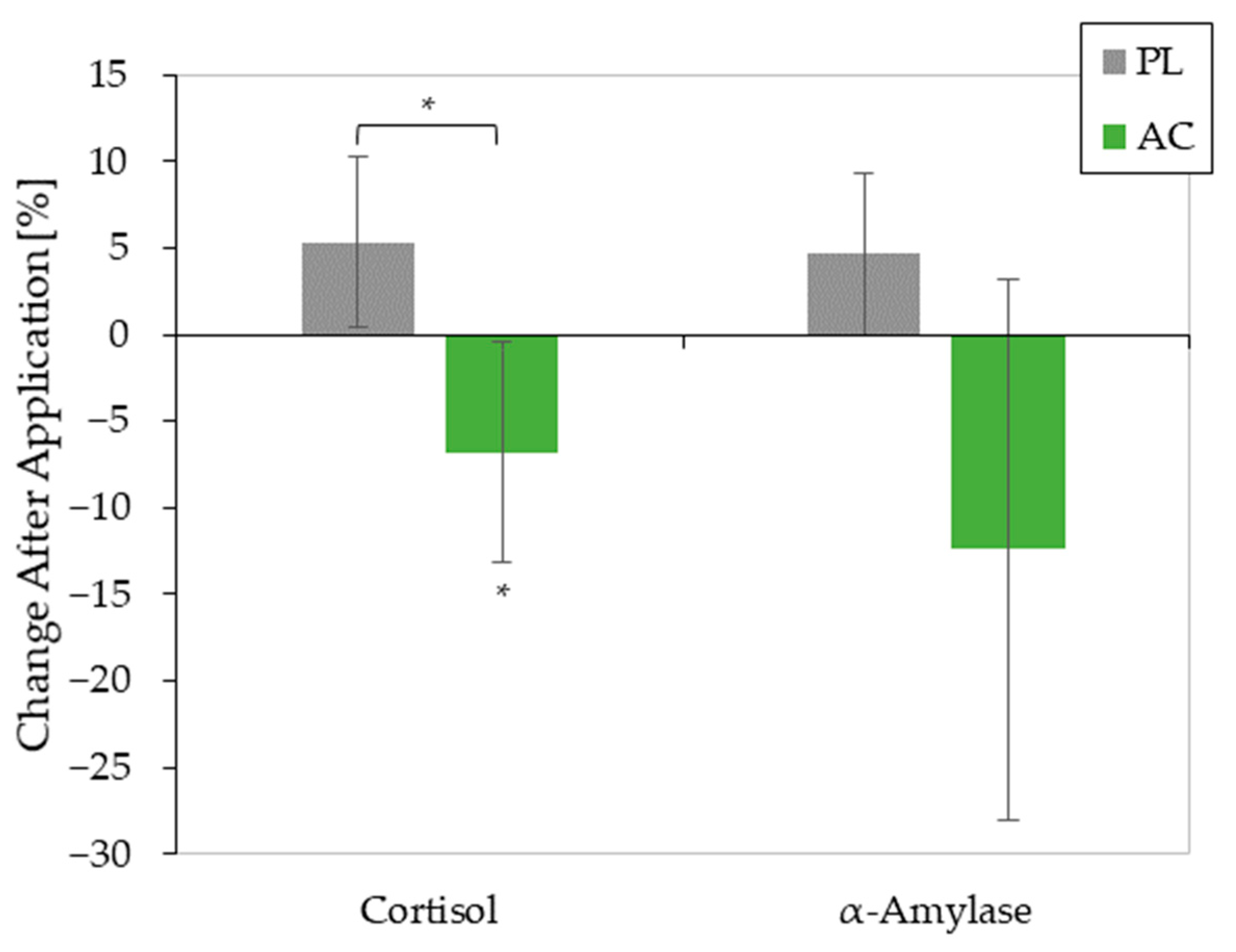
3.3. Effect of AC Application on Stress Hormones
3.4. Effects of AC application on Brain Alpha Waves
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
References
- Zhang, Y.; Ma, Z.F. Impact of the COVID-19 pandemic on mental health and quality of life among local residents in Liaoning Province, China: A cross-sectional study. Int. J. Environ. Res. Public Health 2020, 17, 2381. [Google Scholar] [CrossRef] [PubMed]
- Mazza, C.; Ricci, E.; Biondi, S.; Colasanti, M.; Ferracuti, S.; Napoli, C.; Roma, P. A nationwide survey of psychological distress among Italian people during the COVID-19 pandemic: Immediate psychological responses and associated factors. Int. J. Environ. Res. Public Health 2020, 17, 3165. [Google Scholar] [CrossRef]
- Javeria, S.; Suki, N.M. Perceptions creating stress in cosmetics consumers: Analysis during COVID-19 pandemic in Pakistan. J. Cardiovasc. Dis. Res. 2021, 12, 135–139. [Google Scholar]
- Lee, J.; Kwon, K.H. The Significant Value of Sustainable Cosmetics Fragrance in the Spotlight After COVID-19. J. Cosmet. Dermatol. 2022. [Google Scholar] [CrossRef] [PubMed]
- Russo, M.Z. The Role of Cosmetics During and after the Pandemic Era; Euro Cosmetics: Munich, Germany, 2020. [Google Scholar]
- Available online: https://www.bvl.bund.de/DE/Arbeitsbereiche/03_Verbraucherprodukte/01_Aufgaben/06_Kosmetik/bgs_Kosmetik_node.html (accessed on 2 September 2022).
- Available online: https://www.oekotest.de/kosmetik-wellness/Wie-Hersteller-die-Wirksamkeit-ihrer-Kosmetik-belegen-oder-auch-nicht-_109535_1.html (accessed on 2 September 2022).
- Toda, M.; Morimoto, K. Effect of lavender aroma on salivary endocrinological stress markers. Arch. Oral Biol. 2008, 53, 964–968. [Google Scholar] [CrossRef] [PubMed]
- Hirokawa, K.; Nishimoto, T.; Taniguchi, T. Effects of lavender aroma on sleep quality in healthy Japanese students. Percept. Mot. Ski. 2012, 114, 111–122. [Google Scholar] [CrossRef]
- Kutlu, A.K.; Yılmaz, E.; Çeçen, D. Effects of aroma inhalation on examination anxiety. Teach. Learn. Nurs. 2008, 3, 125–130. [Google Scholar] [CrossRef]
- Bentley, J.; Moore, J.P.; Farrant, J.M. Metabolomic profiling of the desiccation-tolerant medicinal shrub Myrothamnus flabellifolia indicates phenolic variability across its natural habitat: Implications for tea and cosmetics production. Molecules 2019, 24, 1240. [Google Scholar] [CrossRef]
- Bentley, J.; Olsen, E.K.; Moore, J.P.; Farrant, J.M. The phenolic profile extracted from the desiccation-tolerant medicinal shrub Myrothamnus flabellifolia using Natural Deep Eutectic Solvents varies according to the solvation conditions. Phytochemistry 2020, 173, 112323. [Google Scholar] [CrossRef]
- Bentley, J.; Moore, J.P.; Farrant, J.M. Metabolomics as a complement to phylogenetics for assessing intraspecific boundaries in the desiccation-tolerant medicinal shrub Myrothamnus flabellifolia (Myrothamnaceae). Phytochemistry 2019, 159, 127–136. [Google Scholar] [CrossRef]
- Cheikhyoussef, A.; Summers, R.W.; Kahaka, G.K. Qualitative and quantitative analysis of phytochemical compounds in Namibian Myrothamnus flabellifolius. Int. Sci. Technol. J. Namib. 2015, 5, 71–83. [Google Scholar]
- Biscaro, R.C.; Mussi, L.; Sufi, B.; Padovani, G.; Junior, F.B.C.; Magalhães, W.V.; Di Stasi, L.C. Modulation of autophagy by an innovative phytocosmetic preparation (Myrothamnus flabelifolia and Coffea arabica) in human fibroblasts and its effects in a clinical randomized placebo-controlled trial. J. Cosmet. Dermatol. 2022. [Google Scholar] [CrossRef] [PubMed]
- Viljoen, A.M.; Klepser, M.E.; Ernst, E.J.; Keele, D.; Roling, E.; Van Vuuren, S.; Demirci, B.; Baser, K.H.C.; van Wyk, B.-E.; Jäger, A.K. The composition and antimicrobial activity of the essential oil of the resurrection plant Myrothamnus flabellifolius. S. Afr. J. Bot. 2002, 68, 100–105. [Google Scholar] [CrossRef]
- Gescher, K.; Kühn, J.; Lorentzen, E.; Hafezi, W.; Derksen, A.; Deters, A.; Hensel, A. Proanthocyanidin-enriched extract from Myrothamnus flabellifolia Welw. exerts antiviral activity against herpes simplex virus type 1 by inhibition of viral adsorption and penetration. J. Ethnopharmacol. 2011, 134, 468–474. [Google Scholar] [CrossRef] [PubMed]
- Nicoletti, M.; Maggi, F.; Papa, F.; Vittori, S.; Quassinti, L.; Bramucci, M.; Lupidi, G.; Petrelli, D.; Vitali, L.A.; Ralaibia, E.; et al. In vitro biological activities of the essential oil from the ‘resurrection plant’Myrothamnus moschatus (Baillon) Niedenzu endemic to Madagascar. Nat. Prod. Res. 2012, 26, 2291–2300. [Google Scholar] [CrossRef] [PubMed]
- Chagonda, L.S.; Makanda, C.; Chalchat, J.-C. Essential Oils of Four Wild and Semi-Wild Plants from Zimbabwe: Colospermum mopane (Kirk ex Benth.) Kirk ex Leonard, Helichrysum splendidum (Thunb.) Less, Myrothamnus flabellifolia (Welw.) and Tagetes minuta L. J. Essent. Oil Res. 1999, 11, 573–578. [Google Scholar] [CrossRef]
- Kessler, A.; Sahin-Nadeem, H.; Lummis, S.C.R.; Weigel, I.; Pischetsrieder, M.; Buettner, A.; Villmann, C. GABAA receptor modulation by terpenoids from Sideritis extracts. Mol. Nutr. Food Res. 2014, 58, 851–862. [Google Scholar] [CrossRef]
- Hartley, N.; McLachlan, C.S. Aromas Influencing the GABAergic System. Molecules 2022, 27, 2414. [Google Scholar] [CrossRef]
- Nuss, P. Anxiety disorders and GABA neurotransmission: A disturbance of modulation. Neuropsychiatr. Dis. Treat. 2015, 11, 165. [Google Scholar]
- Herz, R.S. Aromatherapy facts and fictions: A scientific analysis of olfactory effects on mood, physiology and behavior. Int. J. Neurosci. 2009, 119, 263–290. [Google Scholar] [CrossRef]
- Angelucci, F.L.; Silva, V.V.; Dal Pizzol, C.; Spir, L.G.; Praes, C.E.; Maibach, H. Physiological effect of olfactory stimuli inhalation in humans: An overview. Int. J. Cosmet. Sci. 2014, 36, 117–123. [Google Scholar] [CrossRef]
- Herz, R.S. Influences of odors on mood and affective cognition. In Olfaction, Taste and Cognition; Rouby, C., Schaal, B., Dubois, D., Gervais, R., Holley, A., Eds.; Cambridge University Press: Cambridge, NY, USA, 2002; pp. 160–177. [Google Scholar]
- Schiffman, S.S.; Sattely-Miller, E.A.; Suggs, M.S.; Graham, B.G. The effect of pleasant odors and hormone status on mood of women at midlife. Brain Res. Bull. 1995, 36, 19–29. [Google Scholar] [CrossRef]
- Loos, H.M.; Schreiner, L.; Karacan, B. A systematic review of physiological responses to odours with a focus on current methods used in event-related study designs. Int. J. Psychophysiol. 2020, 158, 143–157. [Google Scholar] [CrossRef] [PubMed]
- Diego, M.A.; Jones, N.A.; Field, T.; Hernandez-Reif, M.; Schanberg, S.; Kuhn, C.; McAdam, V.; Galamaga, R.; Galamaga, M. Aromatherapy positively affects mood, EEG patterns of alertness and math computations. Int. J. Neurosci. 1998, 96, 217–224. [Google Scholar] [CrossRef]
- Field, T.; Diego, M.; Hernandez-Reif, M.; Cisneros, W.; Feijo, L.; Vera, Y.; Gil, K.; Grina, D.; Claire He, Q. Lavender fragrance cleansing gel effects on relaxation. Int. J. Neurosci. 2005, 115, 207–222. [Google Scholar] [CrossRef]
- Skoric, M.K.; Ivan Adamec, I.; Jerbić, A.B.; Gabelić, T.; Hajnšek, S.; Habek, M. Electroencephalographic response to different odors in healthy individuals: A promising tool for objective assessment of olfactory disorders. Clin. EEG Neurosci. 2015, 46, 370–376. [Google Scholar] [CrossRef] [PubMed]
- Sowndhararajan, K.; Cho, H.; Yu, B.; Kim, S. Effect of olfactory stimulation of isomeric aroma compounds, (+)-limonene and terpinolene on human electroencephalographic activity. Eur. J. Integr. Med. 2015, 7, 561–566. [Google Scholar] [CrossRef]
- Teplan, M. Fundamentals of EEG measurement. Meas. Sci. Rev. 2002, 2, 1–11. [Google Scholar]
- Sanei, S.; Chambers, J.A. EEG Signal Processing; John Wiley & Sons: Hoboken, NJ, USA, 2013. [Google Scholar]
- Klimesch, W.; Schmike, H.; Pfurtscheller, G. Alpha frequency cognitive load and memory performance. Brain Topogr. 1993, 5, 241–251. [Google Scholar] [CrossRef]
- Basar, E. A review of alpha activity in integrative brain function: Fundamental physiology, sensory coding, cognition and pathology. Int. J. Psychophysiol. 2012, 86, 1–24. [Google Scholar] [CrossRef]
- Kim, S.C.; Lee, M.H.; Jang, C.; Kwon, J.W.; Park, J.W. The effect of alpha rhythm sleep on EEG activity and individuals’ attention. J. Phys. Ther. Sci. 2013, 25, 1515–1518. [Google Scholar] [CrossRef] [PubMed]
- Masago, R.; Matsuda, T.; Kikuchi, Y.; Miyazaki, Y.; Iwanaga, K.; Harada, H.; Katsuura, T. Effects of inhalation of essential oils on EEG activity and sensory evaluation. J. Physiol. Anthropol. 2000, 19, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Iijima, M.; Nio, E.; Nashimoto, E.; Iwata, M. Effects of aroma on the autonomic nervous system and brain activity under stress conditions. Auton. Neurosci. 2007, 135, 97–98. [Google Scholar] [CrossRef]
- Sowndhararajan, K.; Kim, S. Influence of fragrances on human psychophysiological activity: With special reference to human electroencephalographic response. Sci. Pharm. 2016, 84, 724–751. [Google Scholar] [CrossRef] [PubMed]
- Balart-Sánchez, S.A.; Vélez-Pérez, H.; Rivera-Tello, S.; Gómez-Velázquez, F.R.; González-Garrido, A.A.; Romo-Vázquez, R. A step forward in the quest for a mobile EEG-designed epoch for psychophysiological studies. Biomed. Eng./Biomed. Tech. 2019, 64, 655–667. [Google Scholar] [CrossRef] [PubMed]
- Mancevska, S.; Gligoroska, J.P.; Todorovska, L.; Dejanova, B.; Petrovska, S. Psychophysiology and the sport science. Res. Phys. Educ. Sport Health 2016, 5, 101–105. [Google Scholar]
- Mavros, P.; Austwick, M.Z.; Smith, A.H. Geo-EEG: Towards the use of EEG in the study of urban behaviour. Appl. Spat. Anal. Policy 2016, 9, 191–212. [Google Scholar] [CrossRef]
- Askamp, J.; van Putten, M.J.A.M. Mobile EEG in epilepsy. Int. J. Psychophysiol. 2014, 91, 30–35. [Google Scholar] [CrossRef]
- Krigolson, O.E.; Williams, C.C.; Norton, A.; Hassall, C.D.; Colino, F.L. Choosing MUSE: Validation of a low-cost, portable EEG system for ERP research. Front. Neurosci. 2017, 11, 109. [Google Scholar] [CrossRef]
- Krigolson, O.E.; Hammerstrom, M.R.; Abimbola, W.; Trska, R.; Wright, B.W.; Hecker, K.G.; Binsted, G. Using Muse: Rapid mobile assessment of brain performance. Front. Neurosci. 2021, 15, 634147. [Google Scholar] [CrossRef]
- Krigolson, O.E.; Williams, C.C.; Colino, F.L. Using portable EEG to assess human visual attention. In Proceedings of the International Conference on Augmented Cognition, Vancouver, Canada, 9–14 July 2017; Springer: Cham, Switzerland, 2017. Available online: http://2017.hci.international/ac (accessed on 2 September 2022).
- Herman, K.; Ciechanowski, L.; Przegalińska, A. Emotional well-being in urban wilderness: Assessing states of calmness and alertness in informal green spaces (IGSs) with muse—Portable EEG headband. Sustainability 2021, 13, 2212. [Google Scholar] [CrossRef]
- Wilkinson, C.M.; Burrell, J.I.; Kuziek, J.W.P.; Thirunavukkarasu, S.; Buck, B.H.; Mathewson, K.E. Predicting stroke severity with a 3-min recording from the Muse portable EEG system for rapid diagnosis of stroke. Sci. Rep. 2020, 10, 18465. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Liu, D.; Wan, Z.; Hu, C. A noninvasive real-time driving fatigue detection technology based on left prefrontal Attention and Meditation EEG. In Proceedings of the 2014 International Conference on Multisensor Fusion and Information Integration for Intelligent Systems (MFI), Beijing, China, 28–29 September 2014. [Google Scholar]
- Purnamasari, P.D.; Fernandya, A. Real time EEG-based stress detection and meditation application with K-nearest neighbor. In Proceedings of the 2019 IEEE R10 Humanitarian Technology Conference (R10-HTC), Depok, Indonesia, 12–14 November 2019; p. 47129. [Google Scholar]
- Sravanth, K.R.; Peddi, A.; Sagar, G.S.; Gupta, B.; Chakraborty, C. Comparison of Attention and Meditation based mobile applications by using EEG signals. In Proceedings of the 2018 Global Wireless Summit (GWS), Chiang Rai, Thailand, 25–28 November 2018. [Google Scholar]
- Rizzi, V.; Gubitosa, J.; Fini, P.; Cosma, P. Neurocosmetics in Skincare—The Fascinating World of Skin–Brain Connection: A Review to Explore Ingredients, Commercial Products for Skin Aging, and Cosmetic Regulation. Cosmetics 2021, 8, 66. [Google Scholar] [CrossRef]
- Gabriel, D.; Merat, E.; Jeudy, A.; Cambos, S.; Chabin, T.; Giustiniani, J.; Haffen, E. Emotional Effects Induced by the Application of a Cosmetic Product: A Real-Time Electrophysiological Evaluation. Appl. Sci. 2021, 11, 4766. [Google Scholar] [CrossRef]
- Nozawa, A.; Uchida, M. Characterization of preference for viscosity and fragrance of cosmetic emulsions by autonomous nervous system activity. In Proceedings of the 2009 ICCAS-SICE, Fukuoka, Japan, 18–21 August 2009. [Google Scholar]
- Barkat, S.; Thomas-Danguin, T.; Bensafi, M.; Rouby, C.; Sicard, G. Odor and color of cosmetic products: Correlations between subjective judgement and autonomous nervous system response. Int. J. Cosmet. Sci. 2003, 25, 273–283. [Google Scholar] [CrossRef] [PubMed]
- Ohira, H.; Hirao, N. Analysis of skin conductance response during evaluation of preferences for cosmetic products. Front. Psychol. 2015, 6, 103. [Google Scholar] [CrossRef]
- Abriat, A.; Barkat, S.; Bensafi, M.; Rouby, C.; Guillou, V. Emotional and psychological effects of fragrance in men’s skin care. Int. J. Cosmet. Sci. 2005, 27, 300. [Google Scholar] [CrossRef]
- Abriat, A.; Barkat, S.; Bensafi, M.; Rouby, C.; Fanchon, C. Psychological and physiological evaluation of emotional effects of a perfume in menopausal women. Int. J. Cosmet. Sci. 2007, 29, 399–408. [Google Scholar] [CrossRef]
- Chrousos, G.P. Stress and disorders of the stress system. Nat. Rev. Endocrinol. 2009, 5, 374–381. [Google Scholar] [CrossRef]
- Kirschbaum, C.; Hellhammer, D.H. Salivary cortisol in psychoneuroendocrine research: Recent developments and applications. Psychoneuroendocrinology 1994, 19, 313–333. [Google Scholar] [CrossRef]
- Nater, U.M.; Rohleder, N. Salivary α-amylase as a non-invasive biomarker for the sympathetic nervous system: Current state of research. Psychoneuroendocrinology 2009, 34, 486–496. [Google Scholar] [CrossRef] [PubMed]
- Skosnik, P.D.; Chatterton, R.T.; Swisher, T.; Park, S. Modulation of attentional inhibition by norepinephrine and cortisol after psychological stress. Int. J. Psychophysiol. 2000, 36, 59–68. [Google Scholar] [CrossRef]
- Chatterton, R.T., Jr.; Vogelsong, K.M.; Lu, Y.C.; Hudgens, G.A. Hormonal responses to psychological stress in men preparing for skydiving. J. Clin. Endocri-Nology Metab. 1997, 82, 2503–2509. [Google Scholar] [CrossRef]
- Takai, N.; Yamaguchi, M.; Aragaki, T.; Eto, K.; Uchihashi, K.; Nishikawa, Y. Effect of psychological stress on the salivary cortisol and amylase levels in healthy young adults. Arch. Oral Biol. 2004, 49, 963–968. [Google Scholar] [CrossRef]
- Watson, D.; Clark, L.A.; Tellegen, A. Development and validation of brief measures of positive and negative affect: The PANAS scales. J. Personal. Soc. Psychol. 1988, 54, 1063. [Google Scholar] [CrossRef]
- Yim, I.S.; Quas, J.A.; Cahill, L.; Hayakawa, C.M. Children’s and adults’ salivary cortisol responses to an identical psychosocial laboratory stress-or. Psychoneuroendocrinology 2010, 35, 241–248. [Google Scholar] [CrossRef]
- Sgoifo, A.; Carnevali, L.; Pattini, E.; Carandina, A.; Tanzi, G.; Del Canale, C.; Goi, P.; Giudice, M.B.D.F.D.; De Carne, B.; Fornari, M.; et al. Psychobiological evidence of the stress resilience fostering properties of a cosmetic routine. Stress 2021, 24, 53–63. [Google Scholar] [CrossRef]
- DIN 10961; Schulung von Prüfpersonen für Sensorische Prüfungen. Deutsches Institut für Normung e.V. Beuth Verlag GmbH: Berlin, Germany, 1996.
- Kurdi, B.; Lozano, S.; Banaji, M.R. Introducing the open affective standardized image set (OASIS). Behav. Res. Methods 2017, 49, 457–470. [Google Scholar] [CrossRef]
- Available online: https://choosemuse.com/ (accessed on 2 September 2022).
- Bird, J.J.; Manso, L.J.; Ribeiro, E.P.; Ekart, A.; Faria, D.R. A study on mental state classification using eeg-based brain-machine interface. In Proceedings of the 2018 International Conference on Intelligent Systems (IS), Funchal, Madeira Island, 25–27 September 2018. [Google Scholar]
- Breyer, B.; Bluemke, M. Deutsche Version der Positive and Negative Affect Schedule PANAS (GESIS Panel). Zusammenstellung sozialwissenschaftlicher Items und Skalen 2016. [Google Scholar] [CrossRef]
- Available online: https://mind-monitor.com/ (accessed on 2 September 2022).
- JASP. version 0.16.3; [Computer Software]; JASP Team: Amsterdam, The Netherlands, 2022. [Google Scholar]
- Python Software Foundation. Available online: https://www.python.org/ (accessed on 2 September 2022).
- Holm, S. A simple sequentially rejective multiple test procedure. Scand. J. Stat. 1979, 6, 65–70. [Google Scholar]
- Available online: https://www.fao.org/food/food-safety-quality/scientific-advice/jecfa/jecfa-flav/details/en/c/1402/ (accessed on 2 September 2022).
- Kontaris, I.; East, B.S.; Wilson, D.A. Behavioral and neurobiological convergence of odor, mood and emotion: A review. Front. Behav. Neurosci. 2020, 14, 35. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Haviland-Jones, J. Rapid mood change and human odors. Physiol. Behav. 1999, 68, 241–250. [Google Scholar] [CrossRef]
- Kirk-Smith, M.D.; Van Toller, C.; Dodd, G.H. Unconscious odour conditioning in human subjects. Biol. Psychol. 1983, 17, 221–231. [Google Scholar] [CrossRef]
- Kaimal, G.; Ray, K.; Muniz, J. Reduction of cortisol levels and participants’ responses following art making. Art Ther. 2016, 33, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Uedo, N.; Ishikawa, H.; Morimoto, K.; Ishihara, R.; Narahara, H.; Akedo, I.; Ioka, T.; Kaji, I.; Fukuda, S. Reduction in salivary cortisol level by music therapy during colonoscopic examination. Hepato-Gastroenterology 2004, 51, 451–453. [Google Scholar] [PubMed]
- Fukui, H.; Komaki, R.; Okui, M.; Toyoshima, K.; Kuda, K. The effects of odor on cortisol and testosterone in healthy adults. Neuroendocrinol. Lett. 2007, 28, 433–437. [Google Scholar] [PubMed]
- Kim, I.H.; Kim, C.; Seong, K.; Hur, M.H.; Lim, H.M.; Lee, M.S. Essential oil inhalation on blood pressure and salivary cortisol levels in prehypertensive and hypertensive subjects. Evid. -Based Complement. Altern. Med. 2012, 2012, 984203. [Google Scholar] [CrossRef]
- Watanabe, E.; Kuchta, K.; Kimura, M.; Rauwald, H.W.; Kamei, T.; Imanishi, J. Effects of bergamot (Citrus bergamia (Risso) Wright & Arn.) essential oil aromatherapy on mood states, parasympathetic nervous system activity, and salivary cortisol levels in 41 healthy females. Complement. Med. Res. 2015, 22, 43–49. [Google Scholar]
- Chaiyasut, C.; Sivamaruthi, B.S.; Wongwan, J.; Thiwan, K.; Rungseevijitprapa, W.; Klunklin, A.; Kunaviktikul, W. Effects of Litsea cubeba (Lour.) persoon essential oil aromatherapy on mood states and salivary cortisol levels in healthy volunteers. Evid. -Based Complement. Altern. Med. 2020, 2020, 4389239. [Google Scholar] [CrossRef]
- Figueiredo, A.C.; Barroso, J.G.; Pedro, L.G.; Scheffer, J.J. Factors affecting secondary metabolite production in plants: Volatile components and essential oils. Flavour Fragr. J. 2008, 23, 213–226. [Google Scholar] [CrossRef]
- Brummer, R.; Godersky, S. Rheological studies to objectify sensations occurring when cosmetic emulsions are applied to the skin. Colloids Surf. A Physicochem. Eng. Asp. 1999, 152, 89–94. [Google Scholar] [CrossRef]
- Doi, T.; Wang, M.; McClements, D.J. Emulsion-based control of flavor release profiles: Impact of oil droplet characteristics on garlic aroma release during simulated cooking. Food Res. Int. 2019, 116, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Ryan, D.; Prenzler, P.D.; Saliba, A.J.; Scollary, G.R. The significance of low impact odorants in global odour perception. Trends Food Sci. Technol. 2008, 19, 383–389. [Google Scholar] [CrossRef]

| AC | PL | |
|---|---|---|
| Ingredients (INCI) | Water, Caprylic/Capric Triglyceride, Glyceryl Stearate Citrate, Cetearyl Alcohol, Phenoxyethanol, Sucrose Stearate, Carbomer, Caprylyl Glycol, Fragrance (Parfum), Xanthan Gum, Myrothamnus Flabellifolia Leaf/Stem Extract, Sodium Hydroxide | Water, Caprylic/Capric Triglyceride, Glyceryl Stearate Citrate, Cetearyl Alcohol, Phenoxyethanol, Sucrose Stearate, Carbomer, Caprylyl Glycol, Fragrance (Parfum), Xanthan Gum, Sodium Hydroxide |
| Active Compound | 3.0% Myrothamnus flabellifolia CO2-extract | 3.0% Water |
| pH-value | 5.8 | 5.8 |
| Density [g/mL] | 0.9–1.0 | 0.9–1.0 |
| Production date | 19 December 2019 | 19 December 2019 |
| Shelf life [months] | 36 | 36 |
| Effect | DFnum | DFden | F | p-Value | ηp2 |
|---|---|---|---|---|---|
| Positive Affect Score | |||||
| Time | 1 | 24 | 0.783 | 0.385 | 0.032 |
| Sample | 1 | 24 | 0.878 | 0.358 | 0.035 |
| Time×Sample | 1 | 24 | 3.442 | 0.076 (*) | 0.125 |
| Negative Affect Score | |||||
| Time | 1 | 24 | 27.615 | <0.001 *** | 0.535 |
| Sample | 1 | 24 | 1.676 | 0.208 | 0.065 |
| Time×Sample | 1 | 24 | 0.741 | 0.398 | 0.030 |
| Effect | DFnum | DFden | F | p-Value | ηp2 |
|---|---|---|---|---|---|
| Cortisol | |||||
| Time | 1 | 23 | 2.017 | 0.169 | 0.081 |
| Sample | 1 | 23 | 0.049 | 0.826 | 0.002 |
| Time×Sample | 1 | 23 | 6.583 | 0.017 * | 0.223 |
| α-amylase | |||||
| Time | 1 | 22 | 0.093 | 0.763 | 0.004 |
| Sample | 1 | 22 | 0.065 | 0.801 | 0.003 |
| Time×Sample | 1 | 22 | 2.743 | 0.112 | 0.111 |
| Effect | DFnum | DFden | F | p-Value | ηp2 |
|---|---|---|---|---|---|
| Time | 1 | 23 | 2.261 | 0.146 | 0.090 |
| Sample | 1 | 23 | 0.005 | 0.944 | <0.001 |
| Time×Sample | 1 | 23 | 3.367 | 0.079 (*) | 0.128 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Springer, A.; Höckmeier, L.; Schicker, D.; Hettwer, S.; Freiherr, J. Measurement of Stress Relief during Scented Cosmetic Product Application Using a Mood Questionnaire, Stress Hormone Levels and Brain Activation. Cosmetics 2022, 9, 97. https://doi.org/10.3390/cosmetics9050097
Springer A, Höckmeier L, Schicker D, Hettwer S, Freiherr J. Measurement of Stress Relief during Scented Cosmetic Product Application Using a Mood Questionnaire, Stress Hormone Levels and Brain Activation. Cosmetics. 2022; 9(5):97. https://doi.org/10.3390/cosmetics9050097
Chicago/Turabian StyleSpringer, Arielle, Laura Höckmeier, Doris Schicker, Stefan Hettwer, and Jessica Freiherr. 2022. "Measurement of Stress Relief during Scented Cosmetic Product Application Using a Mood Questionnaire, Stress Hormone Levels and Brain Activation" Cosmetics 9, no. 5: 97. https://doi.org/10.3390/cosmetics9050097
APA StyleSpringer, A., Höckmeier, L., Schicker, D., Hettwer, S., & Freiherr, J. (2022). Measurement of Stress Relief during Scented Cosmetic Product Application Using a Mood Questionnaire, Stress Hormone Levels and Brain Activation. Cosmetics, 9(5), 97. https://doi.org/10.3390/cosmetics9050097






