A Review of Moisturizing Additives for Atopic Dermatitis
Abstract
1. Introduction
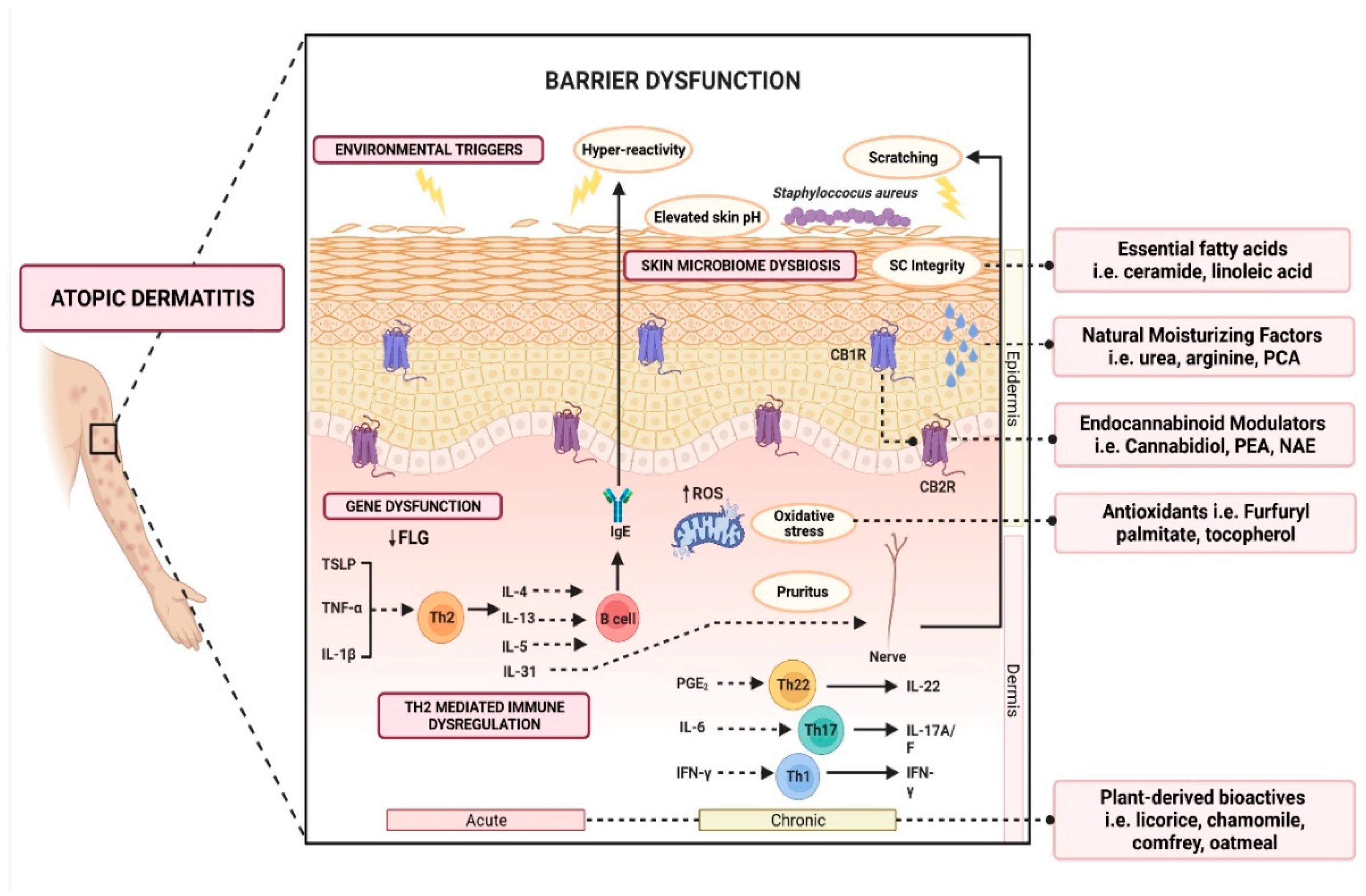
2. Pathophysiology of AD
2.1. Gene Dysfunction and Epidermal Barrier Dysfunction
2.2. Th2 Mediated Immune Dysregulation and Neuroimmune Interactions
2.3. Skin Microbiome Dysbiosis
2.4. Other Factors Affecting Barrier Function
2.4.1. Acid Mantle in Epidermal Barrier Function
2.4.2. Natural Moisturizing Factors
3. Role of Moisturizers in the Treatment of AD
4. Active Ingredients Beneficial in Alleviating Atopic Eczema Inflammation
4.1. Plant-Derived Active Ingredients
4.1.1. Licorice-Derived Bioactives
4.1.2. Chamomile-Derived Bioactives
4.1.3. Comfrey-Derived Bioactive
4.1.4. Colloidal Oatmeal
4.2. Natural Moisturizing Factors
4.3. Ceramides and Fatty Acids Organization in Permeability Barrier Function
4.4. Endocannabinoid System—A New Star in the Therapy of Eczema
4.5. Furfuryl Derivatives and Antioxidants
5. Limitations of Current Cosmeceutical Research on AD
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Langan, S.M.; Irvine, A.D.; Weidinger, S. Atopic dermatitis. Lancet 2020, 396, 345–360. [Google Scholar] [CrossRef]
- Avena-Woods, C. Overview of atopic dermatitis. Am. J. Manag. Care 2017, 23, S115–S123. [Google Scholar] [PubMed]
- Nutten, S. Atopic dermatitis: Global epidemiology and risk factors. Ann. Nutr. Metab. 2015, 66, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Silverberg, J.I.; Barbarot, S.; Gadkari, A.; Simpson, E.L.; Weidinger, S.; Mina-Osorio, P.; Rossi, A.B.; Brignoli, L.; Saba, G.; Guillemin, I.; et al. Atopic dermatitis in the pediatric population: A cross-sectional, international epidemiologic study. Ann. Allergy Asthma Immunol. 2021, 126, 417–428.e412. [Google Scholar] [CrossRef]
- Hanifin, J.M.; Rajka, G. Diagnostic features of atopic dermatitis. Acta Derm. Venereol. 1980, 1980, 44–47. [Google Scholar]
- Patel, K.R.; Immaneni, S.; Singam, V.; Rastogi, S.; Silverberg, J.I. Association between atopic dermatitis, depression, and suicidal ideation: A systematic review and meta-analysis. J. Am. Acad. Dermatol. 2019, 80, 402–410. [Google Scholar] [CrossRef]
- Li, A.W.; Yin, E.S.; Antaya, R.J. Topical corticosteroid phobia in atopic dermatitis: A systematic review. JAMA Dermatol. 2017, 153, 1036–1042. [Google Scholar] [CrossRef]
- Barrett, A.; Hahn-Pedersen, J.; Kragh, N.; Evans, E.; Gnanasakthy, A. Patient-Reported Outcome Measures in atopic dermatitis and chronic hand eczema in adults. Patient 2019, 12, 445–459. [Google Scholar] [CrossRef]
- Sidbury, R.; Davis, D.M.; Cohen, D.E.; Cordoro, K.M.; Berger, T.G.; Bergman, J.N.; Chamlin, S.L.; Cooper, K.D.; Feldman, S.R.; Hanifin, J.M.; et al. Guidelines of care for the management of atopic dermatitis: Section 3. Management and treatment with phototherapy and systemic agents. J. Am. Acad. Dermatol. 2014, 71, 327–349. [Google Scholar] [CrossRef]
- Williams, H.C.; Burney, P.G.; Pembroke, A.C.; Hay, R.J. The U.K. Working Party’s diagnostic criteria for atopic dermatitis. III. Independent hospital validation. Br. J. Dermatol. 1994, 131, 406–416. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef] [PubMed]
- Elias, P.M.; Wakefield, J.S.; Man, M.Q. Moisturizers versus current and next-generation barrier repair therapy for the management of atopic dermatitis. Skin Pharmacol. Physiol. 2019, 32, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Luger, T.; Amagai, M.; Dreno, B.; Dagnelie, M.-A.; Liao, W.; Kabashima, K.; Schikowski, T.; Proksch, E.; Elias, P.M.; Simon, M.; et al. Atopic dermatitis: Role of the skin barrier, environment, microbiome, and therapeutic agents. J. Dermatol. Sci. 2021, 102, 142–157. [Google Scholar] [CrossRef]
- Sörensen, A.; Landvall, P.; Lodén, M. Moisturizers as cosmetics, medicines, or medical device? The regulatory demands in the European Union. In Treatment of dry Skin Syndrome; Springer: Berlin/Heidelberg, Germany, 2011; Volume 8, pp. 3–16. [Google Scholar]
- Wananukul, S.; Chatproedprai, S.; Chunharas, A.; Limpongsanuruk, W.; Singalavanija, S.; Nitiyarom, R.; Wisuthsarewong, W. Randomized, double-blind, split-side, comparison study of moisturizer containing licochalcone A and 1% hydrocortisone in the treatment of childhood atopic dermatitis. J. Med. Assoc. Thai. 2013, 96, 1135–1142. [Google Scholar]
- Nakahara, T.; Kido-Nakahara, M.; Tsuji, G.; Furue, M. Basics and recent advances in the pathophysiology of atopic dermatitis. J. Dermatol. 2021, 48, 130–139. [Google Scholar] [CrossRef] [PubMed]
- Bønnelykke, K.; Sparks, R.; Waage, J.; Milner, J.D. Genetics of allergy and allergic sensitization: Common variants, rare mutations. Curr. Opin. Immunol. 2015, 36, 115–126. [Google Scholar] [CrossRef]
- Eyerich, K.; Novak, N. Immunology of atopic eczema: Overcoming the Th1/Th2 paradigm. Allergy 2013, 68, 974–982. [Google Scholar] [CrossRef]
- Jang, H.; Matsuda, A.; Jung, K.; Karasawa, K.; Matsuda, K.; Oida, K.; Ishizaka, S.; Ahn, G.; Amagai, Y.; Moon, C.; et al. Skin pH Is the master switch of kallikrein 5-mediated skin barrier destruction in a murine atopic dermatitis model. J. Investig. Dermatol. 2016, 136, 127–135. [Google Scholar] [CrossRef]
- Zhu, Y.; Underwood, J.; Macmillan, D.; Shariff, L.; O’Shaughnessy, R.; Harper, J.I.; Pickard, C.; Friedmann, P.S.; Healy, E.; Di, W.L. Persistent kallikrein 5 activation induces atopic dermatitis-like skin architecture independent of PAR2 activity. J. Allergy Clin. Immunol. 2017, 140, 1310–1322.e1315. [Google Scholar] [CrossRef]
- Basu, M.N.; Mortz, C.G.; Jensen, T.K.; Barington, T.; Halken, S. Natural moisturizing factors in children with and without eczema: Associations with lifestyle and genetic factors. J. Eur. Acad. Dermatol. Venereol. 2022, 36, 255–262. [Google Scholar] [CrossRef]
- Nouwen, A.E.M.; Karadavut, D.; Pasmans, S.; Elbert, N.J.; Bos, L.D.N.; Nijsten, T.E.C.; Arends, N.J.T.; Pijnenburg, M.W.H.; Koljenović, S.; Puppels, G.J.; et al. Natural moisturizing factor as a clinical marker in atopic dermatitis. Allergy 2020, 75, 188–190. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Seok, J.K.; Kang, H.C.; Cho, Y.Y.; Lee, H.S.; Lee, J.Y. Skin barrier abnormalities and immune dysfunction in atopic dermatitis. Int. J. Mol. Sci. 2020, 21, 2867. [Google Scholar] [CrossRef] [PubMed]
- Kantor, R.; Silverberg, J.I. Environmental risk factors and their role in the management of atopic dermatitis. Expert Rev. Clin. Immunol. 2017, 13, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Chamlin, S.L.; Kao, J.; Frieden, I.J.; Sheu, M.Y.; Fowler, A.J.; Fluhr, J.W.; Williams, M.L.; Elias, P.M. Ceramide-dominant barrier repair lipids alleviate childhood atopic dermatitis: Changes in barrier function provide a sensitive indicator of disease activity. J. Am. Acad. Dermatol. 2002, 47, 198–208. [Google Scholar] [CrossRef]
- Koppes, S.A.; Brans, R.; Ljubojevic Hadzavdic, S.; Frings-Dresen, M.H.; Rustemeyer, T.; Kezic, S. Stratum corneum tape stripping: Monitoring of inflammatory mediators in atopic dermatitis patients using topical therapy. Int. Arch. Allergy Immunol. 2016, 170, 187–193. [Google Scholar] [CrossRef]
- Somjorn, P.; Kamanamool, N.; Kanokrungsee, S.; Rojhirunsakool, S.; Udompataikul, M. A cream containing linoleic acid, 5% dexpanthenol and ceramide in the treatment of atopic dermatitis. Asian Pac. J. Allergy Immunol. 2021. [Google Scholar] [CrossRef]
- Iwamoto, K.; Moriwaki, M.; Miyake, R.; Hide, M. Staphylococcus aureus in atopic dermatitis: Strain-specific cell wall proteins and skin immunity. Allergol. Int. 2019, 68, 309–315. [Google Scholar] [CrossRef]
- Williams, M.R.; Gallo, R.L. The role of the skin microbiome in atopic dermatitis. Curr. Allergy Asthma Rep. 2015, 15, 65. [Google Scholar] [CrossRef]
- Yamazaki, Y.; Nakamura, Y.; Núñez, G. Role of the microbiota in skin immunity and atopic dermatitis. Allergol Int. 2017, 66, 539–544. [Google Scholar] [CrossRef]
- Byrd, A.L.; Belkaid, Y.; Segre, J.A. The human skin microbiome. Nat. Rev. Microbiol. 2018, 16, 143–155. [Google Scholar] [CrossRef]
- Kong, H.H.; Oh, J.; Deming, C.; Conlan, S.; Grice, E.A.; Beatson, M.A.; Nomicos, E.; Polley, E.C.; Komarow, H.D.; Murray, P.R.; et al. Temporal shifts in the skin microbiome associated with disease flares and treatment in children with atopic dermatitis. Genome Res. 2012, 22, 850–859. [Google Scholar] [CrossRef] [PubMed]
- Eberlein-König, B.; Schäfer, T.; Huss-Marp, J.; Darsow, U.; Möhrenschlager, M.; Herbert, O.; Abeck, D.; Krämer, U.; Behrendt, H.; Ring, J. Skin surface pH, stratum corneum hydration, trans-epidermal water loss and skin roughness related to atopic eczema and skin dryness in a population of primary school children. Acta Derm. Venereol. 2000, 80, 188–191. [Google Scholar] [CrossRef] [PubMed]
- Surber, C.; Humbert, P.; Abels, C.; Maibach, H. The acid mantle: A myth or an essential part of skin health? Curr. Probl. Dermatol. 2018, 54, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Lukić, M.; Pantelić, I.; Savić, S.D. Towards optimal pH of the skin and topical formulations: From the current state of the art to tailored products. Cosmetics 2021, 8, 69. [Google Scholar] [CrossRef]
- Karna, R.V. The investigation on correlation between pH and the likelihood of developing atopic dermatitis on paediatrics and adults population in Indonesia. In Proceedings of the Asian Australasian Regional Conference of Dermatology, Surabaya, Indonesia, 8–11 August 2018. [Google Scholar]
- Sparavigna, A.; Setaro, M.; Gualandri, V. Cutaneous pH in children affected by atopic dermatitis and in healthy children: A multicenter study. Skin Res. Technol. 1999, 5, 221–227. [Google Scholar] [CrossRef]
- Panther, D.J.; Jacob, S.E. The importance of acidification in atopic eczema: An underexplored avenue for treatment. J. Clin. Med. 2015, 4, 970–978. [Google Scholar] [CrossRef]
- Gupta, J.; Margolis, D.J. Filaggrin gene mutations with special reference to atopic dermatitis. Curr. Treat. Options Allergy 2020, 7, 403–413. [Google Scholar] [CrossRef]
- Gunnarsson, M.; Mojumdar, E.H.; Topgaard, D.; Sparr, E. Extraction of natural moisturizing factor from the stratum corneum and its implication on skin molecular mobility. J. Colloid Interface Sci. 2021, 604, 480–491. [Google Scholar] [CrossRef]
- Maeno, K. Direct quantification of natural moisturizing factors in stratum corneum using direct analysis in real time mass spectrometry with inkjet-printing technique. Sci. Rep. 2019, 9, 17789. [Google Scholar] [CrossRef]
- van Zuuren, E.J.; Fedorowicz, Z.; Christensen, R.; Lavrijsen, A.; Arents, B.W.M. Emollients and moisturisers for eczema. Cochrane Database Syst. Rev. 2017, 2, CD012119. [Google Scholar] [CrossRef]
- Xu, S.; Kwa, M.; Lohman, M.E.; Evers-Meltzer, R.; Silverberg, J.I. Consumer Preferences, Product Characteristics, and Potentially Allergenic Ingredients in Best-selling Moisturizers. JAMA Dermatol. 2017, 153, 1099–1105. [Google Scholar] [CrossRef] [PubMed]
- Draelos, Z.D. The science behind skin care: Moisturizers. J. Cosmet. Dermatol. 2018, 17, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Shi, V.Y.; Tran, K.; Lio, P.A. A comparison of physicochemical properties of a selection of modern moisturizers: Hydrophilic index and pH. J. Drugs Dermatol. 2012, 11, 633–636. [Google Scholar] [PubMed]
- Pinter, A.; Thouvenin, M.-D.; Bacquey, A.; Rossi, A.B.; Nocera, T. Tolerability and efficacy of a medical device repairing emollient cream in children and adults with mild to moderate atopic dermatitis. Dermatol. Ther. 2019, 9, 309–319. [Google Scholar] [CrossRef] [PubMed]
- Rossi, A.B.; Bacquey, A.; Nocera, T.; Thouvenin, M.D. Efficacy and tolerability of a medical device repairing emollient cream associated with a topical corticosteroid in adults with atopic dermatitis: An open-label, intra-individual randomized controlled study. Dermatol. Ther. 2018, 8, 217–228. [Google Scholar] [CrossRef]
- European Commission. Medical devices: Guidance Document—Classification of Medical Devices; European Commission: Brussels, Belgium, 2010. [Google Scholar]
- Abramovits, W.; Boguniewicz, M. A multicenter, randomized, vehicle-controlled clinical study to examine the efficacy and safety of MAS063DP (Atopiclair) in the management of mild to moderate atopic dermatitis in adults. J. Drugs Dermatol. 2006, 5, 236–244. [Google Scholar]
- Abramovits, W.; Hebert, A.; Boguniewicz, M.; Kempers, S.; Tschen, E.; Jarratt, M.; Lucky, A.; Cornelison, R.; Swinyer, L.; Jones, T. Patient-reported outcomes from a multicenter, randomized, vehicle-controlled clinical study of MAS063DP (Atopiclair™) in the management of mild-to-moderate atopic dermatitis in adults. J. Dermatol. Treat. 2008, 19, 327–332. [Google Scholar] [CrossRef]
- Boguniewicz, M.; Zeichner, J.A.; Eichenfield, L.F.; Hebert, A.A.; Jarratt, M.; Lucky, A.W.; Paller, A.S. MAS063DP is effective monotherapy for mild to moderate atopic dermatitis in infants and children: A multicenter, randomized, vehicle-controlled study. J. Pediatr. 2008, 152, 854–859. [Google Scholar] [CrossRef]
- Bomstein, Y.; Rozenblat, S. Treatment of atopic dermatitis with KAM-3008, a barrier-based, non-steroidal topical cream. J. Dermatolog. Treat. 2015, 26, 426–430. [Google Scholar] [CrossRef]
- Kowalska, A.; Kalinowska-Lis, U. 18β-Glycyrrhetinic acid: Its core biological properties and dermatological applications. Int. J. Cosmet. Sci. 2019, 41, 325–331. [Google Scholar] [CrossRef]
- Patrizi, A.; Capitanio, B.; Neri, I.; Giacomini, F.; Sinagra, J.; Raone, B.; Berardesca, E. A double-blind, randomized, vehicle-controlled clinical study to evaluate the efficacy and safety of MAS063DP (ATOPICLAIR) in the management of atopic dermatitis in paediatric patients. Pediatr. Allergy Immunol. 2008, 19, 619–625. [Google Scholar] [CrossRef] [PubMed]
- Saeedi, M.; Morteza-Semnani, K.; Ghoreishi, M.R. The treatment of atopic dermatitis with licorice gel. J. Dermatolog. Treat. 2003, 14, 153–157. [Google Scholar] [CrossRef] [PubMed]
- Seiwerth, J.; Tasiopoulou, G.; Hoffmann, J.; Wölfle, U.; Schwabe, K.; Quirin, K.W.; Schempp, C.M. Anti-inflammatory effect of a novel topical herbal composition (VEL-091604) consisting of gentian root, licorice root and willow bark extract. Planta Med. 2019, 85, 608–614. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, Y.; Peng, G.; Han, X. Glycyrrhizin ameliorates atopic dermatitis-like symptoms through inhibition of HMGB1. Int. Immunopharmacol. 2018, 60, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Angelova-Fischer, I.; Neufang, G.; Jung, K.; Fischer, T.W.; Zillikens, D. A randomized, investigator-blinded efficacy assessment study of stand-alone emollient use in mild to moderately severe atopic dermatitis flares. J. Eur. Acad. Dermatol. Venereol. 2014, 28 (Suppl. 3), 9–15. [Google Scholar] [CrossRef] [PubMed]
- Angelova-Fischer, I.; Rippke, F.; Richter, D.; Filbry, A.; Arrowitz, C.; Weber, T.; Fischer, T.W.; Zillikens, D. Stand-alone emollient treatment reduces flares after discontinuation of topical steroid treatment in atopic dermatitis: A double-blind, randomized, vehicle-controlled, left-right comparison study. Acta. Derm. Venereol. 2018, 98, 517–523. [Google Scholar] [CrossRef]
- Udompataikul, M.; Srisatwaja, W. Comparative trial of moisturizer containing licochalcone A vs. hydrocortisone lotion in the treatment of childhood atopic dermatitis: A pilot study. J. Eur. Acad. Dermatol. Venereol. 2011, 25, 660–665. [Google Scholar] [CrossRef]
- Ashfaq, U.A.; Masoud, M.S.; Nawaz, Z.; Riazuddin, S. Glycyrrhizin as antiviral agent against Hepatitis C Virus. J. Transl. Med. 2011, 9, 112. [Google Scholar] [CrossRef]
- Morinaga, O.; Ishiuchi, K.I.; Ohkita, T.; Tian, C.; Hirasawa, A.; Mitamura, M.; Maki, Y.; Yasujima, T.; Yuasa, H.; Makino, T. Isolation of a novel glycyrrhizin metabolite as a causal candidate compound for pseudoaldosteronism. Sci. Rep. 2018, 8, 15568. [Google Scholar] [CrossRef]
- do Nascimento, M.H.M.; de Araújo, D.R. Exploring the pharmacological potential of glycyrrhizic acid: From therapeutic applications to trends in nanomedicine. Future Pharmacol. 2022, 2, 1–15. [Google Scholar] [CrossRef]
- Nokhodchi, A.; Nazemiyeh, H.; Ghafourian, T.; Hassan-Zadeh, D.; Valizadeh, H.; Bahary, L.A. The effect of glycyrrhizin on the release rate and skin penetration of diclofenac sodium from topical formulations. Farmaco 2002, 57, 883–888. [Google Scholar] [CrossRef]
- Teelucksingh, S.; Mackie, A.D.; Burt, D.; McIntyre, M.A.; Brett, L.; Edwards, C.R. Potentiation of hydrocortisone activity in skin by glycyrrhetinic acid. Lancet 1990, 335, 1060–1063. [Google Scholar] [CrossRef]
- Kim, J.-E.; Yoon, G.; Shim, J.-H.; Cho, S.-S. Broad spectrum antimicrobial activity of licochalcones A and E against MDR (Multidrug Resistant) strains of clinical origin. Nat. Prod. Commun. 2017, 12, 1747–1748. [Google Scholar] [CrossRef]
- Yadav, V.R.; Prasad, S.; Sung, B.; Aggarwal, B.B. The role of chalcones in suppression of NF-κB-mediated inflammation and cancer. Int. Immunopharmacol. 2011, 11, 295–309. [Google Scholar] [CrossRef]
- Chen, M.F.; Shimada, F.; Kato, H.; Yano, S.; Kanaoka, M. Effect of glycyrrhizin on the pharmacokinetics of prednisolone following low dosage of prednisolone hemisuccinate. Endocrinol. Jpn. 1990, 37, 331–341. [Google Scholar] [CrossRef]
- MacKenzie, M.A.; Hoefnagels, W.H.; Kloppenborg, P.W. Glycyrrhetinic acid and potentiation of hydrocortisone activity in skin. Lancet 1990, 335, 1534. [Google Scholar] [CrossRef]
- Sigurjónsdóttir, H.A.; Franzson, L.; Manhem, K.; Ragnarsson, J.; Sigurdsson, G.; Wallerstedt, S. Liquorice-induced rise in blood pressure: A linear dose-response relationship. J. Hum. Hypertens. 2001, 15, 549–552. [Google Scholar] [CrossRef] [PubMed]
- Dawid-Pać, R. Medicinal plants used in treatment of inflammatory skin diseases. Postepy Dermatol. Alergol. 2013, 30, 170–177. [Google Scholar] [CrossRef]
- Lee, S.H.; Heo, Y.; Kim, Y.C. Effect of German chamomile oil application on alleviating atopic dermatitis-like immune alterations in mice. J. Vet. Sci. 2010, 11, 35–41. [Google Scholar] [CrossRef]
- Ortiz-Bautista, R.J.; García-González, L.L.; Ocádiz-González, M.A.; Flores-Tochihuitl, J.; García-Villaseñor, A.; González-Hernández, M.; Muñoz-Hernández, L.; Ortiz-Figueroa, M.C.; Ramírez-Anaya, M.; Reyna-Téllez, S.; et al. Matricaria chamomilla (aqueous extract) improves atopic dermatitis-like lesions in a murine model. Rev. Med. Inst. Mex. Seguro Soc. 2017, 55, 587–593. [Google Scholar]
- Wang, W.; Wang, Y.; Zou, J.; Jia, Y.; Wang, Y.; Li, J.; Wang, C.; Sun, J.; Guo, D.; Wang, F.; et al. The mechanism action of German chamomile (Matricaria recutita L.) in the treatment of eczema: Based on dose-effect weight coefficient network pharmacology. Front. Pharmacol. 2021, 12, 706836. [Google Scholar] [CrossRef] [PubMed]
- Aertgeerts, P.; Albring, M.; Klaschka, F.; Nasemann, T.; Patzelt-Wenczler, R.; Rauhut, K.; Weigl, B. Comparative testing of Kamillosan cream and steroidal (0.25% hydrocortisone, 0.75% fluocortin butyl ester) and non-steroidal (5% bufexamac) dermatologic agents in maintenance therapy of eczematous diseases. Z. Hautkr. 1985, 60, 270–277. [Google Scholar] [PubMed]
- Patzelt-Wenczler, R.; Ponce-Pöschl, E. Proof of efficacy of Kamillosan(R) cream in atopic eczema. Eur. J. Med. Res. 2000, 5, 171–175. [Google Scholar]
- Kadir, R.; Barry, B.W. α-Bisabolol, a possible safe penetration enhancer for dermal and transdermal therapeutics. Int. J. Pharm. 1991, 70, 87–94. [Google Scholar] [CrossRef]
- Kamatou, G.P.P.; Viljoen, A.M. A review of the application and pharmacological properties of α-bisabolol and α-bisabolol-rich oils. J. Am. Oil Chem. Soc. 2010, 87, 1–7. [Google Scholar] [CrossRef]
- Arenberger, P.; Arenbergerová, M.; Drozenová, H.; Hladíková, M.; Holcová, S. Effect of topical heparin and levomenol on atopic dermatitis: A randomized four-arm, placebo-controlled, double-blind clinical study. J. Eur. Acad. Dermatol. Venereol. 2011, 25, 688–694. [Google Scholar] [CrossRef]
- Bocchietto, E.; Pecis, L.; Lisi, P.; Angelini, G.; Ayala, F.; Balato, N.; Bonamonte, D.; Camplone, G.; Fabrizi, G.; Marzatico, F.; et al. Furfuryl palmitate: A new topical anti-oxidant effective in the treatment of dermatitis with eczema. G. Ital. Dermatol. Venereol. 2002, 137, 1–13. [Google Scholar]
- Grassi, A.; Palermi, G.; Paradisi, M. Study of tolerance and efficacy of cosmetic preparations with lenitive action in atopic dermatitis in children. Clin. Ter. 2000, 151, 77–80. [Google Scholar]
- Licari, A.; Ruffinazzi, G.M.D.E.F.; Castagnoli, R.; Marseglia, A.; Agostinis, F.; Puviani, M.; Milani, M.; Marseglia, G.L. A starch, glycyrretinic, zinc oxide and bisabolol based cream in the treatment of chronic mild-to-moderate atopic dermatitis in children: A three-center, assessor blinded trial. Minerva Pediatr. 2017, 69, 470–475. [Google Scholar] [CrossRef]
- Marini, A.; Reinelt, K.; Krutmann, J.; Bilstein, A. Ectoine-containing cream in the treatment of mild to moderate atopic dermatitis: A randomised, comparator-controlled, intra-individual double-blind, multi-center trial. Skin Pharmacol. Physiol. 2014, 27, 57–65. [Google Scholar] [CrossRef]
- Nemelka, O.; Bleidel, D.; Fabrizi, G.; Camplone, G.; Occella, C.; Marzatico, F.; Pecis, L.; Bocchietto, E. Experimental survey of a new topical anti-oxidant based on furfuryl palmitate in the treatment of child’s and baby’s dermatitis with eczema: Results from a multicenter clinical investigation. Minerva Pediatr. 2002, 54, 465–474. [Google Scholar] [PubMed]
- Srivastava, J.K.; Shankar, E.; Gupta, S. Chamomile: A herbal medicine of the past with bright future. Mol. Med. Rep. 2010, 3, 895–901. [Google Scholar] [CrossRef] [PubMed]
- Jacob, S.E.; Matiz, C.; Herro, E.M. Compositae-associated allergic contact dermatitis from bisabolol. Dermatitis 2011, 22, 102–105. [Google Scholar] [CrossRef] [PubMed]
- Hausen, B.M. A 6-year experience with compositae mix. Am. J. Contact Dermat. 1996, 7, 94–99. [Google Scholar] [PubMed]
- Horinouchi, C.D.; Otuki, M.F. Botanical briefs: Comfrey (Symphytum officinale). Cutis 2013, 91, 225–228. [Google Scholar]
- Salehi, B.; Sharopov, F.; Boyunegmez Tumer, T.; Ozleyen, A.; Rodríguez-Pérez, C.M.; Ezzat, S.; Azzini, E.; Hosseinabadi, T.; Butnariu, M.; Sarac, I.; et al. Symphytum species: A comprehensive review on chemical composition, food applications and phytopharmacology. Molecules 2019, 24, 2272. [Google Scholar] [CrossRef]
- Staiger, C. Comfrey root: From tradition to modern clinical trials. Wien. Med. Wochenschr. 2013, 163, 58–64. [Google Scholar] [CrossRef]
- Brown, A.W.; Stegelmeier, B.L.; Colegate, S.M.; Gardner, D.R.; Panter, K.E.; Knoppel, E.L.; Hall, J.O. The comparative toxicity of a reduced, crude comfrey (Symphytum officinale) alkaloid extract and the pure, comfrey-derived pyrrolizidine alkaloids, lycopsamine and intermedine in chicks (Gallus gallus domesticus). J. Appl. Toxicol. 2016, 36, 716–725. [Google Scholar] [CrossRef]
- Mazzocchi, A.; Montanaro, F. Observational study of the use of Symphytum 5CH in the management of pain and swelling after dental implant surgery. Homeopathy 2012, 101, 211–216. [Google Scholar] [CrossRef]
- Mei, N.; Guo, L.; Fu, P.P.; Fuscoe, J.C.; Luan, Y.; Chen, T. Metabolism, genotoxicity, annd carcinogenicity of comfrey. J. Toxicol. Environ. Health-B: Crit. Rev. 2010, 13, 509–526. [Google Scholar] [CrossRef]
- Jedlinszki, N.; Balázs, B.; Csányi, E.; Csupor, D. Penetration of lycopsamine from a comfrey ointment through human epidermis. Regul. Toxicol. Pharmacol. 2017, 83, 1–4. [Google Scholar] [CrossRef]
- Kuchta, K.; Schmidt, M. Safety of medicinal comfrey cream preparations (Symphytum officinale s.l.): The pyrrolizidine alkaloid lycopsamine is poorly absorbed through human skin. Regul. Toxicol. Pharmacol. 2020, 118, 104784. [Google Scholar] [CrossRef] [PubMed]
- Koll, R.; Buhr, M.; Dieter, R.; Pabst, H.; Predel, H.G.; Petrowicz, O.; Giannetti, B.; Klingenburg, S.; Staiger, C. Efficacy and tolerance of a comfrey root extract (Extr. Rad. Symphyti) in the treatment of ankle distorsions: Results of a multicenter, randomized, placebo-controlled, double-blind study. Phytomedicine 2004, 11, 470–477. [Google Scholar] [CrossRef] [PubMed]
- Kucera, M.; Barna, M.; Horácek, O.; Kováriková, J.; Kucera, A. Efficacy and safety of topically applied Symphytum herb extract cream in the treatment of ankle distortion: Results of a randomized controlled clinical double blind study. Wien. Med. Wochenschr. 2004, 154, 498–507. [Google Scholar] [CrossRef]
- Predel, H.G.; Giannetti, B.; Koll, R.; Bulitta, M.; Staiger, C. Efficacy of a comfrey root extract ointment in comparison to a diclofenac gel in the treatment of ankle distortions: Results of an observer-blind, randomized, multicenter study. Phytomedicine 2005, 12, 707–714. [Google Scholar] [CrossRef]
- Araújo, L.U.; Grabe-Guimarães, A.; Mosqueira, V.C.; Carneiro, C.M.; Silva-Barcellos, N.M. Profile of wound healing process induced by allantoin. Acta Cir. Bras. 2010, 25, 460–466. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.Y.; Lee, N.H.; Jung, D.; Lee, J.A.; Seo, C.S.; Lee, H.; Kim, J.H.; Shin, H.K. Protective effects of allantoin against ovalbumin (OVA)-induced lung inflammation in a murine model of asthma. Int. Immunopharmacol. 2010, 10, 474–480. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Li, P.; Tang, J.; Guo, Y.; Shen, C.; Chang, J.; Kerrouche, N. Prolonging time to flare in pediatric atopic dermatitis: A randomized, investigator-blinded, controlled, multicenter clinical study of a ceramide-containing moisturizer. Adv. Ther. 2017, 34, 2601–2611. [Google Scholar] [CrossRef] [PubMed]
- Seghers, A.C.; Cai, S.C.; Ho, M.S.; Giam, Y.C.; Tan, L.; Grönhagen, C.M.; Tang, M.B. Evaluation of a pseudoceramide moisturizer in patients with mild-to-moderate atopic dermatitis. Dermatol. Ther. 2014, 4, 83–92. [Google Scholar] [CrossRef][Green Version]
- Luo, C.; Zou, L.; Sun, H.; Peng, J.; Gao, C.; Bao, L.; Ji, R.; Jin, Y.; Sun, S. A review of the anti-inflammatory effects of rosmarinic acid on inflammatory diseases. Front. Pharmacol. 2020, 11, 153. [Google Scholar] [CrossRef]
- Zhou, M.W.; Jiang, R.H.; Kim, K.D.; Lee, J.H.; Kim, C.D.; Yin, W.T.; Lee, J.H. Rosmarinic acid inhibits poly(I:C)-induced inflammatory reaction of epidermal keratinocytes. Life Sci. 2016, 155, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Jang, A.H.; Kim, T.H.; Kim, G.D.; Kim, J.E.; Kim, H.J.; Kim, S.S.; Jin, Y.H.; Park, Y.S.; Park, C.S. Rosmarinic acid attenuates 2,4-dinitrofluorobenzene-induced atopic dermatitis in NC/Nga mice. Int. Immunopharmacol. 2011, 11, 1271–1277. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Jung, E.; Koh, J.; Kim, Y.S.; Park, D. Effect of rosmarinic acid on atopic dermatitis. J. Dermatol. 2008, 35, 768–771. [Google Scholar] [CrossRef] [PubMed]
- Ilnytska, O.; Kaur, S.; Chon, S.; Reynertson, K.; Nebus, J.; Garay, M.; Mahmood, K.; Southall, M. Colloidal oatmeal (Avena Sativa) improves skin barrier through multi-therapy activity. J. Drugs Dermatol. 2016, 15, 684–690. [Google Scholar]
- Lisante, T.A.; Nuñez, C.; Zhang, P. Efficacy and safety of an over-the-counter 1% colloidal oatmeal cream in the management of mild to moderate atopic dermatitis in children: A double-blind, randomized, active-controlled study. J. Dermatol. Treat. 2017, 28, 659–667. [Google Scholar] [CrossRef]
- Lisante, T.A.; Nunez, C.; Zhang, P.; Mathes, B.M. A 1% colloidal oatmeal cream alone is effective in reducing symptoms of mild to moderate atopic dermatitis: Results from two clinical studies. J. Drugs Dermatol. 2017, 16, 671–676. [Google Scholar] [PubMed]
- Celleno, L. Topical urea in skincare: A review. Dermatol. Ther. 2018, 31, e12690. [Google Scholar] [CrossRef]
- Pan, M.; Heinecke, G.; Bernardo, S.; Tsui, C.; Levitt, J. Urea: A comprehensive review of the clinical literature. Dermatol. Online J. 2013, 19, 20392. [Google Scholar] [CrossRef]
- Sasaki, Y.; Tadaki, T.; Tagami, H. The effects of a topical application of urea cream on the function of pathological stratum corneum. Acta Dermatol. Kyoto 1989, 84, 581–586. [Google Scholar]
- Almeyda, J.; Burt, B.W. Double blind controlled study of treatment of atopic eczema with a preparation of hydrocortisone in a new drug delivery system versus betamethasone 17-valerate. Br. J. Dermatol. 1974, 91, 579–583. [Google Scholar] [CrossRef]
- Jacoby, R.H.; Gilkes, J.J. A new urea/hydrocortisone powder-cream compared with other topical corticosteroid preparations: A six-centre study. Curr. Med. Res. Opin. 1974, 2, 474–481. [Google Scholar] [CrossRef] [PubMed]
- Roth, H. Atopic Dermatitis: Treatment with a urea-corticosteriod cream. Cutis 1973, 11, 237–239. [Google Scholar]
- Nistico, S.P.; Del Duca, E.; Tamburi, F.; Pignataro, E.; De Carvalho, N.; Farnetani, F.; Pellacani, G. Superiority of a vitamin B12-barrier cream compared with standard glycerol-petrolatum-based emollient cream in the treatment of atopic dermatitis: A randomized, left-to-right comparative trial. Dermatol. Ther. 2017, 30, e12523. [Google Scholar] [CrossRef] [PubMed]
- de Lucas, R.; García-Millán, C.; Pérez-Davó, A.; Moreno, E.; Redondo, P. New cosmetic formulation for the treatment of mild to moderate infantile atopic dermatitis. Children 2019, 6, 17. [Google Scholar] [CrossRef]
- Hoppe, T.; Winge, M.C.; Bradley, M.; Nordenskjöld, M.; Vahlquist, A.; Törmä, H.; Berne, B. Moisturizing treatment of patients with atopic dermatitis and ichthyosis vulgaris improves dry skin, but has a modest effect on gene expression regardless of FLG genotype. J. Eur. Acad. Dermatol. Venereol. 2015, 29, 174–177. [Google Scholar] [CrossRef]
- Bianchi, P.; Theunis, J.; Casas, C.; Villeneuve, C.; Patrizi, A.; Phulpin, C.; Bacquey, A.; Redoulès, D.; Mengeaud, V.; Schmitt, A.M. Effects of a new emollient-based treatment on skin microflora balance and barrier function in children with mild atopic dermatitis. Pediatr. Dermatol. 2016, 33, 165–171. [Google Scholar] [CrossRef]
- Hon, K.L.; Pong, N.H.; Wang, S.S.; Lee, V.W.; Luk, N.M.; Leung, T.F. Acceptability and efficacy of an emollient containing ceramide-precursor lipids and moisturizing factors for atopic dermatitis in pediatric patients. Drugs R D 2013, 13, 37–42. [Google Scholar] [CrossRef]
- Spada, F.; Harrison, I.P.; Barnes, T.M.; Greive, K.A.; Daniels, D.; Townley, J.P.; Mostafa, N.; Fong, A.T.; Tong, P.L.; Shumack, S. A daily regimen of a ceramide-dominant moisturizing cream and cleanser restores the skin permeability barrier in adults with moderate eczema: A randomized trial. Dermatol. Ther. 2021, 34, e14970. [Google Scholar] [CrossRef]
- Cork, M.J.; Britton, J.; Butler, L.; Young, S.; Murphy, R.; Keohane, S.G. Comparison of parent knowledge, therapy utilization and severity of atopic eczema before and after explanation and demonstration of topical therapies by a specialist dermatology nurse. Br. J. Dermatol. 2003, 149, 582–589. [Google Scholar] [CrossRef]
- Sugarman, J.L.; Parish, L.C. Efficacy of a lipid-based barrier repair formulation in moderate-to-severe pediatric atopic dermatitis. J. Drugs Dermatol. 2009, 8, 1106–1111. [Google Scholar]
- Lee, Y.B.; Park, H.J.; Kwon, M.J.; Jeong, S.K.; Cho, S.H. Beneficial effects of pseudoceramide-containing physiologic lipid mixture as a vehicle for topical steroids. Eur. J. Dermatol. 2011, 21, 710–716. [Google Scholar] [CrossRef] [PubMed]
- McClanahan, D.; Wong, A.; Kezic, S.; Samrao, A.; Hajar, T.; Hill, E.; Simpson, E.L. A randomized controlled trial of an emollient with ceramide and filaggrin-associated amino acids for the primary prevention of atopic dermatitis in high-risk infants. J. Eur. Acad. Dermatol. Venereol. 2019, 33, 2087–2094. [Google Scholar] [CrossRef] [PubMed]
- Kircik, L.H.; Del Rosso, J.Q.; Aversa, D. Evaluating clinical use of a ceramide-dominant, physiologic lipid-based topical emulsion for atopic dermatitis. J. Clin. Aesthet. Dermatol. 2011, 4, 34–40. [Google Scholar] [PubMed]
- Man, M.M.; Feingold, K.R.; Thornfeldt, C.R.; Elias, P.M. Optimization of physiological lipid mixtures for barrier repair. J. Investig. Dermatol. 1996, 106, 1096–1101. [Google Scholar] [CrossRef]
- Mao-Qiang, M.; Elias, P.M.; Feingold, K.R. Fatty acids are required for epidermal permeability barrier function. J. Clin. Investig. 1993, 92, 791–798. [Google Scholar] [CrossRef]
- Avila, C.; Massick, S.; Kaffenberger, B.H.; Kwatra, S.G.; Bechtel, M. Cannabinoids for the treatment of chronic pruritus: A review. J. Am. Acad. Dermatol. 2020, 82, 1205–1212. [Google Scholar] [CrossRef]
- Eagleston, L.R.M.; Kalani, N.K.; Patel, R.R.; Flaten, H.K.; Dunnick, C.A.; Dellavalle, R.P. Cannabinoids in dermatology: A scoping review. Dermatol. Online J. 2018, 24, 13030/qt7pn8c0sb. [Google Scholar] [CrossRef]
- Sheriff, T.; Lin, M.J.; Dubin, D.; Khorasani, H. The potential role of cannabinoids in dermatology. J. Dermatol. Treat. 2020, 31, 839–845. [Google Scholar] [CrossRef]
- Sivesind, T.E.; Maghfour, J.; Rietcheck, H.; Kamel, K.; Malik, A.S.; Dellavalle, R.P. Cannabinoids for the treatment of dermatologic conditions. JID Innov. 2022, 2, 100095. [Google Scholar] [CrossRef]
- Tóth, K.F.; Ádám, D.; Bíró, T.; Oláh, A. Cannabinoid signaling in the skin: Therapeutic potential of the “c(ut)annabinoid” system. Molecules 2019, 24, 918. [Google Scholar] [CrossRef]
- Muller, C.; Morales, P.; Reggio, P.H. Cannabinoid ligands targeting TRP channels. Front. Mol. Neurosci. 2018, 11, 487. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Kim, B.; Park, B.M.; Jeon, J.E.; Lee, S.H.; Mann, S.; Ahn, S.K.; Hong, S.-P.; Jeong, S.K. Topical cannabinoid receptor 1 agonist attenuates the cutaneous inflammatory responses in oxazolone-induced atopic dermatitis model. Int. J. Dermatol. 2015, 54, e401–e408. [Google Scholar] [CrossRef] [PubMed]
- Maekawa, T.; Nojima, H.; Kuraishi, Y.; Aisaka, K. The cannabinoid CB2 receptor inverse agonist JTE-907 suppresses spontaneous itch-associated responses of NC mice, a model of atopic dermatitis. Eur. J. Pharmacol. 2006, 542, 179–183. [Google Scholar] [CrossRef] [PubMed]
- Nam, G.; Jeong, S.K.; Park, B.M.; Lee, S.H.; Kim, H.J.; Hong, S.-P.; Kim, B.; Kim, B.-W. Selective cannabinoid receptor-1 agonists regulate mast cell activation in an oxazolone-induced atopic dermatitis model. Ann. Dermatol. 2016, 28, 22–29. [Google Scholar] [CrossRef]
- Petrosino, S.; Verde, R.; Vaia, M.; Allarà, M.; Iuvone, T.; Di Marzo, V. Anti-inflammatory properties of cannabidiol, a nonpsychotropic cannabinoid, in experimental allergic contact dermatitis. J. Pharmacol. Exp. Ther. 2018, 365, 652–663. [Google Scholar] [CrossRef]
- Maghfour, J.; Rundle, C.W.; Rietcheck, H.R.; Dercon, S.; Lio, P.; Mamo, A.; Runion, T.M.; Fernandez, J.; Kahn, J.; Dellavalle, R.P.; et al. Assessing the effects of topical cannabidiol in patients with atopic dermatitis. Dermatol. Online J. 2021, 27, 13030/qt8h50k2vs. [Google Scholar] [CrossRef]
- Palmieri, B.; Laurino, C.; Vadalà, M. A therapeutic effect of CBD-enriched ointment in inflammatory skin diseases and cutaneous scars. Clin. Ter. 2019, 170, e93–e99. [Google Scholar] [CrossRef]
- Eberlein, B.; Eicke, C.; Reinhardt, H.W.; Ring, J. Adjuvant treatment of atopic eczema: Assessment of an emollient containing N-palmitoylethanolamine (ATOPA study). J. Eur. Acad. Dermatol. Venereol. 2008, 22, 73–82. [Google Scholar] [CrossRef]
- Yuan, C.; Wang, X.M.; Guichard, A.; Tan, Y.M.; Qian, C.Y.; Yang, L.J.; Humbert, P. N-palmitoylethanolamine and N-acetylethanolamine are effective in asteatotic eczema: Results of a randomized, double-blind, controlled study in 60 patients. Clin. Interv. Aging 2014, 9, 1163–1169. [Google Scholar] [CrossRef]
- Pigatto, P.D.; Diani, M. Beneficial Effects of Antioxidant Furfuryl Palmitate in Non-pharmacologic Treatments (Prescription Emollient Devices, PEDs) for Atopic Dermatitis and Related Skin Disorders. Dermatol. Ther. 2018, 8, 339–347. [Google Scholar] [CrossRef]
- Ji, H.; Li, X.-K. Oxidative Stress in Atopic Dermatitis. Oxid. Med. Cell. Longev. 2016, 2016, 2721469. [Google Scholar] [CrossRef]
- Sivaranjani, N.; Rao, S.V.; Rajeev, G. Role of reactive oxygen species and antioxidants in atopic dermatitis. J. Clin. Diagn. Res. 2013, 7, 2683–2685. [Google Scholar] [CrossRef] [PubMed]
- Appiani, E.; Ossola, R.; Latch, D.E.; Erickson, P.R.; McNeill, K. Aqueous singlet oxygen reaction kinetics of furfuryl alcohol: Effect of temperature, pH, and salt content. Environ. Sci. Process. Impacts 2017, 19, 507–516. [Google Scholar] [CrossRef]
- Haag, W.R.; Hoigné, J.; Gassman, E.; Braun, A.M. Singlet oxygen in surface waters—Part I: Furfuryl alcohol as a trapping agent. Chemosphere 1984, 13, 631–640. [Google Scholar] [CrossRef]
- Bocchietto, E.; Pecis, L.; Lisi, P.; Angelini, G.; Ayala, F.; Balato, N.; Bonamonte, D.; Camplone, G.; Fabrizi, G.; Marzatico, F.J. Furfuril palmitato: Un nuovo antiossidante topico efficace nel trattamento di dermatiti eczematose. G. Ital. Dermatol. Venereol. 2002, 137, 1–13. [Google Scholar]
- Pigatto, P.D.; Lauriola, M.M.; Vaccari, G. A single-center, randomized, double-blind, perspective, controlled study of efficacy and safety of a furpalmate-containing cream versus vehicle in the treatment of 40 adult patients with mild to moderate atopic dermatitis. In Proceedings of the 20th European Academy of Dermatology and Venereology Congress, Lisbon, Portugal, 20–24 October 2011. [Google Scholar]
- Lauriola, M.M.; Pigatto, P.D.; Pedrelli, V.A. A single-center, randomized, perspective, investigator blinded controlled trial to examine efficacy and safety of a furpalmate cream in comparison to topical corticosteroid in atopic dermatitis of hands of hands of 40 adult patients. In Proceedings of the 20th European Academy of Dermatology and Venereology Congress, Lisbon, Portugal, 20–24 October 2011. [Google Scholar]
- Patrizi, A.; Raone, B.; Raboni, R.; Neri, I. Efficacy and tolerability of a cream containing AR-GG27® (sorbityl furfural palmitate) in the treatment of mild/moderate childhood atopic dermatitis associated with pityriasis alba. A double-blind, placebo-controlled clinical trial. G. Ital. Dermatol. Venereol. 2012, 147, 1–8. [Google Scholar]
- Flacco, M.E.; Manzoli, L.; Boccia, S.; Capasso, L.; Aleksovska, K.; Rosso, A.; Scaioli, G.; De Vito, C.; Siliquini, R.; Villari, P.; et al. Head-to-head randomized trials are mostly industry sponsored and almost always favor the industry sponsor. J. Clin. Epidemiol. 2015, 68, 811–820. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vanessa, V.V.; Wan Ahmad Kammal, W.S.L.; Lai, Z.W.; How, K.N. A Review of Moisturizing Additives for Atopic Dermatitis. Cosmetics 2022, 9, 75. https://doi.org/10.3390/cosmetics9040075
Vanessa VV, Wan Ahmad Kammal WSL, Lai ZW, How KN. A Review of Moisturizing Additives for Atopic Dermatitis. Cosmetics. 2022; 9(4):75. https://doi.org/10.3390/cosmetics9040075
Chicago/Turabian StyleVanessa, Vincentsia Vienna, Wan Syazween Lyana Wan Ahmad Kammal, Zee Wei Lai, and Kang Nien How. 2022. "A Review of Moisturizing Additives for Atopic Dermatitis" Cosmetics 9, no. 4: 75. https://doi.org/10.3390/cosmetics9040075
APA StyleVanessa, V. V., Wan Ahmad Kammal, W. S. L., Lai, Z. W., & How, K. N. (2022). A Review of Moisturizing Additives for Atopic Dermatitis. Cosmetics, 9(4), 75. https://doi.org/10.3390/cosmetics9040075






