Review on the Use of Kojic Acid—A Skin-Lightening Ingredient
Abstract
:1. Introduction
2. Fungi-Producing Kojic Acid
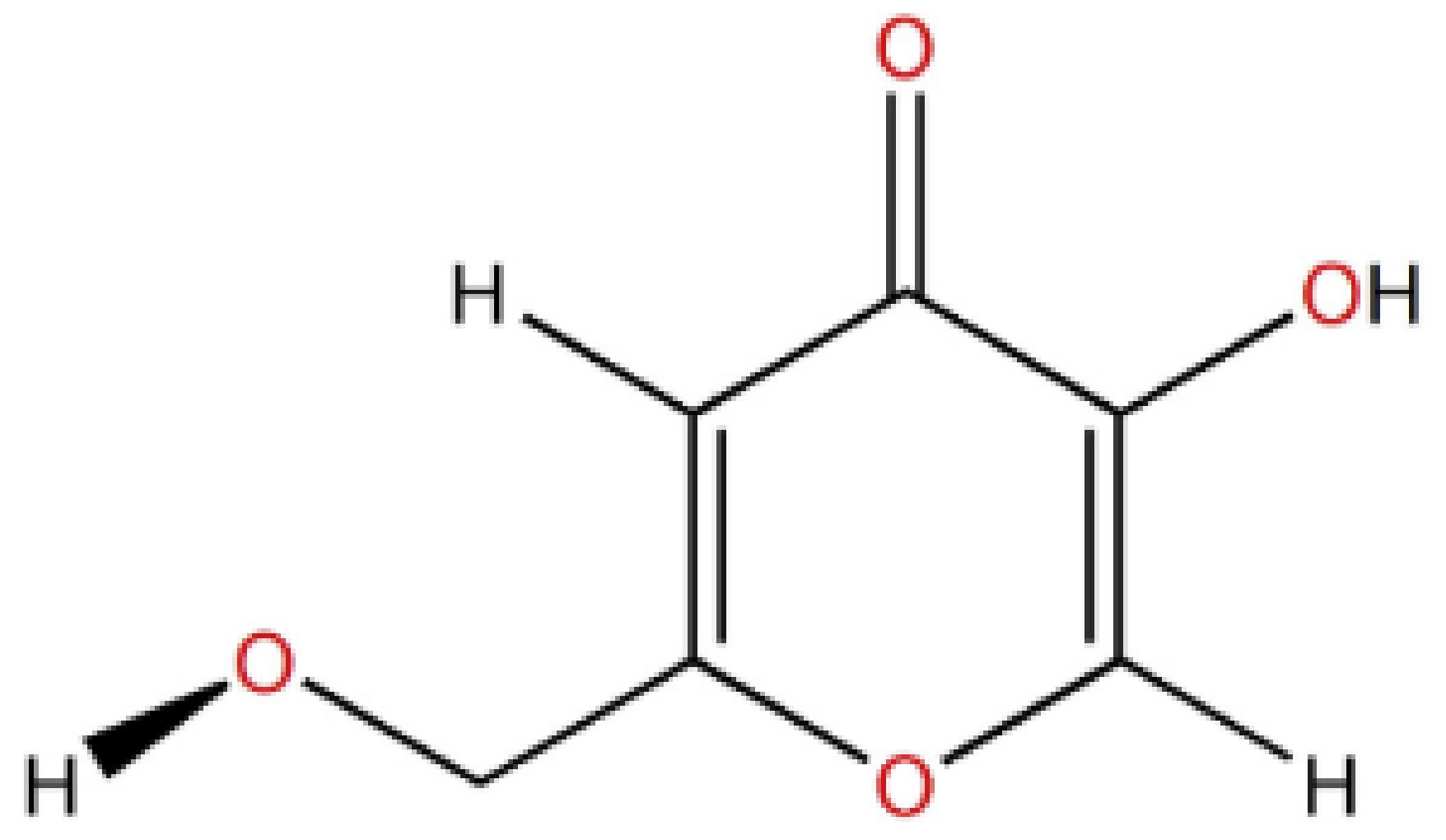
3. Physical and Chemical Properties
4. Safety Assessment of Kojic Acid
5. Kojic Acid Derivatives
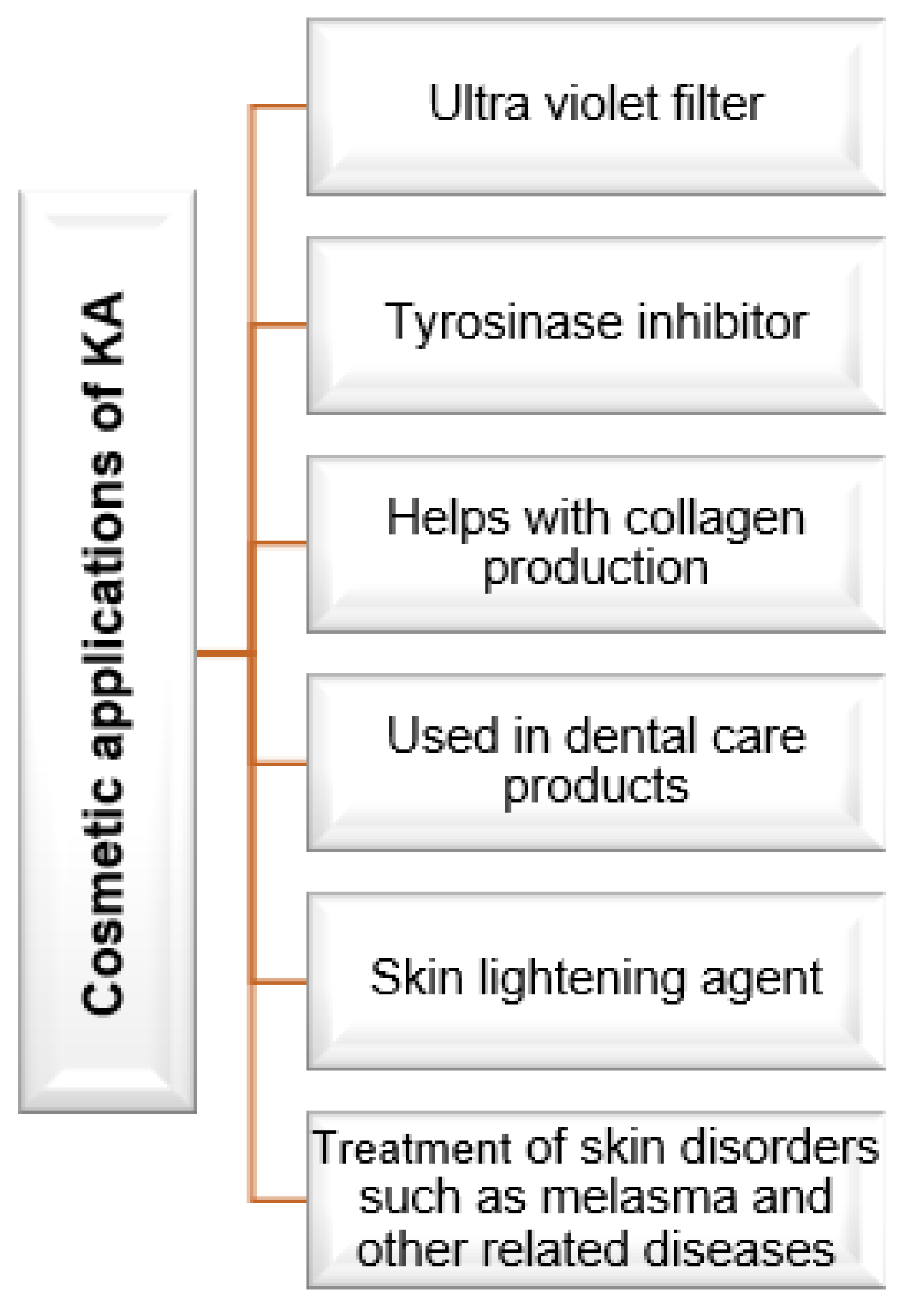
6. Cosmetic Applications of Kojic Acid
7. Biological Activities of Kojic Acid
7.1. Antibacterial and Antimicrobial Activity
7.2. Antioxidant Activity
7.3. Anti-Inflammatory Activity
7.4. Tyrosinase Inhibition Activity
8. Kojic Acid Mechanism of Action
8.1. Assays for Evaluating the Efficacy of Kojic Acid
8.2. Melanin Depigmentation Assays
8.3. Tyrosinase Inhibition Assays
8.4. Mushroom Tyrosinase
8.5. In Vivo Clinical Studies
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Aytemir, D.M.; Karakay, G. Kojic Acid Derivatives. Med. Chem. Drug Des. 2012, 1–27. [Google Scholar]
- Saeedi, M.; Eslamifar, M.; Khezri, K. Kojic acid applications in cosmetic and pharmaceutical preparations. Biomed. Pharmacother. 2019, 110, 582–593. [Google Scholar] [CrossRef] [PubMed]
- Desai, S. Effect of a Tranexamic Acid, Kojic Acid, and Niacinamide Containing Serum on Facial Dyschromia: A Clinical Evaluation. J. Drugs Dermatol. 2019, 18, 454–459. [Google Scholar] [PubMed]
- Lourith, N.; Kanlayavattanakul, M.; Ruktanonchai, U. Formulation and stability of Moringa oleifera Oil Microemulsion. Soft Mater. 2016, 14, 64–71. [Google Scholar] [CrossRef]
- Masum, M.N.; Yamauchi, K.; Mitsunaga, T. Tyrosinase inhibitors from natural and synthetic sources as skin-lightening agents. Rev. Agric. Sci. 2019, 7, 41–58. [Google Scholar] [CrossRef] [Green Version]
- Hasil, A.; Mehmood, A.; Noureen, S.; Ahmed, M. Experimental and theoretical charge density analysis of skin whitening agent kojic acid. J. Mol. Struct. 2020, 1216, 128295. [Google Scholar] [CrossRef]
- Van Tran, V.; Loi Nguyen, T.; Moon, J.Y.; Lee, Y.C. Core-shell materials, lipid particles and nanoemulsions, for delivery of active anti-oxidants in cosmetics applications: Challenges and development strategies. Chem. Eng. J. 2018, 368, 88–114. [Google Scholar] [CrossRef]
- Bashir, F.; Sultana, K.; Khalid, M.; Rabia, H.; Khan, H. Kojic Acid: A Comprehensive Review Abstract: Keywords: The Applications of Kojic Acid Kojic acid. AJAHAS 2021, 6, 13–17. [Google Scholar]
- Chambers, C. Opinion on kojic acid. Sci. Committees Consum. Prod. 2008, 1–79. [Google Scholar]
- Chaudhary, J. Production Technology and Applications of Kojic Acid. Annu. Res. Rev. Biol. 2014, 4, 3165–3196. [Google Scholar] [CrossRef]
- Feng, W.; Liang, J.; Wang, B.; Chen, J. Improvement of kojic acid production in Aspergillus oryzae AR-47 mutant strain by combined mutagenesis. Bioprocess Biosyst. Eng. 2019, 42, 753–761. [Google Scholar] [CrossRef] [PubMed]
- Chib, S.; Dogra, A.; Nandi, U.; Saran, S. Consistent production of kojic acid from Aspergillus sojae SSC-3 isolated from rice husk. Mol. Biol. Rep. 2019, 46, 5995–6002. [Google Scholar] [CrossRef] [PubMed]
- Masse, M.O.; Duvallet, V.; Borremans, M.; Goeyens, L. Identification and quantitative analysis of kojic acid and arbutine in skin-whitening cosmetics. Int. J. Cosmet. Sci. 2001, 23, 219–232. [Google Scholar] [CrossRef] [PubMed]
- Rosfarizan, M.; Mohamed, M.S.; Suhaili, N.; Salleh, M.M.; Ariff, A.B. Kojic acid: Applications and development of fermentation process for production. Biotechnol. Mol. Biol. Rev. 2010, 5, 24–37. [Google Scholar]
- Gomes, C.; Silva, A.C.; Marques, A.C.; Lobo, S.; Amaral, M.H. Biotechnology Applied to Cosmetics and Aesthetic Medicines. Cosmetics 2020, 7, 33. [Google Scholar] [CrossRef]
- Kim, J.H. Enhancement of commercial antifungal agents by Kojic acid. Int. J. Mol. Sci. 2012, 13, 13867–13880. [Google Scholar] [CrossRef]
- Ola, A.R.B. Single production of Kojic acid by Aspergillus flavus and the revision of flufuran. Molecules 2019, 24, 4200. [Google Scholar] [CrossRef] [Green Version]
- Velliou, E.G. In vitro Studies. Model. Optim. Control Biomed. Syst. 2017, 233–264. [Google Scholar] [CrossRef]
- Couteau, C.; Coiffard, L. Overview of Skin Whitening Agents: Drugs and Cosmetic Products. Cosmetics 2016, 3, 27. [Google Scholar] [CrossRef]
- Siddiquee, S. Advancements on the Role of Biologically Active Secondary Metabolites from Aspergillus Microbial Production of Organic Acids for Use in Food; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Zborowski, K.; Gryboś, R.; Proniewicz, L.M. Determination of the most stable structures of selected hydroxypyrones and their cations and anions. J. Mol. Struct. THEOCHEM 2003, 639, 87–100. [Google Scholar] [CrossRef]
- Annan, N.A.; Butler, I.S.; Titi, H.M.; El-Lazeik, Y.; Jean-Claude, B.J.; Mostafa, S.I. DNA interaction and anticancer evaluation of new zinc(II), ruthenium(II), rhodium(III), palladium(II), silver(I) and platinum(II) complexes based on kojic acid; X-ray crystal structure of [Ag(ka)(PPh3)]·H2O. Inorganica Chim. Acta 2019, 487, 433–447. [Google Scholar] [CrossRef]
- Burnett, C.L. Final report of the safety assessment of kojic acid as used in cosmetics. Int. J. Toxicol. 2010, 29. [Google Scholar] [CrossRef] [PubMed]
- Mann, T. Inhibition of Human Tyrosinase Requires Molecular Motifs Distinctively Different from Mushroom Tyrosinase. J. Invest. Dermatol. 2018, 138, 1601–1608. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bergfeld, W.F. Final Report of the Cosmetic Ingredient Review Expert Panel Safety Assessment of Simmondsia Chinensis (Jojoba) Seed Oil , Simmondsia Chinensis (Jojoba) Seed Wax, Hydrogenated Jojoba Oil , Hydrolyzed Jojoba Esters , Isomerized Jojoba Oil, Jojoba Este. Cosmet. Ingred. Rev. 2008, 1–32. Available online: https://online.personalcarecouncil.org/ctfa-static/online/lists/cir-pdfs/FR525.pdf (accessed on 10 May 2022).
- Buschmann, H.J.; Schollmeyer, E. Applications of cyclodextrins in cosmetic products: A review. J. Cosmet. Sci. 2002, 53, 185–191. [Google Scholar] [PubMed]
- Hashemi, H. Climate change and the future of water management in Iran. Middle East Critique 2015, 24, 307–323. [Google Scholar] [CrossRef]
- Kwak, S.Y.; Choi, H.R.; Park, K.C.; Lee, Y.S. Kojic acid-amino acid amide metal complexes and their melanogenesis inhibitory activities. J. Pept. Sci. 2011, 17, 791–797. [Google Scholar] [CrossRef]
- Seyedeh Mahdieh Hashemi, S.E. Kojic acid-derived tyrosinase inhibitors: Synthesis and bioactivity. Pharm. Biomed. Res. 2015, 1, 1–17. Available online: http://pbr.mazums.ac.ir (accessed on 10 May 2022).
- Hariri, R.; Saeedi, M.; Akbarzadeh, T. Naturally occurring and synthetic peptides: Efficient tyrosinase inhibitors. J. Pept. Sci. 2021, 27, 1–10. [Google Scholar] [CrossRef]
- Pillaiyar, T.; Namasivayam, V.; Manickam, M.; Jung, S. H Inhibitors of Melanogenesis: An Updated Review. J. Med. Chem. 2018, 61, 7395–7418. [Google Scholar] [CrossRef]
- De, A. Hyperpigmentation Case Kojic Acid in the Management of Melasma : An Effective Therapeutic Weapon. Indian J. Dermatol. 2019, 1–4. [Google Scholar]
- Davis, E.C.; Calender, V.D. A Review of the Epidemiology, Clinical Features, and Treatment Options in Skin of Color Year study population prevalence rank. J. Clin. Aesthet. Dermatol. 2010, 3, 20–31. [Google Scholar] [PubMed]
- Nurunnabi, T. Antimicrobial activity of kojic acid from endophytic fungus Colletotrichum gloeosporioides isolated from Sonneratia apetala, a mangrove plant of the Sundarbans. Asian Pac. J. Trop. Med. 2018, 11, 350–354. [Google Scholar] [CrossRef] [Green Version]
- Tetali, B.; Fahs, F.M.; Mehregan, D. Popular over-the-counter cosmeceutical ingredients and their clinical efficacy. Int. J. Dermatol. 2020, 59, 393–405. [Google Scholar] [CrossRef]
- El-Kady, I.A.; Zohri, A.N.A.; Hamed, S.R. Kojic Acid Production from Agro-Industrial By-Products Using Fungi. Biotechnol. Res. Int. 2014, 2014, 642385. [Google Scholar] [CrossRef] [Green Version]
- Owolabi, J.O.; Fabiyi, O.S.; Adelakin, L.A.; Ekwerike, M.C. Effects of skin lightening cream agents - hydroquinone and kojic acid, on the skin of adult female experimental rats. Clin. Cosmet. Investig. Dermatol. 2020, 13, 283–289. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.R. Intercalation assembly of kojic acid into Zn-Ti layered double hydroxide with antibacterial and whitening performances. Chinese Chem. Lett. 2019, 30, 919–923. [Google Scholar] [CrossRef]
- Zhang, J. Kojic acid-mediated damage responses induce mycelial regeneration in the basidiomycete Hypsizygus marmoreus. PLoS ONE 2017, 12, e0187351. [Google Scholar] [CrossRef] [Green Version]
- Lajis, A.F.B.; Hamid, M.; Ariff, A.B. Depigmenting effect of kojic acid esters in hyperpigmented B16F1 melanoma cells. J. Biomed. Biotechnol. 2012, 2012, 952452. [Google Scholar] [CrossRef] [Green Version]
- Brtko, J.; Rondahl, L.; Ficková, M.; Hudecová, D.; Eybl, V.; Uher, M. Kojic acid and its derivatives: History and present state of art. Cent. Eur. J. Public Health 2004, 12, S16–S17. [Google Scholar] [CrossRef]
- Lee, M.; Rho, H.S.; Choi, K. Anti-inflammatory Effects of a P-coumaric Acid and Kojic Acid Derivative in LPS-stimulated RAW264.7 Macrophage Cells. Biotechnol. Bioprocess Eng. 2019, 24, 653–657. [Google Scholar] [CrossRef]
- Li, T.; Liang, J.; Liu, L.; Shi, F.; Jia, X.; Li, M. Fitoterapia Novel kojic acid derivatives with anti-inflammatory effects from Aspergillus versicolor. Fitoterapia 2021, 154, 105027. [Google Scholar] [CrossRef] [PubMed]
- Ando, H.; Matsui, M.S.; Ichihashi, M. Quasi-drugs developed in Japan for the prevention or treatment of hyperpigmentary disorders. Int. J. Mol. Sci. 2010, 11, 2566–2575. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J. Recent advances in the design and discovery of synthetic tyrosinase inhibitors. Eur. J. Med. Chem. 2021, 224, 113744. [Google Scholar] [CrossRef]
- Aygun, R.B.; Zengin, G.; Yıldıztugay, E.; Jugreet, S.; Yılmaz, M.A.; Mahomoodally, F.M. Chemical characterization, anti-oxidant and anti-enzymatic properties of extracts from two Silene species: A focus on different plant parts and extraction methods. Process Biochem. 2022, 116, 206–213. [Google Scholar] [CrossRef]
- Bang, E.J. In vitro and in vivo evidence of tyrosinase inhibitory activity of a synthesized (Z)-5-(3-hydroxy-4-methoxybenzylidene)-2-thioxothiazolidin-4-one (5-HMT). Exp. Dermatol. 2019, 28, 734–737. [Google Scholar] [CrossRef] [Green Version]
- Pavic, A.; Ilic-Tomic, T.; Glamočlija, J. Unravelling anti-melanogenic potency of edible mushrooms laetiporus sulphureus and agaricus silvaticus in vivo using the zebrafish model. J. Fungi 2021, 7, 834. [Google Scholar] [CrossRef]
- Meziant, L.; Bachir-bey, M.; Bensouici, C.; Saci, F.; Boutiche, M.; Louaileche, H. Assessment of inhibitory properties of flavonoid-rich fig (Ficus carica L.) peel extracts against tyrosinase, α-glucosidase, urease and cholinesterases enzymes, and relationship with antioxidant activity. Eur. J. Integr. Med. 2021, 43, 101272. [Google Scholar] [CrossRef]
- Dulić, M.; Ciganović, P.; Vujić, L.; Končić, M.Z. Antidiabetic and Cosmeceutical Potential of Common Barbery (Berberis vulgaris L.) Root Bark Extracts Obtained by Optimization of ‘Green’ Ultrasound-Assisted Extraction. Molecules 2019, 24, 3613. [Google Scholar] [CrossRef] [Green Version]
- Khezri, K.; Saeedi, M.; Morteza-Semnani, K.; Akbari, J.; Hedayatizadeh-Omran, A. A promising and effective platform for delivering hydrophilic depigmenting agents in the treatment of cutaneous hyperpigmentation: Kojic acid nanostructured lipid carrier. Artif. Cells Nanomed. Biotechnol. 2021, 49, 38–47. [Google Scholar] [CrossRef]
- Mohiuddin, A.K. Skin Lighteners & Hyperpigmentation Management. ASIO J. Pharm. Herbal Med. Res. 2019, 5, 1–42. [Google Scholar]
- Deri, B. The unravelling of the complex pattern of tyrosinase inhibition. Sci. Rep. 2016, 6, 34993. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lai, X.; Wichers, H.J.; Dijkstra, B.W. Structure and Function of Human Tyrosinase and Tyrosinase- Related Proteins. Chem. A Eur. J. 2018, 24, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Chang, T.S. An updated review of tyrosinase inhibitors. Int. J. Mol. Sci. 2009, 10, 2440–2475. [Google Scholar] [CrossRef] [Green Version]
- Burger, P.; Landreau, A.; Azoulay, S.; Michel, T.; Fernandez, X. Skin whitening cosmetics: Feedback and challenges in the development of natural skin lighteners. Cosmetics 2016, 3, 36. [Google Scholar] [CrossRef] [Green Version]
- Gao, X.H.; Zhang, L.; Wei, H.; Chen, H.D. Efficacy and safety of innovative cosmeceuticals. Clin. Dermatol. 2008, 26, 367–374. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Park, H.Y.; Jung, K.H.; Kim, D.H.; Rho, H.S.; Choi, K. Anti-melanogenic Effects of Kojic Acid and Hydroxycinnamic Acid Derivatives. Biotechnol. Bioprocess Eng. 2020, 25, 190–196. [Google Scholar] [CrossRef]
- Arrowitz, C.; Schoelermann, A.M.; Mann, T.; Jiang, L.I.; Weber, T.; Kolbe, L. Effective Tyrosinase Inhibition by Thiamidol Results in Significant Improvement of Mild to Moderate Melasma. J. Investig. Dermatol. 2019, 139, 1691–1698.e6. [Google Scholar] [CrossRef] [Green Version]
- Kamakshi, R. Fairness via formulations: A review of cosmetic skin-lightening ingredients. J. Cosmet. Sci. 2012, 63, 43–54. [Google Scholar]
- Pillaiyar, T.; Manickam, M.; Namasivayam, V. Skin whitening agents: Medicinal chemistry perspective of tyrosinase inhibitors. J. Enzyme Inhib. Med. Chem. 2017, 32, 403–425. [Google Scholar] [CrossRef] [Green Version]
- Rho, H.S.; Ahn, S.M.; Yoo, D.S.; Kim, M.K.; Cho, D.H.; Cho, J.Y. Kojyl thioether derivatives having both tyrosinase inhibitory and anti-inflammatory properties. Bioorganic Med. Chem. Lett. 2010, 20, 6569–6571. [Google Scholar] [CrossRef] [PubMed]
- Mavon, A.; Bacqueville, D.; De Wever, B. Skin care products. In Handbook of Cosmetic Science and Technology, 3rd ed.; Barel, A.O., Paye, M., Maibach, H.I., Eds.; Informa Healthcare: London, UK, 2009; pp. 347–353. [Google Scholar]
- Gupta, A.K.; Gover, M.D.; Nouri, K.; Taylor, S. The treatment of melasma: A review of clinical trials. J. Am. Acad. Dermatol. 2006, 55, 1048–1065. [Google Scholar] [CrossRef] [PubMed]

| Fungus | Toxins | Characteristics | Production Yield |
|---|---|---|---|
| Aspergillus. flavus | Aflatoxins, Aflatrem, Aspergilic acid, Cydoplazonic acid, β-nitropionic acid, and Serigmatocyctin | Pathogenicity: Generally, a contaminant but also known to cause disease; commonly associated with aflatoxins Macroscopic morphology: Velvety, yellow to green or brown, Reverse goldish to red-brown Macroscopic morphology of conidiophores: Variable length, rough, pitted, spiny Macroscopic morphology of conidiophores: Uni-seriate and bi-seriate, covers entire vesicle, points out in all directions | High |
| Aspergillus orizae | Aspergillus acid, Cycopiazonic acid, Maltoryzine β-nitropropionic acid, Ochtratoxins | Medium to High | |
| Aspergillus parasiticus | Aflatoxins, Aspergillic acid, and Sterigmatocyctin | High | |
| Aspergillus Tamarii | Aflatoxins | Low |
| Benefits | Disadvantages |
|---|---|
|
|
| Study Design and Setting | Concentration | Dosing Regimen (Weeks) | Result | Reference |
|---|---|---|---|---|
| Treatment of freckles, age spots, post-inflammatory hyperpigmentation, and melasma | 1% | - | Effective | [44] |
| KA was combined with two other lightening agents in 40 females to treat epidermal melasma. Treatment on half of the face with KA. The other half was treated with the same application with no KA. | 5% | - | Effective | [32] |
| Skin lightening in patients with dyschromia | Not stated | 12 | Effective | [35] |
| KA was combined with other therapies to treat facial hyperpigmentation and melasma in 39 patients | KA 2% plus glycolic acid (GA) 5% side and HQ 2% plus GA 5% | Effective | [64] | |
| The efficacy of KA and other agents in treating melasma in a randomized clinical study of 55 healthy subjects | 1% KA, 5% niacinamide, and 3% Traxenamic acid | Significant reduction | [3] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Phasha, V.; Senabe, J.; Ndzotoyi, P.; Okole, B.; Fouche, G.; Chuturgoon, A. Review on the Use of Kojic Acid—A Skin-Lightening Ingredient. Cosmetics 2022, 9, 64. https://doi.org/10.3390/cosmetics9030064
Phasha V, Senabe J, Ndzotoyi P, Okole B, Fouche G, Chuturgoon A. Review on the Use of Kojic Acid—A Skin-Lightening Ingredient. Cosmetics. 2022; 9(3):64. https://doi.org/10.3390/cosmetics9030064
Chicago/Turabian StylePhasha, Vivey, Jeremiah Senabe, Phatheka Ndzotoyi, Blessed Okole, Gerda Fouche, and Anil Chuturgoon. 2022. "Review on the Use of Kojic Acid—A Skin-Lightening Ingredient" Cosmetics 9, no. 3: 64. https://doi.org/10.3390/cosmetics9030064
APA StylePhasha, V., Senabe, J., Ndzotoyi, P., Okole, B., Fouche, G., & Chuturgoon, A. (2022). Review on the Use of Kojic Acid—A Skin-Lightening Ingredient. Cosmetics, 9(3), 64. https://doi.org/10.3390/cosmetics9030064







