The Role of Preservatives and Multifunctionals on the Oxidation of Cosmetic O/W Emulsions
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
References
- Matwiejczuk:, N.; Galicka, A.; Brzóska, M.M. Review of the safety of application of cosmetic products containing parabens. J. Appl. Toxicol. 2020, 40, 176–210. [Google Scholar] [CrossRef]
- Matić, M.; Puh, B. Consumers’ Purchase Intentions Towards Natural Cosmetics. Ekon. Vjesn. Econviews-Rev. Contemp. Bus. Entrep. Econ. Issues 2016, 29, 53. [Google Scholar]
- Kale, S.N.; Deore, S.L. Emulsion micro emulsion and nano emulsion: A review. Syst. Rev. Pharm. 2017, 8, S39. [Google Scholar] [CrossRef]
- Köhler, K.; Schuchmann, I.H.P. Emulgiertechnik: Grundlagen, Verfahren und Anwendungen; Behr‘s Verlag: Hamburg, Germany, 2012. [Google Scholar]
- Bährle-Rapp, M. Springer Lexikon Kosmetik und Körperpflege, 5th ed.; Springer: Berlin/Heidelberg, Germany, 2020. [Google Scholar]
- Lagaly, G.; Schulz, O.; Zimehl, R. Dispersionen und Emulsionen: Eine Einführung in die Kolloidik feinverteilter Stoffe einschließlich der Tonminerale; Springer: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Nour, A.H. Emulsion types, stability mechanisms and rheology: A review. Int. J. Innov. Res. Sci. Stud. (IJIRSS) 2018, 1, 14–21. [Google Scholar]
- Halla, N.; Fernandes, I.P.; Heleno, S.A.; Costa, P.; Boucherit-Otmani, Z.; Boucherit, K.; Rodrigues, A.E.; Ferreira, I.C.F.R.; Barreiro, M.F. Cosmetics preservation: A review on present strategies. Molecules 2018, 23, 1571. [Google Scholar] [CrossRef]
- Lück, E. Chemische Lebensmittelkonservierung: Stoffe Wirkungen Methoden; Springer: Berlin/Heidelberg, Germany, 1977. [Google Scholar]
- Rähse, W. Cosmetic Creams: Development, Manufacture and Marketing of Effective Skin Care Products; Wiley-VCH: Weinheim, Germany, 2020. [Google Scholar]
- Das Europäische Parlament und der Rat der Europäischen Union. Verordnung (EG) Nr. 1223/2009 des Europäischen Perlaments und des Rates vom 30. November 2009 Über Kosmetische Mittel: VO (EG) Nr.1223/2009; Das Europäische Parlament und der Rat der Europäischen Union: Bruxelles, Belgium, 2009. [Google Scholar]
- Evonik Dr. Straetmans GmbH. Product Data Sheet Phenethylalcohol Nat; Evonik Dr. Straetmans GmbH: Hamburg, Germany, 2017. [Google Scholar]
- TRI-K, Inc. Product Data Sheet Potassium Sorbate FCC; TRI-K, Inc.: Channahon, IL, USA, 2008. [Google Scholar]
- Kampf, G.; Rudolf, M.; Labadie, J.-C.; Barrett, S.P. Spectrum of antimicrobial activity and user acceptability of the hand disinfectant agent Sterillium Gel. J. Hosp. Infect. 2002, 52, 141–177. [Google Scholar] [CrossRef]
- Merck KGaA. Products Data Sheet RonaCare; Merck KGaA: Darmstadt, Germany, 2020. [Google Scholar]
- Evonik Dr. Straetmans GmbH. Product Data Sheet Dermosoft® GMCY MB; Evonik Dr. Straetmans GmbH: Hamburg, Germany, 2020. [Google Scholar]
- Gunstone, F.D.; Norris, F.A. Lipids in Food Chemistry, Biochemistry and Technology; Pergamon Press: Oxford, UK, 1982. [Google Scholar]
- Werman, M.J.; Neeman, I. Oxidative stability of avocado oil. J. Am. Oil Chem. Soc. 1986, 63, 355–360. [Google Scholar] [CrossRef]
- McClements, D.J.; Decker, E.A. Lipid oxidation in oil-in-water emulsions: Impact of molecular environment on chemical reactions in heterogeneous food systems. J. Food Sci. 2000, 65, 1270–1282. [Google Scholar] [CrossRef]
- Matissek, R.; Baltes, W. Lebensmittelchemie, 8th ed.; Springer Spektrum: Berlin/Heidelberg, Germany, 2016. [Google Scholar]
- Eghbaliferiz, S.; Iranshahi, M. Pro-oxidant activity of polyphenols, flavonoids, anthocyanins and carotenoids: Updated review of mechanisms and catalyzing metals. Phytother. Res. 2016, 30, 1379–1391. [Google Scholar] [CrossRef] [PubMed]
- Oroian, M.; Escriche, I. Antioxidants: Characterization, natural sources, extraction and analysis. Food Res. Int. 2015, 74, 10–36. [Google Scholar] [CrossRef]
- Springer, A.; Destler, E.; Pazurik, B.; Platzer, M.; Kiese, S. Sustainable & Long-lasting: Natural Cosmetics Made from Purely Plant-based Ingredients. Sofwjournal 2022, 148, 32–34. [Google Scholar]
- Decker, E.A.; McClements, D.J.; Bourlieu-Lacanal, C.; Durand, E.; Figueroa-Espinoza, M.C.; Lecomte, J.; Villeneuve, P. Hurdles in Predicting Antioxidant Efficacy in Oil-in-water emulsions. Trends Food Sci. Technol. 2017, 67, 183–194. [Google Scholar] [CrossRef]
- Wu, D.C.; Goldman, M.P.A. Topical Anti-inflammatory Healing Regimen Utilizing Conjugated Linolenic Acid for Use Post-ablative Laser Resurfacing of the Face: A Randomized, Controlled Trial. J. Clin. Aesthetic Dermatol. 2017, 10, 12–17. [Google Scholar]
- Ferreira, M.J.; Fiadeiro, T.; Silvia, M.; Soares, A.P. Topical γ-linolenic acid therapy in atopic dermatitis. Allergo J. 1998, 7, 213–216. [Google Scholar] [CrossRef]
- Nasrollahi, S.A.; Ayatollahi, A.; Yazdanparast, T.; Samadi, A.; Hosseini, H.; Shamsipour, M.; Akhlaghi, A.A.; Yadangi, S.; Abels, C.; Firooz, A.; et al. Comparison of linoleic acid-containing water-in-oil emulsion with urea-containing water-in-oil emulsion in the treatment of atopic dermatitis: A randomized clinical trial. Clin. Cosmet. Investig. Dermatol. 2018, 11, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Girotti, A.W.; Kriska, T. Role of lipid hydroperoxides in photo-oxidative stress signaling. Antioxid. Redox Signal. 2004, 6, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Kohen, R. Skin antioxidants: Their role in aging and in oxidative stress—New approaches for their evaluation. Biomed. Pharmacother. 1999, 53, 181–192. [Google Scholar] [CrossRef]
- Briganti, S.; Picardo, M. Antioxidant activity, lipid peroxidation and skin diseases. What’s new. J. Eur. Acad. Dermatol. Venereol. 2003, 17, 663–669. [Google Scholar] [CrossRef]
- Chiba, K.; Kawakami, K.; Sone, T.; Onoue, M. Characteristics of skin wrinkling and dermal changes induced by repeated application of squalene monohydroperoxide to hairless mouse skin. Ski. Pharmacol. Physiol. 2003, 16, 242–251. [Google Scholar] [CrossRef]
- Ohkido, M.; Yoshino, K.; Matsuo, I. Lipid peroxide of human skin. In Biochem. Norm. Abnorm. Epidermal Differ. 1981, 10, 269–278. [Google Scholar]
- Dey, S.; Nagababu, B.H. Applications of Food Colour and Bio-Preservatives in the Food and Its Effect on the Human Health. Food Chem. Adv. 2022, 100019. [Google Scholar] [CrossRef]
- Gallarate, M.; Carlotti, M.E.; Trotta, M.; Bovo, S. On the stability of ascorbic acid in emulsified systems for topical and cosmetic use. Int. J. Pharm. 1999, 188, 233–241. [Google Scholar] [CrossRef]
- Arnhold, T. Struktur Natürlicher und Synthetischer Retinoide In Untersuchungen zum Metabolismus von Vitamin A Retinoiden im Hinblick auf eine Risikoabschätzung Ihrer Teratogenen Wirkung Beim Menschen. Ph.D. Thesis, Der Tehnichen Universitat Carolo-Wilhelmina, Braunschweig, Germany, 2000. [Google Scholar]
- James, A.M.; Smith, R.A.; Murphy, M.P. Antioxidant and pro-oxidant properties of mitochondrial Coenzyme, Q. Arch. Biochem. Biophys 2004, 423, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Jirovetz, L.; Buchbauer, G.; Schmidt, E.; Denkova, Z.; Slavchev, A.; Stoyanova, A.; Geissler, M. Purity, antimicrobial activities and olfactory evaluations of 2-phenylethanol and some derivatives. J. Essent. Oil Res. 2008, 20, 82–85. [Google Scholar] [CrossRef]
- Lucchini, J.J.; Bonnaveiro, N.; Cremieux, A.; Le Goffic, F. Mechanism of bactericidal action of phenethyl alcohol in Escherichia coli. Curr. Microbiol. 1993, 27, 295–300. [Google Scholar] [CrossRef]
- Zhu, Y.J.; Zhou, H.T.; Hu, Y.H.; Tang, J.Y.; Su, M.X.; Guo, Y.J.; Liu, B. Antityrosinase and antimicrobial activities of 2-phenylethanol, 2-phenylacetaldehyde and 2-phenylacetic acid. Food Chem. 2011, 124, 298–302. [Google Scholar] [CrossRef]
- Molinari, F.; Gandolfi, R.; Aragozzini, F.; Leon, R.; Prazeres, D.M. Biotransformations in two-liquid-phase systems: Production of phenylacetaldehyde by oxidation of 2-phenylethanol with acetic acid bacteria. Enzym. Microb. Technol. 1999, 25, 729–735. [Google Scholar] [CrossRef]
- Sofos, J.N. Sorbate Food Preservatives; CRC Press: Boca Raton, FL, USA, 1989. [Google Scholar]
- Lück, E. Food applications of sorbic acid and its salts. Food Addit. Contam. 1990, 7, 711–715. [Google Scholar] [CrossRef]
- Stopforth, J.D.; Sofos, J.N.; Busta, F.F. Sorbic acid and sorbates. Food Sci. Technol. 2005, 145, 49. [Google Scholar]
- Elez-Martínez, P.; Soliva-Fortuny, R.; Martín-Belloso, O. Oxidative rancidity in avocado purée as affected by α-tocopherol, sorbic acid and storage atmosphere. Eur. Food Res. Technol. 2007, 226, 295. [Google Scholar] [CrossRef]
- Gerschenson, L.N.; Campos, C.A. Sorbic acid stability during processing and storage of high moisture foods. In Food Preservation by Moisture Control; Cánovas, B., Chanes, W., Eds.; Technomic Publishing Co: Lancaster, UK, 1995. [Google Scholar]
- Windholz, M. The Merck Index: An Encyclopedia of Chemicals and Drugs, 9th ed.; Merck: Rahway, NJ, USA, 1976. [Google Scholar]
- Cillard, J.; Cillard, P.; Cormier, M. Effect of experimental factors on the pro-oxidant behavior of α-tocopherol. J. Am. Oil Chem. Soc. 1980, 57, 255–261. [Google Scholar] [CrossRef]
- Jacob, S.E.; Barron, G.S. Benzyl alcohol: A covert fragrance. Dermatitis 2007, 18, 232–233. [Google Scholar] [CrossRef]
- Gottschalck, T.E.; Bailey, J.E. International Cosmetic Ingredient Dictionary and Handbook, 13th ed.; Personal Care Products Council: Washington, DC, USA, 2010. [Google Scholar]
- Mashayekhi, H.A.; Rezaee, M.; Garmaroudi, S.S.; Montazeri, N.; Ahmadi, S.J. Rapid and sensitive determination of benzaldehyde arising from benzyl alcohol used as preservative in an injectable formulation solution using dispersive liquid–liquid microextraction followed by gas chromatography. Anal. Sci. 2011, 27, 865–868. [Google Scholar] [CrossRef] [PubMed]
- Johnson, W.; Bergfeld, W.F.; Belsito, D.V.; Hill, R.A.; Klaassen, C.D.; Liebler, D.C.; Marks, J.G.; Shank, R.C.; Slaga, T.J.; Snyder, P.W.; et al. Safety Assessment of Benzyl Alcohol, Benzoic Acid and its Salts, and Benzyl Benzoate. Int. J. Toxicol. 2017, 36, 5–30. [Google Scholar] [CrossRef]
- Warth, A.D. Mechanism of action of benzoic acid on Zygosaccharomyces bailii: Effects on glycolytic metabolite levels, energy production, and intracellular pH. Appl. Environ. Microbiol. 1991, 57, 3410–3414. [Google Scholar] [CrossRef] [PubMed]
- Medeiros Vinci, R.; De Meulenaer, B.; Andjelkovic, M.; Canfyn, M.; Van Overmeire, I.; Van Loco, J. Factors influencing benzene formation from the decarboxylation of benzoate in liquid model systems. J. Agric. Food Chem. 2011, 59, 12975–12981. [Google Scholar] [CrossRef]
- Frank, S.; Dunkel, A.; Schieberle, P. Model studies on benzene formation from benzaldehyde. Eur. Food Res. Technol. 2020, 246, 901–908. [Google Scholar] [CrossRef]
- Sadighara, P.; Pirhadi, M.; Sadighara, M.; Shavaly-Gilani, P.; Zirak, M.R.; Zeinali, T. Benzene food exposure and their prevent methods: A review. Nutr. Food Sci. 2022, 28. [Google Scholar] [CrossRef]
- Hung, L.C.; Ismail, R.; Basri, M.; Nang, H.L.L.; Tejo, B.A.; Abu Hassan, H.; May, C.Y. Testing of glyceryl monoesters for their anti-microbial susceptibility and their influence in emulsions. J. Oil Palm Res. 2010, 22, 846–855. [Google Scholar]
- Manning, M.; Orawski, P. Food grade esters used as personal care antimicrobials. Cosmet. Toil. 2005, 120, 63–68. [Google Scholar]
- Ahn, G.W.; Choi, M.H.; Woo, Y.T.; Jo, B.K. A Study on the Antimicrobial Effect of Glyceryl Caprylate in Cosmetics. J. Soc. Cosmet. Sci. Korea 2007, 33, 47–52. [Google Scholar]
- Lawan, K.; Kanlayavattanakul, M. Antimicrobial efficacy of caprylyl glycol and ethylhexylglycerine in emulsion. J. Health Res. 2009, 23, 1–3. [Google Scholar]
- Martínez, M.L.; Penci, M.; Ixtaina, V.; Ribbota, P.D.; Maesti, D. Effect of natural and synthetic antioxidants on the oxidative stability of walnut oil under different storage conditions. LWT-Food Sci. Technol. 2013, 51, 44–50. [Google Scholar] [CrossRef]
- Bachofen, R. Gas metabolism of microorganisms. Experientia 1991, 47, 508–513. [Google Scholar] [CrossRef]
- Pascual, O.; Vignault, A.; Gombau, J.; Navarro, M.; Gómez-Alonso, S.; García-Romero, E.; Canals, J.M.; Hermosín-Gutíerrez, I.; Teissedre, P.-L.; Zamora, F. Oxygen consumption rates by different oenological tannins in a model wine solution. Food Chem. 2017, 234, 26–32. [Google Scholar] [CrossRef]
- Navarro, M.; Vignault, A.; Gombau, J.; Navarro, M.; Gómez-Alonso, S.; García-Romero, E.; Canalset, J.M.; Hermosín-Gutíerrez, I.; Teissedre, P.-L.; Zamora, F. Oxygen consumption by oak chips in a model wine solution—Influence of the botanical origin, toast level and ellagitannin content. Food Chem. 2016, 199, 822–827. [Google Scholar] [CrossRef] [PubMed]
- Böhner, N. Einfluss der Handelsbeleuchtung auf die Qualität lichtempfindlicher Lebensmittel am Beispiel von Brühwurst und Kaffeesahne. Ph.D. Thesis, Technische Universität München, München, Germany, 2019. [Google Scholar]
- Malviya, A.; Jadran, V. Henry’s law constant of nitrogen, oxygen, and argon in ternary aqueous alcoholic solvent mixtures. J. Chem. Eng. Data 2019, 65, 1189–1196. [Google Scholar] [CrossRef]
- Tokunaga, J. Solubilities of oxygen, nitrogen, and carbon dioxide in aqueous alcohol solutions. J. Chem. Eng. Data 1975, 20, 41–46. [Google Scholar] [CrossRef]
- Ahlhaus, O.E. Verpackung mit Kunststoffen, 1st ed.; Hanser Verlag: München, Germany, 1997. [Google Scholar]
- Belitz, H.-D.; Grosch, W.; Schieberle, P. Lehrbuch der Lebensmittelchemie, 6th ed.; Springer: Berlin/Heidelberg, Germany, 2008; p. 208. [Google Scholar]
- Fritsch, C.W.; Gale, J.A. Hexanal as a measure of rancidity in low fat foods. J. Am. Oil Chem. Soc. 1977, 54, 225–228. [Google Scholar] [CrossRef]
- Bocharova, O.; Sentyabrina, R.; Vasiliy, E. Toluene and benzyl alcohol formation in fruit juices containing benzoates. J. Food Processing Preserv. 2017, 41, 13054. [Google Scholar] [CrossRef]
- Fennema, O.R. Food Chemistry; CRC Press: Boca Raton, FL, USA, 1996; Volume 76. [Google Scholar]
- Ofosu, F.K.; Daliri, E.B.M.; Elahi, F.; Chelliah, R.; Lee, B.H.; Oh, D.H. New insights on the use of polyphenols as natural preservatives and their emerging safety concerns. Front. Sustain. Food Syst. 2020, 4, 525810. [Google Scholar] [CrossRef]
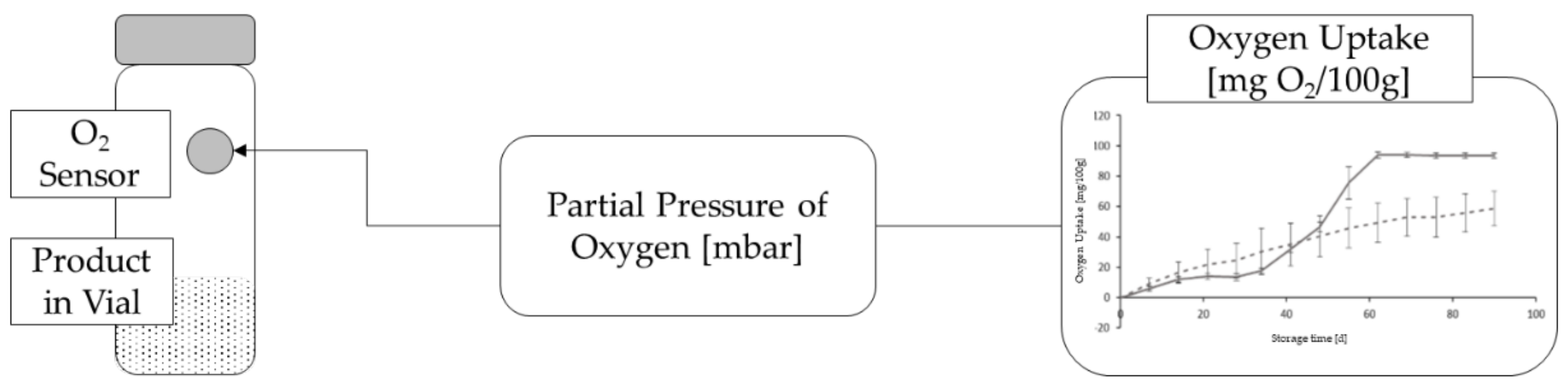
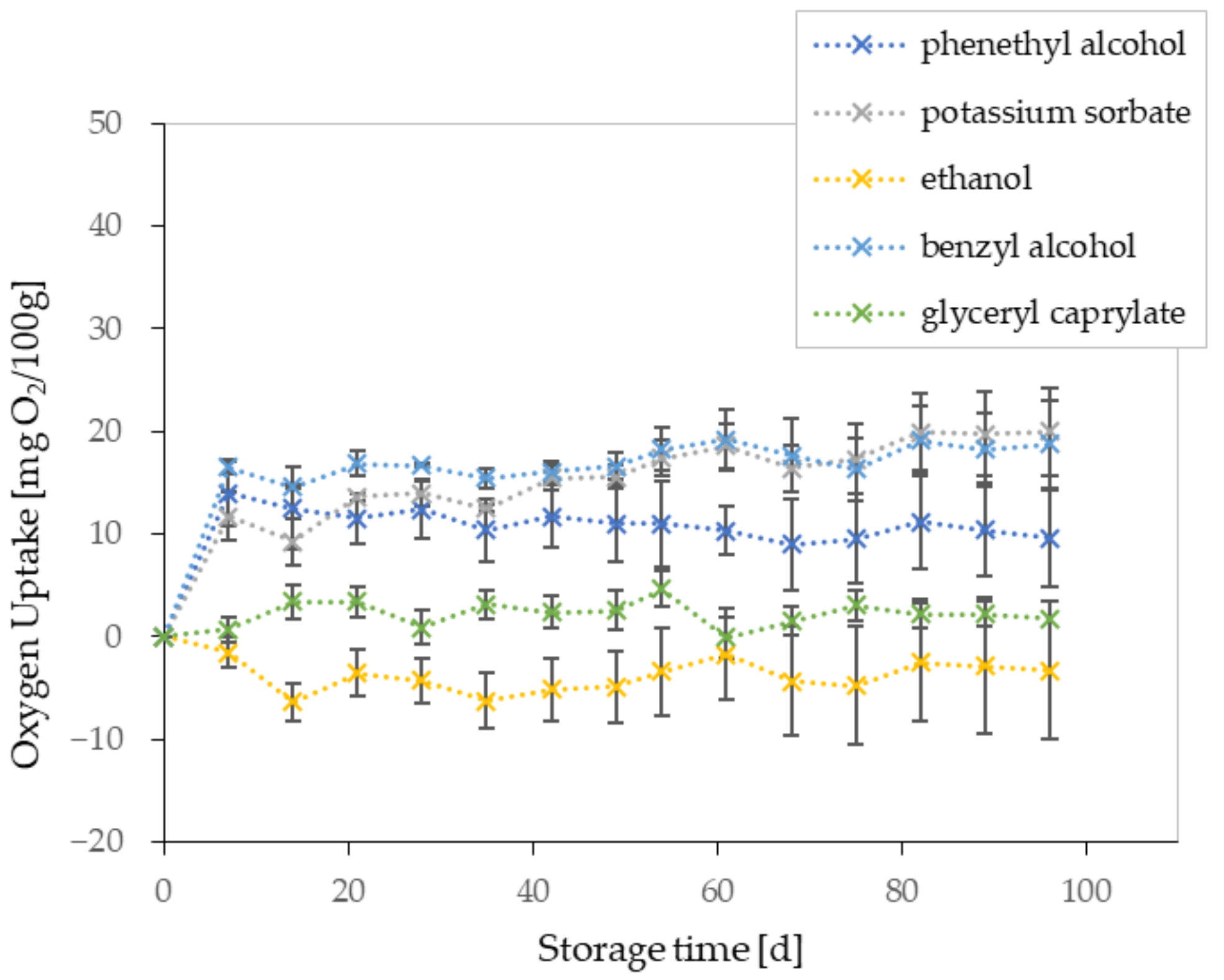

| Preservative | pH-Range | Water Solubility (g/L) | Preservation Spectrum | Max. Quantity [11] (%) |
|---|---|---|---|---|
| Phenethyl alcohol [12] | unlimited | 20 | gram −, mold | 0.7–1.0 |
| Potassium sorbate [13] | <6 | 582 | yeast, mold | 0.6 |
| Ethanol [14] | all | unlimited | broad spectrum | 20.0 |
| Benzyl alcohol [15] | <4.5 | 40 | broad spectrum | 0.5 |
| Glyceryl caprylate [16] | 4.0–7.0 | not specified | gram +, yeast | 0.3–1.0 |
| Preservative | Water (%) | Canola Oil (%) | Glyceryl Stearate SE (%) | Xanthan Gum (%) | pH-Regulator (%) | Preservative (%) |
|---|---|---|---|---|---|---|
| Phenethyl alcohol | 69.3 | 22.8 | 7.0 | 0.2 | 0.2 | 0.7 |
| Potassium sorbate | 69.8 | 22.8 | 7.0 | 0.2 | 0.2 | 0.2 |
| Ethanol | 55.0 | 22.8 | 7.0 | 0.2 | 0.2 | 15.0 |
| Benzyl alcohol | 69.5 | 22.8 | 7.0 | 0.2 | 0.2 | 0.5 |
| Glyceryl caprylate | 69.7 | 22.8 | 7.0 | 0.2 | 0.2 | 0.3 |
| Potassium Sorbate | Ethanol | Benzyl Alcohol | Glyceryl Caprylate | |
|---|---|---|---|---|
| Phenethyl alcohol | n.s. | n.s. | n.s. | n.s. |
| Potassium sorbate | * | n.s. | * | |
| Ethanol | * | n.s. | ||
| Benzyl alcohol | * |
| Potassium Sorbate | Ethanol | Benzyl Alcohol | Glyceryl Caprylate | |
|---|---|---|---|---|
| Phenethyl alcohol | n.s. | n.s. | n.s. | n.s. |
| Potassium sorbate | * | n.s. | * | |
| Ethanol | n.s. | n.s. | ||
| Benzyl alcohol | n.s. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Springer, A.; Ziegler, H. The Role of Preservatives and Multifunctionals on the Oxidation of Cosmetic O/W Emulsions. Cosmetics 2022, 9, 59. https://doi.org/10.3390/cosmetics9030059
Springer A, Ziegler H. The Role of Preservatives and Multifunctionals on the Oxidation of Cosmetic O/W Emulsions. Cosmetics. 2022; 9(3):59. https://doi.org/10.3390/cosmetics9030059
Chicago/Turabian StyleSpringer, Arielle, and Helena Ziegler. 2022. "The Role of Preservatives and Multifunctionals on the Oxidation of Cosmetic O/W Emulsions" Cosmetics 9, no. 3: 59. https://doi.org/10.3390/cosmetics9030059
APA StyleSpringer, A., & Ziegler, H. (2022). The Role of Preservatives and Multifunctionals on the Oxidation of Cosmetic O/W Emulsions. Cosmetics, 9(3), 59. https://doi.org/10.3390/cosmetics9030059





