Cosmetic Potential of a Recombinant 50 kDa Protein
Abstract
:1. Introduction
2. Materials and Methods
2.1. Formulation Compatibility Evaluation
2.2. EpiOcular
2.3. Bacterial Reverse Mutation Assay
2.4. MTT Assay
2.5. Type I Collagen Assay
2.6. Type III Collagen Assays
3. Results
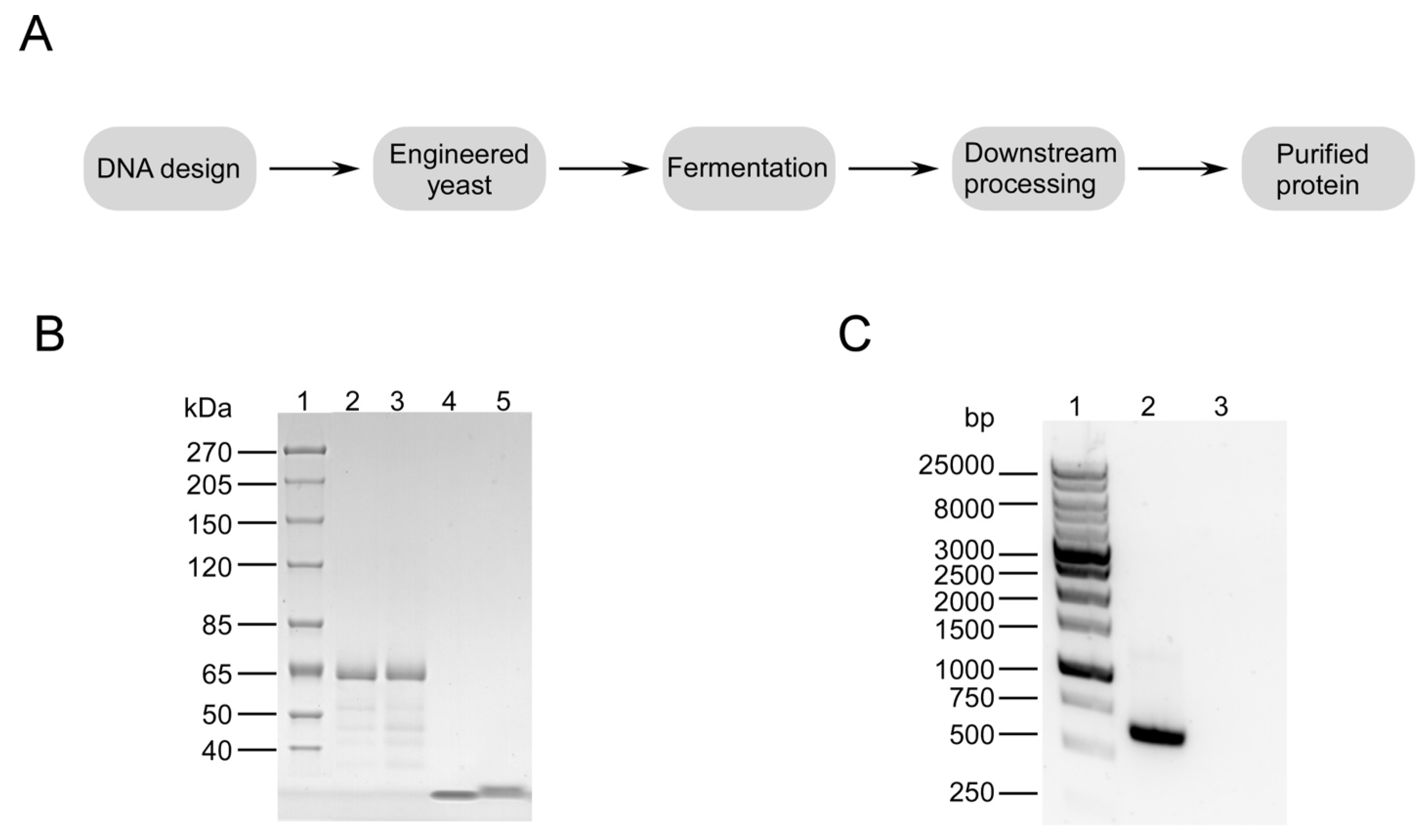
3.1. Characterization of 50 kDa Protein
3.2. Formulation Testing and Potential Applications
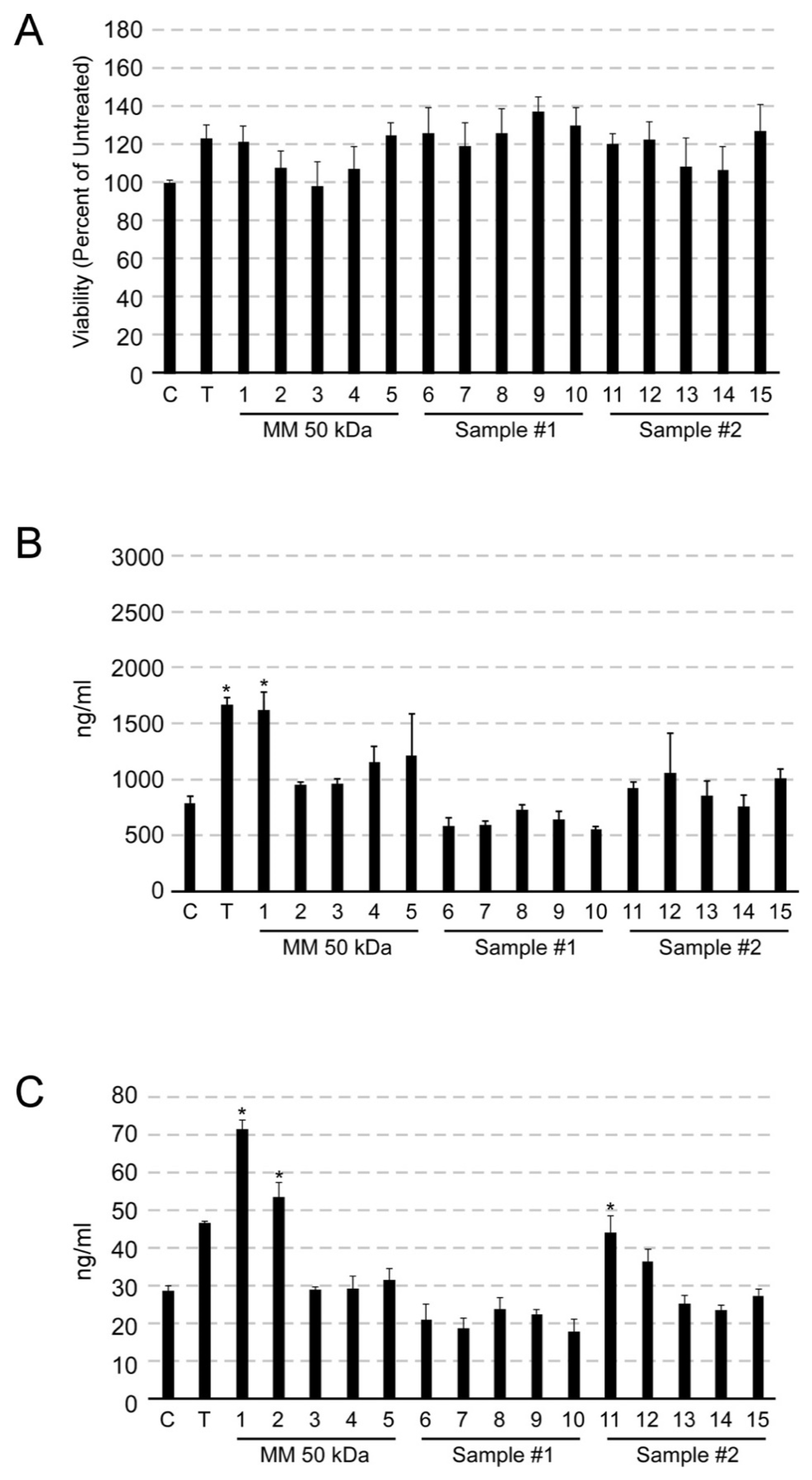
3.3. Safety Testing
3.4. Collagen Synthesis Induction in Human Primary Dermal Fibroblast by 50 kDa Protein
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ricard-Blum, S. The collagen family. Cold Spring Harb. Perspect. Biol 2011, 3, a004978. [Google Scholar] [CrossRef] [Green Version]
- Fleischmajer, R.; MacDonald, E.D.; Perlish, J.S.; Burgeson, R.E.; Fisher, L.W. Dermal collagen fibrils are hybrids of type I and type III collagen molecules. J. Struct. Biol. 1990, 105, 162–169. [Google Scholar] [CrossRef]
- Kuivaniemi, H.; Tromp, G. Type III collagen (COL3A1): Gene and protein structure, tissue distribution, and associated diseases. Gene 2019, 707, 151–171. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, M.J.; Karsdal, M.A. Type III Collagen. In Biochemistry of Collagens, Laminins and Elastin, 1st ed.; Karsdal, M.A., Ed.; Academic Press: Cambridge, MA, USA, 2016; pp. 21–30. [Google Scholar]
- Manturova, G.O.; Smirnova, V.A.; Stupin, E.V.; Silina, N.E. The ratio of collagen types I/III as a marker of skin aging and prognosis of aesthetic facial surgery results. J. Pharm. Sci. Res. 2018, 10, 2543–2546. [Google Scholar]
- Wang, C.; Rong, Y.; Ning, F.; Zhang, G. The content and ratio of type I and III collagen in skin differ with age and injury. Afr. J. Biotechnol. 2011, 10, 2524–2529. [Google Scholar]
- Parkin, J.D.; San Antonio, J.D.; Persikov, A.V.; Dagher, H.; Dalgleish, R.; Jensen, S.T.; Jeunemaitre, X.; Savige, J. The collagen III fibril has a “flexi-rod” structure of flexible sequences interspersed with rigid bioactive domains including two with hemostatic roles. PLoS ONE 2017, 12, e0175582. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Wu, H.; Byrne, M.; Krane, S.; Jaenisch, R. Type III collagen is crucial for collagen I fibrillogenesis and for normal cardiovascular development. Proc. Natl. Acad. Sci. USA 1997, 94, 1852–1856. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Brisson, B.K.; Terajima, M.; Li, Q.; Hoxha, K.; Han, B.; Goldberg, A.M.; Sherry Liu, X.; Marcolongo, M.S.; Enomoto-Iwamoto, M.; et al. Type III collagen is a key regulator of the collagen fibrillar structure and biomechanics of articular cartilage and meniscus. Matrix Biol. 2020, 85–86, 47–67. [Google Scholar] [CrossRef]
- Cliche, S.; Amiot, J.; Avezard, C.; Gariépy, C. Extraction and characterization of collagen with or without telopeptides from chicken skin. Poult. Sci. 2003, 82, 503–509. [Google Scholar] [CrossRef] [PubMed]
- Munasinghe, K.A.; Schwarz, J.G.; Nyame, A.K. Chicken Collagen from Law Market Value By-Products as an Alternate Source. J. Food Process. 2014, 2014, 298295. [Google Scholar] [CrossRef] [Green Version]
- Zhou, C.; Li, Y.; Yu, X.; Yang, H.; Ma, H.; Yagoub, A.E.A.; Cheng, Y.; Hu, J.; Out, P.N.Y. Extraction and characterization of chicken feet soluble collagen. LWT Food Sci. Technol. 2016, 74, 145–153. [Google Scholar] [CrossRef]
- Jafari, H.; Lista, A.; Siekapen, M.M.; Ghaffari-Bohlouli, P.; Nie, L.; Alimoradi, H.; Shavandi, A. Fish Collagen: Extraction, Characterization, and Applications for Biomaterials Engineering. Polymers 2020, 12, 2230. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, A.; Shaw, N.B.; Murphy, S.C.; van de Vis, J.W.; van Pelt-Heerschap, H.; Kerry, J.P. Extraction of Collagen from Fish Skins and Its Use in the Manufacture of Biopolymer Films. J. Aquat. Food Prod. Technol. 2006, 15, 21–32. [Google Scholar] [CrossRef]
- Khong, N.M.H.; Yusoff, F.M.; Jamilah, B.; Basri, M.; Maznah, I.; Chan, K.W.; Armania, N.; Nishikawa, J. Improved collagen extraction from jellyfish (Acromitus hardenbergi) with increased physical-induced solubilization processes. Food Chem. 2018, 251, 41–50. [Google Scholar] [CrossRef]
- Coppola, D.; Oliviero, M.; Vitale, G.A.; Lauritano, C.; D’Ambra, I.; Iannace, S.; de Pascale, D. Marine Collagen from Alternative and Sustainable Sources: Extraction, Processing and Applications. Mar. Drugs 2020, 18, 214. [Google Scholar] [CrossRef] [Green Version]
- Browne, S.; Zeugolis, D.I.; Pandit, A. Collagen: Finding a solution for the source. Tissue Eng. Part A 2013, 19, 1491–1494. [Google Scholar] [CrossRef] [Green Version]
- Asgari, M.; Latifi, N.; Heris, H.K.; Vali, H.; Mongeau, L. In vitro fibrillogenesis of tropocollagen type III in collagen type I affects its relative fibrillar topology and mechanics. Sci. Rep. 2017, 7, 1392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fertala, A. Three Decades of Research on Recombinant Collagens: Reinventing the Wheel or Developing New Biomedical Products? Bioengineering 2020, 7, 155. [Google Scholar] [CrossRef]
- Schnieke, A.; Dziadek, M.; Bateman, J.; Mascara, T.; Harbers, K.; Gelinas, R.; Jaenisch, R. Introduction of the human pro alpha 1(I) collagen gene into pro alpha 1(I)-deficient Mov-13 mouse cells leads to formation of functional mouse-human hybrid type I collagen. Proc. Natl. Acad. Sci. USA 1987, 84, 764–768. [Google Scholar] [CrossRef] [Green Version]
- Olsen, A.S.; Geddis, A.E.; Prockop, D.J. High levels of expression of a minigene version of the human pro alpha 1 (I) collagen gene in stably transfected mouse fibroblasts. Effects of deleting putative regulatory sequences in the first intron. J. Biol. Chem. 1991, 266, 1117–1121. [Google Scholar] [CrossRef]
- Specks, U.; Mayer, U.; Nischt, R.; Spissinger, T.; Mann, K.; Timpl, R.; Engel, J.; Chu, M.L. Structure of recombinant N-terminal globule of type VI collagen alpha 3 chain and its binding to heparin and hyaluronan. EMBO J. 1992, 11, 4281–4290. [Google Scholar] [CrossRef] [PubMed]
- Mazzorana, M.; Gruffat, H.; Sergeant, A.; van der Rest, M. Mechanisms of collagen trimer formation. Construction and expression of a recombinant minigene in HeLa cells reveals a direct effect of prolyl hydroxylation on chain assembly of type XII collagen. J. Biol. Chem. 1993, 268, 3029–3032. [Google Scholar] [CrossRef]
- Fertala, A.; Sieron, A.L.; Ganguly, A.; Li, S.W.; Ala-Kokko, L.; Anumula, K.R.; Prockop, D.J. Synthesis of recombinant human procollagen II in a stably transfected tumour cell line (HT1080). Biochem. J. 1994, 298, 31–37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olsen, D.; Yang, C.; Bodo, M.; Chang, R.; Leigh, S.; Baez, J.; Carmichael, D.; Perälä, M.; Hämäläinen, E.R.; Jarvinen, M.; et al. Recombinant collagen and gelatin for drug delivery. Adv. Drug Deliv. Rev. 2003, 55, 1547–1567. [Google Scholar] [CrossRef] [PubMed]
- Fertala, A.; Shah, M.; Hoffman, R.; Arnold, W.V. Designing recombinant collagens for biomedical applications. Curr. Tissue Eng. 2016, 5, 73–84. [Google Scholar] [CrossRef]
- Bulleid, N.J.; John, D.C.; Kadler, K.E. Recombinant expression systems for the production of collagen. Biochem. Soc. Trans. 2000, 28, 350–353. [Google Scholar] [CrossRef]
- Ruggiero, F.; Exposito, J.Y.; Bournat, P.; Gruber, V.; Perret, S.; Comte, J.; Olagnier, B.; Garrone, R.; Theisen, M. Triple helix assembly and processing of human collagen produced in transgenic tobacco plants. FEBS Lett. 2000, 469, 132–136. [Google Scholar] [CrossRef] [Green Version]
- Xu, X.; Gan, Q.; Clough, R.C.; Pappu, K.M.; Howard, J.A.; Baez, J.A.; Wang, K. Hydroxylation of recombinant human collagen type I alpha 1 in transgenic maize co-expressed with a recombinant human prolyl 4-hydroxylase. BMC Biotechnol. 2011, 11, 69. [Google Scholar] [CrossRef] [Green Version]
- Gellermann, P.; Schneider-Barthold, C.; Bolten, S.N.; Overfelt, E.; Scheper, T.; Pepelanova, I. Production of a Recombinant Non-Hydroxylated Gelatin Mimetic in Pichia pastoris for Biomedical Applications. J. Funct. Biomater. 2019, 10, 39. [Google Scholar] [CrossRef] [Green Version]
- Rutschmann, C.; Baumann, S.; Cabalzar, J.; Luther, K.B.; Hennet, T. Recombinant expression of hydroxylated human collagen in Escherichia coli. Appl. Microbiol. Biotechnol. 2014, 98, 4445–4455. [Google Scholar] [CrossRef] [Green Version]
- John, D.C.; Watson, R.; Kind, A.J.; Scott, A.R.; Kadler, K.E.; Bulleid, N.J. Expression of an engineered form of recombinant procollagen in mouse milk. Nat. Biotechnol. 1999, 17, 385–389. [Google Scholar] [CrossRef] [PubMed]
- Weber, S.C.; Herz, A.H. Method for Recombinant Yeast Expression and Isolation of Water-Soluble Collagen-Type Polypeptides. Application No. 383,748. U.S. Patent 5,710,252, 20 January 1998. [Google Scholar]
- Mashiko, T.; Takada, H.; Wu, S.H.; Kanayama, K.; Feng, J.; Tashiro, K.; Asahi, R.; Sunaga, A.; Hoshi, K.; Kurisaki, A.; et al. Therapeutic effects of a recombinant human collagen peptide bioscaffold with human adipose-derived stem cells on impaired wound healing after radiotherapy. J. Tissue Eng. Regen. Med. 2018, 12, 1186–1194. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Ma, X.; Gao, Y.; Fan, D.; Zhu, C.; Mi, Y.; Xue, W. Hydroxylation of Human Type III Collagen Alpha Chain by Recombinant Coexpression with a Viral Prolyl 4-Hydroxylase in Escherichia coli. Protein J. 2017, 36, 322–331. [Google Scholar] [CrossRef] [PubMed]
- Vuorela, A.; Myllyharju, J.; Nissi, R.; Pihlajaniemi, T.; Kivirikko, K.I. Assembly of human prolyl 4-hydroxylase and type III collagen in the yeast pichia pastoris: Formation of a stable enzyme tetramer requires coexpression with collagen and assembly of a stable collagen requires coexpression with prolyl 4-hydroxylase. EMBO J. 1997, 16, 6702–6712. [Google Scholar] [CrossRef] [Green Version]
- Báez, J.; Olsen, D.; Polarek, J.W. Recombinant microbial systems for the production of human collagen and gelatin. Appl. Microbiol. Biotechnol. 2005, 69, 245–252. [Google Scholar] [CrossRef]
- Sipilä, K.H.; Drushinin, K.; Rappu, P.; Jokinen, J.; Salminen, T.A.; Salo, A.M.; Käpylä, J.; Myllyharju, J.; Heino, J. Proline hydroxylation in collagen supports integrin binding by two distinct mechanisms. J. Biol. Chem. 2018, 293, 7645–7658. [Google Scholar] [CrossRef] [Green Version]
- Rappu, P.; Salo, A.M.; Myllyharju, J.; Heino, J. Role of prolyl hydroxylation in the molecular interactions of collagens. Essays Biochem. 2019, 63, 325–335. [Google Scholar]
- Pihlajaniemi, T.; Myllylä, R.; Kivirikko, K.I. Prolyl 4-hydroxylase and its role in collagen synthesis. J. Hepatol. 1991, 13 (Suppl. S3), S2–S7. [Google Scholar] [CrossRef]
- Kolle, S.N.; Rey Moreno, M.C.; Mayer, W.; van Cott, A.; van Ravenzwaay, B.; Landsiedel, R. The EpiOcular™ Eye Irritation Test is the Method of Choice for the In Vitro Eye Irritation Testing of Agrochemical Formulations: Correlation Analysis of EpiOcular Eye Irritation Test and BCOP Test Data According to the UN GHS, US EPA and Brazil ANVISA Classification Schemes. Altern. Lab. Anim. 2015, 43, 181–198. [Google Scholar]
- Ames, B.N.; Mccann, J.; Yamasaki, E. Methods for detecting carcinogens and mutagens with the Salmonella/mammalian-microsome mutagenicity test. Mutat. Res. 1975, 31, 347–364. [Google Scholar] [CrossRef]
- Myllyharju, J.; Nokelainen, M.; Vuorela, A.; Kivirikko, K.I. Expression of recombinant human type I–III collagens in the yeast Pichia pastoris. Biochem. Soc. Trans. 2000, 28, 353–357. [Google Scholar] [CrossRef]
- He, J.; Ma, X.; Zhang, F.; Li, L.; Deng, J.; Xue, W.; Zhu, C.; Fan, D. New strategy for expression of recombinant hydroxylated human collagen α1(III) chains in Pichia pastoris GS115. Biotechnol. Appl. Biochem. 2015, 62, 293–299. [Google Scholar] [CrossRef]
- Nokelainen, M.; Tu, H.; Vuorela, A.; Notbohm, H.; Kivirikko, K.I.; Myllyharju, J. High-level production of human type I collagen in the yeast Pichia Pastoris. Yeast 2001, 18, 797–806. [Google Scholar] [CrossRef] [Green Version]
- Ouzounov, N.; Lorestani, A.; Bhatia, M. Recombinant collagen and elastin molecules and uses thereof. U.S. Patent WO2019068018, 23 May 2019. [Google Scholar]
- Edgar, S.; Hopley, B.; Genovese, L.; Sibilla, S.; Laight, D.; Shute, J. Effects of collagen-derived bioactive peptides and natural antioxidant compounds on proliferation and matrix protein synthesis by cultured normal human dermal fibroblasts. Sci. Rep. 2018, 8, 10474. [Google Scholar] [CrossRef] [PubMed]
- Chan, C.P.; Lan, W.H.; Chang, M.C.; Chen, Y.J.; Lan, W.C.; Chang, H.H.; Jeng, J.H. Effects of TGF-beta s on the growth, collagen synthesis and collagen lattice contraction of human dental pulp fibroblasts in vitro. Arch. Oral Biol. 2005, 50, 469–479. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.J.; Ha, G.H.; Seo, W.Y.; Kim, C.K.; Kim, K.; Lee, S.B. Human collagen alpha-2 type I stimulates collagen synthesis, wound healing, and elastin production in normal human dermal fibroblasts (HDFs). BMB Rep. 2020, 53, 539–544. [Google Scholar] [CrossRef] [PubMed]
- Bolke, L.; Schlippe, G.; Gerß, J.; Voss, W. A Collagen Supplement Improves Skin Hydration, Elasticity, Roughness, and Density: Results of a Randomized, Placebo-Controlled, Blind Study. Nutrients 2019, 11, 2494. [Google Scholar] [CrossRef] [Green Version]
- Lin, P.; Hua, N.; Hsu, Y.C.; Kan, K.W.; Chen, J.H.; Lin, Y.H.; Kuan, C.M. Oral Collagen Drink for Antiaging: Antioxidation, Facilitation of the Increase of Collagen Synthesis, and Improvement of Protein Folding and DNA Repair in Human Skin Fibroblasts. Oxid. Med. Cell. Longev. 2020, 2020, 8031795. [Google Scholar] [CrossRef] [PubMed]
- Yamanaka, H.; Okada, S.; Sanada, H. A multicenter, randomized, controlled study of the use of nutritional supplements containing collagen peptides to facilitate the healing of pressure ulcers. J. Nutr. Intermed. Metab. 2017, 8, 51–59. [Google Scholar] [CrossRef]
- Asserin, J.; Lati, E.; Shioya, T.; Prawitt, J. The effect of oral collagen peptide supplementation on skin moisture and the dermal collagen network: Evidence from an ex vivo model and randomized, placebo-controlled clinical trials. J. Cosmet. Dermatol. 2015, 14, 291–301. [Google Scholar] [CrossRef] [Green Version]
- Lee, G.; West, V.; Parker, T.; Vollmer, D. A Multi-Type Collagen-Based Drink Supplement Significantly Improved Markers of Aging, both in vitro and in a Human Clinical Study. J. Clin. Exp. Dermatol. Res. 2020, 11, 1000531. [Google Scholar]
| Major Category | Specific Examples |
|---|---|
| Skincare | Eye care—Cream, serum, mask (sheet, pad, cream, etc..), treatment Eyebrow—serums, primer and treatment Eyelash—serums, primer and treatment |
Face care
| |
| Lip care (tinted or not, with/without SPF)—lip oil, lip balm, lip mask, lip treatment | |
Body care
| |
| Haircare—prewash hair treatment, hair mist, scalp treatment and/or oil, serum, primer, leave-in conditioner, hair mask, hair toner, leave on spray, lotion, haircare shampoo and dry shampoo, wash off conditioner, hair dye, scalp scrub and all haircare styling product | |
Shaving—Aftershave balm, razor burn and bumps relief, bear oil
| |
| Makeup |
|
| Supplements/drinks |
| Ingredients: | %wt/wt |
|---|---|
| Alcohol-Based Toner | |
| Water (Aqua) | Remainder Ingredient |
| Recombinant collagen fragment disclosed herein | about 0.0005% to about 25% |
| Alcohol Denat. | 10–20% |
| Pentylene Glycol | 5–10% |
| Glycerin | 1–5% |
| Gluconolactone | 0.1–1% |
| Dipotassium Glycyrrhizate | 0.1–1% |
| Sodium Citrate | 0.1–1% |
| Sodium Benzoate | 0.1–1% |
| Water-Based Toner | |
| Water (Aqua) | Remainder Ingredient |
| Recombinant collagen fragment disclosed herein | about 0.0005% to about 25% |
| Niacinamide | 1–5% |
| Pentylene Glycol | 1–5% |
| Propanediol | 1–5% |
| Glycerin | 1–5% |
| Biosaccharide Gum-1 | 0.1–1% |
| Glyceryl Caprylate | 0.1–1% |
| Sodium Anisate | 0.1–1% |
| Sodium Hydroxide | 0.1–1% |
| Caprylhydroxamic Acid | 0.1–1% |
| Acrylates/C10-30 Alkyl Acrylate Crosspolymer | 0.1–1% |
| Sodium Levulinate | 0.1–1% |
| Caprylyl Glycol | 0.1–1% |
| Cream | |
| Water (Aqua) | Remainder Ingredient |
| Recombinant collagen fragment disclosed herein | about 0.0005% to about 25% |
| Cetearyl Alcohol | 5–10% |
| Glycerin | 1–5% |
| Squalane | 1–5% |
| Butyrospermum Parkii (Shea) Butter | 1–5% |
| Glyceryl Caprylate | 1–5% |
| Microcrystalline Cellulose | 1–5% |
| Glyceryl Stearate Citrate | 0.1–1% |
| Tocopheryl Acetate | 0.1–1% |
| Cetearyl Glucoside | 0.1–1% |
| Sodium Stearoyl Glutamate | 0.1–1% |
| Cellulose Gum | 0.1–1% |
| Xanthan Gum | 0.1–1% |
| Caprylhydroxamic Acid | 0.1–1% |
| Sodium Phytate | 0.01–0.1% |
| Gel | |
| Water (Aqua) | Remainder Ingredient |
| Recombinant collagen fragment disclosed herein | about 0.0005% to about 25% |
| Sodium Phytate | 0.1–1% |
| Sodium Hydroxide | 0.1–1% |
| Carbomer | 0.1–1% |
| Phenoxyethanol | 0.1–1% |
| Serum | |
| Water (Aqua) | Remainder Ingredient |
| Recombinant collagen fragment disclosed herein | about 0.0005% to about 25% |
| Pentylene Glycol | 1–5% |
| Niacinamide | 1–5% |
| Dimethicone | 1–5% |
| Propanediol | 1–5% |
| Tocopherol | 0.1–1% |
| Sodium Hyaluronate | 0.1–1% |
| Linoleic Acid | 0.1–1% |
| Ammonium Acryloyldimethyltaurate/VP Copolymer | 0.1–1% |
| Acrylates/C10-30 Alkyl Acrylate Cross polymer | 0.1–1% |
| Caprylyl Glyceryl Ether | 0.1–1% |
| Tetrasodium Glutamate Diacetate | 0.01–0.1% |
| Sodium Hydroxide | 0.01–0.1% |
| Phenoxyethanol | 0.01–0.1% |
| Linolenic Acid | 0.001–0.0 |
| Shampoo | |
| Water (Aqua) | Remainder Ingredient |
| Recombinant collagen fragment disclosed herein | about 0.0005% to about 25% |
| Cocamidopropyl Betaine | 5–10% |
| Sodium Lauroyl Methyl Isethionate | 5–10% |
| Propanediol | 1–5% |
| Sodium Methyl Oleoyl Taurate | 1–5% |
| Sodium Cocoyl Isethionate | 1–5% |
| Trisodium Ethylenediamine Disuccinate | 0.1–1% |
| Caprylhydroxamic Acid | 0.1–1% |
| Panthenol | 0.1–1% |
| Citric Acid | 0.1–1% |
| Caprylyl Glycol | 0.1–1% |
| Sodium Benzoate | 0.1–1% |
| Conditioner | |
| Water (Aqua) | Remainder Ingredient |
| Recombinant collagen fragment disclosed herein | about 0.0005% to about 25% |
| Cetearyl Alcohol | 5–10% |
| Glyceryl Caprylate | 1–5% |
| Behentrimonium Methosulfate | 1–5% |
| Glycerin | 1–5% |
| Caprylhydroxamic Acid | 0.1–1% |
| Panthenol | 0.1–1% |
| Hydroxyethylcellulose | 0.1–1% |
| Cocos Nucifera (Coconut) Oil | 0.1–1% |
| Citric Acid | 0.01–0.1% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aly, N.; Benoit, E.; Chaubard, J.-L.; Chintalapudi, K.; Choung, S.; de Leeuw, M.; Diaz, M.; Dueppen, D.; Ferraro, B.; Fischetti, V.; et al. Cosmetic Potential of a Recombinant 50 kDa Protein. Cosmetics 2022, 9, 8. https://doi.org/10.3390/cosmetics9010008
Aly N, Benoit E, Chaubard J-L, Chintalapudi K, Choung S, de Leeuw M, Diaz M, Dueppen D, Ferraro B, Fischetti V, et al. Cosmetic Potential of a Recombinant 50 kDa Protein. Cosmetics. 2022; 9(1):8. https://doi.org/10.3390/cosmetics9010008
Chicago/Turabian StyleAly, Nesma, Emilie Benoit, Jean-Luc Chaubard, Kavyasree Chintalapudi, Soojin Choung, Monique de Leeuw, Matthew Diaz, Dan Dueppen, Bryce Ferraro, Valerie Fischetti, and et al. 2022. "Cosmetic Potential of a Recombinant 50 kDa Protein" Cosmetics 9, no. 1: 8. https://doi.org/10.3390/cosmetics9010008
APA StyleAly, N., Benoit, E., Chaubard, J.-L., Chintalapudi, K., Choung, S., de Leeuw, M., Diaz, M., Dueppen, D., Ferraro, B., Fischetti, V., Gassaway, E., Hansenne-Cervantes, I., Heeres, A., Karas, C., Khan, M., Kral, J. M., Lam, S., Lartey, R., Leonard, M., ... Dai, L. (2022). Cosmetic Potential of a Recombinant 50 kDa Protein. Cosmetics, 9(1), 8. https://doi.org/10.3390/cosmetics9010008






