Formula Development of Red Palm (Elaeis guineensis) Fruit Extract Loaded with Solid Lipid Nanoparticles Containing Creams and Its Anti-Aging Efficacy in Healthy Volunteers
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
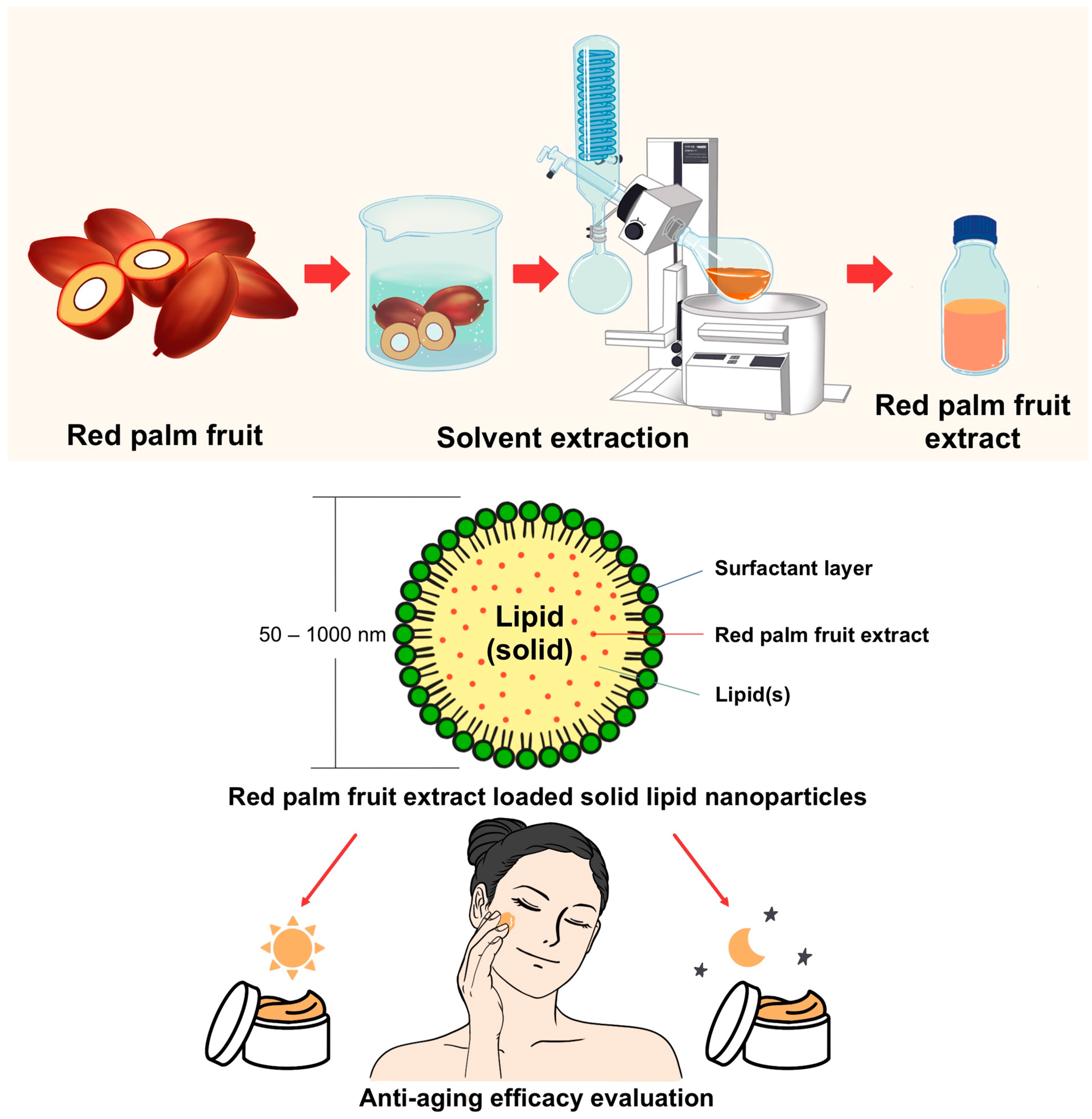
2.2. Preparation of E. guineensis Fruit Extract
2.2.1. Plant Collection
2.2.2. Extraction of E. guineensis
2.3. Evaluation of E. guineensis Fruit Extract
2.3.1. Physical Properties
2.3.2. Determination of Total Vitamin E Content
2.3.3. Determination of β-Carotene Content
2.3.4. Determination of Fatty Acid Content
2.3.5. Determination of DPPH Radical Scavenging Activity
2.4. Preparation of E. guineensis Fruit Extract Loaded SLNs
2.5. Characterization of E. guineensis Fruit Extract Loaded SLNs
2.5.1. Physical Properties
2.5.2. Measurement of Particle Size and Zeta Potential
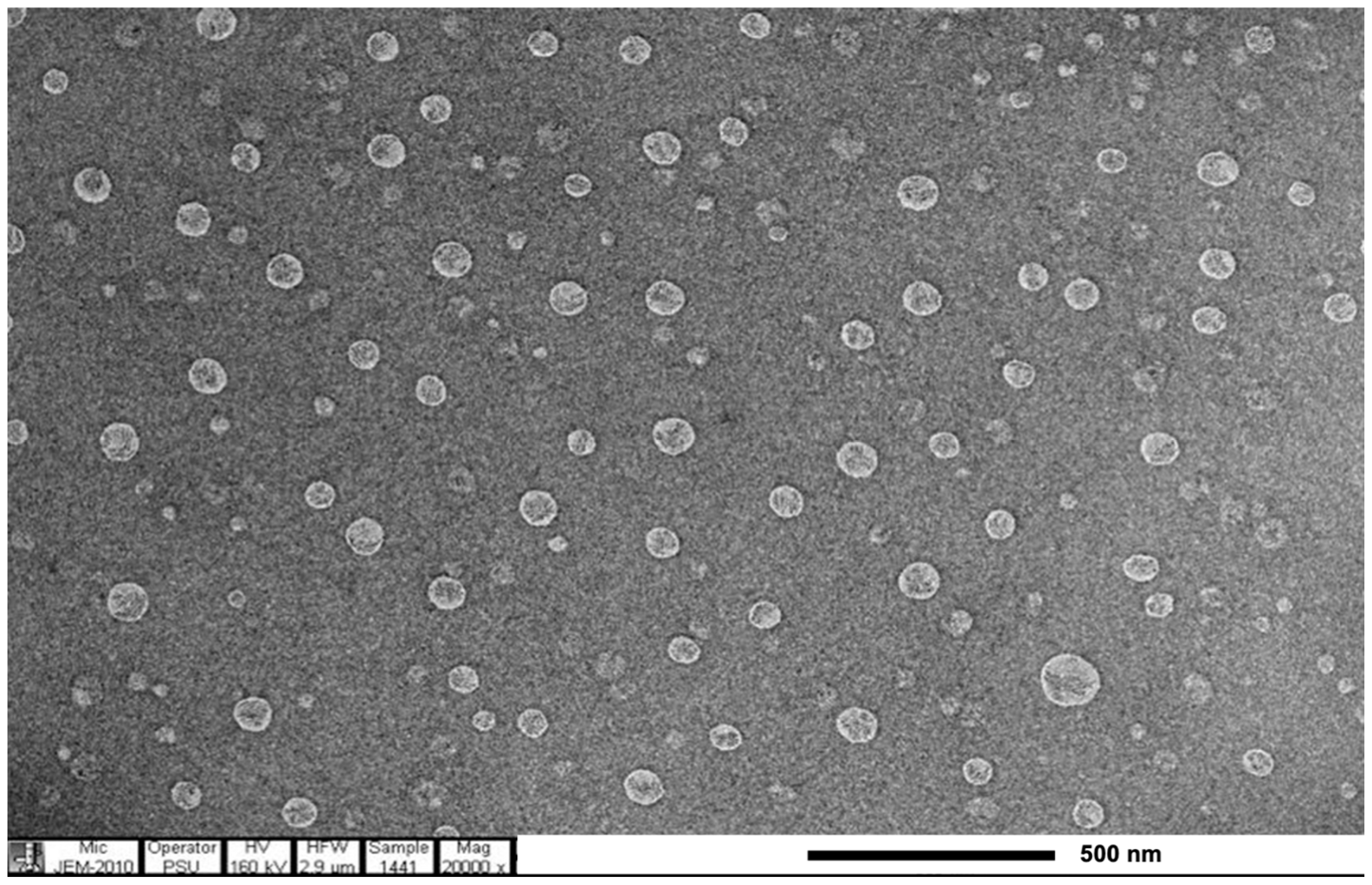
2.5.3. Transmission Electron Microscopy (TEM)
2.6. Preparation of Day and Night Creams Containing E. guineensis Fruit Extract Loaded SLN (Day and Night Creams)
2.7. Evaluation of the Day and Night Creams
2.7.1. Physical Properties
2.7.2. In Vitro Sun Protection Factor (SPF) Evaluation
2.7.3. Stability Study
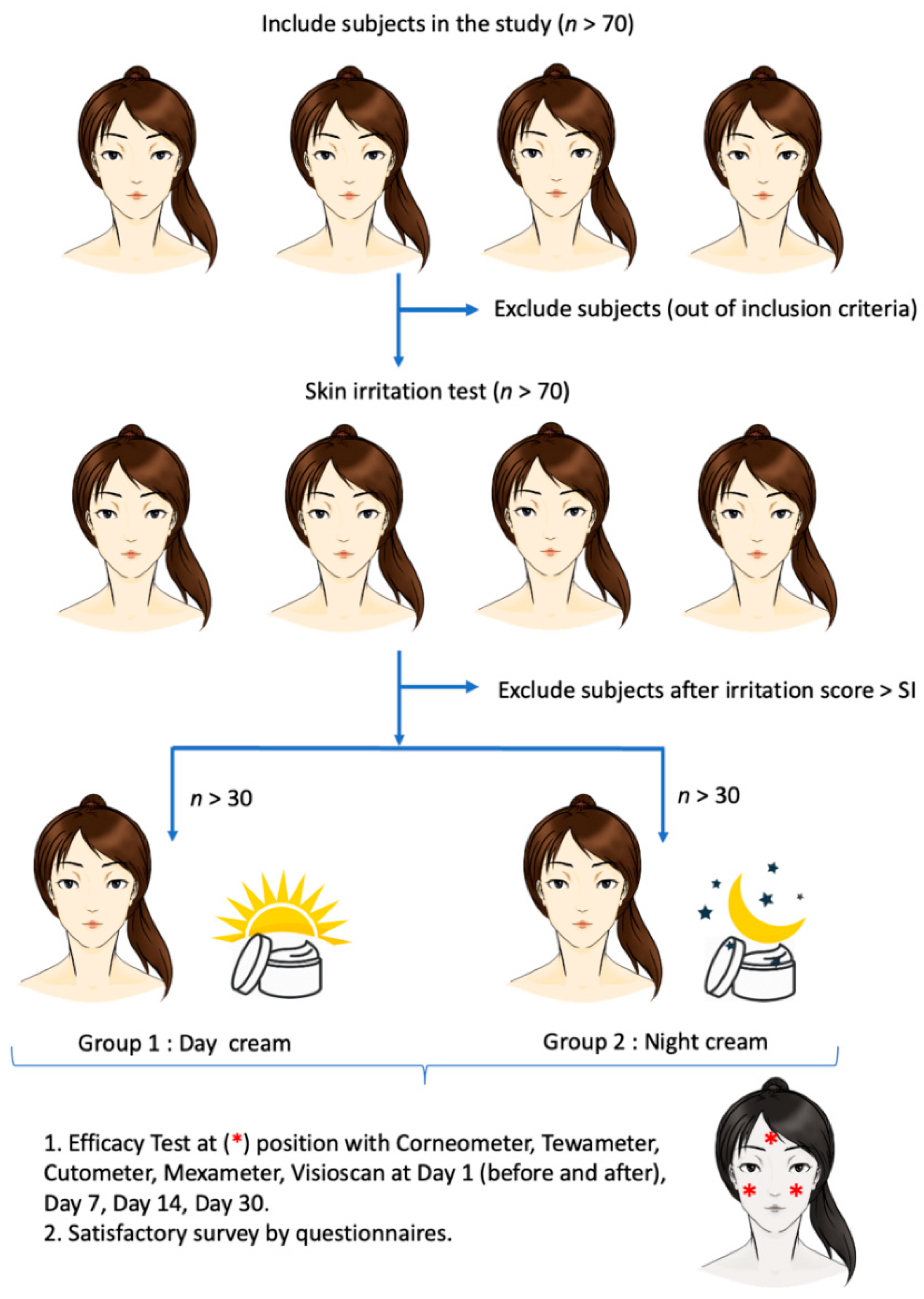
2.8. Clinical Study of Safety and Skin Efficacy of the Day and Night Creams
2.8.1. Ethics Consideration
2.8.2. Subjects
2.8.3. Skin Irritation Protocol
2.8.4. Clinical Efficacy Study Protocol
- The stratum corneum water content and the amount of water accumulated in the epidermis were measured using Corneometer® CM 852 (Courage + Khazaka Electronic Co., Ltd., Köln, Germany).
- TEWL or the amount of water lost from the skin was measured using Tewameter® TM 300 (Courage + Khazaka Electronic Co., Ltd., Germany).
- The skin elasticity was measured using Cutometer® MAP 580 (Courage + Khazaka Electronic Co., Ltd., Germany).
- The melanin index representing pigment amount and skin redness was measured using Mexameter® MX 18 (Courage + Khazaka Electronic Co., Ltd., Germany).
2.8.5. Satisfactory Survey by Questionnaires
2.9. Statistical Analysis
3. Results and Dissuasion
3.1. Extraction of E. guineensis Fruit
3.2. Formulation Development of Creams Containing E. guineensis Fruit Extract Loaded Solid Lipid Nanoparticles
3.3. Stability Study
3.4. Skin Irritation Study of Day and Night Creams
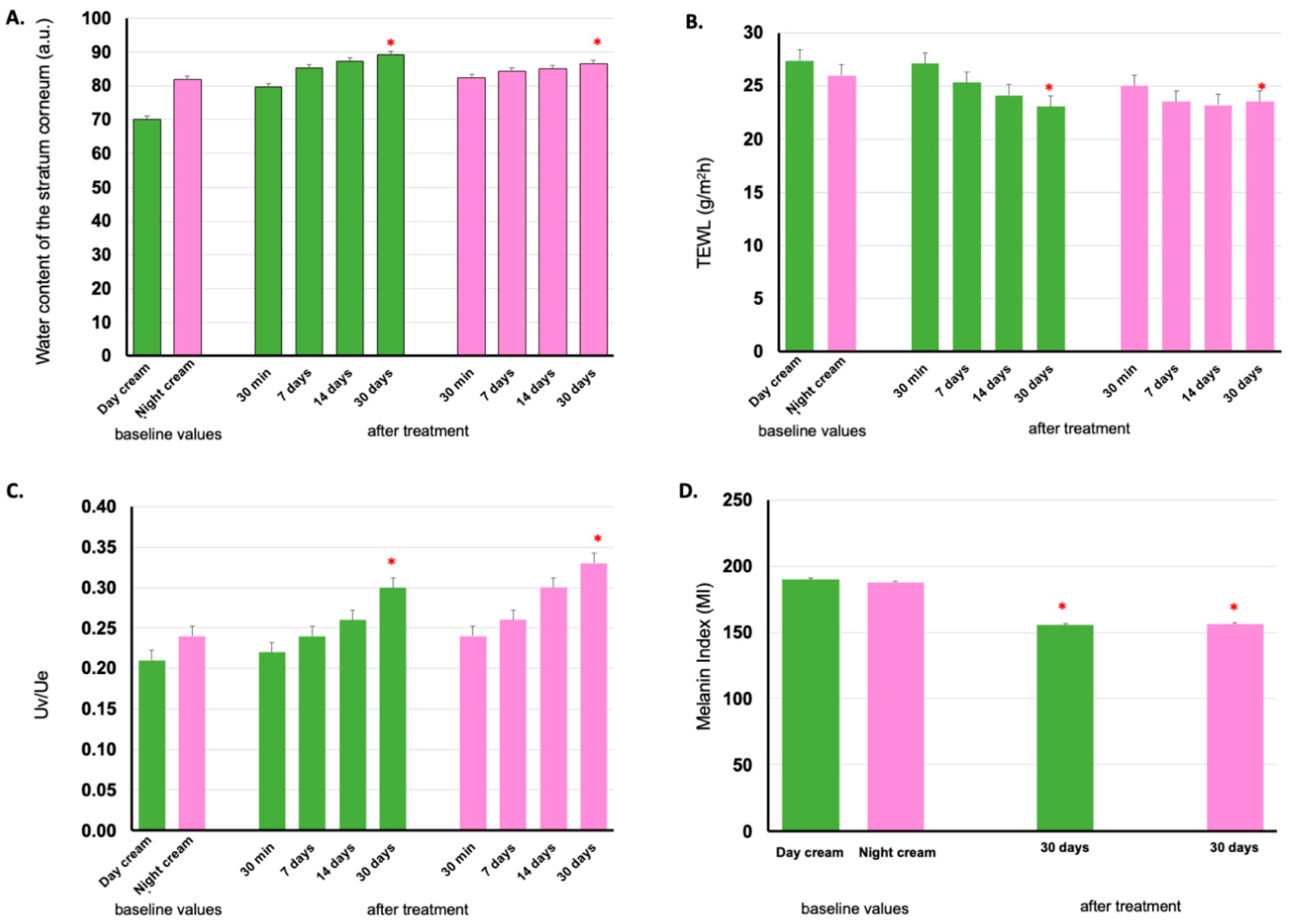
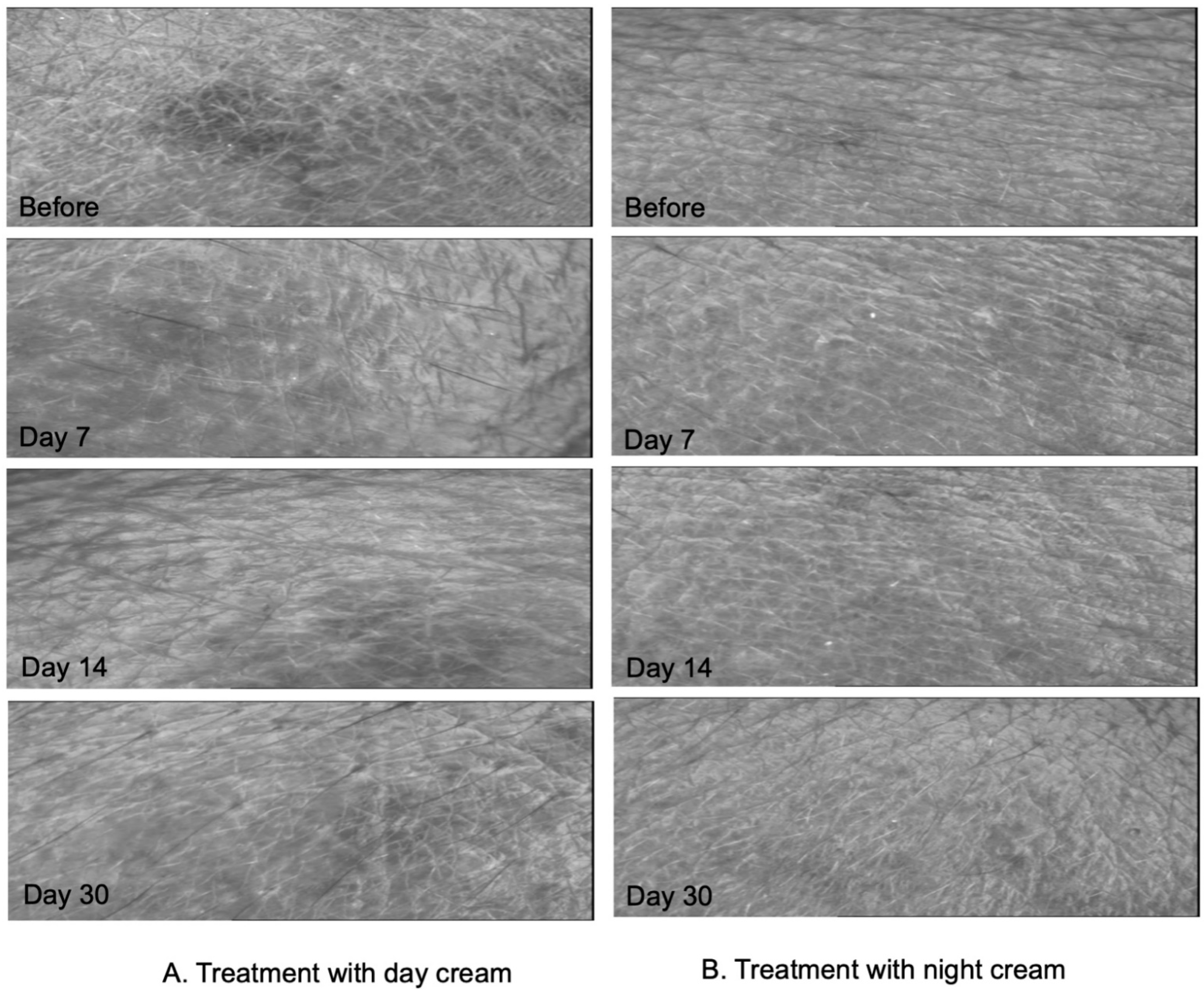
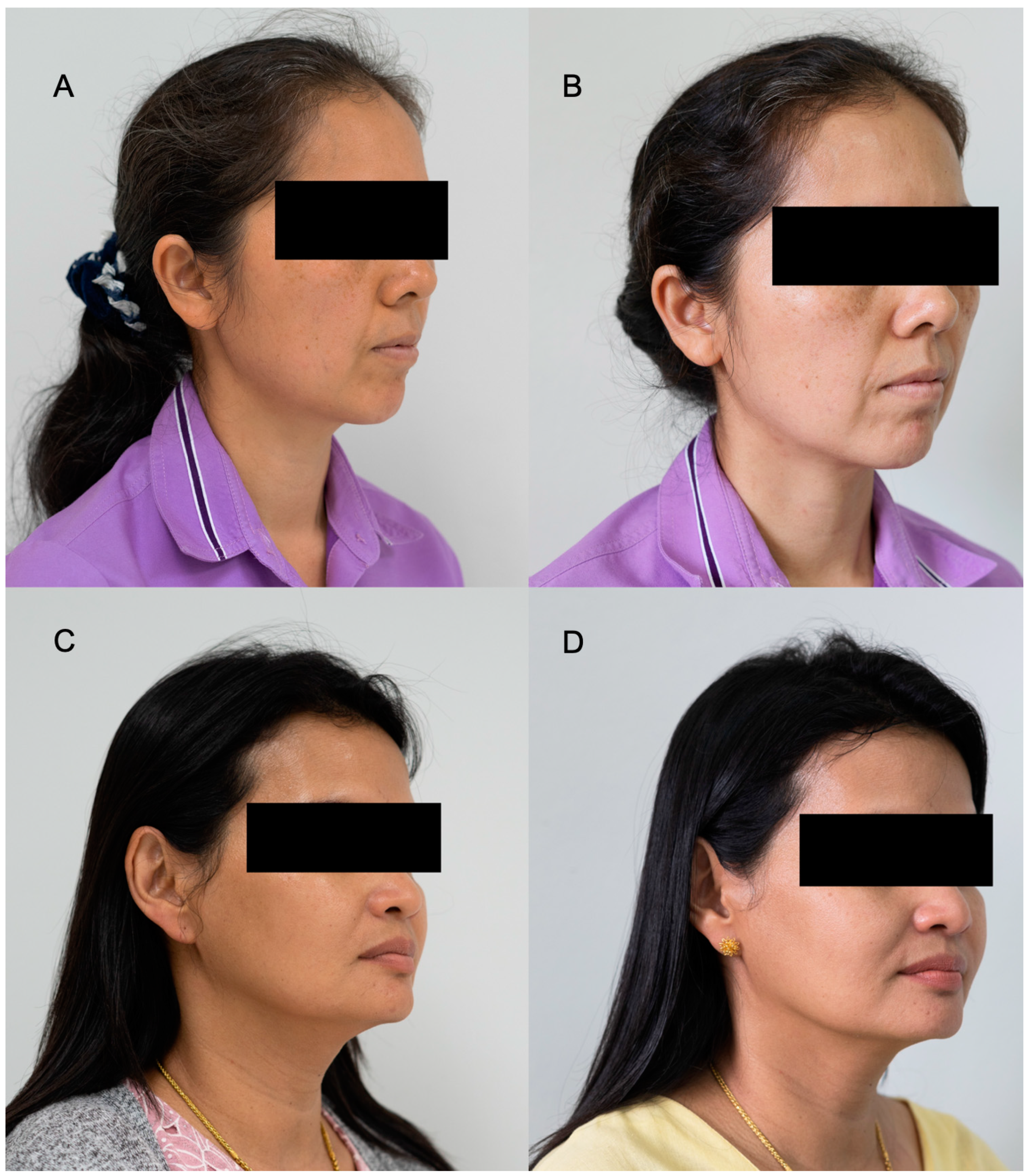
3.5. Clinical Efficacy by Skin Assessment
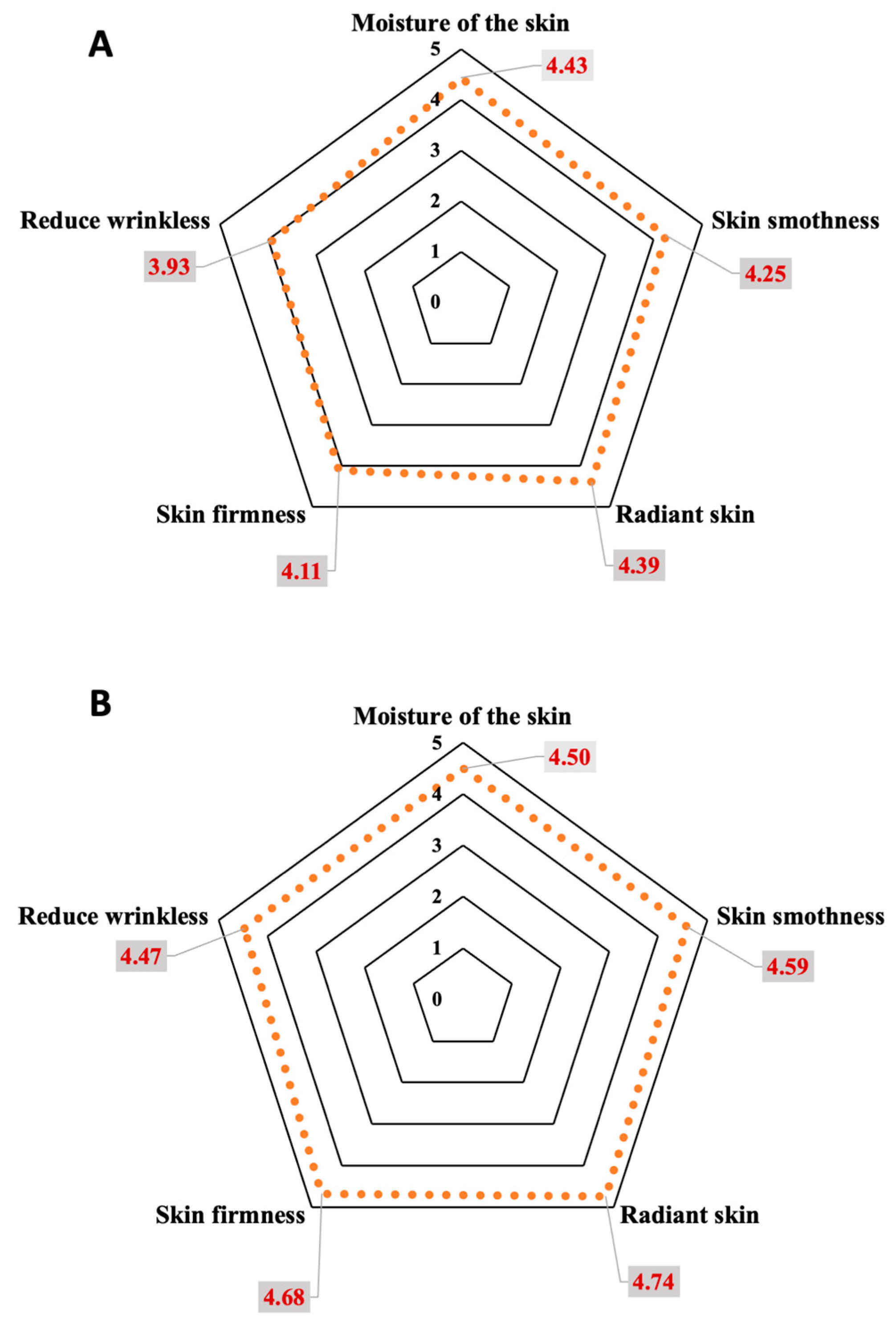
3.6. Satisfaction Survey of the Participants
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mesa-Arango, A.C.; Flórez-Munoz, S.V.; Sanclemente, G. Mechanism of skin aging. Iatreia 2017, 30, 160–170. [Google Scholar] [CrossRef]
- Letsiou, S. Tracing skin aging process: A mini-review of in vitro approaches. Biogerontology 2021, 22, 261–272. [Google Scholar] [CrossRef]
- Sarkar, R.; Podder, I.; Gokhale, N.; Jagadeesan, S.; Garf, V.K. Use of vegetable oils in dermatology: An overview. Int. J. Dermatol. 2017, 56, 1080–1086. [Google Scholar] [CrossRef]
- Leow, S.; Fairus, S.; Sambanthamurthi, R. Water-soluble palm fruit extract: Composition, biological properties, and molecular mechanisms for health and non-health applications. Crit. Rev. Food Sci. Nutr. 2021, in press. [Google Scholar] [CrossRef] [PubMed]
- Lademann, J.; Schanzer, S.; Meinke, M.; Sterry, W.; Darvin, M.E. Interaction between carotenoids and free radicals in human skin. Skin Pharmacol. Physiol. 2011, 24, 238–244. [Google Scholar] [CrossRef] [PubMed]
- Osuala, N.; Ezechukwu, L.A.; Tony, P.O. Screening of wound healing effect of Elaeis guineensis Oil, extract of Vernonia amygdalina mixed with dried egg albumin on burn wound inflicted guinea pig. World J. Biol. Pharm. Health Sci. 2021, 8, 13–28. [Google Scholar] [CrossRef]
- Imoisi, O.; Ilori, G.; Agho, I.; Ekhator, J. Palm oil, its nutritional and health implications (review). J. Appl. Sci. Environ. Manag. 2015, 19, 127–133. [Google Scholar] [CrossRef]
- Tan, C.H.; Lee, C.J.; Tan, S.N.; Poon, D.T.S.; Chong, C.Y.E.; Pui, L.P. Red palm oil; a review on processing, health benefits and its application in food. J. Oleo Sci. 2021, 70, 1201–1210. [Google Scholar] [CrossRef]
- Owoyele, B.V.; Owolabi, G.O. Traditional oil palm (Elaeis guineensis jacq.) and its medicinal uses: A review. Tang Humanit. Med. 2014, 4, e16. [Google Scholar] [CrossRef]
- Ramli, S.; Norhman, N.; Zainuddin, N.; Ja’afar, S.M.; Rahman, I.A. Nanoemulsion based palm olein as vitamin E carrier. Malays. J. Anal. Sci. 2017, 21, 1399–1408. [Google Scholar]
- Mahdi, E.S.; Noor, A.M.; Sakeena, M.H.; Abdullah, G.; Abdulkarim, M.F.; Sattar, M.A. Formulation and in vitro release evaluation of newly synthesized palm kernel oil esters-based nanoemulsion delivery system for 30% ethanolic dried extract derived from local Phyllanthus urinaria for skin antiaging. Int. J. Nanomed. 2011, 6, 2499–2512. [Google Scholar] [CrossRef] [PubMed]
- Ilomuanya, M.O.; Oforkaja, C.A. Formulation and characterization of palm olein/caprylic triglycerides ester based emulgel for topical use. Afr. J. Pharm. Res. Dev. 2020, 12, 172–180. [Google Scholar]
- Abbasalipourkabir, R.; Salehzadeh, A.; Abdullah, R. Characterization and stability of nanostructured lipid carriers as drug delivery system. Pak. J. Biol. Sci. 2012, 15, 141–146. [Google Scholar] [CrossRef][Green Version]
- Abdulkarim, M.F.; Abdullah, G.Z.; Chitneni, M.; Mahdi, E.S.; Yam, M.F.; Faisal, A.; Salman, I.M.; Ameer, O.Z.; Sahib, M.N.; Absulsattar, M.Z.; et al. Formulation and characterization of palm oil esters based nano-cream for topical delivery of piroxicam. Int. J. Drug Deliv. 2010, 2, 287–298. [Google Scholar] [CrossRef][Green Version]
- Deepa, M.K.; Karthikeyan, M.; Abhay, A.D.; Shruti, P.C.; Omkar, S.D.; Satish, R.C. Comprehensive review on solid lipid nanoparticles. Ann. Pharmacol. Pharm. 2020, 5, 1190. [Google Scholar]
- Bhushan, P. (Ed.) Encyclopedia of Nanotechnology; Springer: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Madan, J.R.; Khude, P.A.; Dua, K. Development and evaluation of solid lipid nanoparticles of mometasone furoate for topical delivery. Int. J. Pharm. Investig. 2014, 4, 60–64. [Google Scholar] [CrossRef] [PubMed]
- Mishra, V.; Bansal, K.K.; Verma, A.; Yadev, N.; Thakur, S.; Sudhakar, K.; Rosenholm, J.M. Solid lipid nanoparticles: Emerging colloidal nano drug delivery systems. Pharmaceutics 2018, 10, 191. [Google Scholar] [CrossRef]
- Silva, J.C.; Borrin, T.R.; Ruy, P.; Brito, T.C.; Pinheiro, A.C.; Vicente, A.A.; Pinho, S.C. Characterization, physicochemical stability, and evaluation of in vitro digestibility of solid lipid microparticles produced with palm kernel oil and tristearin. Food Sci. Technol. Camp. 2014, 34, 532–538. [Google Scholar] [CrossRef]
- Goon, D.E.; Kadir, S.H.S.A.; Latip, N.A.; Rahim, S.A.; Mazlan, M. Palm oil in lipid-based formulations and drug delivery systems. Biomolecules 2019, 9, 64. [Google Scholar] [CrossRef]
- Nahum, V.; Domb, A.J. Recent developments in solid lipid microparticles for food ingredients delivery. Foods 2021, 10, 400. [Google Scholar] [CrossRef]
- Lim, W.H.; Tan, Y.J.; Lee, C.S.; Er, H.M.; Wong, S.F. Peparation and optimization of palm-based lipid nanoparticles loaded with griseofulvin. Iran. J. Pharm. Res. 2017, 16, 451–461. [Google Scholar]
- Loganathan, R.; Subramaniam, K.M.; Radhakrishnan, A.K.; Choo, Y.; Teng, K. Health-promoting effects of red palm oil: Evidence from animal and human studies. Nutr. Rev. 2017, 75, 98–113. [Google Scholar] [CrossRef]
- Ng, M.H.; Choo, Y.M.; Ma, A.N.; Chuah, C.H.; Hashim, N.A. Separation of Vitamin E (tocopherol, tocotrienol, and tocomonoenol) in Palm Oil. Lipids 2004, 39, 1031–1035. [Google Scholar] [CrossRef] [PubMed]
- Ng, M.H.; Choo, Y.M. Chromatographic analyses of tocopherols and tocotrienols in palm oil. J. Chromatogr. Sci. 2012, 50, 283–286. [Google Scholar] [CrossRef]
- Weissenberg, M.; Levy, A.; Schaeffler, I.; Menagem, E.; Barzilai, M. Rapid isocratic HPLC analysis of beta-carotene in red peppers (Capsicum annuum L.) and food preparations. Chromatographia 1997, 46, 399–403. [Google Scholar] [CrossRef]
- Taufik, M.; Lioe, H.N.; Yuliana, N.D. Evaluation of major fatty acids determination in palm oil by gas chromatography-flame ionization detection. Agritech 2016, 36, 308–316. [Google Scholar] [CrossRef]
- Shah, R.; Eldridge, D.; Palombo, E.; Harding, I. Optimisation and stability assessment of solid lipid nanoparticles using particle size and zeta potential. J. Phys. Sci. 2014, 25, 59–75. [Google Scholar]
- Wei, L.; Yang, Y.; Shi, K.; Wu, J.; Zhao, W.; Mo, J. Preparation and characterization of loperamide-loaded dynasan 114 solid lipid nanoparticles for increased oral absorption in the treatment of diarrhea. Front. Pharmacol. 2016, 7, 332. [Google Scholar] [CrossRef]
- Phetmung, H.; Sawatdee, S. Development of cosmetic cream containing a pharmaceutical cocrystal of salicylic and phenylalanine. Int. J. Appl. Pharm. 2019, 11, 1–11. [Google Scholar] [CrossRef]
- Gaspar, L.R.; Camargo, F.B., Jr.; Gianeti, M.D.; Maia Campos, P.M.B.G. Evaluation of dermatological effects of cosmetic formulations containing Saccharomyces cerevisiae extract and vitamins. Food Chem. Toxicol. 2008, 46, 3493–3500. [Google Scholar] [CrossRef] [PubMed]
- Lademann, J.; Vergou, T.; Darvin, M.E.; Patzelt, A.; Meinke, M.C.; Voit, C.; Papakostas, D.; Zastrow, L.; Sterry, W.; Doucet, O. Influence of topical, systemic and combined application of antioxidants on the barrier properties of the human skin. Skin Pharmacol. Physiol. 2016, 29, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Zurko Research SL. Assessment in Human with Sensitive Skin of the Cutaneous Compatibility of a Cosmetical Product after a Single under Patch Application under Dermatological Control. (Test Element References 02/TC-PCS_150_15-001). 2015. Available online: https://nutritape.com/web/wp-content/themes/nutritape_theme/pdf/Report_02-TC-PTS_150_15-003_V1.pdf (accessed on 1 March 2019).
- Mwaurah, P.W.; Kumar, S.; Kumar, N.; Attkan, A.K.; Panghal, A.; Singh, V.K.; Garg, M.K. Novel oil extraction technologies: Process conditions, quality parameters, and optimization. Compr. Rev. Food Sci. Food Saf. 2020, 19, 3–20. [Google Scholar] [CrossRef] [PubMed]
- Badawi, N.; El-Say, K.; Attia, D.; El-Nabarawi, M.; Elmazar, M.; Teaima, M. Development of pomegranate extract-loaded solid lipid nanoparticles; quality by design approach to screen the variables affecting the quality attributes and characterization. ACS Omega 2020, 5, 21712–21721. [Google Scholar] [CrossRef] [PubMed]
- Kashanian, S.; Azandaryani, A.H.; Derakhshandeh, K. New surface-modified solid lipid nanoparticles using N-glutaryl phosphatidylethanolamine as the outer shell. Int. J. Nanomed. 2011, 6, 2393–2401. [Google Scholar]
- Mura, P.; Maestrelli, F.; D’Ambrosio, M.; Luceri, C.; Cirri, M. Evaluation and comparison of solid lipid nanoparticles (SLNs) and nanostructured lipid carriers (NLCs) as vectors to develop hydrochlorothiazide effective and safe pediatric oral liquid formulations. Pharmaceutics 2021, 13, 437. [Google Scholar] [CrossRef]
- Makoni, P.A.; Wa Kasongo, K.; Walker, R.B. Short term stability testing of efavirenz-loaded solid lipid nanoparticle (SLN) and nanostructured lipid carrier (NLC) dispersions. Pharmaceutics 2019, 11, 397. [Google Scholar] [CrossRef]
- Khater, D.; Nsairat, H.; Odeh, F.; Saleh, M.; Jaber, A.; Alshaer, W.; Bawab, A.A.; Mubarak, M.S. Design, preparation, and characterization of effective dermal and transdermal lipid nanoparticles: A review. Cosmetics 2021, 8, 39. [Google Scholar] [CrossRef]
- Understanding Skin—Skin pH. Available online: https://int.eucerin.com/about-skin/basic-skin-knowledge/skins-ph (accessed on 1 March 2021).
- Sundram, K.; Sambanthamurthi, R.; Tan, Y. Palm fruit chemistry and nutrition. Asia Pac. J. Clin. Nutr. 2003, 12, 355–362. [Google Scholar]
- Iwata, H.; Shimada, K. Formulas, Ingredients and Production of Cosmetics: Technology of Skin- and Hair-Care Products in Japan; Springer: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Constantin, M.; Poenaru, E.; Poenaru, C.; Constantin, T. Skin hydration assessment through modern non-invasive bioengineering technologies. Maedica J. Clin. Med. 2014, 9, 33–38. [Google Scholar]
- Akdeniz, M.; Gabriel, S.; Lichterfeld-Kottner, A.; Blume-Peytavi, U.; Kottner, J. Transepidermal water loss in healthy adults: A systematic review and meta-analysis update. Br. J. Dermatol. 2018, 179, 1049–1055. [Google Scholar] [CrossRef]
- Ganceviciene, R.; Liakou, A.I.; Theodoridis, A.; Makrantonaki, E.; Zouboulis, C.C. Skin anti-aging strategies. Derm.-Endocrinol. 2012, 4, 308–319. [Google Scholar] [CrossRef] [PubMed]
- Dumoulin, M.; Gaudout, D.; Lemaire, B. Clinical effects of an oral supplement rich in antioxidants on skin radiance in women. Clin. Cosmet. Investig. Dermatol. 2016, 9, 315–324. [Google Scholar] [CrossRef]
- Bino, S.D.; Duval, C.; Bernerd, F. Clinical and biological characterization of skin pigmentation diversity and its consequences on UV impact. Int. J. Mol. Sci. 2018, 19, 2668. [Google Scholar] [CrossRef] [PubMed]
- Kao, Y.; Chuang, T.; Chao, S.; Yang, J.; Lin, Y.; Huang, H. Evaluation of the Antioxidant and Melanogenesis Inhibitory Properties of Pracparatum Mungo (Lu-Do Huang). J. Tradit. Complement. Med. 2013, 3, 163–170. [Google Scholar] [CrossRef] [PubMed][Green Version]


| Ingredients | Amount (%) |
|---|---|
| Elaeis guineensis fruit extract | 6.00 |
| Sorbitan monooleate (Span 80) | 1.12 |
| Polysorbate 80 (Tween 80) | 0.83 |
| Glyceryl monostearate | 0.30 |
| Purified water | 91.75 |
| Total weight | 100.00 |
| Ingredients | Amount (% w/w) | Function | |
|---|---|---|---|
| Day Cream | Night Cream | ||
| E. guineensis fruit extract loaded with solid lipid nanoparticles | 50.00 | 50.00 | Active ingredient |
| Octyl methoxy cinnamate | 7.00 | - | Sunscreening agent |
| Liquid paraffin | 5.00 | 10.00 | Skin conditioning agent, Emollient |
| Propylene glycol | 5.00 | 5.00 | Humectant |
| Glycerin | 5.00 | 5.00 | Humectant |
| Glyceryl monostearate-SE | 4.40 | - | Thickening agent, Emulsifier |
| White soft paraffin | 4.00 | 5.00 | Skin conditioning agent, Emollient |
| Polysorbate 80 (Tween® 80) | 3.60 | - | Emulsifier |
| Isopropyl myristate | 3.00 | 3.00 | Skin conditioning agent, Emollient |
| Cetyl alcohol | 3.00 | 5.00 | Thickening agent, Emollient |
| Titanium dioxide | 3.00 | - | Sunscreening agent |
| Stearic acid | 2.00 | 2.00 | Thickening agent, Emollient |
| Grape seed extract | 1.00 | 1.00 | Antioxidant |
| Phenoxyethanol | 0.50 | 0.50 | Preservative |
| Tocopherol acetate | 0.50 | 0.50 | Antioxidant |
| Carbomer 940 | 0.20 | 0.20 | Viscosity increasing agent |
| Triethanolamine | 0.10 | 0.10 | Neutralizing carbomer |
| Citric acid | 0.05 | - | pH adjustment |
| Disodium EDTA | 0.005 | 0.005 | Chelating agent |
| Purified water | 2.645 | 12.695 | Vehicle |
| Total weight | 100.00 | 100.00 | |
| Inclusion Criteria | Exclusion Criteria |
|---|---|
|
|
| Score | Assessment of Reaction | Parameter Evaluated | |
|---|---|---|---|
| Erythema (E) | Oedema (O) | ||
| 0 | Absent | No erythema | No oedema |
| 0.5 | Doubtful | Barely perceptible: like pink color of one part of the tested area) | Palpable, barely visible |
| 1 | Slight | Quiet pink color of the complete tested area or rather visible on one part of the tested area | Palpable, visible |
| 2 | Obvious | Obvious erythema covering the whole tested area | Obvious oedema (thickness < 1 mm) with or without papule(s) or vesicle(s) |
| 3 | Important | Obvious erythema diffusing outside the tested area | Severe oedema (thickness ≥ 1 mm or diffusing outside the tested area) with or without papule(s) or vesicle(s) |
| Mean Irritation Index (M.I.I.) | Product Classification |
|---|---|
| M.I.I. = 0.0 | Non-Irritating (NI)/Very Good Cutaneous Compatibility |
| M.I.I. < 0.20 | Non-Irritating (NI)/Good Cutaneous Compatibility |
| 0.20 ≤ M.I.I. < 0.50 | Slightly Irritating (SI)/Intermediate Cutaneous Compatibility |
| 0.50 ≤ M.I.I. < 1 | Moderately Irritating (NI)/Bad Cutaneous Compatibility |
| M.I.I. > 1 | Irritating (I)/Very Bad Cutaneous Compatibility |
| Test | Results |
|---|---|
| Appearance | Yellow to orange semi-solid or paste |
| * IC50 (μg/mL) | 49.96 ± 12 |
| * IC50 (μg/mL) (ascorbic acid as control) | 1.57 ± 0.04 |
| Tocopherol (mg/100 g) | 5.34 ± 3.22 |
| Tocotrienol (mg/100 g) | 4.23 ± 0.15 |
| β-Carotene (mg/100 g) | 21.89 ± 0.23 |
| Palmitic acid (g/100 g) | 74.53 ± 0.68 |
| Lauric acid (g/100 g) | 8.65 ± 0.14 |
| Stearic acid (g/100 g) | 6.48 ± 0.76 |
| Myristic acid (g/100 g) | 3.33 ± 0.21 |
| Caprylic acid (g/100 g) | 2.18 ± 0.12 |
| Capric acid (g/100 g) | 0.45 ± 0.01 |
| Attribute | Acceptance Criteria |
|---|---|
| 1. Appearance | Yellow to orange semi-solid or paste |
| 2. Vitamin A content (β-carotene) | Not less than 3 mg/100 g |
| 3. Total Vitamin E content | Not less than 1.5 mg/100 g |
| 4. Palmitic acid (C16:0) | 30–85 g/100 g |
| Products | Test | Storage Conditions | ||
|---|---|---|---|---|
| Initial | 4 °C (1 Month) | Ambient (1 Month) | ||
| Blank solid lipid nanoparticles | Appearance |  |  |  |
| pH | 4.75 ± 0.02 | 4.76 ± 0.02 | 4.73 ± 0.01 | |
| Size (nm) | 507.53 ± 69.99 | 537.67 ± 36.01 | 561.47 ± 36.66 | |
| Polydispersity index | 0.18 | 0.24 | 0.32 | |
| Zeta potential (mV) | −32.4 ± 2.1 | −30.6 ± 2.8 | −31.9 ± 1.9 | |
| E. guineensis fruit extract loaded solid lipid nanoparticles | Appearance |  |  |  |
| pH | 4.77 ± 0.01 | 4.82 ± 0.01 | 4.90 ± 0.01 | |
| Size (nm) | 609.70 ± 54.48 | 619.53 ± 22.42 | 640.11 ± 26.12 | |
| Polydispersity index | 0.22 | 0.32 | 0.40 | |
| Zeta potential (mV) | −28.3 ± 1.0 | −27.1 ± 1.4 | −28.1 ± 1.1 | |
| Products | Test | Storage Conditions | ||
|---|---|---|---|---|
| Initial | Freeze-Thaw (6 Cycles) | 30 °C/75% RH (6 Months) | ||
| Day cream | Appearance | Light yellow smooth cream with characteristic odor. | Conform | Conform |
| pH | 5.72 ± 0.02 | 5.82 ± 0.01 | 5.71 ± 0.03 | |
| Viscosity (mPaS) | 55,406.67 ± 480.87 | 80,663.33 ± 461.99 | 63,549.18 ± 412.55 | |
| SPF value | 15.32 ± 0.77 | 14.57 ± 0.33 | 15.02 ± 0.10 | |
| Tocopherol (mg/100 g) | 0.18 ± 0.01 | 0.21 ± 0.02 | 0.17 ± 0.01 | |
| Tocotrienol (mg/100 g) | 0.14 ± 0.04 | 0.12 ± 0.02 | 0.15 ± 0.01 | |
| β-Carotene (mg/100 g) | 0.60 ± 0.05 | 0.71 ± 0.01 | 0.63 ± 0.03 | |
| Night cream | Appearance | Light yellow to yellow smooth cream with characteristic odor. | Conform | Conform |
| pH | 5.06 ± 0.01 | 4.60 ± 0.02 | 5.12 ± 0.02 | |
| Viscosity (mPaS) | 843,600.00 ± 23,618.43 | 366,933.33 ± 17,778.73 | 674,129.02 ± 16,220.12 | |
| Tocopherol (mg/100 g) | 0.22 ± 0.01 | 0.22 ± 0.02 | 0.19 ± 0.01 | |
| Tocotrienol (mg/100 g) | 0.15 ± 0.02 | 0.16 ± 0.01 | 0.12 ± 0.02 | |
| β-Carotene (mg/100 g) | 0.68 ± 0.02 | 0.66 ± 0.03 | 0.60 ± 0.01 | |
| Characteristics | Day Cream | Night Cream |
|---|---|---|
| Total participants (n) | 34 | 34 |
| Gender | Female | Female |
| Nationality | Thai | Thai |
| Age range (years) | 25–50 | 25–50 |
| History of allergy to chemicals or substances from nature (n) | None | None |
| History of skin disease | None | None |
| The appearance of open wounds, blisters and skin lesions at the test site was found (n) | None | None |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Plyduang, T.; Atipairin, A.; Sae Yoon, A.; Sermkaew, N.; Sakdiset, P.; Sawatdee, S. Formula Development of Red Palm (Elaeis guineensis) Fruit Extract Loaded with Solid Lipid Nanoparticles Containing Creams and Its Anti-Aging Efficacy in Healthy Volunteers. Cosmetics 2022, 9, 3. https://doi.org/10.3390/cosmetics9010003
Plyduang T, Atipairin A, Sae Yoon A, Sermkaew N, Sakdiset P, Sawatdee S. Formula Development of Red Palm (Elaeis guineensis) Fruit Extract Loaded with Solid Lipid Nanoparticles Containing Creams and Its Anti-Aging Efficacy in Healthy Volunteers. Cosmetics. 2022; 9(1):3. https://doi.org/10.3390/cosmetics9010003
Chicago/Turabian StylePlyduang, Thipapun, Apichart Atipairin, Attawadee Sae Yoon, Namfa Sermkaew, Pajaree Sakdiset, and Somchai Sawatdee. 2022. "Formula Development of Red Palm (Elaeis guineensis) Fruit Extract Loaded with Solid Lipid Nanoparticles Containing Creams and Its Anti-Aging Efficacy in Healthy Volunteers" Cosmetics 9, no. 1: 3. https://doi.org/10.3390/cosmetics9010003
APA StylePlyduang, T., Atipairin, A., Sae Yoon, A., Sermkaew, N., Sakdiset, P., & Sawatdee, S. (2022). Formula Development of Red Palm (Elaeis guineensis) Fruit Extract Loaded with Solid Lipid Nanoparticles Containing Creams and Its Anti-Aging Efficacy in Healthy Volunteers. Cosmetics, 9(1), 3. https://doi.org/10.3390/cosmetics9010003






