Effect of Sodium Lauryl Sulfate (SLS) Applied as a Patch on Human Skin Physiology and Its Microbiota
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Skin Clinical Evaluation, Sample Collection and Analysis
2.3. Clinical Evaluation
2.4. Skin Bacterial 16S rRNA Gene Metabarcoding
2.5. Statistical Analysis
3. Results
3.1. Clinical Evaluation
Transepidermal Water Loss
3.2. Skin Color, Luminosity and Moisturization
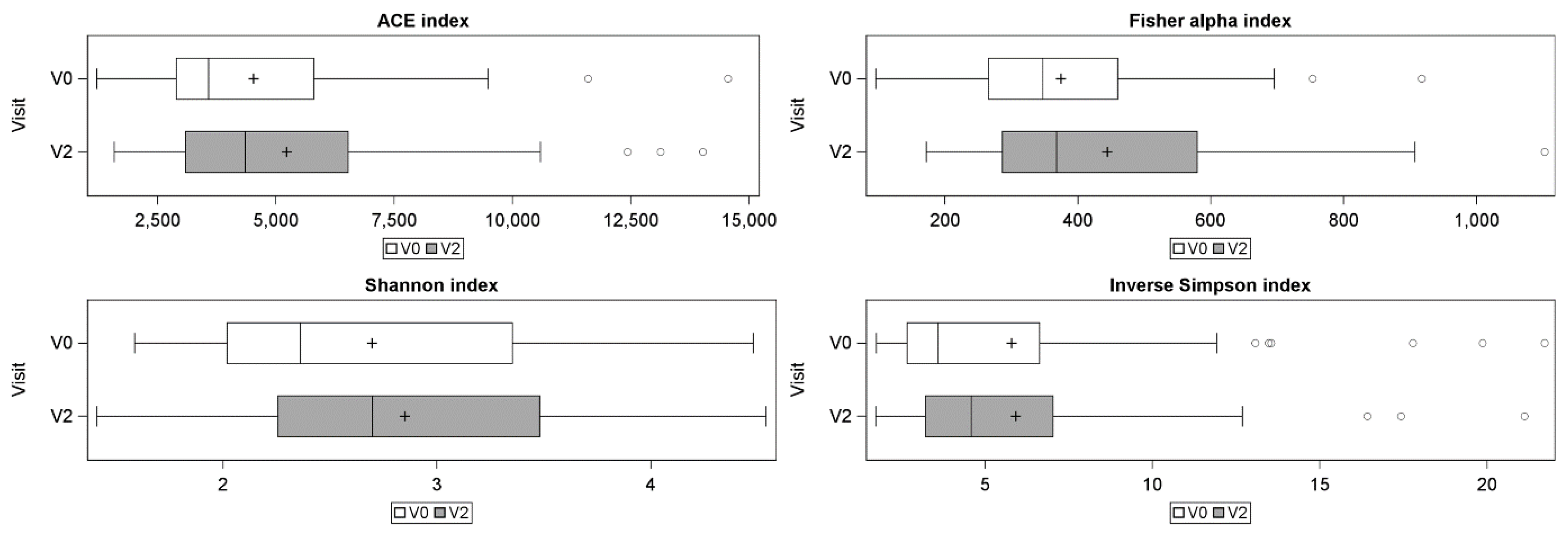
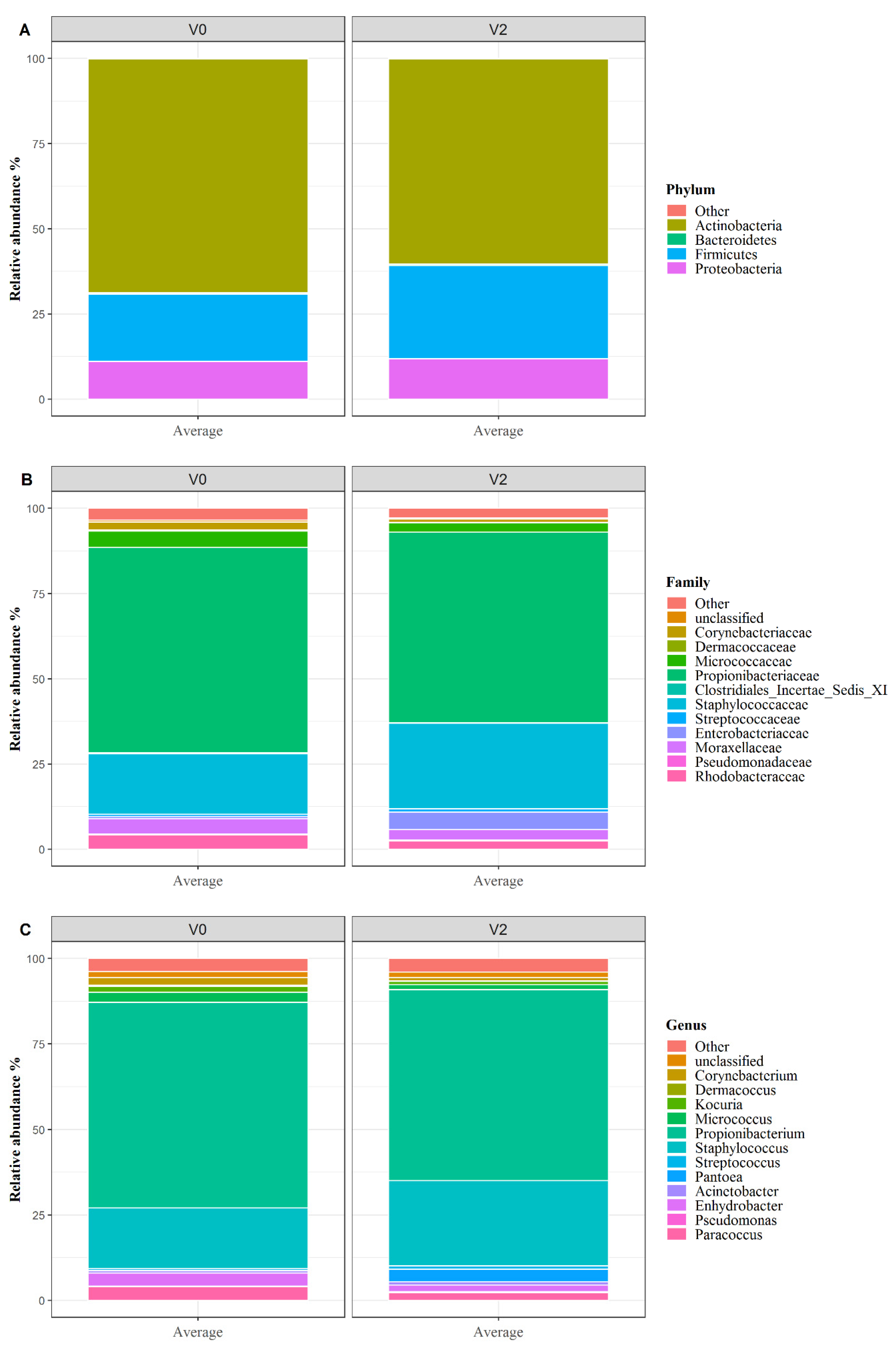
3.3. Skin Microbiota Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schommer, N.N.; Gallo, R.L. Structure and function of the human skin microbiome. Trends Microbiol. 2013, 21, 660–668. [Google Scholar] [CrossRef] [PubMed]
- Grice, E.A.; Segre, J.A. The skin microbiome. Nat. Rev. Microbiol. 2011, 9, 244–253. [Google Scholar] [CrossRef] [PubMed]
- Perez Perez, G.I.; Gao, Z.; Jourdain, R.; Ramirez, J.; Gany, F.; Clavaud, C.; Demaude, J.; Breton, L.; Blaser, M.J. Body Site Is a More Determinant Factor than Human Population Diversity in the Healthy Skin Microbiome. PLoS ONE 2016, 11, e0151990. [Google Scholar] [CrossRef] [PubMed]
- Cundell, A.M. Microbial Ecology of the Human Skin. Microb. Ecol. 2018, 76, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Grice, E.A.; Kong, H.H.; Conlan, S.; Deming, C.B.; Davis, J.; Young, A.C.; NISC Comparative Sequencing Program; Bouffard, G.G.; Blakesley, R.W.; Murray, P.R.; et al. Topographical and temporal diversity of the human skin microbiome. Science 2009, 324, 1190–1192. [Google Scholar] [CrossRef]
- Vandegrift, R.; Bateman, A.C.; Siemens, K.N.; Nguyen, M.; Wilson, H.E.; Green, J.L.; Van Den Wymelenberg, K.G.; Hickey, R.J. Cleanliness in context: Reconciling hygiene with a modern microbial perspective. Microbiome 2017, 5, 76. [Google Scholar] [CrossRef]
- Branco, C.T.; Guimaraes, J.P. Modulation of skin microbiota by topical prebiotics. TKS Publ. 2015, 10, 21–27. [Google Scholar]
- Sanford, J.A.; Gallo, R.L. Functions of the skin microbiota in health and disease. Semin. Immunol. 2013, 25, 370–377. [Google Scholar] [CrossRef]
- Draelos, Z.D. The science behind skin care: Cleansers. J. Cosmet. Dermatol. 2018, 17, 8–14. [Google Scholar] [CrossRef]
- Bondi, C.A.; Marks, J.L.; Wroblewski, L.B.; Raatikainen, H.S.; Lenox, S.R.; Gebhardt, K.E. Human and Environmental Toxicity of Sodium Lauryl Sulfate (SLS): Evidence for Safe Use in Household Cleaning Products. Environ. Health Insights 2015, 9, 27–32. [Google Scholar] [CrossRef]
- Fiume, M.; Bergfeld, W.F.; Belsito, D.V.; Klaassen, C.D.; Marks, J.G.; Shank, R.C.; Slaga, T.J.; Snyder, P.W.; Alan Andersen, F. Final report on the safety assessment of sodium cetearyl sulfate and related alkyl sulfates as used in cosmetics. Int. J. Toxicol. 2010, 29, 115S–132S. [Google Scholar] [CrossRef]
- Larrouy, M. Le Sodium Laury Sulfate. 2015. Available online: https://www.scc-quebec.org/wp-content/uploads/2017/08/Monographie-Sodium-Lauryl-Sulfate-Malaury-Larrouy-2015.pdf (accessed on 1 December 2015).
- Piret, J.; Lamontagne, J.; Bestman-Smith, J.; Roy, S.; Gourde, P.; Désormeaux, A.; Omar, R.F.; Juhász, J.; Bergeron, M.G. In vitro and in vivo evaluations of sodium lauryl sulfate and dextran sulfate as microbicides against herpes simplex and human immunodeficiency viruses. J. Clin. Microbiol. 2000, 38, 110–119. [Google Scholar] [PubMed]
- Gabard, B.; Chatelain, E.; Bieli, E.; Haas, S. Surfactant irritation: In vitro corneosurfametry and in vivo bioengineering. Skin Res. Technol. 2001, 7, 49–55. [Google Scholar] [CrossRef]
- Lee, E.; Kim, S.; Lee, J.; Cho, S.-A.; Shin, K. Ethnic differences in objective and subjective skin irritation response: An international study. Skin Res. Technol. 2014, 20, 265–269. [Google Scholar] [CrossRef] [PubMed]
- Antonov, D.; Schliemann, S.; Elsner, P. Methods for the Assessment of Barrier Function. Curr. Probl. Dermatol. 2016, 49, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Pinnagoda, J.; Tupker, R.A.; Agner, T.; Serup, J. Guidelines for transepidermal water loss (TEWL) measurement. A report from the Standardization Group of the European Society of Contact Dermatitis. Contact Dermat. 1990, 22, 164–178. [Google Scholar] [CrossRef] [PubMed]
- Clarys, P.; Clijsen, R.; Taeymans, J.; Barel, A.O. Hydration measurements of the stratum corneum: Comparison between the capacitance method (digital version of the Corneometer CM 825®) and the impedance method (Skicon-200EX®). Skin Res. Technol. 2012, 18, 316–323. [Google Scholar] [CrossRef]
- Klindworth, A.; Pruesse, E.; Schweer, T.; Peplies, J.; Quast, C.; Horn, M.; Glöckner, F.O. Evaluation of general 16S ribosomal RNA gene PCR primers for classical and next-generation sequencing-based diversity studies. Nucleic Acids Res. 2013, 41, e1. [Google Scholar] [CrossRef]
- Schloss, P.D.; Westcott, S.L.; Ryabin, T.; Hall, J.R.; Hartmann, M.; Hollister, E.B.; Lesniewski, R.A.; Oakley, B.B.; Parks, D.H.; Robinson, C.J.; et al. Introducing mothur: Open-Source, Platform-Independent, Community-Supported Software for Describing and Comparing Microbial Communities. Appl. Environ. Microbiol. 2009, 75, 7537–7541. [Google Scholar] [CrossRef]
- Edgar, R.C.; Haas, B.J.; Clemente, J.C.; Quince, C.; Knight, R. UCHIME improves sensitivity and speed of chimera detection. Bioinform. Oxf. Engl. 2011, 27, 2194–2200. [Google Scholar] [CrossRef]
- Wang, Q.; Garrity, G.M.; Tiedje, J.M.; Cole, J.R. Naïve Bayesian Classifier for Rapid Assignment of rRNA Sequences into the New Bacterial Taxonomy. Appl. Environ. Microbiol. 2007, 73, 5261–5267. [Google Scholar] [CrossRef] [PubMed]
- Angelova-Fischer, I.; Dapic, I.; Hoek, A.-K.; Jakasa, I.; Fischer, T.W.; Zillikens, D.; Kezic, S. Skin barrier integrity and natural moisturising factor levels after cumulative dermal exposure to alkaline agents in atopic dermatitis. Acta. Derm. Venereol. 2014, 94, 640–644. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-C.; Wang, P.-R.; Lai, T.-J.; Lu, L.-H.; Dai, L.-W.; Wang, C.-H. Using therapeutic ultrasound to promote irritated skin recovery after surfactant-induced barrier disruption. Ultrasonics 2019, 91, 206–212. [Google Scholar] [CrossRef]
- Törmä, H.; Lindberg, M.; Berne, B. Skin barrier disruption by sodium lauryl sulfate-exposure alters the expressions of involucrin, transglutaminase 1, profilaggrin, and kallikreins during the repair phase in human skin in vivo. J. Investig. Dermatol. 2008, 128, 1212–1219. [Google Scholar] [CrossRef]
- Stettler, H.; Kurka, P.; Lunau, N.; Manger, C.; Böhling, A.; Bielfeldt, S.; Wilhelm, K.-P.; Dähnhardt-Pfeiffer, S.; Dähnhardt, D.; Brill, F.H.H.; et al. A new topical panthenol-containing emollient: Results from two randomized controlled studies assessing its skin moisturization and barrier restoration potential, and the effect on skin microflora. J. Dermatol. Treat. 2017, 28, 173–180. [Google Scholar] [CrossRef]
- Wilhelm, K.P.; Freitag, G.; Wolff, H.H. Surfactant-induced skin irritation and skin repair: Evaluation of a cumulative human irritation model by noninvasive techniques. J. Am. Acad. Dermatol. 1994, 31, 981–987. [Google Scholar] [CrossRef]
- Two, A.M.; Nakatsuji, T.; Kotol, P.F.; Arvanitidou, E.; Du-Thumm, L.; Hata, T.R.; Gallo, R.L. The Cutaneous Microbiome and Aspects of Skin Antimicrobial Defense System Resist Acute Treatment with Topical Skin Cleansers. J. Investig. Dermatol. 2016, 136, 1950–1954. [Google Scholar] [CrossRef] [PubMed]
- Larson, E.L.; Hughes, C.A.; Pyrek, J.D.; Sparks, S.M.; Cagatay, E.U.; Bartkus, J.M. Changes in bacterial flora associated with skin damage on hands of health care personnel. Am. J. Infect. Control 1998, 26, 513–521. [Google Scholar] [CrossRef]
- Rocha, L.A.; de Borges, L.F.D.A.; Gontijo Filho, P.P. Changes in hands microbiota associated with skin damage because of hand hygiene procedures on the health care workers. Am. J. Infect. Control 2009, 37, 155–159. [Google Scholar] [CrossRef]
- Staudinger, T.; Pipal, A.; Redl, B. Molecular analysis of the prevalent microbiota of human male and female forehead skin compared to forearm skin and the influence of make-up: Skin microbiota of human forehead compared to forearm. J. Appl. Microbiol. 2011, 110, 1381–1389. [Google Scholar] [CrossRef]
- Li, X.; Yuan, C.; Xing, L.; Humbert, P. Topographical diversity of common skin microflora and its association with skin environment type: An observational study in Chinese women. Sci. Rep. 2017, 7, 18046. [Google Scholar] [CrossRef]
- Shibagaki, N.; Suda, W.; Clavaud, C.; Bastien, P.; Takayasu, L.; Iioka, E.; Kurokawa, R.; Yamashita, N.; Hattori, Y.; Shindo, C.; et al. Aging-related changes in the diversity of women’s skin microbiomes associated with oral bacteria. Sci. Rep. 2017, 7, 10567. [Google Scholar] [CrossRef]
- Mukherjee, S.; Mitra, R.; Maitra, A.; Gupta, S.; Kumaran, S.; Chakrabortty, A.; Majumder, P.P. Sebum and Hydration Levels in Specific Regions of Human Face Significantly Predict the Nature and Diversity of Facial Skin Microbiome. Sci. Rep. 2016, 6, 36062. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.E.; Fischbach, M.A.; Belkaid, Y. Skin microbiota-host interactions. Nature 2018, 553, 427–436. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, H.E.; Bhatia, N.D.; Friedman, A.; Eng, R.M.; Seite, S. The Role of Cutaneous Microbiota Harmony in Maintaining a Functional Skin Barrier. J. Drugs Dermatol. JDD 2017, 16, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Dutkiewicz, J.; Mackiewicz, B.; Kinga Lemieszek, M.; Golec, M.; Milanowski, J. Pantoea agglomerans: A mysterious bacterium of evil and good. Part III. Deleterious effects: Infections of humans, animals and plants. Ann. Agric. Environ. Med. AAEM 2016, 23, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Bilal, J.A.; Ahmad, M.I.; Robaee, A.A.A.; Alzolibani, A.A.; Shobaili, H.A.A.; Al-Khowailed, M.S. Pattern of bacterial colonization of atopic dermatitis in saudi children. J. Clin. Diagn. Res. JCDR 2013, 7, 1968–1970. [Google Scholar] [CrossRef]
- Christensen, G.J.M.; Brüggemann, H. Bacterial skin commensals and their role as host guardians. Benef. Microbes 2014, 5, 201–215. [Google Scholar] [CrossRef]
- Leung, M.H.Y.; Tong, X.; Bastien, P.; Guinot, F.; Tenenhaus, A.; Appenzeller, B.M.R.; Betts, R.J.; Mezzache, S.; Li, J.; Bourokba, N.; et al. Changes of the human skin microbiota upon chronic exposure to polycyclic aromatic hydrocarbon pollutants. Microbiome 2020, 8, 100. [Google Scholar] [CrossRef]
- Basílico, G.; Roger, C.A.; Seigelchifer, M.; Kerner, N. UV-specific DNA repair recombinant fusion enzyme: A new stable pharmacologically active principle suitable for photoprotection. J. Dermatol. Sci. 2005, 39, 81–88. [Google Scholar] [CrossRef]
- Shukla, M.R.; Yadav, R.; Desai, A. Catalase and superoxide dismutase double staining zymogram technique for Deinococcus and Kocuria species exposed to multiple stresses: Catalase and superoxide dismutase double staining zymogram technique for Deinococcus and Kocuria species exposed to multiple stresses. J. Basic Microbiol. 2009, 49, 593–597. [Google Scholar] [CrossRef] [PubMed]
- Ramsey, M.M.; Freire, M.O.; Gabrilska, R.A.; Rumbaugh, K.P.; Lemon, K.P. Staphylococcus aureus Shifts toward Commensalism in Response to Corynebacterium Species. Front. Microbiol. 2016, 7, 1230. [Google Scholar] [CrossRef] [PubMed]
- Jurek, I.; Góral, I.; Mierzyńska, Z.; Moniuszko-Szajwaj, B.; Wojciechowski, K. Effect of synthetic surfactants and soapwort (Saponaria officinalis L.) extract on skin-mimetic model lipid monolayers. Biochim. Biophys. Acta Biomembr. 2019, 1861, 556–564. [Google Scholar] [CrossRef] [PubMed]
- Teng, Y.; Luo, Y.; Sun, M.; Liu, Z.; Li, Z.; Christie, P. Effect of bioaugmentation by Paracoccus sp. strain HPD-2 on the soil microbial community and removal of polycyclic aromatic hydrocarbons from an aged contaminated soil. Bioresour. Technol. 2010, 101, 3437–3443. [Google Scholar] [CrossRef] [PubMed]
- Leow, Y.-H.; Maibach, H. Effect of occlusion on skin. J. Dermatol. Treat. 1997, 8, 139–142. [Google Scholar] [CrossRef]
- GBD 2015 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990-2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet Lond. Engl. 2016, 388, 1545–1602. [Google Scholar] [CrossRef]
- Bryld, L.E.; Agner, T.; Kyvik, K.O.; Brøndsted, L.; Hindsberger, C.; Menné, T. Hand eczema in twins: A questionnaire investigation. Br. J. Dermatol. 2000, 142, 298–305. [Google Scholar] [CrossRef]
- Kampf, G.; Löffler, H. Prevention of irritant contact dermatitis among health care workers by using evidence-based hand hygiene practices: A review. Ind. Health 2007, 45, 645–652. [Google Scholar] [CrossRef][Green Version]
- Platts-Mills, T.A.E.; Erwin, E.; Heymann, P.; Woodfolk, J. Is the hygiene hypothesis still a viable explanation for the increased prevalence of asthma? Allergy 2005, 60, 25–31. [Google Scholar] [CrossRef]
| Baseline(V0) Mean (SD) | 24 h after SLS Patch Removal (V2) Mean (SD) | Estimated Mean Change (V2-V0) [95% CI] | p-Value | |
|---|---|---|---|---|
| TEWL 1 score (g m−2 h−1) | 5.1 (2.28) | 42.6 (6.79) | 37.5 [36.1 to 39.0] | <0.0001 |
| Colorimetry score L* (a.u. 2) | 65.3 (2.98) | 60.7 (2.92) | −4.7 [5.2 to 4.1] | <0.0001 |
| Colorimetry score a* (a.u.) | 10.7 (1.61) | 17.5 (1.86) | 6.8 [6.4 to 7.2] | <0.0001 |
| Corneometry score (a.u.) | 45.1 (8.19) | 39.7 (10.70) | −5.4 [−8.0 to −2.8] | <0.0001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leoty-Okombi, S.; Gillaizeau, F.; Leuillet, S.; Douillard, B.; Le Fresne-Languille, S.; Carton, T.; De Martino, A.; Moussou, P.; Bonnaud-Rosaye, C.; André, V. Effect of Sodium Lauryl Sulfate (SLS) Applied as a Patch on Human Skin Physiology and Its Microbiota. Cosmetics 2021, 8, 6. https://doi.org/10.3390/cosmetics8010006
Leoty-Okombi S, Gillaizeau F, Leuillet S, Douillard B, Le Fresne-Languille S, Carton T, De Martino A, Moussou P, Bonnaud-Rosaye C, André V. Effect of Sodium Lauryl Sulfate (SLS) Applied as a Patch on Human Skin Physiology and Its Microbiota. Cosmetics. 2021; 8(1):6. https://doi.org/10.3390/cosmetics8010006
Chicago/Turabian StyleLeoty-Okombi, Sabrina, Florence Gillaizeau, Sébastien Leuillet, Benoit Douillard, Sophie Le Fresne-Languille, Thomas Carton, Alessandra De Martino, Philippe Moussou, Catherine Bonnaud-Rosaye, and Valérie André. 2021. "Effect of Sodium Lauryl Sulfate (SLS) Applied as a Patch on Human Skin Physiology and Its Microbiota" Cosmetics 8, no. 1: 6. https://doi.org/10.3390/cosmetics8010006
APA StyleLeoty-Okombi, S., Gillaizeau, F., Leuillet, S., Douillard, B., Le Fresne-Languille, S., Carton, T., De Martino, A., Moussou, P., Bonnaud-Rosaye, C., & André, V. (2021). Effect of Sodium Lauryl Sulfate (SLS) Applied as a Patch on Human Skin Physiology and Its Microbiota. Cosmetics, 8(1), 6. https://doi.org/10.3390/cosmetics8010006





