Melanogenesis Effect of 7-acetoxy-4-methylcoumarin in B16F10 Melanoma Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Acetylation of 4MUMB
2.2. Methylation of 4MUMB
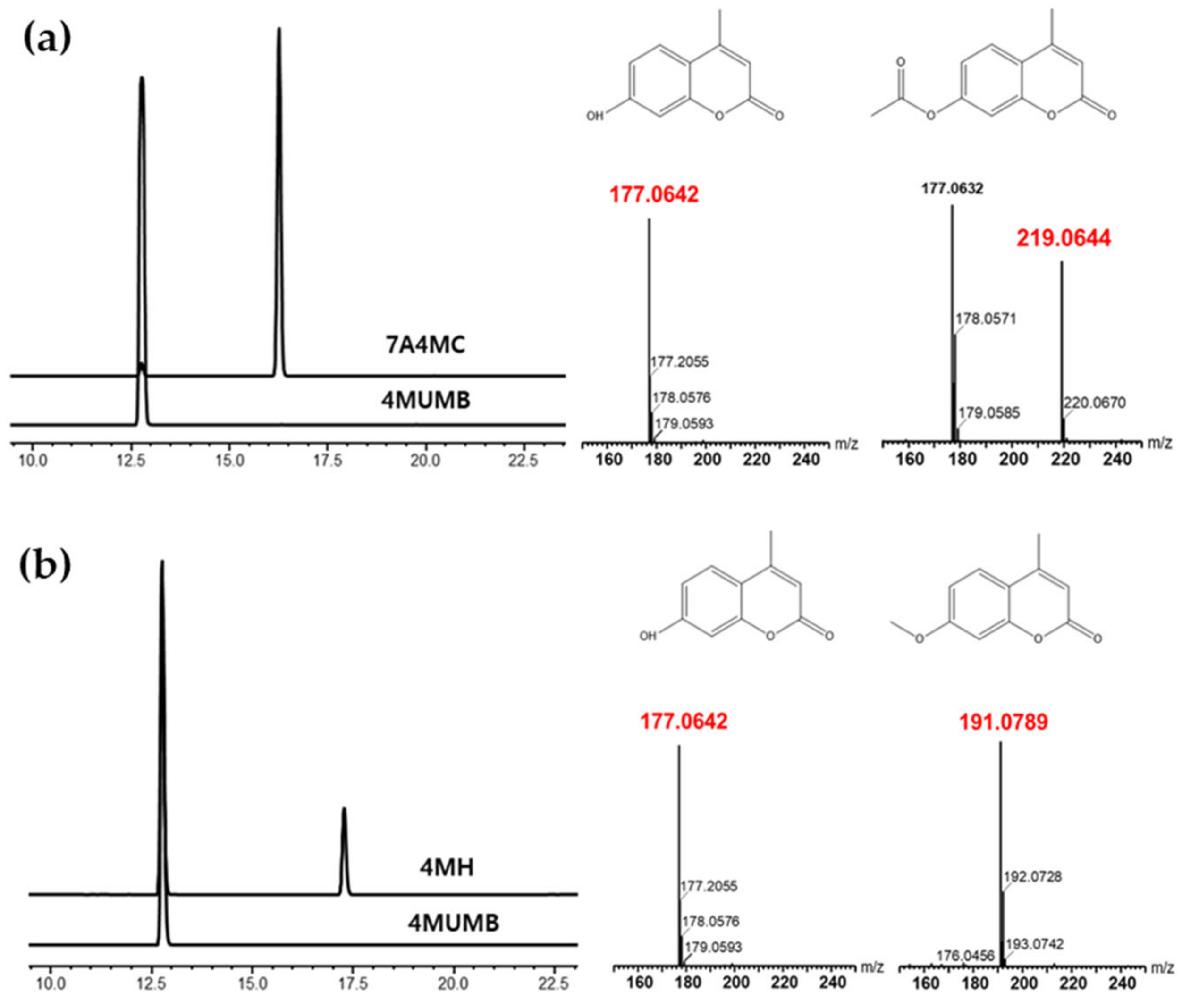
2.3. High-Pressure Liquid Chromatography (HPLC) Analysis and Purification of 4MUMB, 7A4MC, and 4MH
2.4. Liquid Chromatography-Mass Spectrometry (LC-MS) Analysis 7A4MC
2.5. Cell Culture and Viability Assay
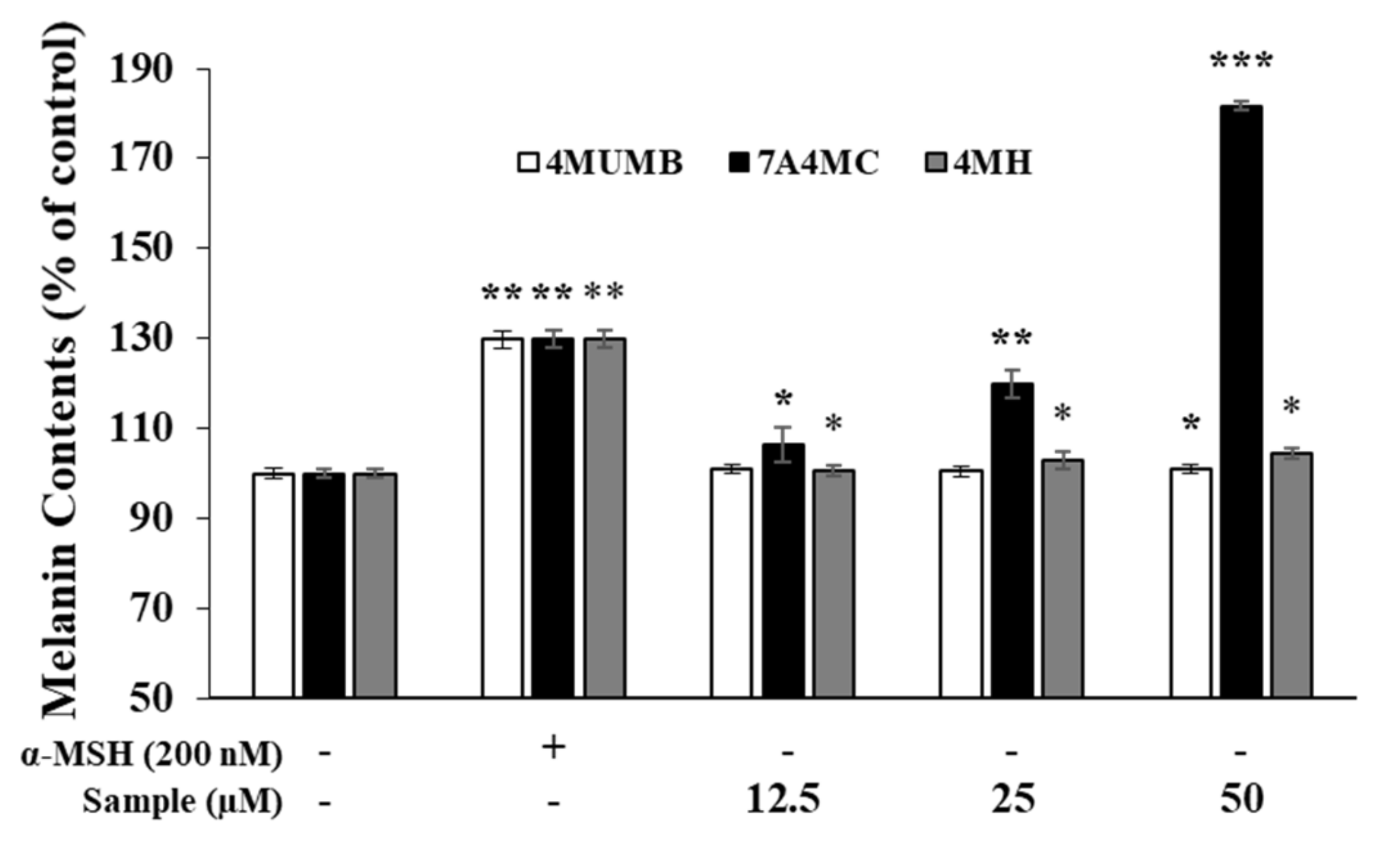
2.6. Measurement of Melanin Content
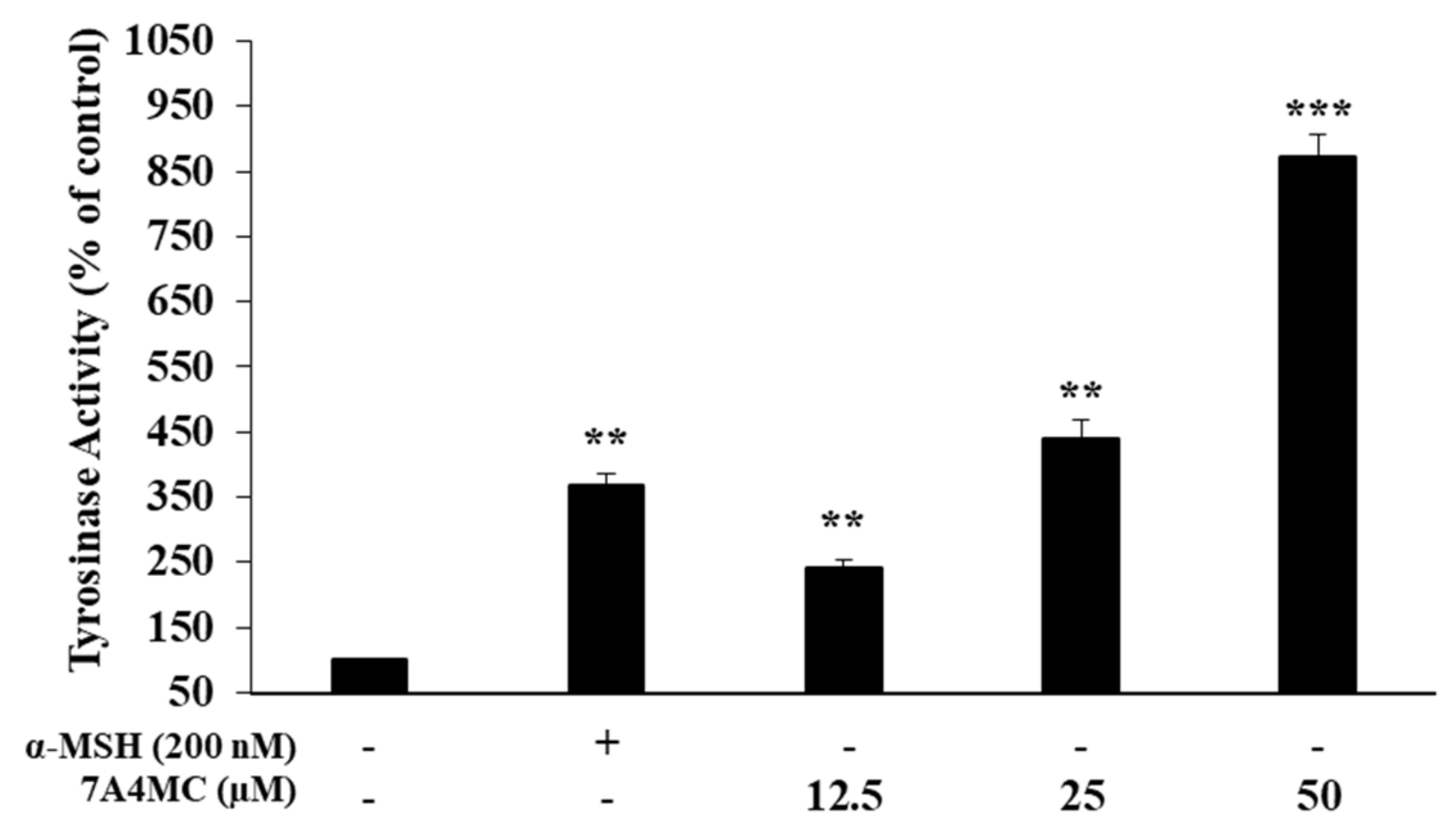
2.7. Assessment of Intracellular Tyrosinase (TYR) Activity
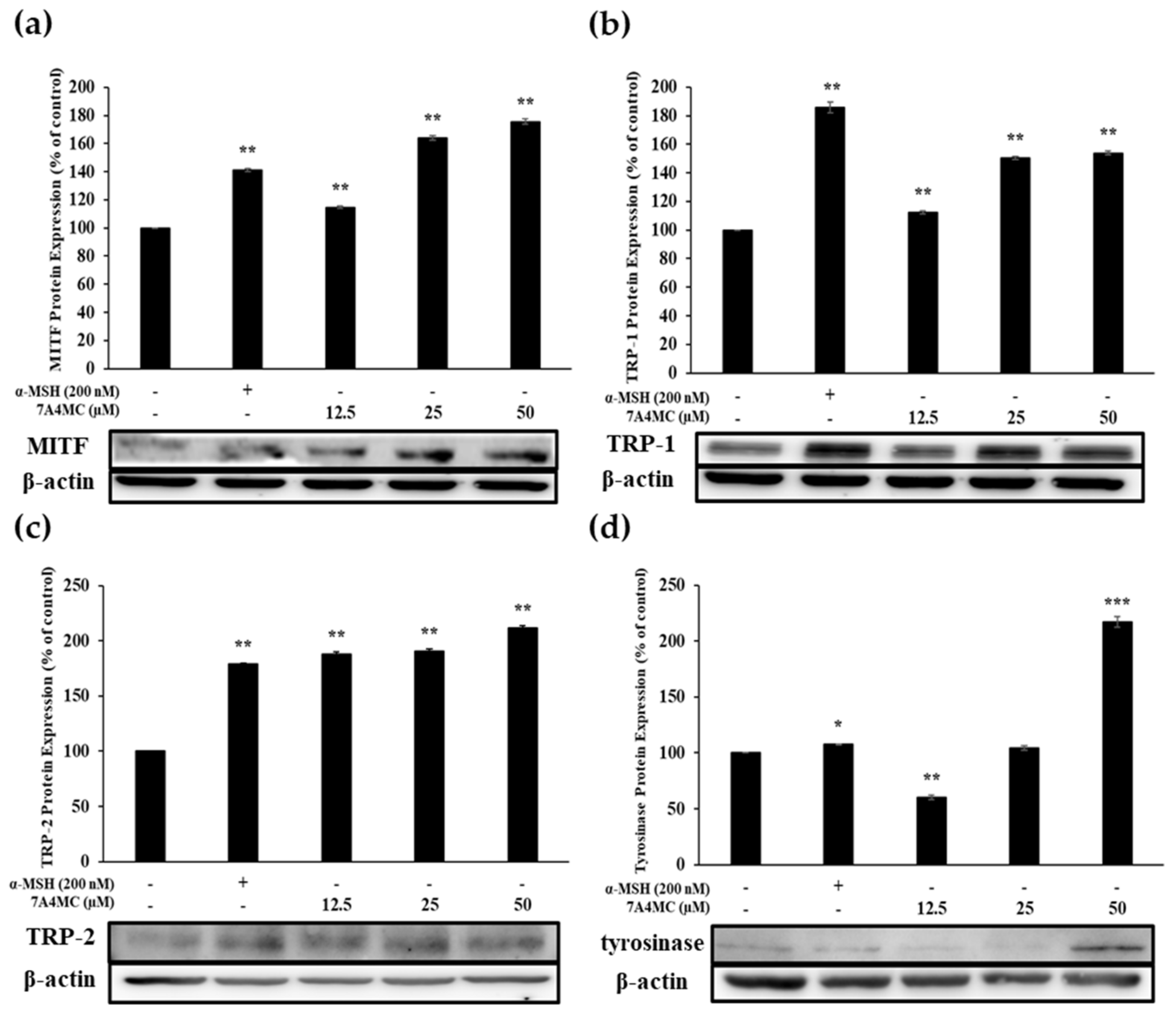
2.8. Western Blotting
2.9. Statistical Analysis
3. Results
3.1. Acetylation of 4MUMB and Methylation of 4MUMB
3.2. Effects of 4MUMB, 7A4MC, and 4MH on the Viability of B16F10 Cells
3.3. Effects of 4MUMB, 7A4MC, and 4MH on Melanin Production
3.4. Effect of 7A4MC on TYR Activity in B16F10 Melanoma Cells
3.5. Effect of 7A4MC on the Protein Expression of MITF and Melanogenic Enzymes
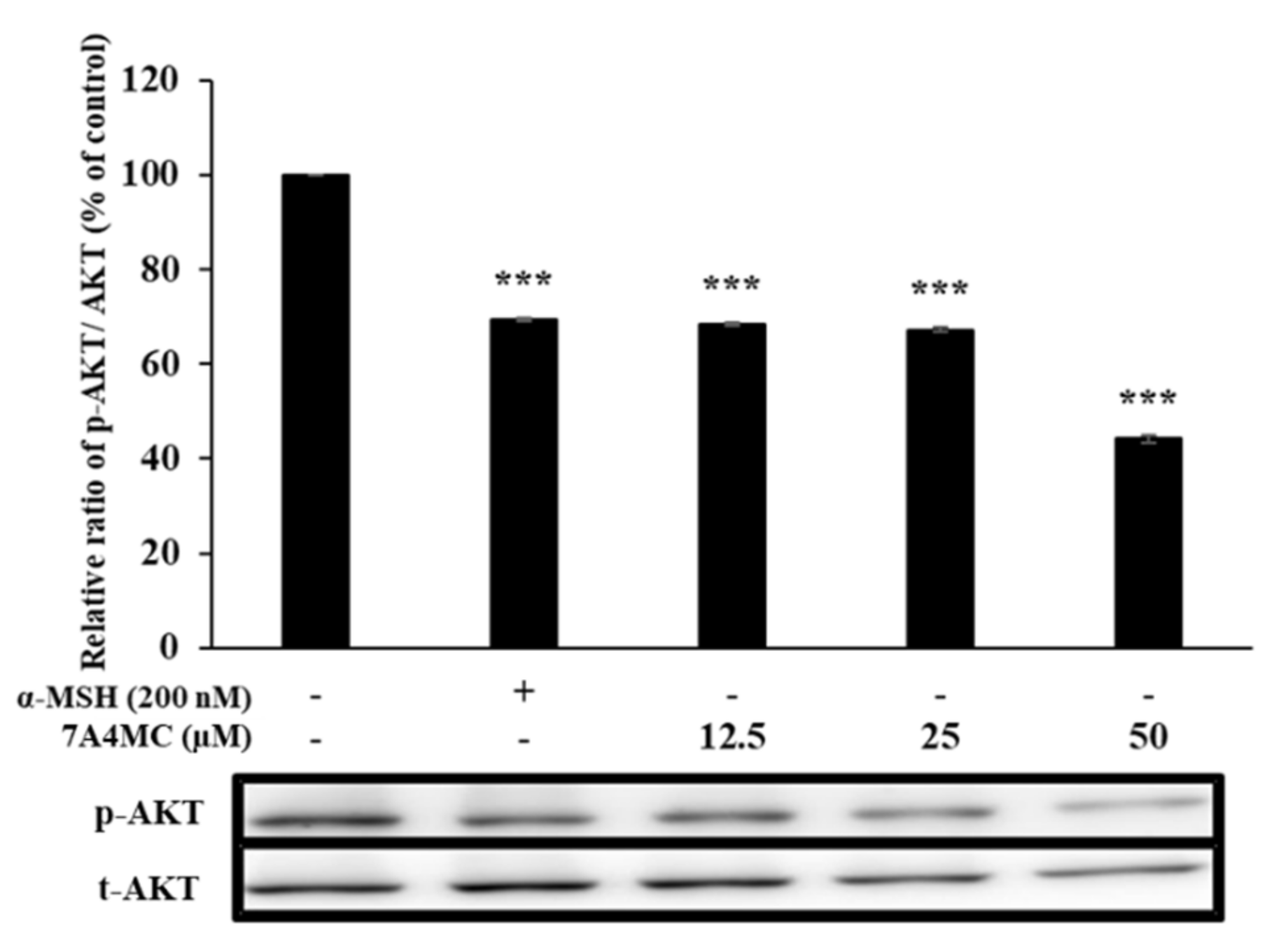
3.6. 7A4MC Suppresses p-AKT Expression in B16F0 Melanoma Cells
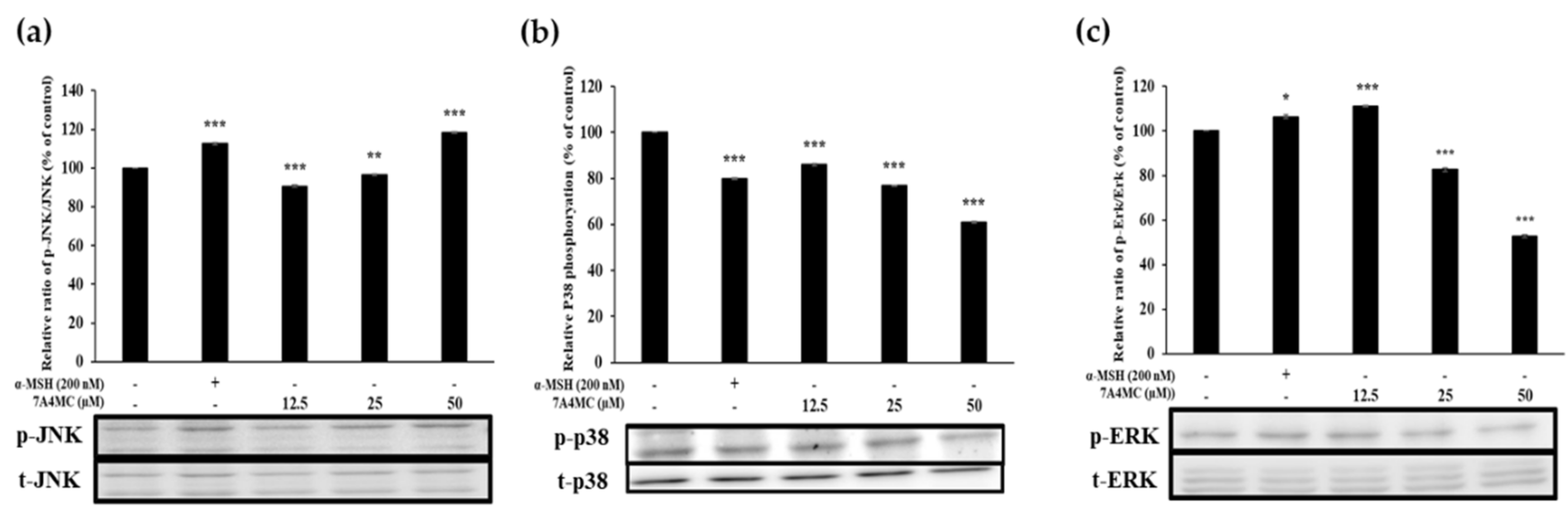
3.7. 7A4MC Increases p-JNK, p-p38, and p-ERK Expression in the MAPK Pathway
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- González, S.; Fernández-lorente, M.; Gilaberte-Calzada, Y. The latest on skin photoprotection. Clin Dermatol. 2008, 26, 614–626. [Google Scholar] [CrossRef]
- Maverakis, E.; Miyamura, Y.; Bowen, M.P.; Correa, G.; Ono, Y.; Goodarzi, H. Light, including ultraviolet. J. Autoimmu. 2010, 34, J247–J257. [Google Scholar] [CrossRef]
- Jiang, Z.; Xu, J.; Long, M.; Tu, Z.; Yang, G.; He, G. 2, 3, 5, 40-tetrahydroxystillbene-2-O-d-glucoside (THSG) induces melanogenesis in B16 cells by MAP kinase activation and tyrosinase upregulation. Life Sci. 2009, 85, 345–350. [Google Scholar] [CrossRef]
- Moon, S.H.; You, C.C.; Hyun, C.G. Tobramycin promotes melanogenesis by upregulating p38 MAPK protein phosphorylation in B16F10 melanoma cells. Antibiotics 2019, 8, 140. [Google Scholar] [CrossRef]
- Aroca, P.; Urabe, K.; Kobayashi, T.; Tsukamoto, K.; Hearing, V.J. Melanin biosynthesis patterns following hormonal stimulation. J. Biol. Chem. 1993, 268, 25650–25655. [Google Scholar]
- Slominski, A.; Wortsman, J.; Plonka, P.M.; Schallreuter, K.U.; Paus, R.; Tobin, D.J. Hair follicle pigmentation. J. Investig. Dermatol. 2005, 124, 13–21. [Google Scholar] [CrossRef]
- Imesch, P.D.; Wallow, I.H.; Albert, D.M. The color of the human eye: A review of morphologic correlates and of some conditions that affect iridial pigmentation. Surv. Ophthalmol. 1997, 41, S117–S123. [Google Scholar] [CrossRef]
- Kim, D.S.; Kim, S.Y.; Chung, J.H.; Kim, K.H.; Eun, H.C.; Park, K.C. Delayed ERK activation by ceramide reduces melanin synthesis in human melanocytes. Cell Signal. 2002, 14, 779–785. [Google Scholar] [CrossRef]
- Yao, C.; Jin, C.L.; Oh, J.H.; Oh, I.G.; Park, C.H.; Chung, J.H. Ardisia crenata extract stimulates melanogenesis in B16F10 melanoma cells through inhibition ERK1/2 and Akt activation. Mol. Med. Rep. 2015, 11, 653–657. [Google Scholar] [CrossRef]
- Ahn, J.H.; Jin, S.H.; Kang, H.Y. LPS induces melanogenesis through p38 MAPK activation in human melanocytes. Arch. Dermatol. Res. 2008, 300, 325–329. [Google Scholar] [CrossRef]
- Widlude, H.R.; Fisher, D.E. Microphthalmia-associated transcription factor: A critical regulator of pigment cell development and survival. Oncoqene 2003, 22, 3035–3041. [Google Scholar]
- Anagha, B.K.; Huma, S.; Umashankar, N. Premature Graying of Hair: Review with Updates. Int. J. Trichology 2018, 10, 198–203. [Google Scholar]
- Bing, Z.; Sai, M.; Inbal, R.; Megan, H.; Pankaj, B.; Sekyu, C.; William, A.G.; Yulia, S.; Eva, M.F.; Yiqun, S.; et al. Hyperactivation of sympathetic nerves drives depletion of melanocyte stem cells. Nature 2020, 577, 676–681. [Google Scholar]
- Saha, B.; Singh, S.K.; Sarkar, C.; Bera, R.; Ratha, J.; Tobin, D.J.; Bhadra, R. Activation of the Mitf promoter by lipid-stimulated activation of p38-stress signaling to CREB. Pigment Cell Res. 2006, 19, 595–605. [Google Scholar] [CrossRef]
- Borges, F.; Roleira, F.; Milhazes, N.; Uriarte, E.; Santana, L. Simple coumarins: Priviled scaffolds in medicinal chemistry. Front. Med. Chem. 2009, 4, 23–85. [Google Scholar]
- Matos, M.J.; Terán, C.; Pérez-Castillo, Y.; Uriarte, E.; Santana, L.; Viña, D. Synthesis and study of a series of 3-arylcoumarins as potent and selective monoamine oxidase B inhibitors. J. Med. Chem. 2011, 54, 7127–7137. [Google Scholar] [CrossRef]
- Matos, M.J.; Viña, D.; Vázquez-Rodríguez, S.; Uriarte, E.; Santana, L. Focusing on new monoamine oxidase inhibitors: Differently substituted coumarins as an interesting scaffold. Curr. Top. Med. Chem. 2012, 12, 2210–2239. [Google Scholar] [CrossRef]
- Pérez-Cruz, F.; Serra, S.; Delogu, G.; Lapier, M.; Maya, J.D.; Olea-Azar, C.; Santana, L.; Uriarte, E. Antitrypanosomal and antioxidant properties of 4-hydroxycoumarins derivatives. Bioorg. Med. Chem. Lett. 2012, 22, 5569–5573. [Google Scholar] [CrossRef]
- Khan, Z.A.; Rama, N.H.; Hussain, M.T.; Khan, G.S. Synthesis and antimicrobial evaluation of some new 3-alkyl-,3-(2’-naphthyl)isocoumarins and their (dl)-3,4 dihydroderivatives. Indian J. Chem. 2007, 46B, 1322–1327. [Google Scholar] [CrossRef]
- Seth, J.O.; Li, N.K. Coumarin compounds as melanogenesis modifiers and uses thereof. United. States Patent Application No. 0220545 A1, 30 August 2012. [Google Scholar]
- Balaji, P.N.; Sreevani, M.S.; Harini, P.; Johnsi, R.P.; Prathusha, K.; Chandu, T.J. Antimicrobial activity of some novel synthesized heterocyclic compounds from substituted chalcones. J. Chem. Pharm. Res. 2010, 2, 754–758. [Google Scholar]
- Piccioni, F.; Malvicini, M.; Garcia, M.G.; Rodriguez, A.; Atorrasagasti, C.; Kippes, N.; Buena, I.T.P.; Rizzo, M.M.; Bayo, J.; Aquino, J.; et al. Antitumor effects of hyaluronic acid inhibitor 4-methylumbelliferone in an orthotopic hepatocellular carcinoma model in mice. Glycobiology 2011, 22, 400–410. [Google Scholar] [CrossRef]
- Piccioni, F.; Fiore, E.; Bayo, J.; Atorrasagasti, C.; Peixoto, E.; Rizzo, M.; Malvicini, M.; Tirado-González, I.; García, M.G.; Alaniz, L.; et al. 4-methylumbelliferone inhibits hepatocellular carcinoma growths by decreasing IL-6 production and angiogenesis. Glycobiology 2015, 25, 825–835. [Google Scholar] [CrossRef]
- Al-Amiery, Y.K.; Al-Amiery, A.; Kadhum, A.A.H.; Mohamad, A.B. Antioxidant activities of 4-methylumbelliferone derivatives. PLoS ONE 2016, 11, e0156625. [Google Scholar]
- Balaji, P.N.; Seleena, S.K.P.; Vidya, G.; Himateja, T.; Neelima, B.; Lakshmiteja, T. Synthesis and charecterisation of Schiff bases derived from acetyl coumarin and evaluated for anti-micribial activity. Int. J. Drug Dev. Res. 2013, 4, 0975–8585. [Google Scholar]
- Musiliyu, A.M.; Jonh, S.C.; Khan, M.O.F. A review of coumarin derivatives in pharmacotherapy of breast cancer. Curr. Med. Chem. 2008, 15, 2664–2679. [Google Scholar]
- Zhao, D.Y.; Hao, Q.X.; Kang, L.P.; Zhang, Y.; Chen, M.L.; Wang, T.L.; Guo, L.P. Advance in Studying Early Bolting of Umbelliferae Medicinal Plant. China J. Chin. Mater. Med. 2016, 41, 20–23. [Google Scholar]
- Musiliyu, A.M.; Veera, L.D.B.; Lekan, M.L.; John, C.; Andre, S.; Ahkinyala, A. Cytotoxic activity of new acetoxycoumarin derivatives in cancer cell lines. Anticancer Res. 2011, 31, 2017–2022. [Google Scholar]
- Bernini, R.; Crisante, F.; Ginnasi, M.C. A comvenient and safe O-methylation of flavonoids with dimethyl carbonate (DMC). Molecules 2011, 16, 1418. [Google Scholar] [CrossRef]
- Tao, H.; Mian, L.; Bo, H. Competitive binding to cuprous lons of protein and BCA in the bicinchoninic acid protein assay. Biomed. Eng. J. 2010, 4, 271–278. [Google Scholar]
- Gratacap, B.; Attard, A.; Laurent, A.; Stoebner, P.; Smirou, D.; Charachon, R. Melanin in the inner ear. An experimental study with control and kanamycin-intoxicated colored guinea-pigs. Arch. Oto Rhino Laryngol. 1989, 246, 235–237. [Google Scholar] [CrossRef]
- Zecca, L.; Bellei, C.; Costi, P.; Albertini, A.; Monzani, E.; Casella, L.; Gallorini, M.; Bergamaschi, L.; Moscatelli, A.; Turro, N.J.; et al. New melanic pigments in the human brain that accumulate in aging and block environmental toxic metals. Proc. Natl. Acad. Sci. USA 2008, 105, 17567–17572. [Google Scholar] [CrossRef]
- Chung, Y.C.; Kim, J.H.; Lee, G.S.; Lee, J.N.; Lee, N.H.; Hyun, C.G. Pratol, an O-methylated flavone, induces melanogenesis in B16F10 melanoma cells via p-p38 and p-jnk upregulation. Molecules 2017, 22, 1704. [Google Scholar] [CrossRef]
- Jiang, L.; Wang, D.; Zhang, Y.; Li, J.; Wu, Z.; Wang, Z.; Wang, D. Investigation of the pro-apoptotic effects of arbutin and its acetylated derivative on murine melanoma cells. Int. J. Mol. Med. 2018, 41, 1048–1054. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sim, J.-H.; Jang, S.-C.; Park, T.-J.; Chi, W.-J.; Kim, S.-Y. Melanogenesis Effect of 7-acetoxy-4-methylcoumarin in B16F10 Melanoma Cells. Cosmetics 2020, 7, 94. https://doi.org/10.3390/cosmetics7040094
Sim J-H, Jang S-C, Park T-J, Chi W-J, Kim S-Y. Melanogenesis Effect of 7-acetoxy-4-methylcoumarin in B16F10 Melanoma Cells. Cosmetics. 2020; 7(4):94. https://doi.org/10.3390/cosmetics7040094
Chicago/Turabian StyleSim, Ji-Han, Sung-Chan Jang, Tae-Jin Park, Won-Jae Chi, and Seung-Young Kim. 2020. "Melanogenesis Effect of 7-acetoxy-4-methylcoumarin in B16F10 Melanoma Cells" Cosmetics 7, no. 4: 94. https://doi.org/10.3390/cosmetics7040094
APA StyleSim, J.-H., Jang, S.-C., Park, T.-J., Chi, W.-J., & Kim, S.-Y. (2020). Melanogenesis Effect of 7-acetoxy-4-methylcoumarin in B16F10 Melanoma Cells. Cosmetics, 7(4), 94. https://doi.org/10.3390/cosmetics7040094





