Abstract
This aim of this study is to prepare four novel oil-in-water creams from 100% naturally sourced oil ingredients such as jojoba, baobab and coconut oil, and compare the effect of the oils on the physico-chemical properties of the creams and their short- and long-term stability. Four 100 g each oil-in-water active containing creams and their controls (without the active ingredient) were formulated and stored in eight separate glass jars. The short-term stability of the creams was assessed via phase separation resistance, pH, microscopic size analysis, globule size, zeta potential, conductivity and microbial challenge evaluation after 8, 14 and 28 days, under three different storage temperature conditions (4 °C, 25 °C and 40 °C) and at ambient relative humidity. Model creams IA, IB, IIA, and IIB containing 1:1 of jojoba and baobab oil mix, all had good shelf-life or stability at the end of the 28 days after storage at 4 °C, 25 °C and 40 °C, compared to models IIIA, IVA and pairs. The long-term stability of creams stored at 25 °C for 28 days, was subsequently assessed using the Dynamic Vapor Sorption system. Model creams IB, IIB, IA and IIA showed the lowest percentage moisture loss or change in mass during a period of desorption steps. Therefore, the creams containing a mixture of jojoba and baobab oils are capable of retaining moisture easily for an extended period of time when compared to the creams containing jojoba and coconut oil or baobab and coconut oil combinations, thus they were proven to be the best products in terms of stability and quality. The stability ranking of the creams using the novel DVS method was in congruence with the results from the short-term stability experiments. This novel DVS method can, therefore, be generically applied in the cosmetic, food and pharmaceutical industries for the evaluation of the long-term stability of semisolids.
1. Introduction
In recent times, the health and beauty industry has tended to lean towards the use of natural ingredients such as herbal or natural oils because of their low toxicity profile [1]. Some of these oils e.g., jojoba oil, are superior compared to others (e.g., castor, olive oil etc.) due to their structural and chemical similarity to the human skin’s sebum [2,3]. Jojoba (pronunciation; hohoba) oil is extracted from the jojoba seed plant called Simmondsia Chinensis, also known as desert gold. It mostly comprises of straight-chain monoesters within the C20–C22 range, and at each end of the acidic and alcoholic ester bond, two double bonds exist. Jojoba plant originated from South-Western North America and the oil has found applications in food, pharmaceuticals, electrical insulators, lubricants, plasticizers, and cosmetics. This range of applications can be attributed to its unique properties [4]; in the medical and pharmaceutical industries, the oil is used for treating a wide range of skin diseases (e.g., eczema, seborrheic dermatitis, acne, sores and inflammation), as an anaesthetic for severe pain [5] and for the manufacture of penicillin-G.
When used on the skin, jojoba oil reduces the appearance of fine lines by inhibiting the aging process due to its antioxidant effect and promotes cutaneous healing or rejuvenation through the stimulation of collagen synthesis [2,3,4,5,6,7,8]. Jojoba oil has a refractive index of 1.46, is highly stable and resistant to oxidation both under normal and extreme temperature conditions [8,9].
Another natural seed oil that is unique and nontoxic in nature is the baobab oil. Baobab oil is derived from the baobab plant, also known as Adansonia digitate. It is largely distributed across the semi-arid and sub humid province of sub-Sahara Africa and Western Madagascar [10,11,12]. Baobab oil is composed of 0.2–3% of Omega 3 (linolenic acid), 25–37% of Omega 6 (linoleic acid), 23–44% of Omega 9 (oleic acid), 1.5–6.0% of Stearic acid and 18–30% of Palmitic acid [12]. In many African regions, the oil is used as an analgesic for the treatment of pain (e.g., toothache), as anti-inflammatory, antioxidant, immune-stimulant, insect repellent, for treating wounds, varicose veins, muscle spasm, dysentery and diarrhea [13,14]. When used with coconut oil, the mixture can be used for the production of antibacterial soap to be applied on skin lesions such as eczema, acne, rashes and sunburn. Baobab oil is a powerful emollient that nourishes, hydrates and conditions the skin, scalp and nails, it helps reduce stretchmarks and improves skin elasticity through the restoration of the skin and rejuvenation of the epithelial tissue. This explains why the oil is recommended during pregnancy [14,15,16]; the high concentrations of Omega 6 and 9 found in the oil stimulates synthesis of collagen and elastin fibers, promoting flexibility, firmness and smoothness while inhibiting inflammation [17,18]. Baobab oil has a strong oxidative stability and a long shelf life, its refractive index and iodine value range from 1.4596 to 1.4633 and 55 to 96 mg/100 g, respectively [17,18,19].
Coconut oil, extracted from coconut kernel also called the white endosperm, is obtained from Cocos nucifera of the family of Arecaceae and subfamily of Cocoideae. Cocos nucifera tree grows in tropical countries of Africa, Asia, South and Central America and the Pacific, [20,21]. It is composed of 95% saturated fatty acids, most of which are medium-chain fatty acids (MCFA), C8–C12 i.e., 44% lauric, 18% myristic, 11% palmitic, 6% capric and 6% caprylic acids, and 15% long-chain fatty acid (LCFA) i.e., 7% oleic, 6% stearic and 2% linoleic acids. MCFAs do not require the action of carnitine for mitochondrial transportation; instead, they are directly oxidized and thus decreasing cholesterol synthesis and fat deposition in the adipose tissue and other body organs [21,22,23,24,25]. MCFAs such as monoglyceride monolaurin largely make up human breast milk and function to protect infants from viral, bacterial and fungal infections, thereby, boosting the immune system. Monoglyceride monolaurin has also been seen to be effective in the reduction of degenerative diseases such as premature aging and skin inflammation, as well as increasing insulin secretion and blood glucose utilization [25,26]. Studies have revealed the oil’s ability to heal the skin by elevating the levels of pepsin-soluble collagen and by increasing collagen cross-linking through glycohydrolase activity [27].
Testing of the physical and chemical stability of natural cosmetics is an essential quality control step due to their highly degradable nature [28]. Various analytical techniques can be used to determine product stability, such methods involve: (i) differential scanning calorimetry (DSC)—to detect changes in heat flow related to material changes (crystallization or melting); (ii) solution calorimetry (SC)—to detect changes in enthalpy dependent on the structure of solid and liquid and their interactions; (iii) HPLC—to measure residual content of specific compounds in a semisolid after extraction by an organic or inorganic solvent [29,30]. As water is ubiquitous in most semisolid cosmetic formulations, the dynamic vapor sorption (DVS) technique could be a useful technique for new product development and processing [31,32]. The DVS analyzer was first invented in the 1990s [33]. It is a computer-automated gravimetric method, i.e., measures mass changes that translate to water vapour sorption isotherms—a phase change of H2O molecules from vapour to condensed phase, involving the production or interruption of strong intermolecular bonds between the H2O molecule and solids or other H2O molecules in the sample. Sorption occurs in two ways; adsorption (water interaction only with molecules at the surface) and absorption (interaction both within and on the surface of the sample); and desorption isotherms—involves the transition of moisture absorbed into the vapour phase, over a wide variety of temperatures (5–85 °C) and humidity (0–98% RH) against time [34,35,36].
In contrast to the aforementioned techniques, the DVS is a multifaceted system due to its ability to provide long-term temperature stability and maximum level of humidity precision and accuracy; allowing generated and delivered vapour to occur typically within ±0.02 °C and ±0.1% RH of target temperature and humidity, respectively. It has a highly-sensitive microbalance that measures mass changes at 0.1 µg resolution, with sample size ranging from 1 mg to 1.5 g [36,37] alongside a unified resolution for generating and capturing Raman spectra in sorption analysis. This combination allows for a thorough comprehension of the chemical and structural properties of materials in relation to their vapour–solid interaction. It also provides an optional vapour permeability measurement and moisture vapour transmission rates via porous elements; microscopic visualization at 200× zoom lens, 5 megapixel camera for well-defined images; and an analysis software that generates a single key result from over 20 various models for stability prediction, surface characterization, and understanding hysteresis (difference in H2O vapor uptake between the isotherms) and solvent interaction [36,37,38].
The focus of this work was to prepare four novel, active oil-in-water creams and their controls (without active) from 100% naturally sourced oil ingredients, and to compare their effects on the physico-chemical properties of creams over short- and long-term storage. All short-term stability assessment was carried out in agreement with the ICH guideline over a period of 28 days. The long-term stability assessment involved the development of a novel method, using the Dynamic Vapor Sorption system to help provide information on the percentage change in mass, in a cycle of drying and sorption. In a previous paper, we demonstrated that stability can be measured using the oscillatory amplitude sweep rheological test, showing changes in the Linear Viscosity Region, LVR (where the complex modulus is independent of stress applied i.e., the longer the LVR, the more stable the structure) [39].
2. Materials and Methods
2.1. Materials
The active ingredient (X), cholesterol, span65 and solutol HS-15 were obtained from Sigma-Aldrich, Inc. (Gillingham, UK). Baobab oil was purchased from Aromatic Natural Skin Care (Forres, UK), Jojoba and Coconut oil from SouthernCross Botanicals (Knockrow, Australia). The Emulsifying Wax was obtained from CRODA International Plc (Goole, East Yorkshire, UK). Other excipients of the cream and Tris buffer solutions were of analytical grade.
2.2. Methods
2.2.1. Preparation of Creams
The formulation of the creams was according to the method described in our previous paper [39]. Four novel active oil-in-water creams and their controls (without active) were prepared. Each model cream contained oils combined as follows: I (1:1 of 8% jojoba and baobab oils)—water phase (85%), oil phase (10%) and emulsifier (5%); II (1:1 of 10% jojoba and baobab oil), III (1:1 of 10% jojoba oil and coconut oil) and IV (1:1 of 10% baobab and coconut oil)—water phase (83%), oil phase (12%) and emulsifier (5%).
2.2.2. Short-Term Stability Studies
The short-term stability assessment was carried out in agreement with the ICH guideline. Creams with actives (model IA-IVA) and without actives (model IB-IVB) were roughly divided into 3 equal portions in similar glass jars and stored at 4 ± 1 °C in the refrigerator, 25 ± 1 °C ambient room temperature and 40 ± 1 °C in an incubator. Physical (appearance, odor, color, phase separation resistance, globule size), chemical (pH, zeta potential measurement) and microbial changes were assessed after 8, 14 and 28 days. The creams were made to acclimatize at room temperature 2 h before assessment.
Cream Separation Resistance
This experiment was conducted using an automated centrifuge after 8 days of product formulation at an rpm of 3000 for 15 min to assess the physical stability of the formulations. The study was repeated after 14 and 28 days. All measurements were conducted at room temperature.
pH Determination
The determination of pH value for each formulated cream model was performed using a benchtop pH meter with a single electrode, measuring temperature and pH, constantly stored in 0.1 M HCl solution. After 8 days of product preparation, the measurement was attained by rinsing the probe with deionized H2O after it was removed from the 0.1 M HCl storage solution and placed into the diluted test sample (0.05 mL of cream, using a 1 mL graduated syringe, was dissolved in 5 mL deionized H2O). The probe was kept in place until a steady pH value was reached. All measurements were done in triplicate, taken at a temperature of 23 °C and repeated after 14 and 28 days of storage.
Microscopic Size Examination
Globule size analysis was conducted using the Olympus microscope, AxioVision® Rel. software version 4.4. This was done by placing a dot of each product on a glass slide and viewed in nonpolarized light (angle 90), 40× magnification. All measurements were carried out at room temperature, 25 ± 1 °C and humidity of 33%, and repeated after 14 and 28 days.
Globule Size and Zeta Potential Measurement
Globule size analysis was performed after 8 days of product preparation using the photon correlation principle of the Malvern® ZETASIZER NANO Instrument (Malvern, UK) by dissolving 10 µL of each cream in 990 µL of distilled H2O (100× dilution) in a cuvette cell. Globule charge analysis was also performed using the same instrument by mixing 10 µL of each cream with 990 µL of distilled H2O and injecting the mix into a double folded capillary cell. All measurements were done in triplicate, at 25 ± 1 °C, a humidity of 33% and repeated after 14 and 28 days.
Microbial Challenge Test
The microbial challenge test was performed using Schulke+ mikrocount® duo dipslides containing two agar surfaces (the yellow agar surface promotes bacteria growth, i.e., Staphylococcus spp and Escherichia coli, while the pink agar surface promotes yeast and fungi growth).
The test sample was transferred onto the yellow agar surface via a wet swab in a unidirectional motion and a different swab was used the same way for the pink agar surface. The slides were then enclosed and left for 72 h to allow optimum fungi growth. The products were made to acclimatize at room temperature 2 h before assessment. All measurements were done at room temperature 25 ± 1 °C and a humidity of 33%.
2.2.3. Long-Term Stability Study
The Dynamic Vapour Sorption (DVS) analyser was used to determine the long-term stability of the creams, by measuring changes in sample mass by uptake (sorption) and loss (desorption) of moisture content, at 25 °C constant temperature. The instrument was initially calibrated, the device preheated, and a new method was created as follows—the nitrogen tank was set at a constant pressure of 2 mbar, a full cycle (sorption and desorption) was selected on the software, at 200 sccm gas flow, dm/dt was 0.0005 for 100 mg sample weight (DMDT% mass change of rate in time, to determine equilibrium) and a pair of 9 mm glass pans were used. The sample was exposed to an increasing and decreasing step size of 10 in humidity ranging from 0% up to 90% RH, and 90% down to 0% RH. Following the short-term stability studies, the test samples initially stored at 25 °C room temperature was used to perform this study.
2.2.4. Statistical Analysis
Statistical evaluation of results obtained for all formulated creams was carried out using the IBM SPSS software. Analysis of variance (one-way ANOVA) was conducted to observe differences between attributes of the oil-in-water creams stored under 4, 25 and 40 °C, after 8, 14 and 28 days, where p < 0.05 indicates a significant difference between the emulsions stored under the three different storage conditions.
3. Results and Discussion
3.1. Short-Term Stability Studies
3.1.1. Cream Separation Resistance
The centrifuge test showed that all models remained completely intact without separating into different layers, i.e., no phase separation was observed in the formulations (Figure 1). This implies that all products (with equal ratio oil combinations jojoba and baobab oil; jojoba and coconut oil; and baobab and coconut oils) were physically stable in terms of macroscopic stability.
Figure 1.
(a) Image of newly prepared oil-in-water cream formulation. (b) An active model and its baseline after centrifuging for 15 min at 3000 rpm.
3.1.2. pH Determination
The surface of human integument has a slightly acidic pH ranging from 4.0 to 5.5 [40,41], this value is said to be slightly higher in old age (i.e., >80 years) [41]. An acidic pH range of 4.0 to 5.5 on the surface of the skin is essential for colonizing microbiota metabolites, i.e., Propioni, Staphylococcus epidermidis and Corynebacteria, which have no harmful effect but serve to inhibit non-resident bacteria and fungi growth, ultimately acting as a biological barrier. Constant use of cosmetic products that are alkaline in nature tamper with the acidic mantle, allowing multiplication of non-resident bacteria and fungi on the surface [42,43]. These microorganisms can then penetrate the surface of the skin and become harmful or toxic to human health; therefore, the formulation of cosmetic products between pH 4.0/±0.10 and 5.5/±0.10 could help maintain the acidic barrier and prevent toxic reactions [43].
Nearly all products containing actives showed higher pH values in comparison to their baseline (Table 1, Figure 2); this difference in pH could be attributed to the basic nature of the active vehicle. Models stored under 4 °C, at the end of 28 days were observed to have the highest pH increase than those stored at 25 and 40 °C. This shows that the products are not suitable for prolonged storage under 4 °C. Model IVA stored at 25 °C had high pH values after 14 days of 5.66 and after 28 days the pH value was 5.78; however, pH remained low at 40 °C storage, i.e., 5.02 and 5.16 after 14 days and 28 days, respectively.

Table 1.
Mean pH and standard deviation values after 8, 14, 28 days measurements for each product stored at 4 °C, 25 °C and 40 °C.
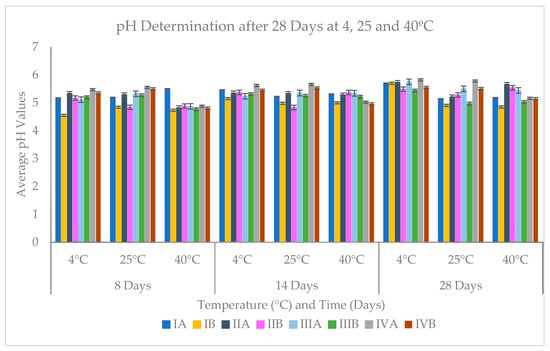
Figure 2.
A graphical representation of the average pH values after 8, 14, 28 days measurements.
Table 2 shows the average cumulative pH values and/or standard deviation of 8, 14, 28 days measurements for each cream under the different storage conditions. Nearly all creams stored at 25 °C room temperature, exhibited very low changes in pH < 0.1, and only products IIB and IVB showed low changes in pH after 28 days. However, all products stored at 40 °C after the end of the 4 weeks’ measurement showed variations in pH > 0.1. This implies that all products stored at room temperature had very good shelf-life or stability and could be safe for use. On the contrary, products stored at 4 °C (except for model IIIB and IVB) and 40 °C did not exhibit good shelf-life due to large variations in average pH values after 4 weeks. This may mean that the control creams containing coconut oil (models IIIB and IVB) are suitable for low-temperature storage but, the differences observed in their active-containing pairs (model IIIA and IVA), under the same storage temperature, could be attributed to the effect of the active ingredient on the overall stability of the products after 28 days.

Table 2.
Average cumulative pH values/deviation after 28 days for each product stored at 4 °C, 25 °C and 40 °C.
3.1.3. Microscopic Size Examination
Overall, from the microscopic data obtained, models IIA and IIB (containing 1:1 of 10% jojoba and baobab oils) appeared to have the highest stability under low, normal or high temperatures, followed by models IA and IB (1:1 of 8% jojoba and baobab oils), and IVA and IVB (1:1 of 10% baobab and coconut oils), while models IIIA and IIIB (containing 1:1 of 10% jojoba and coconut oils) showed the least stability. The increase in temperature presumably led to the swelling, and eventually rupturing, of the niosome vesicles causing the active drug to leak out into the cream base. An increase in temperature resulted in an increase in thermal energy, allowing the globules and niosome particles to move faster, colliding with each other and thereby coalescing.
3.1.4. Globule Size and Zeta Potential Measurement
In an oil-in-water emulsion, oil globule sizes range from 100 to 15,000 nm [44,45]. Globule size and zeta potential of the prepared oil-in-water cream models were performed to assess their stability (Table 3, Table 4 and Table 5) [46,47]. Zeta potential value is influenced by the pH of the test sample, i.e., an addition of alkali medium to a negative zeta potential causes the negative charge to increase, while the addition of an acidic medium to a negative zeta potential causes a reduction in the negative charge until a neutral state is obtained, and additional acid will result in a positive charge [48].

Table 3.
Globule size and zeta values of model IA to IVA and their controls at 4 °C, 25 °C and 40 °C after 8 days.

Table 4.
Globule size and zeta values of model IA to IVA and their controls at 4 °C, 25 °C and 40 °C after 14 days.

Table 5.
Globule size and zeta values of model IA to IVA and their controls at 4 °C, 25 °C and 40 °C after 28 days.
An increase in globule size, i.e., a globule size approaching 10,000 nm is due to coalescence [49,50,51]. In this study, an emulsion with average globule size ≥6000 nm was classified as having poor stability. Charges > −25 were termed poor stability, −25 to −29.9 as average stability, −30 to −44.9 were said to have good stability and ≤−45 indicated excellent stability.
Overall, the oil-in-water creams demonstrated positive but weak conductivity values, which was expected as water (the dispersion medium) is a good conductor of electricity. The size and zeta measurement revealed all formulations, i.e., model IA, IB, IIA, IIB, IIIA and IIIB showed physical and chemical stability or shelf-life when stored at 25 °C, with the exception of models IVA and IVB which showed good stability only at storage conditions of 4 °C. The average size and zeta potential result (Figure 3) revealed that models IA, IB, IIA, IIB and IIIB were the most stable in comparison to other cream models. This result also correlates with the pH and globule size analysis.
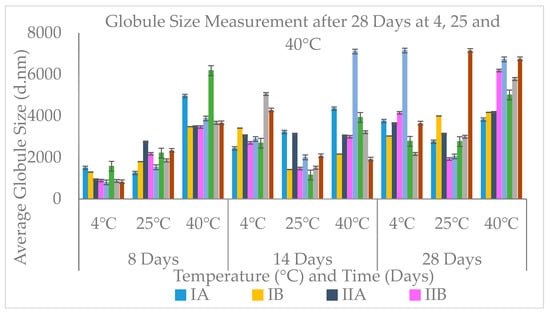
Figure 3.
Globule size measurement after 8, 14 and 28 days at 4, 25 and 40 °C.
The one-way ANOVA test revealed a statistically significant difference in globule size between samples stored under the different temperature conditions, i.e., 4 °C, 25 °C and 40 °C after 8, 14 and 28 days. The total highest change was observed after 28 days measurement. At the end of the first week of measurement, 8 days, a significant difference in globule size of p value = 0.03 between 4 °C and 25 °C was seen, samples in 4 °C and 40 °C, and 40 °C and 25 °C had p values of <0.001. After 14 days measurement, samples stored under 40 °C and 25 °C showed a significant difference in globule size of p value = 0.033. A p value of 0.034 was observed between those stored under 40 °C and 25 °C, after 28 days measurement. However, there was no statistically significant difference in charge.
Based on the results from the short-term stability tests, creams IA, IB, IIA and IIB (1:1 jojoba and baobab oil) and IIIB (1:1 jojoba and coconut oil) all had good shelf-life or stability, particularly with samples stored at room temperature, with cream IA, IB and IIA showing little to no changes in all parameters compared to creams IIIA, IVA and IVB, over the period of evaluation. The variations observed in the short-term stability data of cream IIIA and IIIB with the same oil combination formulation, under the same storage temperatures (4 °C, 25 °C and 40 °C after 8, 14 and 28 days) could be attributed to the effect of the active ingredient on the overall stability of the product IIIA. This implies that the 1:1 of jojoba and baobab oil combinations (regardless of the amount used in formulation i.e., 8% or 10%) appeared to be the best oil combinations in terms of short-term stability compared to 1:1 of jojoba and coconut oil or baobab and coconut oil combinations.
3.1.5. Microbial Challenge Evaluation
Microbial challenge test is a safety assessment of formulated products, indicating the ability of the product to promote or inhibit bacteria and fungi growth. In other words, it determines the effectiveness or efficiency of the preservative and the compatibility of the preservative with the other ingredients in the creams [52]. The challenge test performed using Schulke+ mikrocount® duo dipslides containing two agar surfaces (the yellow agar surface promotes bacteria growth, i.e., Staphylococcus spp. and Escherichia coli while the pink agar surface promotes yeast and fungi growth).
The density and type of colony formed on the nutrient plate is determined using the colony density charts specified by Schulke+. After 72 h, there was an absence of microbial growth in all formulations. Model IVA stored at 25 °C room temperature, in reference to the colony density charts, as shown in Figure 4, had <1 CFU/cm2 total plate count (TPC) on the bottom of the yellow agar surface after 28 days of evaluation, indicating bacteria contamination, although, the preservative was effective against yeast or fungi growth. It is important that the preservative is capable of inhibiting microbial growth as this may cause changes in the product, i.e., color, smell, viscosity and stability, and acne, desquamation or infections to the skin [52,53]. The bacteria contamination observed in model IVA, in contrast to the absence of bacterial growth in model IVB, could be attributed to the packing material, as no sterilization of the containers were done prior to packaging.
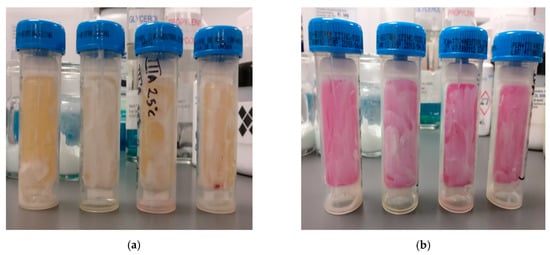
Figure 4.
Model IVA under 25 °C storage temperature after 28 days evaluation showing (a) TPC of bacteria <1 CFU/cm2 (b) TCP of yeast or fungi, no colonies formed.
3.2. Long-Term Stability Studies
The Dynamic Vapour Sorption (DVS) analyser was evaluated as a potential technique for the determination of the long-term stability of the creams (Figure 5). Using the DVS-Advantage-1 system, the percentage change in sample mass was achieved by measuring sample uptake (sorption or absorption) and loss (desorption or drying) of moisture content, at % RH ranging from 0 to 90, at 25 °C constant temperature.
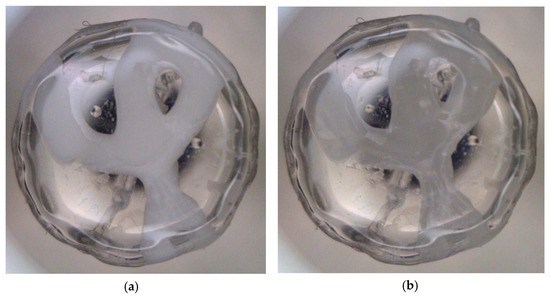
Figure 5.
Images produced by the dynamic vapour sorption system of sample on 9 mm glass pan after (a) moisture content uptake or absorption and (b) drying phase, at 90% RH and 25 °C steady temperature.
The uptake and loss of moisture content (and organic vapour) data of all eight creams were derived from the DVS software and analysed using Microsoft Excel® (Figure 6) Although moisture (and organic vapour) was absorbed in the hydration phase by each test cream (Table 6), a reduction in moisture (mass) at the end of the analysis compared to their initial moisture content (%) was observed i.e., IA, IB, IIA, IIB, IIIA, IIIB, IVA and IVB had a % moisture content decrease of 37.37%, 27.04%, 52.75%, 37.08%, 72.75%, 74.09%, 56.25% and 56.41%, respectively, at the end of the analysis. This could be as a result of the cream becoming too saturated, making the test sample very runny that it flows over the 9 mm measuring pan, rendering the sorption (uptake) method of the DVS for semi-solid materials less valid. However, the method can be applied to a more structured material (e.g., lipsticks, patches and solid dosage forms) to observe changes in swelling or expansion of the solid material during moisture uptake.
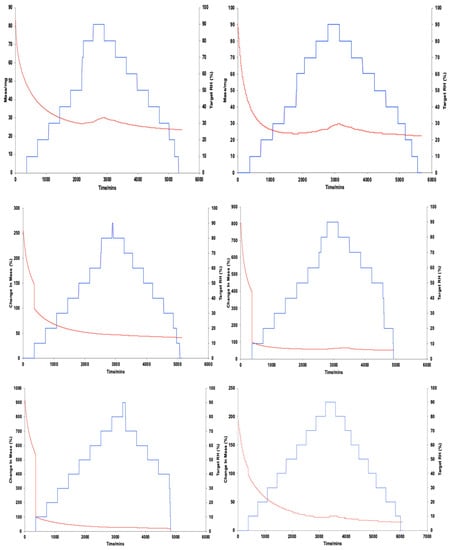
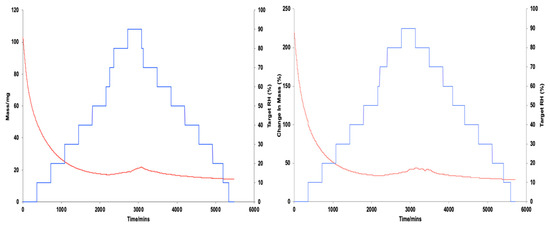
Figure 6.
Graphical illustrations of moisture sorption and desorption kinetics at constant temperature of 25 °C, showing change in mass, DM (red) and % relative humidity, RH (blue) plotted against time/min, DT, of all model creams.

Table 6.
Target relative humidity (increasing steps) and % change in sample mass through moisture content uptake (sorption).
The desorption (drying) method of the DVS in Table 7 revealed more reliable data for the long-term stability analysis of semi-solid formulations. Models IA, IB, IIA, IIB, IIIA, IIIB, IVA and IVB showed a decrease in moisture content (mass change) % by 51.52%, 45.34%, 58.60%, 47.91%, 80.06%, 85.73%, 71.53% and 71.68%, respectively, as shown in Figure 6. This means that the moisture content at equilibrium is greater for the creams in the following order IB, IIB, IA and IIA; therefore, they have the ability to retain more moisture and for an extended period. Models IIIB, IIIA, IVB and IVA were unable to retain more moisture when compared to IB, IIB, IA and IIA. The cream IVB and IVA initially contained the highest amount of moisture 109.03% and 102.72%, respectively, compared to other cream models (except for IIA, 105.21%) but as humidity decreased, <90% over time, both samples experienced a high loss of moisture content. Therefore, creams IB, IIB, IA and IIA—1:1 jojoba and baobab oil combinations showed good long-term stability compared to IIIB and IIIA—1:1 jojoba and coconut oil combinations; and IVB and IVA—1:1 of baobab and coconut oil. This result correlates with the short-term stability studies where, creams IA, IB, IIA and IIB all had good shelf-life or stability based on the data derived from the phase separation resistance, pH, microscopic size analysis, globule size, zeta potential, and conductivity measurements.

Table 7.
Target relative humidity (decreasing steps) and % change in sample mass through moisture content loss (desorption).
The chemical structure of jojoba oil inhibits free radical buildup, because its double bonds are not in close proximity to each other. Formulations containing jojoba oil have extensive storage life even in the absence of preservative resulting in no color change and odor development of the product, making the oil highly suitable for use in the cosmetic industry [54,55].
The ability of creams containing a mixture of jojoba and baobab oil to exhibit good short- and long-term stability can be attributed to the strong oxidative stability of the individual oils, i.e., both jojoba and baobab oils are less prone to photooxidation (a common cause of degradation in oil quality) during manufacturing and storage, due to the presence of high amounts of monounsaturated fatty acids (FA) chains in them. Coconut oil is made up of 95% saturated FA, which allows it to absorb light and become more susceptible to deterioration by photooxidation during processing or storage [56]. Therefore, the validity of the DVS method in determining long-term stability was proven as all products formulated with jojoba and baobab oil combination showed extensive stability compared to those containing coconut oil.
In this study, the long-term stability evaluation performed using the DVS desorption method proved to be very effective. The procedure has high accuracy due to the ability to detect mass changes of <1 part in 10 million. The method was time consuming as it took a period of 5 days to run a full cycle (both sorption and desorption process), see Figure 6. However, this can be overcome when a smaller sample size is applied because the equipment is capable of determining percentage mass changes in samples as small as 1 mg to 1.5 g, and when a half cycle procedure is selected (either, desorption for the accurate determination of mass change in a semi-solid material, or sorption for a more structured test sample). These attributes make the technique highly economical, reliable and less complex than other techniques (e.g., HPLC, DSC and SC).
4. Conclusions
As consumers begin to become increasingly aware of the harsh chemicals contained in commercially available products, there has been a recent surge for natural and organic ingredients [57]. Four novel cream formulas with natural oils, containing actives (model IA-IVA) and their controls (model IB-IVB) were evaluated for safety and quality in accordance with the ICH guideline.
The microbial challenge result revealed the products were capable of inhibiting bacterial, yeast and fungal growth; therefore, there was no color change or odor development over the period of storage. The phase separation resistance, pH, microscopic size analysis, globule size, charge, conductivity data revealed creams containing 1:1 jojoba and baobab oil, and 1:1 jojoba and coconut oil all had good shelf-life, especially with samples stored at 25 °C.
The proposed long-term stability test using the DVS system, desorption method, demonstrated a more comprehensive stability profile by indicating the ability of the creams to retain moisture.
In conclusion, Models IA, IB and IIA containing 1:1 of jojoba and baobab oil mix, were stable over short-term storage at 4 °C, 25 °C and 40 °C. Their stability was validated after long-term storage at 0 to 90 %RH using the proposed DVS method. Whereas, the creams containing coconut oil (IIIA and IVA) were less stable. The proposed DVS method was, therefore, reliable and can be universally applied in the food, cosmetic and pharmaceutical industries to determine the long-term stability of semi-solids.
Author Contributions
Conceptualization, K.D., and D.A.A.; methodology, D.A.A., and K.D.; validation, K.D., and D.A.A.; formal analysis, D.A.A.; investigation, D.A.A.; resources, K.D.; data curation, D.A.A.; writing—original draft preparation, D.A.A.; writing—review and editing, K.D.; visualization, K.D., and D.A.A.; supervision, K.D.; project administration, K.D.; funding acquisition, D.A.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research was self-funded by the researcher (self-funded Ph.D.)
Acknowledgments
The authors would like to thank the teaching formulation laboratory for supplying some of the materials used in the study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Chanchal, D.; Swarnlata, S. Novel approaches in herbal cosmetics. J. Cosmet. Dermatol. 2008, 7, 89–95. [Google Scholar] [CrossRef]
- Harsh, L.N.; Tewari, J.C.; Bohra, M.; Tripathi, D. Standardization of agronomic practices of Jojoba cultivation in arid regions. In Proceedings of the National Seminar on Production, Marketing and Processing of Jojoba (Simmondsia chinensis), Jaipur (Rajasthan), India, 19–20, February 2001. [Google Scholar]
- What You Need to Know about Jojoba Oil-Health Benefits. Available online: http://thejojobaoil.com/ (accessed on 1 December 2017).
- Abu-Arabi, M.; Allawzi, M.; Al-Zoubi, H.; Tamimi, A.; Abu-Arabi, M.; Al-Zoubi, H.S. Extraction of jojoba oil by pressing and leaching. Chem. Eng. J. 2000, 76, 61–65. [Google Scholar] [CrossRef]
- Kim, Y.-J.; Lee, M.S.; Yang, Y.S.; Hur, M.-H. Self-aromatherapy massage of the abdomen for the reduction of menstrual pain and anxiety during menstruation in nurses: A placebo-controlled clinical trial. Eur. J. Integr. Med. 2011, 3, e165–e168. [Google Scholar] [CrossRef]
- Ruggeri, C. Jojoba Oil-Skin & Hair Healer and Moisturizer-Dr. Axe. 2016. Available online: https://draxe.com/jojoba-oil/ (accessed on 29 March 2019).
- Kubitschek-KM, A.R.; Zero, J.M. Development of jojoba oil (Simmondsia chinensis (Link) CK Schneid.) based nanoemulsions. Lat. Am. J. Pharm. 2014, 33, 459–463. [Google Scholar]
- Arya, D.; Khan, S. A Review of Simmondsia chinensis (Jojoba)" The Desert gold": A multipurpose oil seed crop for industrial uses. J. Pharm. Sci. Res. 2016, 8, 381. [Google Scholar]
- Knoepfler, N.B.; Vix, H.L.E. Vegetable oils, review of chemistry and research potential of Simmondsia Chinensis (Jojoba) Oil. J. Agric. Food Chem. 1958, 6, 118–121. [Google Scholar] [CrossRef]
- Adenekan, M.K.; Fadimu, G.J.; Odunmbaku, L.A.; Nupo, S.S.; Oguntoyinbo, S.I.; Oke, E.K. Chemical and functional characterization of baobab (Adansonia Digitata, L.) seed protein concentrate using alcohol extraction method. Int. J. Environ. Agric. Biotechnol. 2017, 2, 2554–2558. [Google Scholar] [CrossRef]
- Abubakar, S.; Etim, V.; Bwai, D.; Afolayan, M. Nutraceutical evaluation of baobab (Adansonia digitata L.) seeds and physicochemical properties of its oil. Ann. Biol. Sci. 2015, 3, 13–19. [Google Scholar]
- Organic Baobab Oil Nourishing Skin Naturally. Available online: https://protecbotanica.com/downloads/baobab.pdf (accessed on 7 March 2019).
- Kamatou, G.; Vermaak, I.; Viljoen, A.M. An updated review of Adansonia digitata: A commercially important African tree. South Afr. J. Bot. 2011, 77, 908–919. [Google Scholar] [CrossRef]
- Cissé, M.; Sow, A.; Poucheret, P.; Margout, D.; Ayessou, N.C.; Faye, P.G.; Sakho, M.; Diop, C.M.G. Impact of extraction method on physicochemical characteristics and antioxidant potential of Adansonia digitata oil. Food Nutr. Sci. 2018, 9, 937–955. [Google Scholar] [CrossRef]
- Komane, B.M.; Vermaak, I.; Kamatou, G.P.; Summers, B.; Viljoen, A.M. Beauty in Baobab: A pilot study of the safety and efficacy of Adansonia digitata seed oil. Rev. Bras. de Farm. 2017, 27, 1–8. [Google Scholar] [CrossRef]
- Baobab Oil. Available online: https://slichemicals.com/wp-content/uploads/sites/2/2015/02/BAOBAB-OIL-Mail.pdf (accessed on 7 March 2019).
- Babiker, S.; Mirghani, M.E.; Matar, S.M.; Kabbashi, N.A.; Alam, M.Z.; Marikkar, J.M. Evaluation of antioxidant capacity and physicochemical properties of Sudanese Baobab (Adansonia digitata) seed-oil. Int. Food Res. J. 2017, 24. [Google Scholar]
- Osman, M.A. Chemical and nutrient analysis of Baobab (Adansonia digitata) fruit and seed protein solubility. Plant Foods Hum. Nutr. 2004, 59, 29–33. [Google Scholar] [CrossRef] [PubMed]
- Zahra’u, B.; Mohammed, A.S.; Ghazali, H.M.; Karim, R. Baobab tree (Adansonia digitata L) parts: Nutrition, applications in food and uses in ethno-medicine-A review. Ann Nutr Disord Ther. 2014, 1, 1011. [Google Scholar]
- Resende, N.M.; Félix, H.R.; Soré, M.R.; Neto, A.M.M.; Campos, K.E.; Volpato, G.T. The effects of coconut oil supplementation on the body composition and lipid profile of rats submitted to physical exercise. Anais Acad. Bras. Ciênc. 2016, 88, 933–940. [Google Scholar] [CrossRef]
- Bawalan, D.D.; Chapman, K.R. Virgin Coconut oil Production: Manual for Micro-and Village-Scale Processing; FAO Regional Office for Asia and the Pacific: Bangko, Thailand, 2006. [Google Scholar]
- Buderwitz, P. Health risks and benefits of coconut oil. Pharm. Today 2013, 19, 27. [Google Scholar] [CrossRef]
- Boemeke, L.; Marcadenti, A.; Busnello, F.M.; Gottschall, C.B.A. Effects of coconut oil on human health. Open J. Endocr. Metab. Dis. 2015, 5, 84–87. [Google Scholar] [CrossRef]
- Lockyer, S.; Stanner, S. Coconut oil-A nutty idea? Nutr. Bull. 2016, 41, 42–45. [Google Scholar] [CrossRef]
- Mikołajczak, N. Coconut oil in human diet-nutrition value and potential health benefits. J. Educ. Health Sport. 2017, 7, 307–319. [Google Scholar]
- Rethinam, P. Health and nutritional aspects of coconut oil. Inform 2002, 13. [Google Scholar]
- Kappally, S.; Shirwaikar, A.; Shirwaikar, A. Coconut oil-A review of potential applications. Hygeia JD Med. 2015, 7, 34–41. [Google Scholar]
- Denyer, S.P.; Baird, R.M. Microbial Contamination. In Guide to microbiological control in pharmaceuticals and medical devices, 2nd ed.; CRS Press: Boca Raton, FL, USA, 2007. [Google Scholar] [CrossRef]
- Sheokand, S.; Modi, S.R.; Bansal, A.K. Dynamic vapor sorption as a tool for characterization and quantification of amorphous content in predominantly crystalline materials. J. Pharm. Sci. 2014, 103, 3364–3376. [Google Scholar] [CrossRef]
- Bilia, A.R.; Bergonzi, M.C.; Mazzi, G.; Vincieri, F.F. Development and stability of semisolid preparations based on a supercritical CO2 Arnica extract. J. Pharm. Biomed. Anal. 2006, 41, 449–454. [Google Scholar] [CrossRef] [PubMed]
- Penner, E. Comparison of the New Vapor Sorption Analyzer to the Traditional Saturated Salt Slurry Method and the Dynamic Vapor Sorption Instrument. Master’s Thesis, University of Illinois at Urbana-Champaign, Urbana, IL, USA, 2013. [Google Scholar]
- Driemeier, C.; Mendes, F.M.; Oliveira, M.M. Dynamic vapor sorption and thermoporometry to probe water in celluloses. Cellulose 2012, 19, 1051–1063. [Google Scholar] [CrossRef]
- Wimmer, R.; Schmid, T. Dynamic Vapor Sorption of Analyses of Wood-A New Approach to Classical Problems. In Proceedings of the International Convention of Society of Wood Science and Technology and United Nations Economic Commission for Europe–Timber Committee, Geneva, Switzerland, 11–14 October 2010. [Google Scholar]
- Thybring, E.E.; Glass, S.V.; Zelinka, S.L. Kinetics of water vapor sorption in wood cell walls: State of the art and research needs. Forests 2019, 10, 704. [Google Scholar] [CrossRef]
- Sheokand, S.; Modi, S.R.; Bansal, A.K. Quantification of low levels of amorphous content in crystalline celecoxib using dynamic vapor sorption (DVS). Eur. J. Pharm. Biopharm. 2016, 102, 77–86. [Google Scholar] [CrossRef]
- Automated Multi-Vapor Gravimetric Sorption Analyzer for Advanced Research Applications. Available online: https://www.micromeritics.com/Repository/Files/DVS_Advantage_Brochure.pdf (accessed on 23 October 2020).
- Galdeano, M.C.; Tonon, R.V.; Carvalho, C.W.P.; Menezes, N.S.; Nogueira, R.I.; Leal-Junior, W.F.; Minguita, A.P.S. Moisture sorption isotherms of raw and extruded wholemeal sorghum flours studied by the dynamic and salt slurry methods. Braz. J. Food Technol. 2018, 21. [Google Scholar] [CrossRef]
- Glass, S.V.; Boardman, C.R.; Zelinka, S.L. Short hold times in dynamic vapor sorption measurements mischaracterize the equilibrium moisture content of wood. Wood Sci. Technol. 2016, 51, 243–260. [Google Scholar] [CrossRef]
- Adejokun, D.A.; Dodou, K. Quantitative sensory interpretation of rheological parameters of a cream formulation. Cosmetics 2019, 7, 2. [Google Scholar] [CrossRef]
- Dikstein, S.; Zlotogorski, A. Measurement of skin pH. Acta Derm. -Venereol. Suppl. 1994, 185, 18–20. [Google Scholar]
- Zlotogorski, A. Distribution of skin surface pH on the forehead and cheek of adults. Arch. Dermatol. Res. 1987, 279, 398–401. [Google Scholar] [CrossRef]
- Fluhr, J.W.; Elias, P.M. Stratum corneum pH: Formation and function of the ‘acid mantle’. Exog. Dermatol. 2002, 1, 163–175. [Google Scholar] [CrossRef]
- Lambers, H.; Piessens, S.; Bloem, A.; Pronk, H.; Finkel, P. Natural skin surface pH is on average below 5, which is beneficial for its resident flora. Int. J. Cosmet. Sci. 2006, 28, 359–370. [Google Scholar] [CrossRef]
- Argov, N.; Lemay, D.G.; German, J.B. Milk fat globule structure and function: Nanoscience comes to milk production. Trends Food Sci. Technol. 2008, 19, 617–623. [Google Scholar] [CrossRef] [PubMed]
- Michalski, M.-C.; Camier, B.; Briard, V.; Leconte, N.; Gassi, J.-Y.; Goudédranche, H.; Michel, F.; Fauquant, J. The size of native milk fat globules affects physico-chemical and functional properties of Emmental cheese. Le Lait 2004, 84, 343–358. [Google Scholar] [CrossRef]
- Instruments, M. Zeta potential: An Introduction in 30 minutes. Zetasizer Nano Serles Tech. Note. MRK654. 2011, 1, 1–6. [Google Scholar]
- Seleci, D.A.; Seleci, M.; Walter, J.-G.; Stahl, F.; Scheper, T. Niosomes as nanoparticular drug carriers: Fundamentals and recent applications. J. Nanomater. 2016, 2016, 1–13. [Google Scholar] [CrossRef]
- Clogston, J.D.; Patri, A.K. Zeta Potential Measurement. In Characterization of Nanoparticles Intended for Drug Delivery; Humana Press: New York, NY, USA, 2011; Volume 697, pp. 63–70. [Google Scholar]
- Milk Homogenization-HORIBA. Available online: http://www.horiba.com/uk/scientific/products/particle-characterization/applications/milk-homogenization/ (accessed on 31 January 2019).
- Walstra, P.; Geurts, T.J.; Noomen, A.; Jellema, A.; van Boekel, M.A.J.S. Dairy Technology: Principles of Milk Properties and Processes; Marcel Dekker, Inc.: New York, NY, USA, 1999. [Google Scholar]
- Walstra, P.; Jenness, R. Dairy Chemistry and Physics; John Wiley & Sons: New York, NY, USA, 1984. [Google Scholar]
- Orús, P.; Leranoz, S. Current trends in cosmetic microbiology. Int. Microbiol. 2005, 8, 77–79. [Google Scholar]
- .Budecka, A.; Kunicka-Styczyńska, A. Microbiological contaminants in cosmetics–isolation and characterization. Biotechnol. Food Sci. 2014, 78, 15–23. [Google Scholar]
- Chemistry-Jojoba Naturals. Available online: https://www.jojobanaturals.com/learn/jojoba-chemistry/ (accessed on 7 August 2020).
- Jojoba Oil: Boosting Stability, Advancing the Beauty Business. Available online: https://www.personalcaremagazine.com/story/30658/jojoba-oil-boosting-stability-advancing-the-beauty-business (accessed on 7 August 2020).
- Madhujith, T.; Sivakanthan, S. Oxidative stability of edible plant oils. Ref. Series Phytochem. 2018, 1–23. [Google Scholar]
- Vermaak, I.; Kamatou, G.; Komane-Mofokeng, B.; Viljoen, A.; Beckett, K. African seed oils of commercial importance-Cosmetic applications. S. Afr. J. Bot. 2011, 77, 920–933. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).