Collagenase and Melanogenesis Inhibitory Effects of Perilla Frutescens Pomace Extract and Its Efficacy in Topical Cosmetic Formulations
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Extracts
2.2. Total Phenolic Content
2.3. Total Flavonoids Content
2.4. Antioxidant Properties
2.4.1. DPPH Radical Scavenging Assay
2.4.2. ABTS radical Scavenging Assay
2.5. Collagenase Activity Assay
2.6. Cell Viability
2.7. Melanin Content Assay
2.8. Clinical Evaluation
2.8.1. Skin Irritation Patch Test
2.8.2. Efficacy Testing
2.9. Statistical Analysis
3. Results and Discussion
3.1. Total Phenolic Content, Total Flavonoid Content, and Free Radical Scavenging Activity
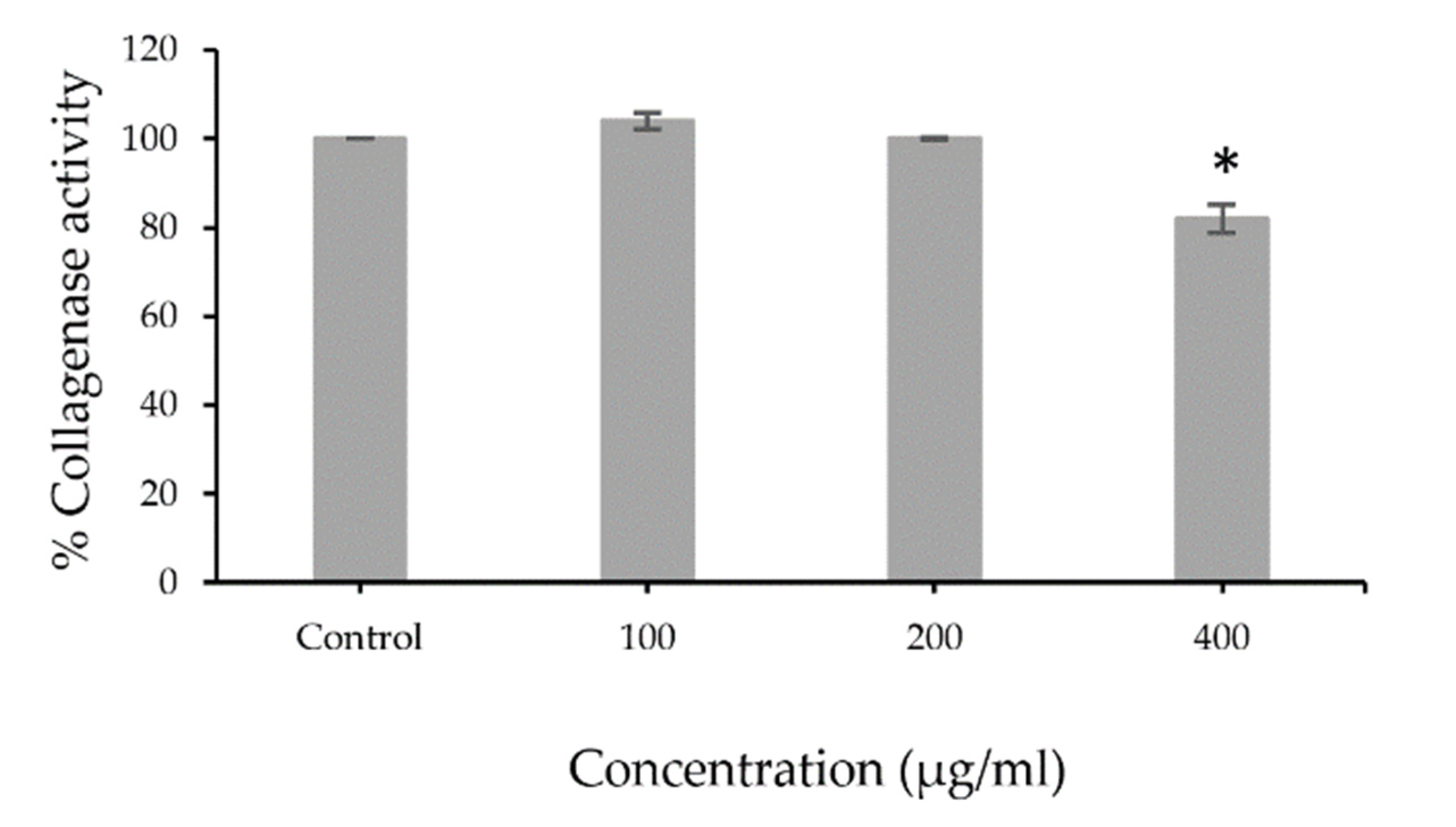
3.2. Collagenase Inhibition Activity
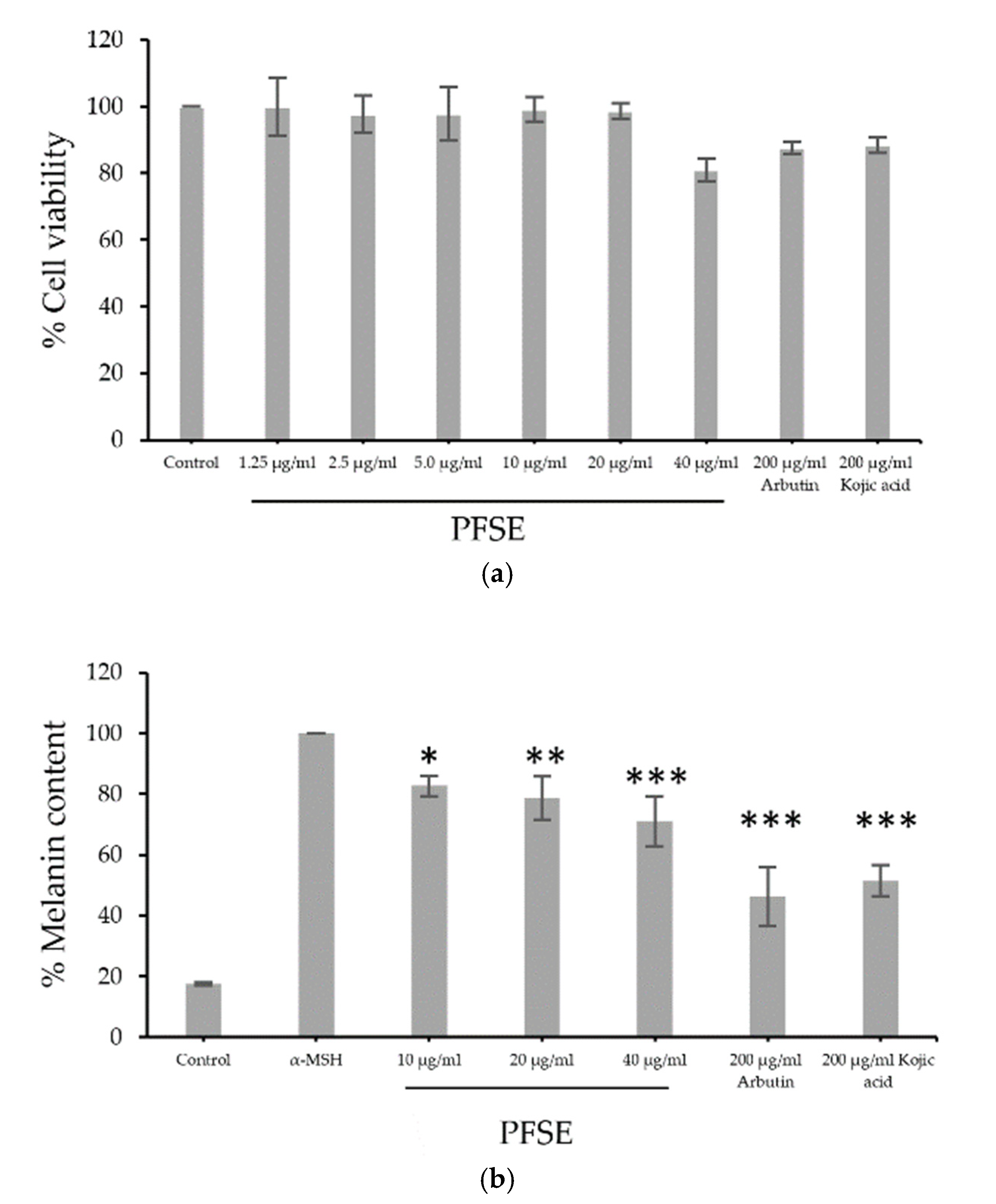
3.3. Cell Viability and Assessment of Melanin Content
3.4. Clinical Evaluation
3.4.1. Skin Irritation Patch Testing
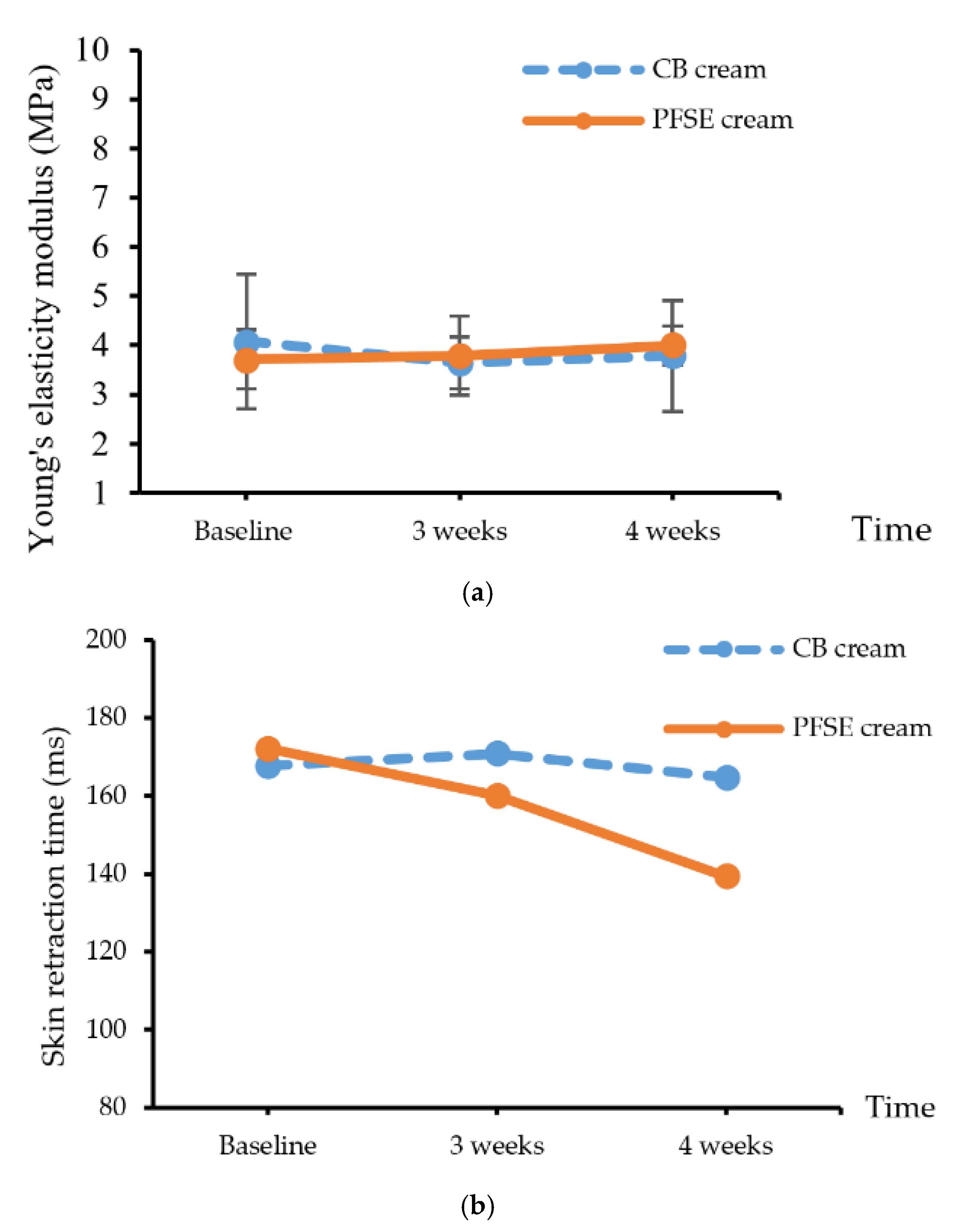
3.4.2. Efficacy Testing
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zhou, X.-J.; Yan, L.-L.; Yin, P.-P.; Shi, L.-L.; Zhang, J.-H.; Liu, Y.; Ma, C. Structural characterisation and antioxidant activity evaluation of phenolic compounds from cold-pressed Perilla frutescens var. arguta seed flour. Food Chem. 2014, 164, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Povilaityee, V.; Venskutonis, P.R. Antioxidative activity of purple peril (Perilla frutescens L.), moldavian dragonhead (Dracocephalum moldavica L.), and roman chamomile (Anthemis nobilis L.) extracts in rapeseed oil. J. Am. Oil Chem. Soc. 2000, 77, 951–956. [Google Scholar] [CrossRef]
- Ahmed, H.M. Ethnomedicinal, Phytochemical and Pharmacological Investigations of Perilla frutescens (L.) Britt. Molecules 2018, 24, 102. [Google Scholar] [CrossRef] [PubMed]
- Mungmai, L.; Preedalikit, W.; Aunsri, N.; Peerakam, N. Bioactivity test and GC–MS analysis of different solvent extracts from Perilla frutescens (Linn.) Britton and cosmetic product application for sensitive skin. Sci. Tech. RMUTT J. 2019, 9, 78–93. [Google Scholar]
- Mungmai, L.; Preedalikit, W.; Aunsri, N.; Amornlerdpison, D. Efficacy of cosmetic formulation containing Perilla frutescens leaves extract for irritation and aging skin. Biomed. Pharmacol. J. 2020, 13, 779–787. [Google Scholar] [CrossRef]
- Pintathong, P.; Chaiwut, P.; Thitipramote, N.; Thitilertdecha, N.; Nantitanont, W.; Sangthong, S.; Tiensri, N. Simultaneous extraction of oil and protein from perilla seed by three-phase partitioning and their application in serum. J. Appl. Sci. 2018, 17, 73–84. [Google Scholar] [CrossRef]
- Ding, Y.; Neo, C.M.; Hu, Y.; Shi, L.; Chao, M.; Liu, Y. Characterization of fatty acid composition from five perilla seed oils in China and its relationship to annual growth temperature. J. Med. Plants Res. 2012, 6, 1645–1651. [Google Scholar] [CrossRef][Green Version]
- Izumi, Y.; Matsumura, A.; Wakita, S.; Akagi, K.-I.; Fukuda, H.; Kume, T.; Irie, K.; Takada-Takatori, Y.; Sugimoto, H.; Hashimoto, T.; et al. Isolation, identification, and biological evaluation of Nrf2-ARE activator from the leaves of green perilla (Perilla frutescens var. crispa f. viridis). Free. Radic. Biol. Med. 2012, 53, 669–679. [Google Scholar] [CrossRef]
- Dhyani, A.; Chopra, R.; Garg, M. A Review on Nutritional Value, Functional Properties and Pharmacological Application of Perilla (Perilla Frutescens L.). Biomed. Pharmacol. J. 2019, 12, 649–660. [Google Scholar] [CrossRef]
- Peng, Y.; Ye, J.; Kong, J. Determination of Phenolic Compounds inPerilla frutescensL. by Capillary Electrophoresis with Electrochemical Detection. J. Agric. Food Chem. 2005, 53, 8141–8147. [Google Scholar] [CrossRef]
- Meng, L.; Lozano, Y.; Bombarda, I.; Gaydou, E.M.; Li, B. Polyphenol extraction from eight Perilla frutescens cultivars. Comptes Rendus Chim. 2009, 12, 602–611. [Google Scholar] [CrossRef]
- Sirilun, S.; Sivamaruthi, B.S.; Pengkumsri, N.; Saelee, M.; Chaiyasut, K.; Tuntisuwanno, N.; Suttajit, M.; Peerajan, S.; Chaiyasut, C. Impact of different pre-treatment strategies on the quality of fatty acid composition, tocols content and metabolic syndrome related activities of Perilla frutescens seed oil. J. Appl. Pharm. Sci. 2016, 6, 1–8. [Google Scholar] [CrossRef]
- Guan, Z.; Li, S.; Lin, Z.; Yang, R.; Zhao, Y.; Liu, J.; Yang, S.-M.; Chen, A. Identification and Quantitation of Phenolic Compounds from the Seed and Pomace of Perilla frutescens Using HPLC/PDA and HPLC-ESI/QTOF/MS/MS. Phytochem. Anal. 2014, 25, 508–513. [Google Scholar] [CrossRef] [PubMed]
- Chandra, S.; Khan, S.; Avula, B.; Lata, H.; Yang, M.H.; ElSohly, M.A.; Khan, I.A. Assessment of Total Phenolic and Flavonoid Content, Antioxidant Properties, and Yield of Aeroponically and Conventionally Grown Leafy Vegetables and Fruit Crops: A Comparative Study. Evid. Based Complement. Altern. Med. 2014, 2014, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Tepe, B.; Daferera, D.; Sokmen, A.; Sökmen, M.; Polissiou, M. Antimicrobial and antioxidant activities of the essential oil and various extracts of Salvia tomentosa Miller (Lamiaceae). Food Chem. 2005, 90, 333–340. [Google Scholar] [CrossRef]
- Duan, X.; Jiang, Y.; Su, X.; Zhang, Z.; Shi, J. Antioxidant properties of anthocyanins extracted from litchi (Litchi chinenesis Sonn.) fruit pericarp tissues in relation to their role in the pericarp browning. Food Chem. 2007, 101, 1365–1371. [Google Scholar] [CrossRef]
- Sridhar, K.; Charles, A.L. In vitro antioxidant activity of Kyoho grape extracts in DPPH and ABTS assays: Estimation methods for EC50 using advanced statistical programs. Food Chem. 2019, 275, 41–49. [Google Scholar] [CrossRef]
- Zinger, A.; Adir, O.; Alper, M.; Simon, A.; Poley, M.; Tzror, C.; Yaari, Z.; Krayem, M.; Kasten, S.; Nawy, G.; et al. Proteolytic Nanoparticles Replace a Surgical Blade by Controllably Remodeling the Oral Connective Tissue. ACS Nano 2018, 12, 1482–1490. [Google Scholar] [CrossRef]
- Preedalikit, W.; Pintha, K.; Tantipaiboonwong, P.; Aunsri, N.; Vivattanaseth, P.; Mungmai, L. Inhibitory Effect of Perilla frutescens L. Leaves Extract on Melanogenesis and Skin Whitening Efficacy in the Underarm Whitening Product Application. Key Eng. Mater. 2020, 859, 166–171. [Google Scholar] [CrossRef]
- Lim, J.W.; Ha, J.H.; Jeong, Y.J.; Park, S.N. Anti-melanogenesis effect of dehydroglyasperin C through the downregulation of MITF via the reduction of intracellular cAMP and acceleration of ERK activation in B16F1 melanoma cells. Pharmacol. Rep. 2018, 70, 930–935. [Google Scholar] [CrossRef]
- World Health Organization. Handbook for Good Clinical Research Practice (GCP): Guidance for Implementation; World Health Organization: Geneva, Switzerland, 2005. [Google Scholar]
- Acute Dermal Irritation/Corrosion 404. OECD Guideline for the Testing Chemicals; OECD Publishing: Paris, France, 2002. [Google Scholar]
- Francis, M.A.; Rew, W.V.; Awah, F.M.; Verla, R.W. Antioxidant activity, nitric oxide scavenging activity and phenolic contents of Ocimum gratissimum leaf extract. J. Med. Plants Res. 2010, 4, 2479–2487. [Google Scholar] [CrossRef]
- Mandrone, M.; Lorenzi, B.; Venditti, A.; Guarcini, L.; Bianco, A.; Sanna, C.; Ballero, M.; Poli, F.; Antognoni, F. Antioxidant and anti-collagenase activity of Hypericum hircinum L. Ind. Crop. Prod. 2015, 76, 402–408. [Google Scholar] [CrossRef]
- Mansauda, K.L.R.; Anwar, E.; Nurhayati, T. Antioxidant and Anti-Collagenase Activity of Sargassum plagyophyllum Extract as an Anti-Wrinkle Cosmetic Ingredient. Pharmacogn. J. 2018, 10, 932–936. [Google Scholar] [CrossRef]
- Junlatat, J.; Fangkrathok, N.; Sripanidkulchai, B. Antioxidative and melanin production inhibitory effects of Syzygium cumini extracts. Songklanakarin J. Sci. Technol. 2018, 40, 1136–1143. [Google Scholar]
- Kao, Y.-Y.; Chuang, T.-F.; Chao, S.-H.; Yang, J.-H.; Lin, Y.-C.; Huang, H.-Y. Evaluation of the Antioxidant and Melanogenesis Inhibitory Properties of Pracparatum Mungo (Lu-Do Huang). J. Tradit. Complement. Med. 2013, 3, 163–170. [Google Scholar] [CrossRef][Green Version]
- Matwiejczuk, N.; Galicka, A.; Zareba, I.; Brzoska, M.M. The Protective Effect of Rosmarinic Acid against Unfavorable Influence of Methylparaben and Propylparaben on Collagen in Human Skin Fibroblasts. Nutrients 2020, 12, 1282. [Google Scholar] [CrossRef]

| Test Materials | Total Phenolic Content (mg GAE/g Extract ± SD) | Total Flavonoids Content (mg CE/g Extract ± SD) | DPPH• Assay | ABTS• Assay | ||
|---|---|---|---|---|---|---|
| Ascorbic Acid IC50 ± SD (µg/mL) | IC50 ± SD (µg/mL) | Ascorbic Acid IC50 ± SD (µg/mL) | IC50 ± SD (µg/mL) | |||
| PFSE | 92.79 ± 1.19 | 56.02 ± 2.83 | 3.07 ± 0.18 | 86.06 ± 5.65 | 2.68 ± 0.12 | 24.84 ± 2.21 |
| Test Materials | PDII Value | Classification of Irritancy |
|---|---|---|
| 1% w/v SLS | 1.53 | Mild irritant |
| DIW | 0.00 | Non-irritant |
| CB cream | 0.00 | Non-irritant |
| PFSE cream | 0.00 | Non-irritant |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mungmai, L.; Preedalikit, W.; Pintha, K.; Tantipaiboonwong, P.; Aunsri, N. Collagenase and Melanogenesis Inhibitory Effects of Perilla Frutescens Pomace Extract and Its Efficacy in Topical Cosmetic Formulations. Cosmetics 2020, 7, 69. https://doi.org/10.3390/cosmetics7030069
Mungmai L, Preedalikit W, Pintha K, Tantipaiboonwong P, Aunsri N. Collagenase and Melanogenesis Inhibitory Effects of Perilla Frutescens Pomace Extract and Its Efficacy in Topical Cosmetic Formulations. Cosmetics. 2020; 7(3):69. https://doi.org/10.3390/cosmetics7030069
Chicago/Turabian StyleMungmai, Lapatrada, Weeraya Preedalikit, Komsak Pintha, Payungsak Tantipaiboonwong, and Nattapol Aunsri. 2020. "Collagenase and Melanogenesis Inhibitory Effects of Perilla Frutescens Pomace Extract and Its Efficacy in Topical Cosmetic Formulations" Cosmetics 7, no. 3: 69. https://doi.org/10.3390/cosmetics7030069
APA StyleMungmai, L., Preedalikit, W., Pintha, K., Tantipaiboonwong, P., & Aunsri, N. (2020). Collagenase and Melanogenesis Inhibitory Effects of Perilla Frutescens Pomace Extract and Its Efficacy in Topical Cosmetic Formulations. Cosmetics, 7(3), 69. https://doi.org/10.3390/cosmetics7030069






