Abstract
After characterization of the reactive skin microbiota, we investigated whether the active Halymenia durvillei (HD), rich in polysaccharides, could modulate this microbiota after 28 days of treatment, act on neuroinflammation parameters, and calm feelings of discomfort and redness. Skin microbiota was assessed using next-generation sequencing experiments (16S RNA gene fragment sequencing) on samples collected from 30 volunteers suffering from reactive, sensitive skin. To evaluate the effect of the HD extract on neuroinflammation, we used an ex vivo model. Finally, an in vivo study was performed using a clinical assessment (blood microcirculation via videocapillaroscopy) of functional signs employing the Sensitive Scale and the soothing effect was evaluated and compared to a placebo treatment. At the phylum level, the samples were mostly composed of Actinobacteria, Proteobacteria, Firmicutes, and Bacteroidetes, which accounted for more than 97% of the total sequencing read in all samples, with no differences before or after treatment with the HD active ingredient. The Shannon Diversity index indicated lower microbial communities compared to healthy skin. Maintenance of the Shannon Diversity index was reported after 28 days of HD active ingredient treatment, wherein microbial communities continued to decrease in number during treatment with the placebo. The average taxonomic composition of associated skin microbial communities showed that reactive skin is characterized by a low proportion of the Chryseobacterium genus compared to a high proportion of the Corynebacterium genus. At the species level, Actinobacteria are mainly represented by Propionibacterium acnes (72.13%) and Corynebacterium kroppenstedtii (13.23%), representing species typically observed in clinical cases of redness, the main criteria for volunteer inclusion. Corynebacterium kroppenstedtii, with increased levels being associated with skin redness, decreased with HD treatment. This decrease coincided with the clinical improvement observed after 7 weeks of treatment. The ex vivo study revealed that the HD extract induced a significant decrease in the expression of TRPV-1 (−67%; p < 0.001) and NK1-R (−43%; p < 0.01) compared to the control after 6 days of treatment. These data support the use of polysaccharides, found in red alga, in the treatment of reactive and sensitive skin related to the modulation of skin microbiota.
1. Introduction
The skin is colonized by hundreds of diverse bacterial species from the four phyla Actinobacteria, Proteobacteria, Firmicutes, and Bacteriodetes, which act as part of the body’s first line of defense against the external environment [1]. These microorganisms are generally classified into two groups: resident and transient microbes [2]. Resident microbes are not harmful under most conditions and may provide some benefits to the host. The diversity of these transient and resident microbes is determined by different characteristics of local surface areas, such as moisture, the pH value, internal factors (such as age, sex, and genotype), and external factors (such as nutrition, lifestyle, the climate pattern, and cosmetics) [3]. Desequilibrum, or dysbiosis, of the skin microbiome, and decreased microbial biodiversity have been linked to many diseases, including acne, eczema [4], rosacea [5], and psoriasis [6]. More recently, Seite et al. [7] hypothesized that bacteria could affect the pathophysiology of sensitive skin syndrome. However, data concerning this relationship is scarce. The term “sensitive skin” has been used to describe the phenomenon of hyperreactivity, wherein exaggerated skin reactions develop after exposure to stressful factors [8]. “Sensitive skin” is also defined by the self-reported presence of different sensory perceptions, including tightness, stinging, burning, tingling, pain, and pruritus in response to stimuli that normally should not provoke such sensations [9]. Sensitive skin’s diagnosis, pathophysiology, and treatment are still under discussion, but it has become evident that all environmental factors, such as sun radiation (i.e., ultraviolet, visible light, and infrared wavelengths) and air pollution contribute to skin alterations or “skin aging exposome” and need to be considered in relation to sensitive skin [10]. Potential mechanisms of sensitive skin involve skin neurosensory dysfunction, neurogenic inflammation, epidermal barrier disruption, immune cell activity (transient receptor potential (TRP) channels), and hyperreaction of the skin blood vessels [8]. More specifically, the skin is the largest neuroendocrine organ of the human body. Many cutaneous neurohormones diffuse in the skin’s upper epidermal layers and skin microbiota are permanently exposed to these factors [11]. Cutaneous nerve fibres, such as unmyelinated C fibres mediating pain, itch, and warmth, are equipped with sensory neuroreceptors such as endothelin and TRP channels. Interestingly, the activation of TRP family members results in a combination of afferent functions with effector roles. Among them, TRPV-1, initially named “capsaicin”, or “vanilloid 1 receptor”, was identified on nociceptive sensory nerve endings and is known to mediate sensations of pain, itching, warmth, and afferent functions in response to chemical stimuli. Additionally, TRPV-1 controls the release of neuropeptides in local neurogenic inflammation, such as substance P (SP), which subsequently activates different types of cells in the skin (i.e., keratinocytes, mast cells, antigen-presenting cells, and T cells located in close vicinity to the sensory nerve endings) [12]. Generally speaking, SP is considered a major mediator of neurogenic inflammation and itching, through activation of tissular Neurokinin 1 receptors (NK1-R), inducing the release of pro-inflammatory cytokines and chemokines, resulting in the recruitment of additional immune cell subsets to the skin. The activation of TRP sensory proteins via triggering factors also induces the release of other neurotransmitters, such as vasoactive intestinal polypeptide (VIP) and calcitonin gene-related polypeptide (CGRP), triggering vasodilatation and cell degranulation.
It appears that non-specific inflammation is linked to the release of cytokines (IL-8) and tumor necrosis factor (TNF) [13]. Lower concentrations of pyrrolidone carboxylic acid (PCA) and transglutaminase (TG) activities, coupled with a greater number of smaller and immature corneocytes indicating altered stratum corneum (SC) maturation in sensitive subjects, also characterize sensitive skin [14].
As mentioned above, the role of cutaneous microbiota in skin sensitivity has recently been hypothesized, wherein SP and CGRP have a direct effect on the skin microbiota in relation to the modulation of bacterial virulence [15]. SP stimulates the biofilm formation activity of Pseudomonas fluorescens and the cytotoxicity of Bacillus cereus. It also increases the adhesion potential of Staphylococcus aureus (S. aureus) and Staphylococcus epidermidis (S. epidermidis) on keratinocytes [15].
Considering the high prevalence of sensitive skin around the world [16], its treatment represents an excellent target for active ingredients in cosmetics. Various naturally-derived complex mixtures such as botanical extracts have been used for centuries. The application of algae in cosmetic products has recently received more attention in the skin treatment community. Algae are rich sources of structurally novel and biologically active metabolites with great industrial potential and accessibility. Among them, Halymenia durvillei (HD) is a red alga belonging to the Rhodophyceae family, and is abundant in a vast area of the Indian Ocean. Red algae are often small and can live in the deepest known parts of the ocean for organisms containing chlorophyll. Halymenia durvillei contains phycocolloids, which are the constituent polysaccharides of cell membranes. The current interest in these polysaccharides is due to their known bioactivities, conferred by their anti-allergic, neuroprotective, cytotoxic, anti-nociceptive, and immunomodulatory properties. This has made them promising bioactive products and biomaterials [17].
The concept of the human skin exposome was introduced more than a decade ago, inducing cutaneous aging, sensitive, and reactive skin [18]. Hyperreactivity of the skin’s neural response is postulated to play a role in sensitive skin [19]. The treatment of reactive skin is challenging and generally based on the continuous and topical application of antisensitive, moisturizing, and extreme tolerance products that improve skin features. After characterization of the reactive skin microbiota, we investigated whether the polysaccharide-rich Halymenia durvillei (HD) active ingredient could modulate this microbiota after 28 days of treatment, as well as reduce feelings of discomfort and redness.
2. Material and Methods
Marine Extracts
Production of the Halymenia durvillei extract occurred in several steps. First, polysaccharides of the seaweed were extracted at a high temperature (80 °C) in water for 2 h, with stirring at 180 rpm. Then, these native polysaccharides, analysed as β-(1,3) and α-(1,4)-linked sulphated galactosic residues, were cut by a radical hydrolysis reaction for 3 h. Biomass waste was removed by centrifugation and filtrations (Westfalia separator, Oelde, Germany, SA7-06-076). Several successive filtrations on a plate filter (Eaton, Dublin, Ireland, Beco compact plate A400/2SF) allowed clarification of the supernatant. A first filtration was established with 15 µm retention level cellulosic membranes (Beco, CP07S) and a second with a 1 µm retention level (Beco, KD7). The extract was then ultra-filtered at 5 kDa (IMECA, 1M2-040871) until permeate dry matter reached 6%. The concentration of product was finalized via evaporation under vacuum at 40 °C (Auriol, B-115-V) as the total sugar content exceed 65 g.L−1 [20]. By this method, specific low molecular weight polysaccharides were obtained.
The characterization of these new small polysaccharides was determined and showed that they consist of 93% galactose and, to a lesser extent, xylose (1%) and glucose (6%).
3. Microbiota
3.1. Sampling and DNA Extraction
The aim of this project was to study the effect of the HD extract versus placebo on bacterial skin microflora after 28 days of treatment by using next-generation sequencing experiments (16S RNA gene fragment sequencing) on samples collected from 25 volunteers.
At Day 0 (D0), two samples were collected from each volunteer (one on each cheek: D0-No Treatment (NT) and D0-Treatment (T)). Similarly, two samples were taken after 28 days of treatment. Sampling was performed for a duration of ≈30 s over an area of 12 cm2 using “PurFlock Ultra, Puritan” sterile swabs previously soaked in a physiological saline solution (NaCl 0.85%) containing Tween 20 (0.1%, v/v). Swabs were frozen at −80 °C immediately after sampling.
On the day of extraction, the tip of each swab was cut and placed in a “PowerBead Tube” included in the kit “DNeasy PowerSoil” (QIAGEN), and DNA extraction was then performed according to the supplier recommendations. To ensure that all reagents and materials used were completely free of contaminant bacterial DNA, DNA extraction of the different solutions and consumable material used (physiological saline water, Tween 20, active product, placebo, sterile swab, and column belonging to the extraction kit, for a total of six negative controls) was performed.
3.2. Sequencing and Raw Data Processing
Microbiota composition analysis of samples was performed by amplifying the hypervariable regions V1–V3 of the 16S RNA gene by using primers 24F-533R which target the conserved regions of this gene common to all bacteria. The libraries were generated by means of the Nextera kit, and 250 paired-end sequencing reactions were performed on a MiSeq Illumina platform. The trimming of sequences was performed by an informatic pipeline developed internally, which was run under the Mothur program (version 1.36.1). Chimeras and unique sequences were removed using UCHIME. Filtered sequences with a 100% homology were kept and classified using the default method. Finally, Operational Taxonomic Units (OTUs) assignation was performed by interrogating the Greengenes database. This was done using bioinformatic tools for processing large amounts of sequence data (Mothur). In total, the amplicon dataset included 2,930,605 reads, with an average of 16,102 reads per sample (min: 8534 reads; maximum: 23,042 reads). To estimate the species richness and diversity, the Shannon Diversity index was calculated, which combines the variety (the species richness) and evenness (equitability) components of diversity.
4. Ex Vivo Study
Twenty-one skin explants were prepared from an arm plasty of a Black woman aged 31 years (reference P1822-BN31). The explants were kept alive in BIO-EC’s Explants Medium (BEM) at 37 °C in a humid atmosphere, enriched with 5% CO2. The explants were divided into four batches as follows: Product, Placebo, Control plasty, and Control (T0). At Day 0, Day 2, and Day 5, the Placebo and HD active ingredient were applied to the surface of the explant at a rate of 2 μL.cm−2 and spread with a spatula. The control group received no treatment, except for the renewal of the medium (2 mL) on Day 0 and Day 2.
At D0, the explants of batch T0 were removed and cut in half. One half was fixed in buffered formalin and one half was frozen at −80 °C. On Day 2 and Day 6, three explants of each lot were taken and treated in the same way as on Day 0. Culture media from all lots on D2 (2 mL) and D6 (2 mL) were removed and frozen at −80 °C for assays.
4.1. Measurement of TNF-α
The TNFα assay was performed with the human Elisa TNFα kit (ref 589201, Cayman). The culture medium and the TNFα standard were incubated with acetylcholinesterase (AChE): TNFα-binding Fab’s conjugate in the wells containing the immobilized TNFα antibody, overnight at 4 °C. According to the manufacturer’s instructions, after washing the plate, the reaction was revealed for 80 min by a solution containing the AChE substrate. Absorbance at 412 nm was measured with the Tecan M200Pro microplate reader and Magellan7 software.
4.2. Immunostaining of TRPV1
After 24 h in the buffered formalin, the samples were dehydrated and impregnated in paraffin using a Leica PEARL dehydration automaton. They were packaged using a Leica EG 1160 coating station. Sections of 5 μm were made using a Minot microtome, Leica RM 2125, and mounted on Superfrost® histological glass slides.
TRPV1 was scored on formalin paraffin sections with an anti-TRPV1 polyclonal antibody (Abcam, Cambridge, UK, ref.ab3487), diluted 1:100 in PBS-BSA 0.3–0.05% Tween 20 for 1 h at room temperature, amplified with a biotin/streptavidin system, and revealed in a violet substrate of peroxidase (VIP) (Vector, SK-4600). Immunostaining was performed using an immunostaining automaton (Dako Autostainer Plus: Holly, MI, USA).
4.3. Immunostaining of NK1-R
NK1-R was scored on paraffin sections with an anti-NK1-R polyclonal antibody (Santa-Cruz, ref sc-365091); diluted 1/50 in PBS-BSA 0.3–0.05% Tween 20 for 1 h at room temperature with a Universal VECTASTain RTU VECTOR streptavidin/peroxidase system; and revealed in VIP, a purple peroxidase substrate (Vector, SK-4600). Immunostaining was performed using an immunostaining automaton (Dako, AutostainerPlus) and evaluated by microscopic observation and image analysis. Finally, Vascular Endothelial Growth Factor (VEGF) was scored on paraffin sections with an anti-VEGF polyclonal antibody (Santa-Cruz, ref sc-7269). TRPV1 was scored on formalin paraffin sections with an anti-TRPV1 polyclonal antibody (Abcam, ref.ab3487).
4.4. Immunostaining of VEGF
VEGF was scored on paraffin sections with an anti-VEGF polyclonal antibody (Santa-Cruz, ref sc-7269); diluted 1/100 in PBS-BSA0.3–0.05%Tween 20 for 1 h at room temperature with the Vectastain RTU Universal VECTOR streptavidin/peroxidase system; and revealed in VIP, a purple peroxidase substrate (Vector, SK-4600). Immunostaining was performed using an immunostaining automaton (Dako, AutostainerPlus) and evaluated by microscopic observation and image analysis.
5. In Vivo Study
To go further at the clinical level, a clinical study was carried out in order to analyze the effect of the extract on reactive skin at an in vivo level. For that, 25 female volunteers aged 41 ± 10 years old with skin phototypes II to IV and dry, sensitive skin were enrolled in this study, according to the recommendation of the current Helsinki Declaration. They gave their written informed consent. The study was performed by a comparison of before and after the hemi-face application of HD active ingredient 3% or a placebo. To be enrolled in this study, the subjects were either not smokers or smoked no more than eight cigarettes per day, presented sensitive and reactive skin as defined from the stinging test on Day 7 (total score ≥ 3), and presented with mild to moderate erythrosis (score ≥2 and <7m, as defined from the clinical score).
Exclusion criteria were the presence of any skin-related pathologies and abnormalities (eczema, psoriasis, etc.), allergies, systemic treatment (antihistaminic, antibiotics, corticoids, or retinoids) for more than 5 consecutive days within the 4 weeks before inclusion, acute and/or chronic inflammation or infection of facial skin, exposure to sunlight or artificial UV rays within 15 days of the experiment, pregnancy, and nursing. Subjects were advised to avoid the application of other similar products during the whole study.
Evaluations were performed at the baseline (D0) and 28 days (D28) after twice-daily application. Subjects were evaluated in the standard skin situation (last face washing the night before the visit, without any cosmetics, water, and makeup application until the measures). Clinical scoring of erythrosis (measurement and analysis of blood microcirculation by videocapillaroscopy: Moritex MS500 with a ×50 magnification: exploration area = 13.6 mm2), assessment of functional signs using the Sensitive Scale [21], and the soothing effect were evaluated.
Pictures were taken using a VISIA from CANFIELD® imaging system equipped with a Canon EOS 5D Mark II. Women were also asked to report their overall opinion about the product, with a three sentence maximum for remarks after the 28 days of application.
5.1. Stinging Test
To determine if the volunteer had sensitive skin, a stinging test was performed during the inclusion visit (D-7) by the dermatologist who recorded the subject’s sensations. To induce a burning or stinging sensation, subjects were stimulated on both nasolabial folds with a 10% lactic acid aqueous solution for at least 2 min until a stinging sensation was triggered. The side of application of lactic acid was defined by randomization. At 30 s, 2.5 min, and 5 min post application, subjects were asked to describe the sensations they were experiencing on a 10-tiered visual analogue scale (VAS) (0 = no stinging, 2 = moderate stinging, and 3 = strong stinging). The cumulative stinging score for one volunteer was the sum of the three grades obtained with lactic acid minus the sum of the three grades obtained with physiological saline solution. Those individuals whose values were equal to or greater than 3, were regarded as “positive stingers” and were eligible to participate in the study. The physiological saline ran concurrently on the opposite site, ensuring that volunteers could reliably distinguish a stinging response.
The instant soothing effect was estimated. After lactic acid application and stinging induction, a placebo or active ingredient was simultaneously applied to either the right or left nasolabial fold. The side of the face was permutated from subject to subject by means of a randomization scheme. The stinging test was carried out under a controlled temperature and relative humidity (temperature: 21 ± 1 °C, hygrometry: 45 ± 5%). A significant decrease in the score meant an improvement in the sensitivity of the skin.
At D28, the same procedure was administered.
5.2. Quantification of the Blood Microcirculation by Videocapillaroscopy
The equipment used was a video microscope MORITEX MS500 series (magnification ×50) connected to a video capture card for a PC computer. Videocapillaroscopy is a method that allows direct visualization of the capillary network in vivo. The system consists of a video signal control unit and a CCD camera. A manually adjusted focusing system coupled with the camera head provides sharp imaging of the capillary network. The subject was in a lying position. Magnification of 50× was used, which corresponds to an exploration range of about 13.6 mm2. “Neural” filtering was applied to the images in order to separate the erythrosis from the “noise” in the pictures. The result of the filtering was then thresholded, allowing us to define a surface parameter expressed in pixels, as well as a number of erythrosis areas [22]. Acquisitions took place on D0 and D28.
5.3. The Sensitive Scale Test
The Sensitive Scale is a new scale with 10-item scoring used to measure the severity of sensitive skin and variations pre- and post-treatment [21].. The scale allows the quantification of each symptom from to 0 to 10 on a numeric scale. The highest possible score is 100. This test was performed at D0 and D28.
5.4. Self-Assessment
To self-assess the evolution of their facial skin sensitivity after 28 days, volunteers had to answer the following questions:
- 1
- Do you think that your facial skin is less sensitive?
- 2
- Do you think that your facial skin is less prone to irritation?
- 3
- Would you say that your facial skin is less reactive? (reactive = stinging, burning sensations, or itching, whether associated or not with redness/flushes)
- 4
- Would you say that your facial skin is less prone to general discomfort (tautness sensations, tingling sensations...)?
- 5
- Does your facial skin less react * less to emotions or stress?
- 6
- Does your facial skin less react * less to external aggressions (cold, heat, wind, sun, temperature changes...). The result must be ticked “YES or NO”.
5.5. Statistical Analyses
The analyses were carried out with SPSS 21. Descriptive statistics are provided for each in vitro and ex vivo parameter. A statistical comparison was carried out with the Student-t Test or Wilcoxon test (depending on the normality of the distribution). A p value < 0.05 was considered statistically significant. For the microbiota data, we evaluated the changes between the data noted at the beginning and end of the study using the percentage of change.
6. Results and Discussion
6.1. Microbiota
Several skin disorders are characterized by shifts in the skin microbiome, most notably, the loss of protective bacteria, overgrowth of pathogenic bacteria, and increased severity correlating with decreased microbial diversity [23]. At the phylum level, our data showed that reactive and sensitive skin samples were mostly composed of Actinobacteria, Proteobacteria, Firmicutes, and Bacteroidetes, accounting for more than 97% of the total sequencing read in all samples. We also noted that 15 days of application of the HD extract maintained these major phyla (Figure 1).
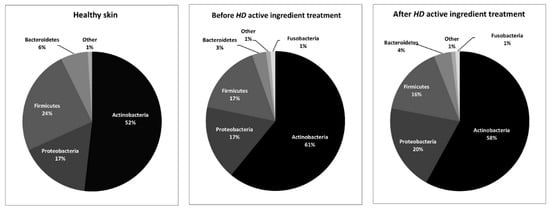
Figure 1.
Comparison of the main phyla in healthy skin [20] and before and after Halymenia durvillei (HD) active ingredient treatment.
This observation is in accordance with the literature that described these phyla as common and dominant on healthy human skin [24] and more particularly in facial cheek skin, suggesting that reactive and sensitive skin is not characterized by a modification of the phyla level.
We also observed that reactive skin was characterized by a low Shannon index when compared to healthy skin [2]. After 15 days of HD active ingredient, this index only decreased in areas treated by the placebo and remained stable for cheeks treated with the active product (Figure 2).
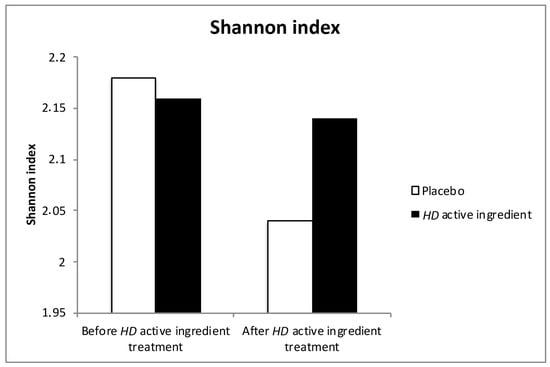
Figure 2.
Evolution of the Shannon Diversity index before and after HD active ingredient treatment. White bar for Placebo, black bar for HD active ingredient
Genus level analysis revealed significant abundance variations by age, disease severity, locality, and immune response. Among the genii, Streptococcus, Cutibacterium, and Corynebacterium appeared to be specific signatures for atopic dermatitis in children, adolescents, and adults, respectively [25]. The genus Corynebacterium currently comprises almost 100 species and represents a very diverse collection of taxonomically-related Gram-positive bacteria. Corynebacteria promote a dramatic increase in the number and activation of a defined subset of γδ T-cells. This effect is long-lasting, occurs independently of other microbes, and is partly mediated by interleukin (IL-23). Under steady-state conditions, the impact of Corynebacterium is discrete and non-inflammatory. However, under specific conditions, Corynebacterium alone promotes inflammation [26]. Other genus members, such as Chryseobacterium, are able to produce a variety of enzymes, including the relatively heat-stable keratinases. They also produce flexirubins, unique types of bacterial pigments, which are used for the chronic skin disease eczema [27].
To our knowledge, no data in line with this genus is available for sensitive skin. Our study showed that reactive and sensitive skin is characterized by a low proportion of the Chryseobacterium genus and high proportion of the Corynebacterium genus. The average taxonomic composition of skin microbial communities with HD active ingredient treatment over 28 days decreased in terms of the amount of Corynebacterium genus and increased in terms of the amount of Chryseobacterium genus (Figure 3).
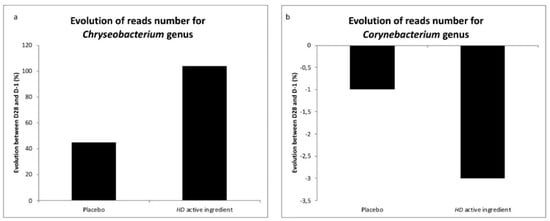
Figure 3.
Evolution of the reads number for the Chryseobacterium and Corynebaterium genii during the investigation. a: Evolution of reads number for Chryseobacterium placebo versus HD active ingredient. b: Evolution of reads number for Corynebacterium placebo versus HD active ingredient.
At a species level, Corynebacterium kroppenstedtii (C. kroppenstedtii) has been classified by chemotaxonomic data as a member of the genus Corynebacterium. This species is part of the normal skin microbiota, but increased levels have been associated with skin redness. S. epidermidis is considered a bacterium that is beneficial to skin health. Indeed, an increase in its concentration on the skin can lead to an increased lipid content and reduced water evaporation, improving skin moisture [28]. Moreover, S. epidermidis is involved in the inhibition of Cutibacterium acnes. (C. acnes) inflammation and may enhance the production of antimicrobial peptides to defend against infections [29]. Our results showed that Actinobacteria are mainly represented by C. acnes (72.13%) and C. kroppenstedtii (13.23%), a species particularly observed in the case of redness, the main criteria for volunteer inclusion. The HD active ingredient decreased the amount of this species by 10% (Figure 4). Firmicutes were mainly represented by Staphylococcus epidermidis (35%), with our active ingredient improving this species (12%).
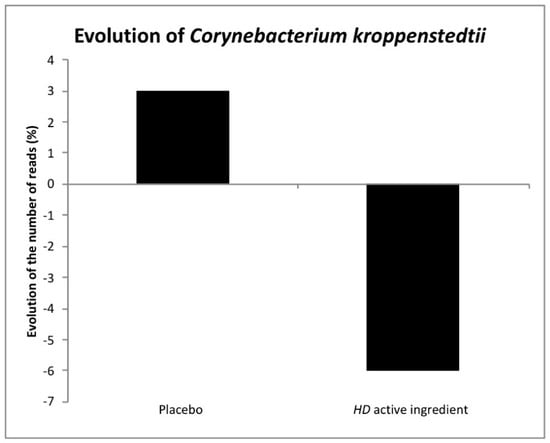
Figure 4.
Evolution of Corynebacterium kroppenstedtii after the use of the HD active ingredient extract.
6.2. Ex Vivo
Pathomechanisms of sensitive skin are very complicated. It is generally accepted that the development of sensitive skin could be attributable to epidermal dysfunction, increased neural and/or vascular reactivity, and inflammation. The involvement of transient receptor potential (TRP) vanilloid 1 and TRP melastatin 8 in the sensations of stinging, pain, and itching has also been recognized [30]. In patients with sensitive skin, the increase in the sensorineural impulse is interpreted as unpleasant sensations. Neurotransmitters and their receptors that regulate the neuroendocrine system of the skin, present in keratinocytes, recognize the stimuli and lead to the release of neurotransmitters as substance P, neurotransmitters inducing the vasodilatation and degranulation of mast cells, which also act on sensory perception [9]. The HD active ingredient induced a non-significant decrease of TNF-α (-21%) compared to the control after 6 days of treatment. Simultaneously, the expression of TRPV-1 in the epidermis significantly decreased (−67%; p < 0.001). A significant decrease of NK1-R (−43%; p < 0.01) and a decrease of VEGF (−16%) were also reported when compared to the control. All these results suggested that the HD active ingredient has an effect on pain and itching.
6.3. In Vivo Study
6.3.1. Stinging Test
This test has been widely employed as a diagnostic tool of sensitive skin. A total of 90% of the women declared that the product had a soothing effect when applied just after the stinging test at D0.
After 28 days of HD application, the subject’s skin was less susceptible to the irritant action of lactic acid in comparison with the initial conditions, showing that the HD ingredient has a protective effect (data not shown).
6.3.2. Blood Microcirculation
Erythrosis takes the form of diffuse, persistent red patches on the face. It appears as the result of exposome, such as shaving or temperature fluctuations. It mainly occurs around the nose, chin, forehead, and cheeks, and is associated with sensitive skin. In this study, a significant decrease of the surface of erythrosis after 28 days of application of the HD active ingredient was reported (−15%; p < 0.05) (Figure 5).
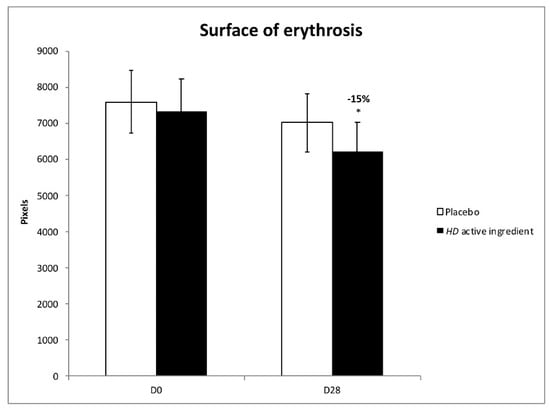
Figure 5.
Effect of the HD active ingredient on erythrosis (* p < 0.05 vs. D0). White bar for Placebo, black bar for HD active ingredient
6.3.3. Sensitive Scale
Patients with sensitive skin often experience unpleasant sensations, such as burning, stinging, itching, and tautness, but without objective signs [31]. The methods used to evaluate sensitive skin include subjective, semi-subjective, and objective evaluation. Subjective evaluation mainly relies on self-assessment questionnaires. The Sensitive Scale (SS-10) used in this study is a new method employed to measure the severity of skin sensitivity and therapeutic efficacy [21]. Significant decreases of skin sensitivity, irritability, heat sensations, pain, flushes, and redness were noted after 28 days of repeated applications of the HD active ingredient (Table 1).

Table 1.
Evolution of skin irritability, sensitivity, heating, pain, flushes, and redness state from D0 to D28 using the SS-10 scale [21] (* p < 0.05, *** p < 0.001 vs. D0).
Finally, a self-assessment relating to skin sensitivity has been proposed. The percentage of women who described their skin as less sensitive increased at D28 (Table 2), compared with the placebo. This result is in line with the results obtained with the SS-10.

Table 2.
Percentage of women who stated that they had sensitive or reactive skin at D0 and percentage of women who stated that they had less sensitive or reactive skin at D28.
7. Conclusions
Subjects with sensitive skin present discomfort such as itching, stinging, burning, or pain, which has a high impact on quality of life. Our data demonstrated that specific bacterial genii and species are associated with sensitive and reactive skin. The HD active ingredient is able to modulate the skin microbiota, reducing specific species, decreasing redness, and maintaining bacterial diversity and the proportion of individuals in each phylum. In vivo tests also showed that the HD active ingredient calms feelings of discomfort and redness, and controls the microvascularization. Lastly, the HD active ingredient fights against red skin conditions by reducing the levels of Corynebacterium kroppendenstedtii, which is a liphophilic bacteria associated with age and skin redness if at increased levels [23] (Figure 4).
Author Contributions
Conceptualization, E.F. and J.-Y.B.; data curation, J.-P.C., C.M., and S.G.; formal analysis, C.V. and A.D.-Z.; funding acquisition, J.-Y.B.; investigation, E.F., A.D.-Z., and S.G.; methodology, E.F. and S.G.; software, E.F.; supervision, J.-Y.B.; writing—original draft, E.F.; writing—review and editing, E.F.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Natsuga, K. Epidermal Barriers. Cold Spring Harb. Perspect. Med. 2014, 4, a018218. [Google Scholar] [CrossRef] [PubMed]
- Grice, E.A.; Kong, H.H.; Renaud, G.; Young, A.C.; NISC Comparative Sequencing Program; Bouffard, G.G.; Blakesley, R.W.; Wolfsberg, T.G.; Turner, M.L.; Segre, J.A. A diversity profile of the human skin microbiota. Genome Res. 2008, 18, 1043–1050. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, H.E.; Bhatia, N.D.; Friedman, A.; Eng, R.M.; Seite, S. The Role of Cutaneous Microbiota Harmony in Maintaining a Functional Skin Barrier. J. Drugs Dermatol. JDD 2017, 16, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Baviera, G.; Leoni, M.C.; Capra, L.; Cipriani, F.; Longo, G.; Maiello, N.; Ricci, G.; Galli, E. Microbiota in Healthy Skin and in Atopic Eczema. BioMed Res. Int. 2014, 436921. [Google Scholar] [CrossRef]
- Zaidi, A.K.; Spaunhurst, K.; Sprockett, D.; Thomason, Y.; Mann, M.W.; Fu, P.; Ammons, C.; Gerstenblith, M.; Tuttle, M.S.; Popkin, D.L. Characterization of the facial microbiome in twins discordant for rosacea. Exp. Dermatol. 2018, 27, 295–298. [Google Scholar] [CrossRef]
- Benhadou, F.; Mintoff, D.; Schnebert, B.; Thio, H. Psoriasis and Microbiota: A Systematic Review. Diseases 2018, 6, 47. [Google Scholar] [CrossRef]
- Seite, S.; Misery, L. Skin sensitivity and skin microbiota: Is there a link? Exp. Dermatol. 2018, 27, 1061–1064. [Google Scholar] [CrossRef]
- Roussaki-Schulze, A.V.; Zafiriou, E.; Nikoulis, D.; Klimi, E.; Rallis, E.; Zintzaras, E. Objective biophysical findings in patients with sensitive skin. Drugs Exp. Clin. Res. 2005, 31, S17–S24. [Google Scholar]
- Duarte, I.; Silveira, J.E.P.S.; Hafner, M.F.S.; Toyota, R.; Pedroso, D.M.M. Sensitive skin: Review of an ascending concept. An. Bras. Dermatol. 2017, 92, 521–525. [Google Scholar] [CrossRef]
- Krutmann, J.; Bouloc, A.; Sore, G.; Bernard, B.A.; Passeron, T. The skin aging exposome. J. Dermatol. Sci. 2017, 85, 152–161. [Google Scholar] [CrossRef]
- N’Diaye, A.; Mijouin, L.; Hillion, M.; Diaz, S.; Konto-Ghiorghi, Y.; Percoco, G.; Chevalier, S.; Lefeuvre, L.; Harmer, N.J.; Lesouhaitier, O.; et al. Effect of Substance P in Staphylococcus aureus and Staphylococcus epidermidis Virulence: Implication for Skin Homeostasis. Front. Microbiol. 2016, 7, 506. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.M.; Kim, Y.K.; Kim, K.H.; Park, S.J.; Kim, S.J.; Chung, J.H. A novel role for the TRPV1 channel in UV-induced matrix metalloproteinase (MMP)-1 expression in HaCaT cells. J. Cell. Physiol. 2009, 219, 766–775. [Google Scholar] [CrossRef] [PubMed]
- Taieb, C.; Auges, M.; Georgescu, V.; Cullell, N.P.; Miséry, L. Sensitive skin in Brazil and Russia: An epidemiological and comparative approach. Eur. J. Dermatol. 2014, 24, 372–376. [Google Scholar] [CrossRef] [PubMed]
- Raj, N.; Voegeli, R.; Rawlings, A.V.; Doppler, S.; Imfeld, D.; Munday, M.R.; Lane, M.E. A fundamental investigation into aspects of the physiology and biochemistry of the stratum corneum in subjects with sensitive skin. Int. J. Cosmet. Sci. 2017, 39, 2–10. [Google Scholar] [CrossRef] [PubMed]
- N’Diaye, A.; Gannesen, A.; Borrel, V.; Maillot, O.; Enaut, J.; Racine, P.J.; Plakunov, V.; Chevalier, S.; Lesouhaitier, O.; Feuilloley, M.G.J. Substance P and Calcitonin Gene-Related Peptide: Key Regulators of Cutaneous Microbiota Homeostasis. Front. Endocrinol. 2017, 8, 15. [Google Scholar] [CrossRef] [PubMed]
- Guinot, C.; Malvy, D.; Mauger, E.; Ezzedine, K.; Latreille, J.; Ambroisine, L.; Tenenhaus, M.; Preziosi, P.; Morizot, F.; Galan, P.; et al. Self-reported skin sensitivity in a general adult population in France: Data of the SU.VI.MAX cohort. J. Eur. Acad. Dermatol. Venereol. 2006, 20, 380–390. [Google Scholar] [CrossRef] [PubMed]
- Fenoradosoa, T.A.; Delattre, C.; Laroche, C.; Wadouachi, A.; Dulong, V.; Picton, L.; Andriamadio, P.; Michaud, P. Highly sulphated galactan from Halymenia durvillei (Halymeniales, Rhodophyta), a red seaweed of Madagascar marine coasts. Int. J. Biol. Macromol. 2009, 45, 140–145. [Google Scholar] [CrossRef]
- Salsberg, J.; Andriessen, A.; Abdulla, S.; Ahluwalia, R.; Beecker, J.; Sander, M.; Schachter, J. A review of protection against exposome factors impacting facial skin barrier function with 89% mineralizing thermal water. J. Cosmet. Dermatol. 2019, 18, 815–820. [Google Scholar] [CrossRef]
- Schoelermann, A.M.; Jung, K.A.; Buck, B.; Grönniger, E.; Conzelmann, S. Comparison of skin calming effects of cosmetic products containing 4-t-butylcyclohexanol or acetyl dipeptide-1 cetyl ester on capsaicin-induced facial stinging in volunteers with sensitive skin. J. Eur. Acad. Dermatol. Venereol. 2016, 30, 18–20. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric Method for Determination of Sugars and Related Substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Misery, L.; Jean-Decoster, C.; Mery, S.; Georgescu, V.; Sibaud, V. A new ten-item questionnaire for assessing sensitive skin: The Sensitive Scale-10. Acta Derm.-Venereol. 2014, 94, 635–639. [Google Scholar] [CrossRef] [PubMed]
- Sainthillier, J.-M.; Gharbi, T.; Muret, P.; Humbert, P. Skin capillary network recognition and analysis by means of neural algorithms. Skin Res. Technol. 2005, 11, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Van Rensburg, J.J.; Lin, H.; Gao, X.; Toh, E.; Fortney, K.R.; Ellinger, S.; Zwickl, B.; Janowicz, D.M.; Katz, B.P.; Nelson, D.E.; et al. The Human Skin Microbiome Associates with the Outcome of and Is Influenced by Bacterial Infection. mBio 2015, 6, e01315-15. [Google Scholar] [CrossRef] [PubMed]
- Grice, E.A.; Segre, J.A. The skin microbiome. Nat. Rev. Microbiol. 2011, 9, 244–253. [Google Scholar] [CrossRef]
- Ramadan, M.; Solyman, S.; Yones, M.; Abdallah, Y.; Halaby, H.; Hanora, A. Skin Microbiome Differences in Atopic Dermatitis and Healthy Controls in Egyptian Children and Adults, and Association with Serum Immunoglobulin, E. OMICS J. Integr. Biol. 2019, 23, 247–260. [Google Scholar] [CrossRef]
- Ridaura, V.K.; Bouladoux, N.; Claesen, J.; Chen, Y.E.; Byrd, A.L.; Constantinides, M.G.; Merrill, E.D.; Tamoutounour, S.; Fischbach, M.A.; Belkaid, Y. Contextual control of skin immunity and inflammation by Corynebacterium. J. Exp. Med. 2018, 215, 785–799. [Google Scholar] [CrossRef]
- Venil, C.K.; Zakaria, Z.A.; Ahmad, W.A. Optimization of culture conditions for flexirubin production by Chryseobacterium artocarpi CECT 8497 using response surface methodology. Acta Biochim. Pol. 2015, 62, 185–190. [Google Scholar] [CrossRef]
- Nodake, Y.; Matsumoto, S.; Miura, R.; Honda, H.; Ishibashi, G.; Matsumoto, S.; Dekio, I.; Sakakibara, R. Pilot study on novel skin care method by augmentation with Staphylococcus epidermidis, an autologous skin microbe—A blinded randomized clinical trial. J. Dermatol. Sci. 2015, 79, 119–126. [Google Scholar] [CrossRef]
- Xia, X.; Li, Z.; Liu, K.; Wu, Y.; Jiang, D.; Lai, Y. Staphylococcal LTA-Induced miR-143 Inhibits Propionibacterium acnes-Mediated Inflammatory Response in Skin. J. Investig. Dermatol. 2016, 136, 621–630. [Google Scholar] [CrossRef]
- Jain, A.; Brönnecke, S.; Kolbe, L.; Stäb, F.; Wenck, H.; Neufang, G. TRP-channel-specific cutaneous eicosanoid release patterns. Pain 2011, 152, 2765–2772. [Google Scholar] [CrossRef]
- Ding, D.M.; Tu, Y.; Man, M.Q.; Wu, W.J.; Lu, F.Y.; Li, Y.; Yang, J.T.; Jin, Y.M.; Yang, C.Y.; He, L. Association between lactic acid sting test scores, self-assessed sensitive skin scores and biophysical properties in Chinese females. Int. J. Cosmet. Sci. 2019, 41, 398–404. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).