Abstract
In this study, we examined the effect of a hot-water extract of coccolithophore Pleurochrysis carterae on melanogenesis in B16F1 and B16F10 melanoma cells. P. carterae extract inhibited the α-melanocyte-stimulating hormone (α-MSH)-enhanced melanin synthesis in B16F1 melanoma cells. P. carterae also inhibited unstimulated melanin synthesis in B16F10 melanoma cells. Western blotting showed that the P. carterae extract inhibited tyrosinase and microphthalmia-associated transcription factor (MITF) in a dose-dependent manner. The reporter assay also revealed a decline in the tyrosinase promoter activity in the presence of P. carterae extract. Furthermore, quantitative real-time RT-PCR analysis showed that P. carterae extract downregulated the mRNA levels of tyrosinase and MITF. Finally, our study demonstrated that the hot-water extract of P. carterae inhibits melanin synthesis via the down-regulation of MITF mRNA level. Our findings indicate that P. carterae extract could be a possible cosmetic ingredient.
1. Introduction
Coccolithophore (Haptophyta) are a group of unicellular marine microalgae that have unique calcium carbonate scales called coccoliths [1]. The coccolithophore, Pleurochrysis carterae (Figure 1), is currently regarded as one of the most promising candidates for biodiesel production as it has a high lipid content [2]. Previously, Takenaka et al. demonstrated the safety of the freeze-dried powder of algal cells using oral administration in rats [3]. The calcium-rich P. carterae has already been used as a calcium supplement [3].
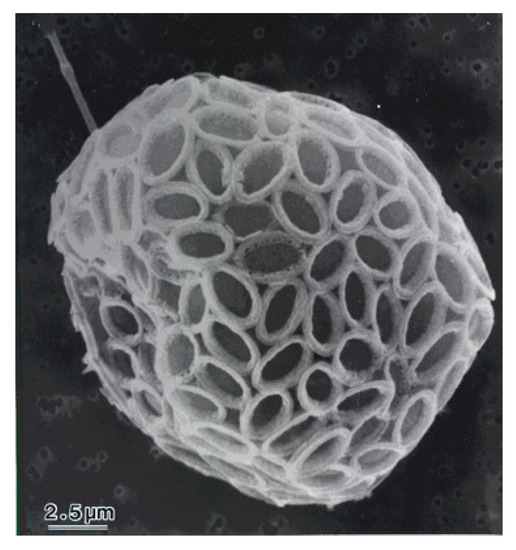
Figure 1.
SEM image of Pleurochrysis carterae.
Melanin has various important biological functions, including protection of the skin from ultraviolet light, and is one of the skin color determinants [4]. Tyrosinase is a rate-limiting enzyme and plays a crucial role in melanin synthesis under the control of various signals. cAMP-protein kinase A (PKA) dependent pathway is one of the most important pathways for activation of melanogenesis. UV and α-MSH activate adenyl cyclase to increase the intracellular cAMP level, which triggers the activation of PKA. PKA phosphorylates the cAMP response element-binding protein (CREB), which binds to the CRE consensus motif in the microphthalmia-associated transcription factor (MITF) promoter to upregulate MITF gene expression [5]. MITF is a transcription factor belonging to the family of basic helix-loop-helix leucine zipper proteins that can transactivate genes involved in melanin synthesis [6].
Many anti-melanogenic agents have been reported. For example, we previously demonstrated that several nonsteroidal anti-inflammatory drugs can inhibit melanin synthesis via downregulation of gene transcription [7]. However, there are no reports available on the effect of coccolithophore extract on melanin synthesis to date. Therefore, in this study, we attempted to elucidate the effect of P. carterae extract on melanogenesis.
2. Materials and Methods
2.1. Preparation of the Hot-Water Extract of Pleurochrysis carterae
P. carterae (Haptophyta, collected from Naha, Okinawa, Japan) was grown in outdoor open raceway ponds at the MAC Miyako farm in Okinawa (Figure 2). The culture medium contained 50.5 mg of KNO3, 8.7 mg of K2HPO4, 10 mg of Fe-EDTA, 3.6 mg of MnCl2·4H2O, 44 µg of ZnSO4·7H2O, 20 µg of CoCl2·6H2O, 19.6 µg of CuSO4·5H2O, 12.6 µg of Na2MoO4·2H2O, 0.2 mg of Thiamine HCl, 1 µg of Biotin, and 0.2 µg of Cyanocobalamin in 1 L of seawater.

Figure 2.
Raceway pond used for cultivation of P. carterae on Miyako Island, Okinawa, Japan.
The cells were harvested by centrifugation (10,000 rpm for 15 min), immediately sterilized by heating (90 °C, 30 min), and then dehydrated by freeze-drying. Thirty grams of P. carterae powder was mixed with 1000 mL water at 95 °C for 60 min and then filtered (GA-100, ADVANTEC, Tokyo, Japan) after cooling. This aqueous extract was concentrated using an evaporator. The yield of this extract from dry algae was 39.5%. Before starting the experiments, the hot-water extract was sterilized by filtration using a Millex filter unit (Millipore, Burlington, MA, USA).
2.2. Cell Culture
B16F1 and B16F10 melanoma cells (Riken BioResource Center, Tsukuba, Japan) were cultured in Dulbecco’s modified Eagle’s medium (DMEM; Sigma, St. Louis, MO, USA) supplemented with 5% fetal bovine serum, 50 U/mL penicillin, and 100 µg/mL streptomycin at 37 °C in a humidified atmosphere containing 5% CO2.
2.3. Crystal Violet Assay
B16F1 cells were seeded in a 96-well plate (1000 cells per well), and cell growth was assessed for 4 days in the absence or presence of the P. carterae extract. After treatment, cells were washed twice with 200 µL phosphate buffered saline (PBS) and fixed with 40 µL of 20% methanol in PBS containing 0.1% crystal violet (Sigma). The crystal violet solution was discarded, the cells were washed twice with PBS, and solubilized in 200 µL DMSO. The color intensity was evaluated at 595 nm absorbance.
2.4. Melanin Content
The melanin content was measured as described previously [8]. B16F1 and B16F10 cells were incubated in 60 mm dishes at a density of 1 × 105 cells for 24 h, then P. carterae extract was added in the presence or absence of 10 nM α-MSH for 4 days. After treatment, the cells were solubilized in boiling 2 M NaOH for 20 min. Spectrophotometric analysis of the melanin content was performed at 405 nm absorbance, and melanin content was normalized using synthetic melanin (Sigma).
2.5. Mushroom Tyrosinase Activity
The direct effect of P. carterae extract on the tyrosinase activity was determined with a cell-free assay system using mushroom tyrosinase [9]. First, 300 µL of 0.5 mg/mL L-3,4-dihydroxyphenylalanine (L-DOPA) solution, 50 µL of P. carterae hot-water extract, and 1.1 mL of 0.1 M phosphate buffer (pH 6.8) were mixed. The mixture was preincubated at 25 °C for 10 min before 50 µL of 1000 units/mL mushroom tyrosinase (Sigma) in aqueous solution was added, and the reaction was monitored at 475 nm. The percentage of tyrosinase activity was calculated as follows: 100 − [(A − B)/A × 100], where A represents the difference in the absorbance of the control sample between an incubation time of 0.5 and 1.0 min, and B represents the difference in the absorbance of the test sample over the same incubation period.
2.6. Western Blotting
Western blot analyses were performed to determine protein expression. For this, P. carterae extract-treated B16F1 melanoma cells were lysed using the RIPA buffer system (Santa Cruz Biotechnology, St. Louis, MO, USA). Whole-cell lysates were separated using 7.5% SDS-PAGE gel electrophoresis and transferred onto polyvinylidene fluoride membranes (Millipore). After blocking with 5% skimmed milk containing 0.1% Tween 20, the membranes were probed overnight with specific primary antibodies at 4 °C and then incubated with horseradish peroxidase-conjugated secondary antibodies. Bound antibodies were detected using ImmunoStar® Zeta (Fujifilm Wako, Osaka, Japan). Antibodies against tyrosinase, Mitf, β-actin, and anti-goat IgG and anti-mouse IgG were purchased from Santa Cruz Biotechnology. Chemiluminescence signal was observed using WSE-6100 LuminoGraph I (ATTO, Tokyo, Japan).
2.7. Luciferase Reporter Assay
A reporter plasmid containing the mouse tyrosinase promoter was constructed according to the procedure described by Shirasugi et al. [10]. B16F1/pTyr-Luc cells were incubated at a density of 2 × 103 cells in a 96-well plate for 24 h and then treated with P. carterae extract in the presence of α-MSH for another 24 h. After treatment, the cells were washed with PBS and the luciferase reporter assay was performed using the Bright-GloTM Luciferase assay system (Promega, Madison, WI, USA). The luciferase value was normalized to that of the protein concentration to obtain the relative luciferase activity (%) against the control that was set as 100%. The protein concentration in the supernatant was determined using the XL-Bradford reagent (APRO Life Science Institute, Naruto, Japan).
2.8. Quantitative RT-PCR
To investigate the effects of P. carterae extract on the melanogenic gene expression, qRT-PCR analysis was performed. B16F1 melanoma cells were treated with or without P. carterae extract and stimulated with α-MSH (10 nM).
After treatment, the total RNA was extracted from the cells using ISOGEN 2 (NIPPON GENE, Tokyo, Japan). Reverse-transcription and cDNA amplification were carried out using the TAKARA RT-PCR kit (TaKaRa, Kyoto, Japan).
Primers used for quantitative RT-PCR were as follows: MITF upstream 5’-CGC CTG ATC TGG TGA ATC G-3’, downstream 5’-CCT GGC TGC AGT TCT CAA GAA-3’; tyrosinase upstream 5’-TTG CCA CTT CAT GTC ATC ATA GAA TAT T-3’, downstream 5’-TTT ATC AAA GGT GTG ACT GCT ATA CAA AT-3’; GAPDH upstream 5’-CGT CCC GTA GAC AAA ATG GT-3’, downstream 5’-TTG ATG GCA ACA ATC TCC AC-3’. PCR amplification was conducted using a 7500 fast real-time PCR system (Thermo Fisher Scientific, Waltham, MA, USA), Power UP SYBR Green Master Mix (Thermo Fisher Scientific), synthesized cDNA, primers, and distilled water according to the manufacturer’s instructions. The real-time PCR cycle conditions were as follows: 1 cycle at 95 °C for 2 min, followed by 40 cycles at 95 °C for 10 s and 60 °C for 30 s. All samples were run in triplicates, and all data are expressed relative to the corresponding calculated GAPDH threshold cycle (ΔCt) values. The calibrated ΔCt values (ΔΔCt) for each sample and for the internal control (GAPDH) were calculated according to the 2−ΔΔCt method [11] [ΔΔCt = ΔCt treated sample −ΔCt control sample]. Results are expressed as the relative mRNA levels compared to controls.
2.9. Statistical Analysis
Unless otherwise specified, the results are expressed as the mean ± standard deviation (SD) of the data collected from at least three independent experiments. Data were analyzed using Dunnett’s test.
3. Results and Discussion
In the present study, we attempted to clarify the effect of the coccolithophore alga P. carterae on melanin synthesis. In this study, we used the B16F1 murine melanoma cells that can produce melanin in the presence of cAMP inducers such as α-MSH, forskolin, and 3-isobutyl-1-methylxanthine [12].
First, we tested the effect of P. carterae extract on cell proliferation. B16F1 melanoma cells were cultured in the presence of P. carterae extract for 4 days, and cell growth was determined using the crystal violet assay. As shown in Figure 3, P. carterae did not show any proliferation arrest in the treated group up to a concentration of 1.6 mg/mL. This result suggests that P. carterae does not have a harmful effect on B16F1 melanoma cells.
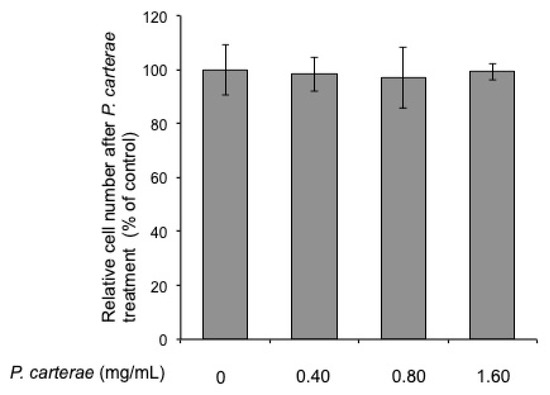
Figure 3.
Effect of P. carterae extract on cell proliferation. B16F1 melanoma cells were cultured in the presence of P. carterae extract for 4 days. After treatment, the crystal violet assay was performed as described in Materials and Methods.
Next, to investigate the effect of P. carterae on melanin synthesis, B16F1 melanoma cells were treated with P. carterae extract in the presence of α-MSH for 4 days. As shown in Figure 4a, the melanin synthesis was enhanced significantly in the α-MSH-only treated cells (approximately 97 pg/cell), whereas after treatment with P. carterae extract, the α-MSH-enhanced melanin contents were decreased (approximately 77% at 0.80 mg/mL, and 84% at 1.60 mg/mL; IC50 = 0.26 mg/mL). Moreover, the color of the lysate of the pelleted cells also became lighter (Figure 4b). Additionally, we also measured melanin contents in B16F10 melanoma cells that were treated with the P. carterae extract. In this assay, B16F10 melanoma cells were not stimulated by α-MSH. Nevertheless, the melanin contents of unstimulated B16F10 cells were extremely high (approximately 587 pg/cell). As shown in Figure 4c, the P. carterae extract inhibited melanin synthesis significantly. These results demonstrated that P. carterae extract can inhibit not only α-MSH-enhanced melanogenesis but also the baseline melanin content. We also assessed the effect of P. carterae on mushroom tyrosinase activity. As shown in Figure 4d, P. carterae extract slightly inhibited mushroom tyrosinase. These results demonstrate that the direct inhibitory effect of P. carterae on the tyrosinase activity does not contribute significantly to the anti-melanogenic effect on the melanoma cells.
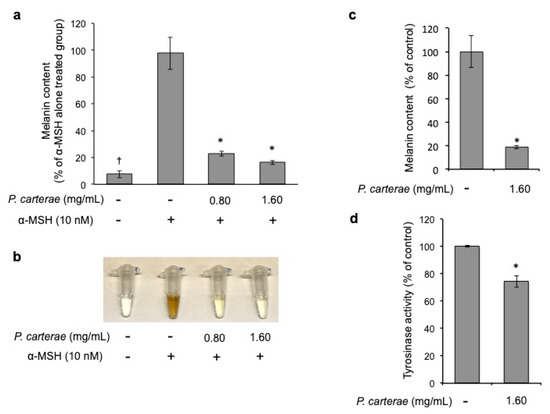
Figure 4.
Effect of P. carterae extract on melanin synthesis and mushroom tyrosinase activity. (a) B16F1 melanoma cells were treated with or without P. carterae extract in the presence of α-melanocyte-stimulating hormone (α-MSH) (10 nM) for 4 days, and the melanin content was measured. † p < 0.01 versus vehicle control, * p < 0.01 versus α-MSH-only- treated. (b) After treatment, the cells were solubilized in 2 M NaOH and then photographed. (c) B16F10 melanoma cells were treated with or without P. carterae extract for 4 days, and the melanin content was measured. * p < 0.01 versus vehicle control. (d) Effect of P. carterae extract on mushroom tyrosinase activity was measured. Results are expressed as the percentage of the control.
Thus, we hypothesize that P. carterae extract might exert its anti-melanogenic effect through the inhibition of the melanogenic protein and/or gene expression. Therefore, we performed the western blotting, luciferase reporter assay, and quantitative real-time RT-PCR to clarify the effect of P. carterae extract on protein expression and gene transcription. As shown in Figure 5, α-MSH enhanced tyrosinase protein amount significantly. In the presence of P. carterae extract, α-MSH-enhanced tyrosinase level was decreased in a dose-dependent manner. MITF protein amount was also decreased following P. carterae treatment. In this experiment, the reason why α-MSH did not affect MITF level is that MITF protein level perks at 24 h after α-MSH stimulation [8]. In the presence of the P. carterae extract, the tyrosinase promoter activity was downregulated (Figure 6). We also performed luciferase reporter assay using B16F1 melanoma cells harboring reporter plasmid containing TRP-1 promoter. In this assay, TRP-1 promoter activity was slightly inhibited by P. carterae treatment (data not shown). As shown in Figure 7a, tyrosinase mRNA was significantly decreased after treatment with P. carterae extract for 48 h (approximately 56% of control). MITF mRNA was also decreased after treatment with P. carterae extract for both treatment duration (Figure 7a,b). These results show P. carterae downregulates melanogenesis by inhibiting MITF gene transcription.
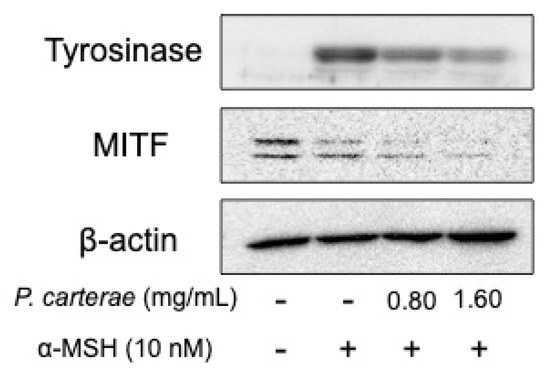
Figure 5.
Western blot analysis of the effect of P. carterae extract on melanogenic protein expression. B16F1 melanoma cells were treated with P. carterae extract for 3 days. After treatment, western blotting of tyrosinase and microphthalmia-associated transcription factor (MITF) was performed. The loading control was assessed using anti-β-actin antibody.
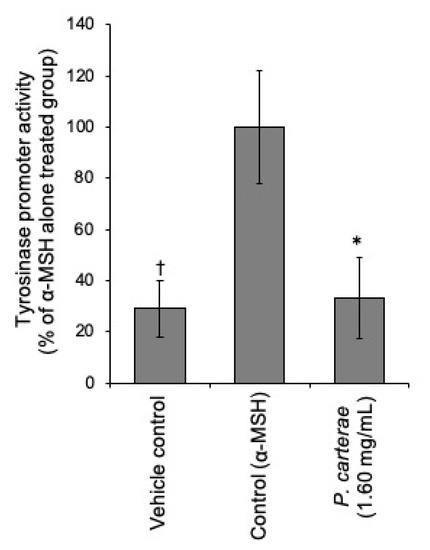
Figure 6.
Effect of P. carterae on tyrosinase promoter activity. B16F1/pTyr-Luc cells were treated with or without P. carterae extract and stimulated with α-MSH (10 nM) for 24 h before a luciferase reporter assay was performed. † p < 0.01 versus control, * p < 0.01 versus α-MSH-only-treated.
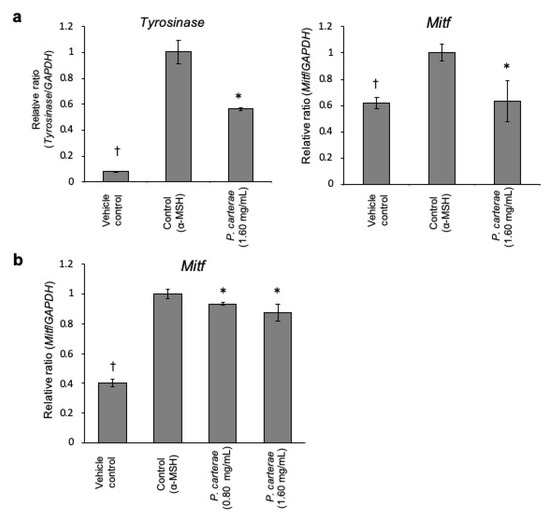
Figure 7.
Melanogenic gene mRNA levels in P. carterae-treated B16F1 melanoma cells. (a) qRT-PCR was performed to determine the tyrosinase and MITF mRNA level in B16F1 melanoma cells after treatment with P. carterae in the presence of 10 nM α-MSH for 48 h. (b) Cells were treated with P. carterae and stimulated with 10 nM α-MSH for 6 h. After treatment, qRT-PCR was carried out to detect MITF mRNA level. * p < 0.01 versus α-MSH-only-treated.
4. Conclusions
In this study, we demonstrated that hot-water extract of P. carterae can inhibit melanin synthesis in both B16F1 and B16F10 melanoma cells. P. carterae inhibits tyrosinase expression and subsequent melanogenesis because of MITF mRNA downregulation. To date, no studies have elucidated the anti-melanogenic effect of the coccolithophore algae. To the best of our knowledge, this is the first study to reveal the potential role of the coccolithophore P. carterae as an inhibitor of melanin synthesis. Our results suggest that P. carterae extract may be a suitable active ingredient in the formulation of cosmetics. However, further studies are necessary to identify the chemical structures of anti-melanin synthesis agents. Efforts are now underway to clarify the anti-melanogenic mechanism of P. carterae and other marine microalgae.
Author Contributions
Conceptualization, K.S. and H.T.; methodology, K.S.; cultivation and subsequent extraction of P. carterae, Y.Y., S.S., and H.T.; formal analysis, K.S. and H.T.; investigation, K.S.; writing—original draft preparation, K.S.; writing—review and editing, Y.Y., S.S., and H.T.; project administration, K.S. and H.T.
Funding
This research received no external funding.
Acknowledgments
We are grateful to Kazuki Chiyoda and Ayaka Hosoya for their contributions to our work.
Conflicts of Interest
The authors declare that they have no conflict of interest.
References
- Gal, A.; Sorrentino, A.; Kahil, K.; Pereiro, E.; Faivre, D.; Scheffel, A. Native-state imaging of calcifying and noncalcifying microalgae reveals similarities in their calcium storage organelles. Proc. Natl. Acad. Sci. USA 2018, 115, 11000–11005. [Google Scholar] [CrossRef] [PubMed]
- Endo, H.; Yoshida, M.; Uji, T.; Saga, N.; Inoue, K.; Nagasawa, H. Stable nuclear transformation system for the cocolithophorid alga Pleurochrysis carterae. Sci. Rep. 2016, 6, 22252. [Google Scholar] [CrossRef] [PubMed]
- Takenaka, H.; Yamaguchi, Y.; Teramoto, S.; Tanaka, N.; Hori, M.; Seki, H.; Nishimori, T.; Morinaga, T. Safety evaluation of Pleurochrysis carterae as a potential food supplement. J. Mar. Biotechnol. 1996, 3, 274–277. [Google Scholar]
- Komer, A.; Pawelek, J. Mammalian tyrosinase catalyzes three reactions in the biosynthesis of melanin. Science 1982, 217, 1163–1165. [Google Scholar]
- Corre, S.; Primot, A.; Sviderskaya, E.; Bennett, D.C.; Vaulont, S.; Goding, C.R.; Galibert, M.D. UV-induced expression of key component of the tanning process, the POMC and MC1R genes, is dependent on the p-38-activated upstream stimulating factor-1 (USF-1). J. Biol. Chem. 2004, 279, 51226–51233. [Google Scholar] [CrossRef] [PubMed]
- Yasumoto, K.; Yokoyama, K.; Takahashi, K.; Tomita, Y.; Shibahara, S. Functional analysis of microphthalmia-associated transcription factor in pigment cell-specific transcription of the human tyrosinase family genes. J. Biol. Chem. 1997, 272, 503–509. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Takahashi, H.; Toriyama, M. Depigmenting mechanism of NSAIDs on B16F1 melanoma cells. Arch. Dermatol. Res. 2011, 303, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Takei, M.; Iyota, R.; Muraoka, Y.; Yoshimura, Y. Indomethacin inhibits melanogenesis via down-regulation of MITF mRNA transcription. Biosci. Biotechnol. Biochem. 2017, 81, 2307–2313. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Morita, M.; Ichikawa, C.; Takahashi, H.; Toriyama, M. Depigmenting mechanisms of all-trans retinoic acid and retinol on B16 melanoma cells. Biosci. Biotechnol. Biochem. 2008, 72, 2589–2597. [Google Scholar] [CrossRef] [PubMed]
- Shirasugi, I.; Sakakibara, Y.; Yamasaki, M.; Nishiyama, K.; Matsui, T.; Liu, M.C.; Suiko, M. Novel screening method for potential skin-whitening compounds by a luciferase reporter assay. Biosci. Biotechnol. Biochem. 2010, 74, 2253–2258. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔC(T) method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Jiménez, M.; Kameyama, K.; Maloy, W.L.; Tomita, Y.; Hearing, V.J. Mammalian tyrosinase: Biosynthesis, processing, and modulation by melanocyte-stimulating hormone. Proc. Natl. Acad. Sci. USA 1988, 11, 3830–3834. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).