Known and Unknown Features of Hair Cuticle Structure: A Brief Review
Abstract
1. Introduction
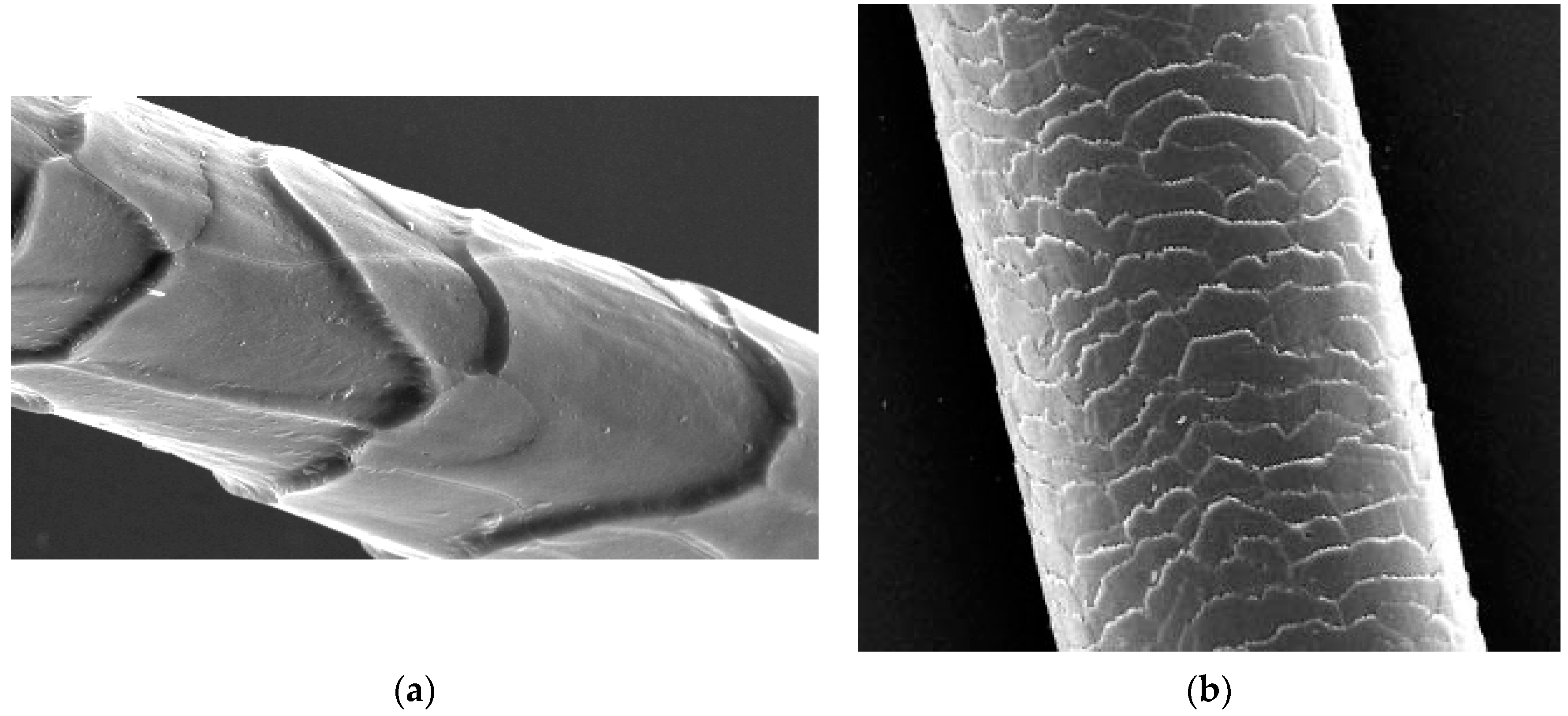
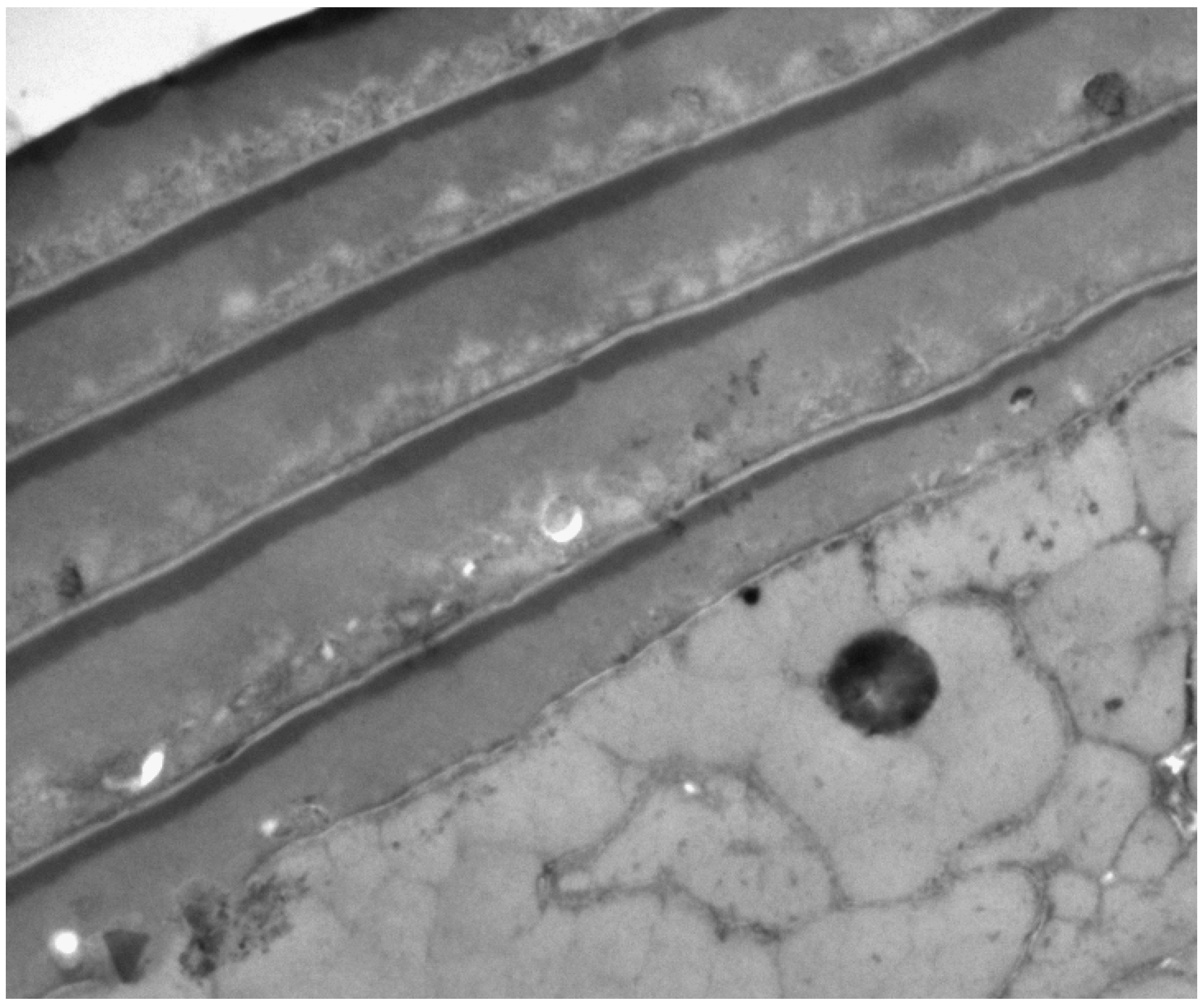
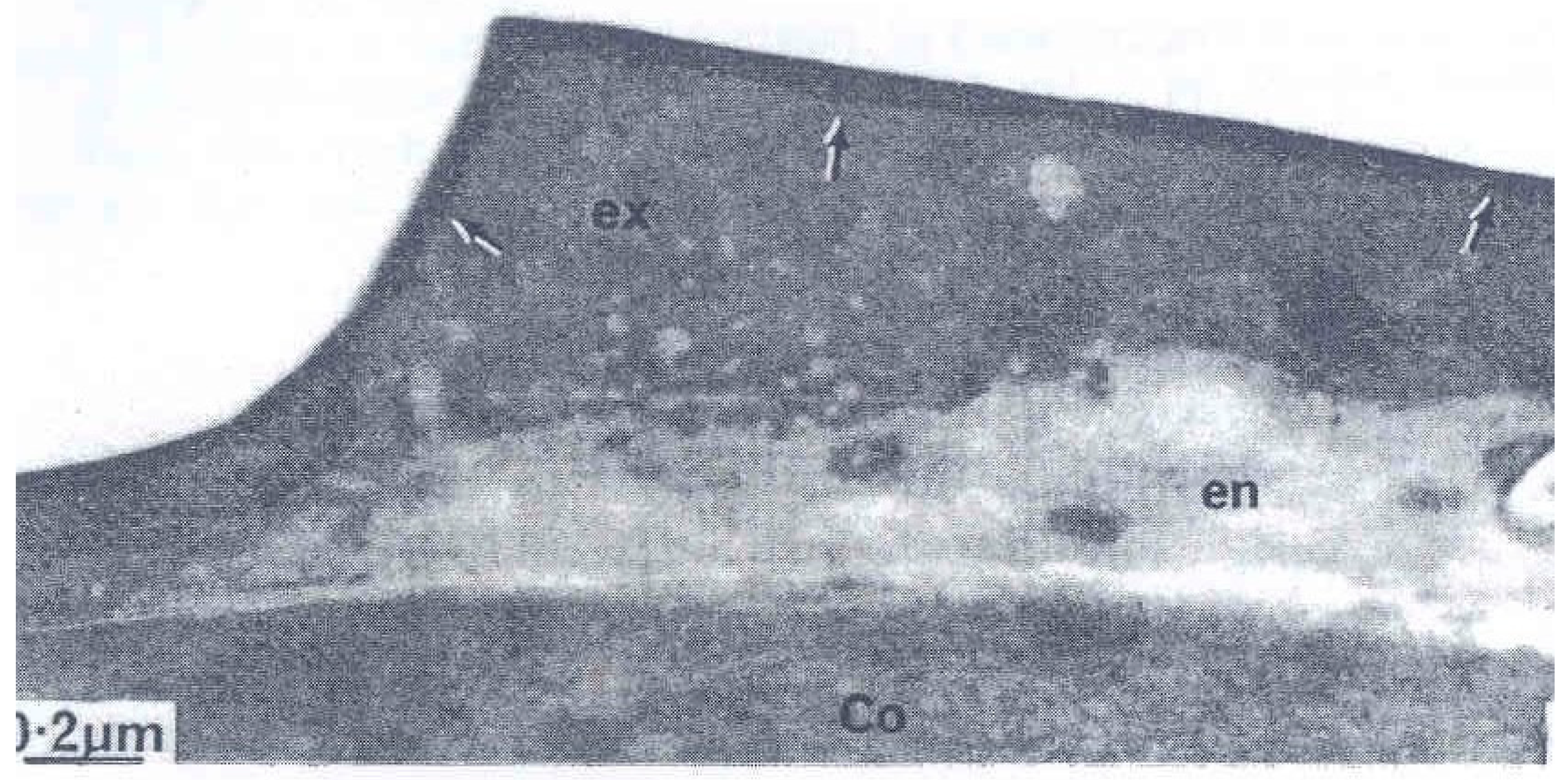
2. The Three Layers in a Cuticle Cell
3. The Epicuticle
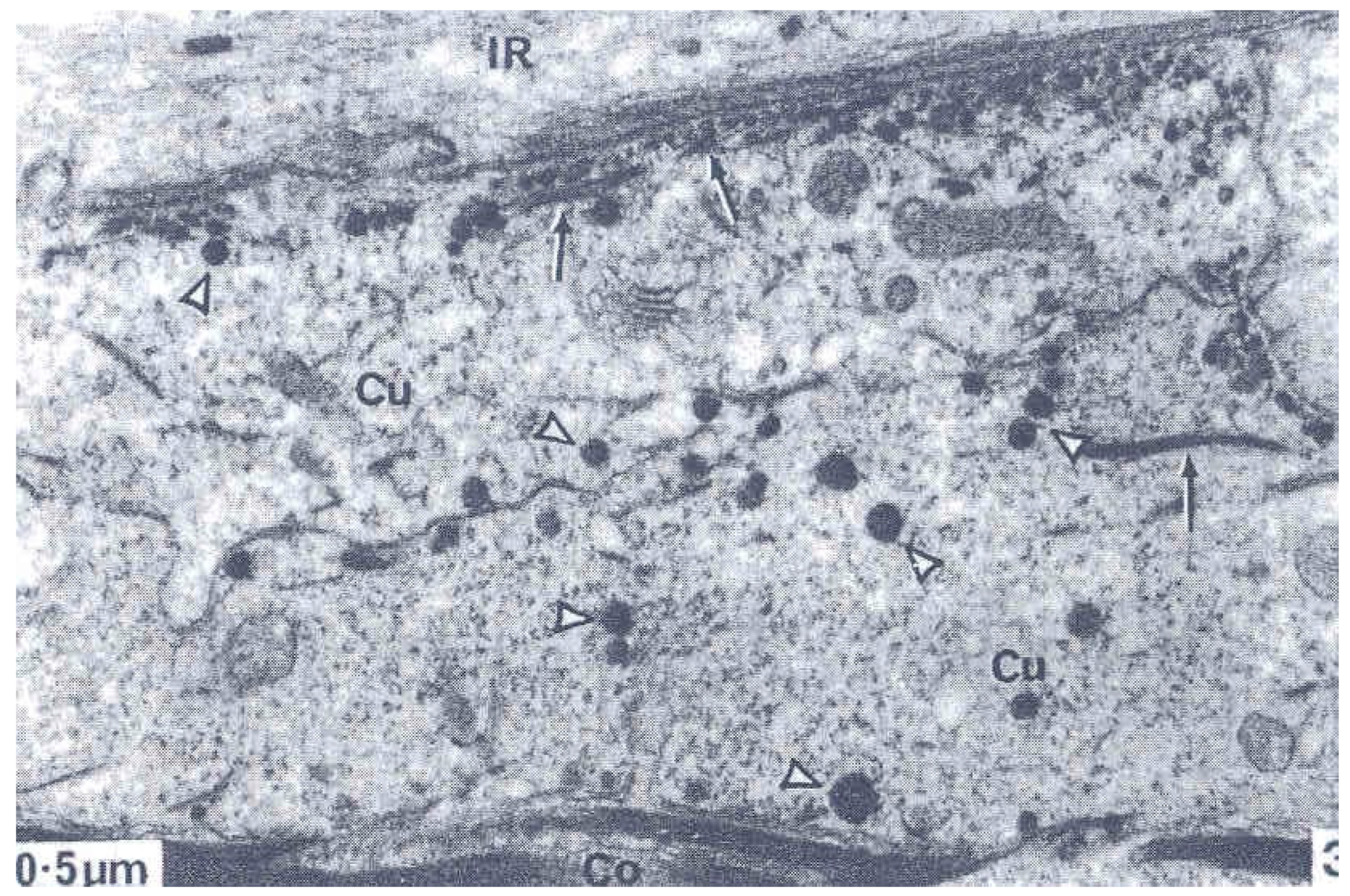
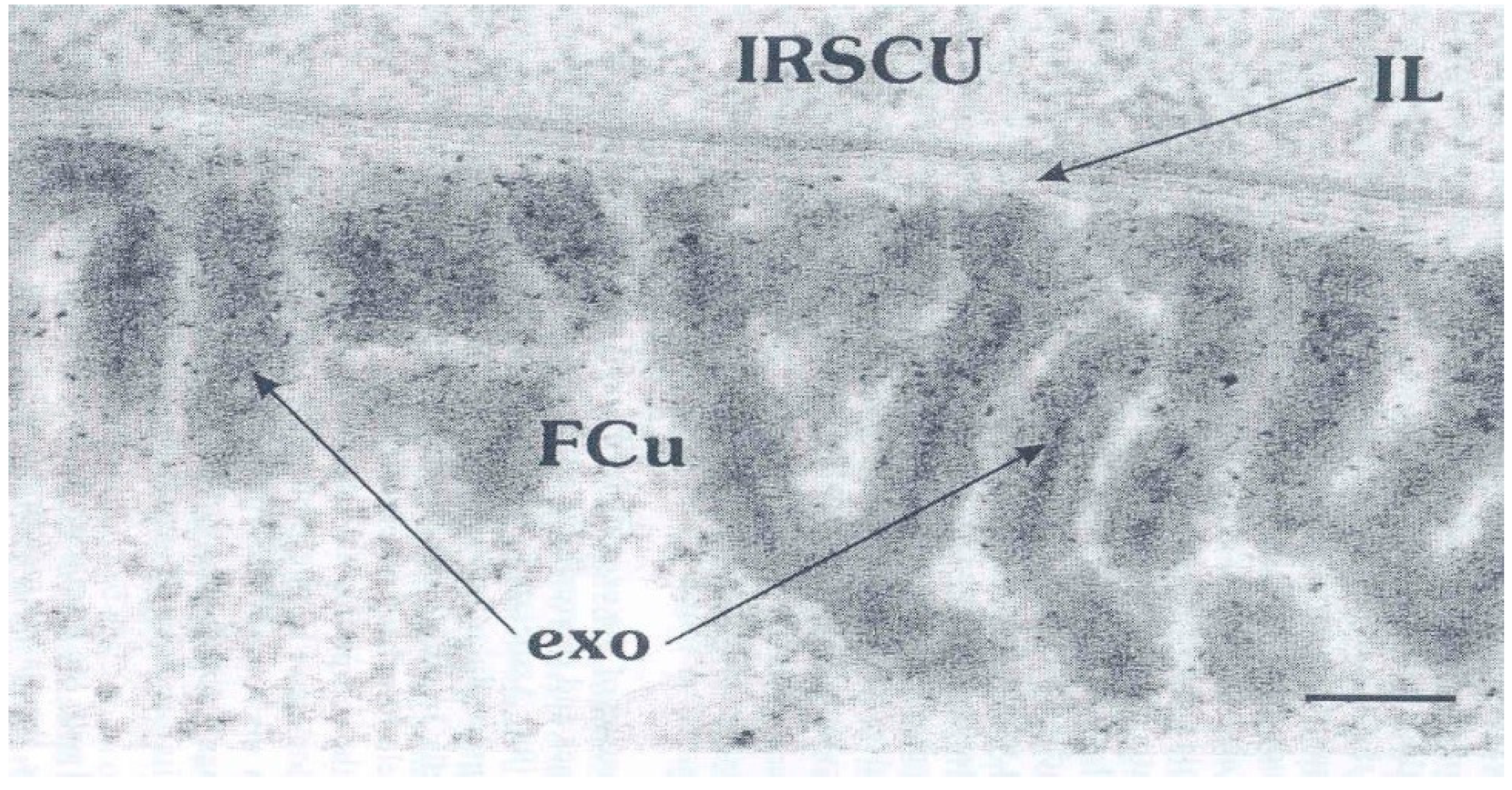
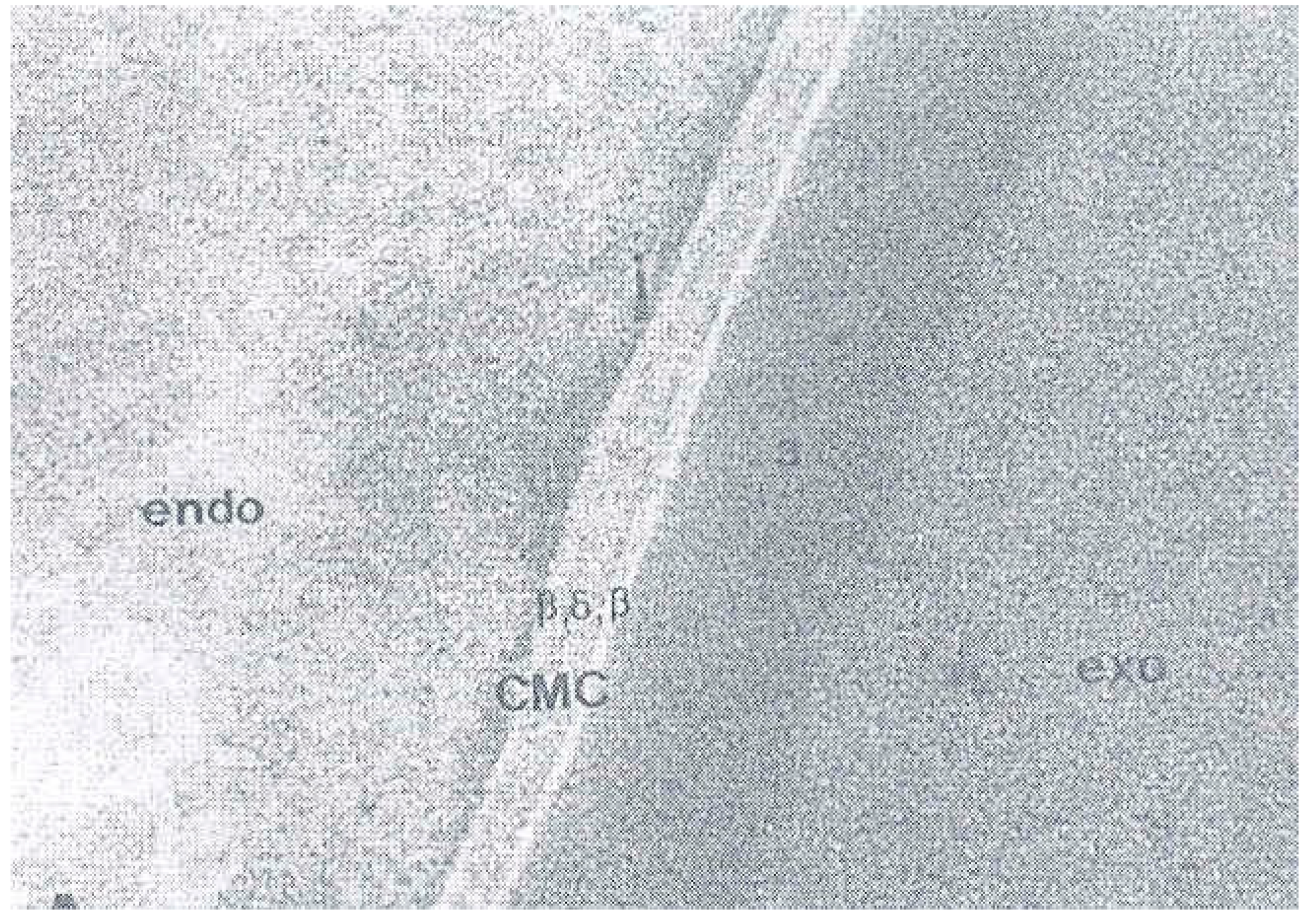
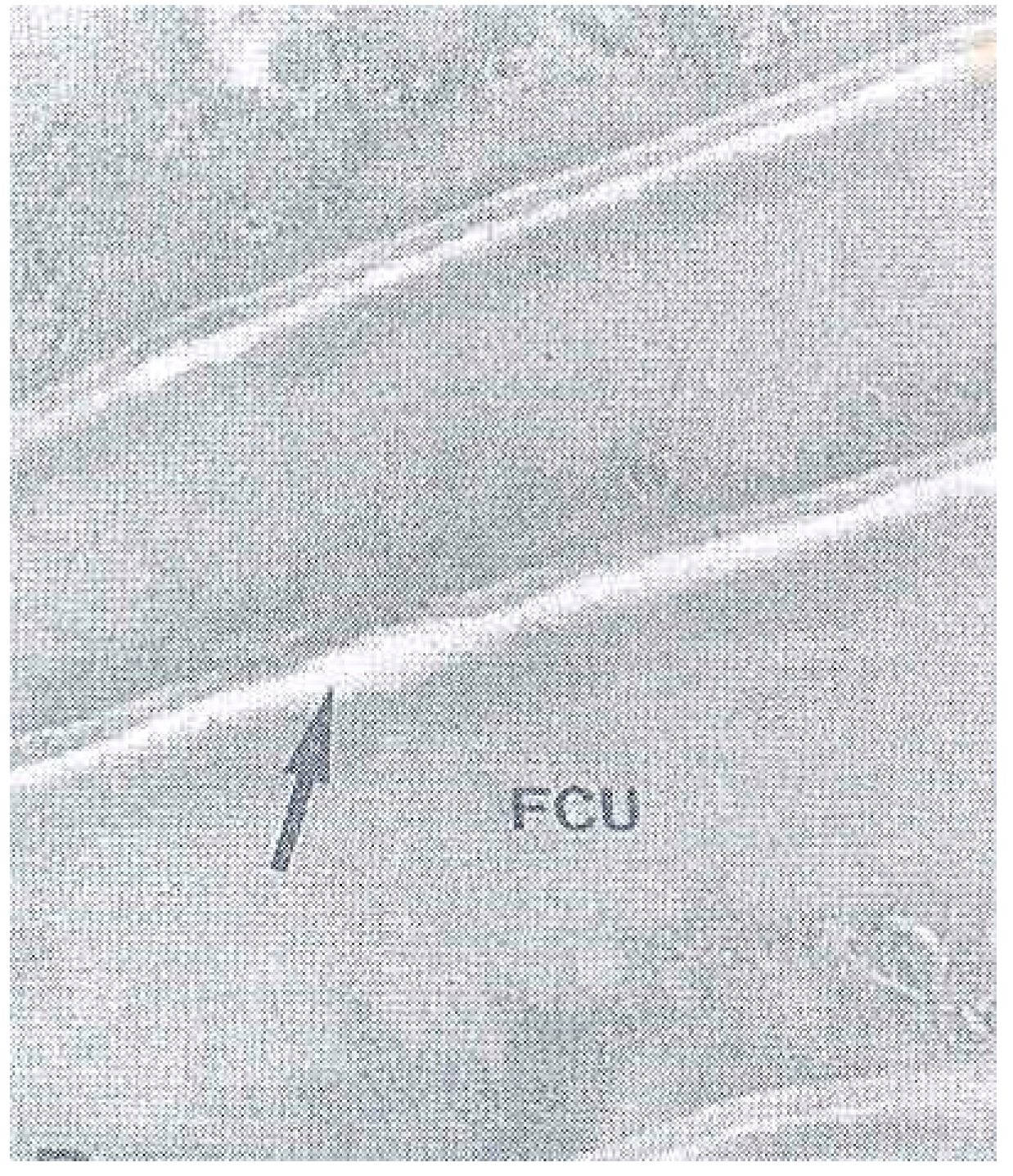
4. The Cell Membrane Complex (CMC)
5. Discussion: Some Unknown Aspects of Cuticle Structure that Need Innovative Techniques to Resolve
6. At What Stage of Hair Growth is 18-MEA Attached to the Cuticle and How Does This Occur?
7. Summary
Funding
Conflicts of Interest
References
- Auber, L. The anatomy of follicles producing wool fibres with special reference to keratinization. Trans. R. Soc. Edinb. 1951, 62 Pt 1, 191–254. [Google Scholar] [CrossRef]
- Rogers, G.E. Electron microscope studies of hair and wool. Ann. N. Y. Acad. Sci. 1959, 83, 378–399. [Google Scholar] [CrossRef] [PubMed]
- Rogers, G.E. Electron. microscopy of wool. J. Ultrastruct. Res. 1959, 2, 309–330. [Google Scholar] [CrossRef]
- Woods, L.J.; Orwin, D.F.G. Studies on the surface layers of the wool fibre cuticle. In Fibrous Proteins: Scientific, Medical and Industrial Aspects; Parry, D.A.D., Creamer, L.K., Eds.; Academic Press: London, UK, 1980; pp. 141–150. [Google Scholar]
- Orwin, D.F.G. The cytology and cytochemistry of the wool follicle. Int. Rev. Cytol. 1979, 60, 331–374. [Google Scholar]
- Jones, L.N. The hair fibre surface. In Skin, Hair and Nails; Forslind, B., Lindberg, M., Eds.; CRC: Boca Raton, FL, USA, 2003; pp. 285–316. [Google Scholar]
- Evans, D.J.; Leeder, J.D.; Rippon, J.A.; Rivett, D.E. Separation and analysis of surface lipids of the wool fibre. In Proceedings of the 7th International Wool Textile Research Conference, Tokyo, Japan, 28 August–3 September 1985; pp. 181–193. [Google Scholar]
- Negri, A.P.; Cornell, H.J.; Rivett, D.E. The nature of covalently bound fatty acids in wool fibres. Aust. J. Agric. Res. 1991, 42, 1285–1292. [Google Scholar] [CrossRef]
- Negri, A.P.; Cornell, H.J.; Rivett, D.E. A model for the surface of keratin fibres. Text. Res. J. 1993, 63, 109–115. [Google Scholar] [CrossRef]
- Ward, R.J.; Willis, H.A.; George, G.A.; Guise, G.B.; Denning, R.J.; Evans, D.J.; Short, R.D. Surface analysis of wool by X-ray photoelectron spectroscopy and static secondary ion mass spectrometry. Text. Res. J. 1993, 63, 362–368. [Google Scholar] [CrossRef]
- Jones, N.L.; Rivett, D.E. The role of 18-methyleicosanoic acid in the structure and formation of mammalian hair fibres. Micron 1997, 28, 469–485. [Google Scholar] [CrossRef]
- Zahn, H.; Messinger, H.; Hocker, H. Covalently-linked fatty acids at the surface of wool: Part of the “cuticle cell envelope. Text. Res. J. 1994, 64, 554–555. [Google Scholar] [CrossRef]
- Kizawa, K.; Uchiwa, H.; Murakami, U. Highly expressed S100A3, a calcium binding protein in human hair cuticle. Biochim. Biophys. Acta 1996, 13, 94–98. [Google Scholar] [CrossRef][Green Version]
- Lindberg, J.; Philip, B.; Gralen, N. Occurrence of thin membranes in the structure of wool. Nature 1948, 162, 458. [Google Scholar] [CrossRef]
- vonAllworden, K. The properties of wool and a new chemical method for detecting damaged wool. Angew. Chem. 1916, 29, 77. [Google Scholar]
- Bradbury, H.J.; Leeder, J.D. Keratin fibres. IV. Structure of the cuticle. Aust. J. Biol. Sci. 1970, 23, 843–854. [Google Scholar] [CrossRef] [PubMed][Green Version]
- King, R.N.L.; Bradbury, J.H. The chemical composition of wool. V: The epicuticle. Aust. J. Biol. Sci. 1968, 21, 375–384. [Google Scholar] [CrossRef]
- Rogers, G.E.; Harding, H.W.J.; Llewellyn-Smith, I.J. The origin of citrulline-containing proteins in the hair follicle and the chemical nature of trichohyalin, an intracellular precursor. Biochim. Biophys. Acta 1977, 495, 159–175. [Google Scholar] [CrossRef]
- Rogers, G.E. Some observations on the proteins of the inner root sheath cells of hair follicles. Biochim. Biophys. Acta 1958, 29, 33–43. [Google Scholar] [CrossRef]
- Rogers, G.E. Newer findings on the enzymes and proteins of hair follicles. Ann. N. Y. Acad. Sci. 1959, 83, 408–428. [Google Scholar] [CrossRef]
- Leeder, J.D. Wool-Nature’s Wonder Fibre; Australasian Textile Publishers: Ocean Grove, Australia, 1984. [Google Scholar]
- Jones, L.N.; Horr, T.J.; Kaplin, I.J. Formation of surface membranes in developing mammalian hair fibres. Micron 1994, 25, 589–595. [Google Scholar] [CrossRef]
- Swift, A.J.; Bews, B. The chemistry of human hair cuticle- II: The isolation and amino acid analysis of the cell membranes and A-layer. J. Soc. Cosmet. Chem. 1974, 25, 355–366. [Google Scholar]
- Jones, L.N.; Peet, D.J.; Danks, D.M.; Negri, A.P.; Rivett, D.E. Hairs from patients with maple syrup urine disease show a structural defect in the fiber cuticle. J. Investig. Dermatol. 1996, 106, 461–464. [Google Scholar] [CrossRef] [PubMed]
- Fujikawa, H.; Fujimoto, A.; Farooq, M.; Ito, M.; Shimomura, Y. Characterization of the human hair shaft cuticle-specific keratin-associated protein 10 family. J. Investig. Dermatol. 2013, 133, 2780–2782. [Google Scholar] [CrossRef] [PubMed]
- Bringans, S.D.; Plowman, J.E.; Dyer, J.M.; Clerens, S.; Vernon, J.A.; Bryson, W.G. Characterization of the exocuticle a-layer proteins of wool. Exp. Dermatol. 2007, 16, 951–960. [Google Scholar] [CrossRef] [PubMed]
- Drisdel, C.R.; Green, W.N. Labeling and quantifying sites of protein palmitoylation. Biotechniques 2004, 36, 276–285. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rogers, G.E. Known and Unknown Features of Hair Cuticle Structure: A Brief Review. Cosmetics 2019, 6, 32. https://doi.org/10.3390/cosmetics6020032
Rogers GE. Known and Unknown Features of Hair Cuticle Structure: A Brief Review. Cosmetics. 2019; 6(2):32. https://doi.org/10.3390/cosmetics6020032
Chicago/Turabian StyleRogers, George E. 2019. "Known and Unknown Features of Hair Cuticle Structure: A Brief Review" Cosmetics 6, no. 2: 32. https://doi.org/10.3390/cosmetics6020032
APA StyleRogers, G. E. (2019). Known and Unknown Features of Hair Cuticle Structure: A Brief Review. Cosmetics, 6(2), 32. https://doi.org/10.3390/cosmetics6020032



