Contact Allergy to Fragrances: In Vitro Opportunities for Safety Assessment
Abstract
1. Introduction
2. Quantitative Risk Assessment for Skin Sensitization
3. In Vitro Possibility to Establish a NESIL
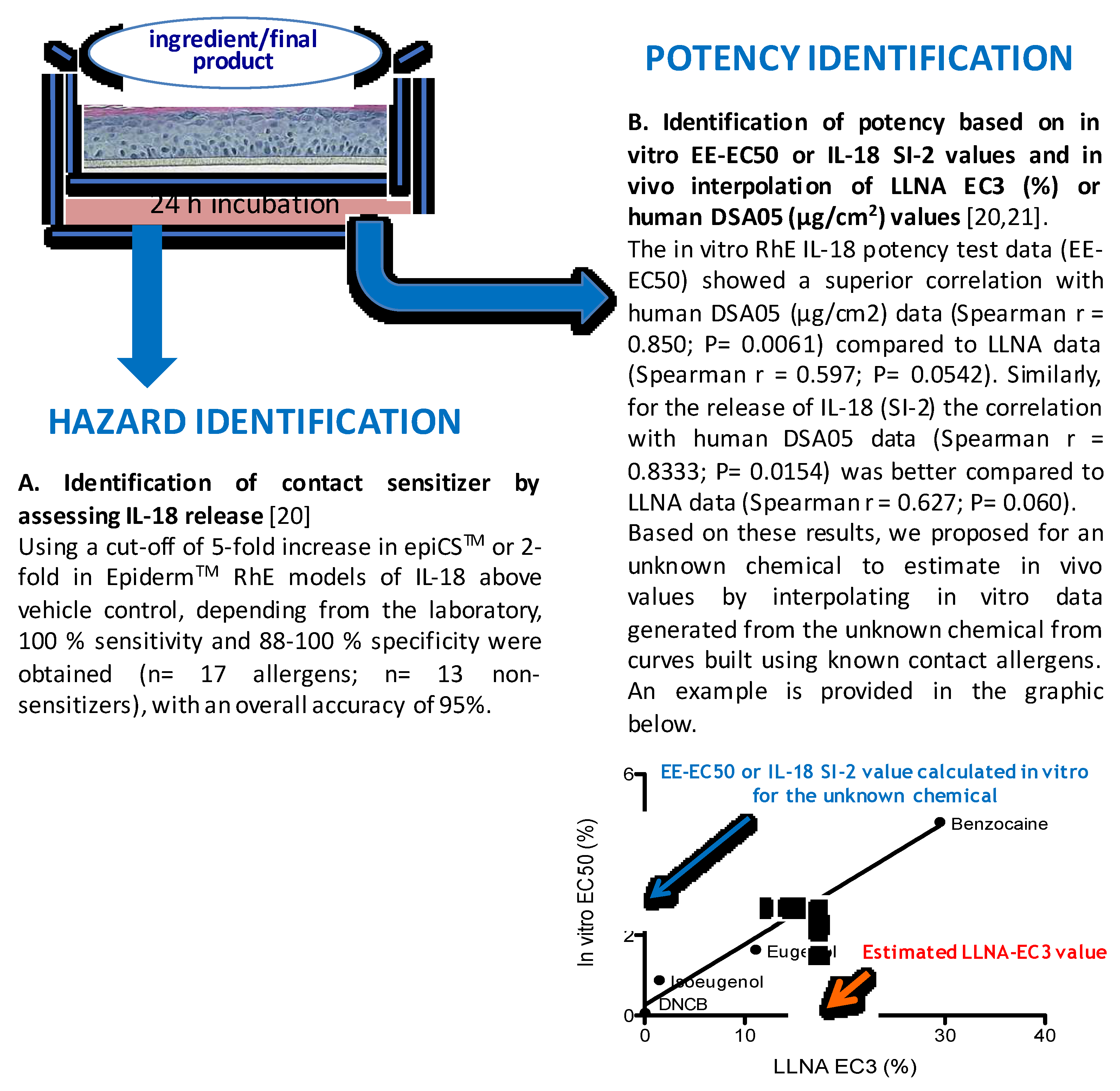
4. In Vitro Methods, Fragrances, and Hazard Identification
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bråred Christensson, J.; Hagvall, L.; Karlberg, A.-T. Fragrance Allergens, Overview with a Focus on Recent Developments and Understanding of Abiotic and Biotic Activation. Cosmetics 2016, 3, 19. [Google Scholar] [CrossRef]
- Schnuch, A.; Ute, W.; Lessmann, H.; Geier, J. Risk of sensitization to fragrances estimated on the basis of patch test data and exposure, according to volume used and a sample of 5451 cosmetic products. Flavour Fragr. J. 2015, 30, 208–217. [Google Scholar] [CrossRef]
- Corsini, E.; Engin, A.B.; Neagu, M.; Galbiati, V.; Nikitovic, D.; Tzanakakis, G.; Tsatsakis, A.M. Chemical-induced contact allergy: From mechanistic understanding to risk prevention. Arch. Toxicol. 2018, 92, 3031–3050. [Google Scholar] [CrossRef] [PubMed]
- Koppes, S.A.; Engebretsen, K.A.; Agner, T.; Angelova-Fischer, I.; Berents, T.; Brandner, J.; Brans, R.; Clausen, M.L.; Hummler, E.; Jakasa, I.; et al. Current knowledge on biomarkers for contact sensitization and allergic contact dermatitis. Contact Dermat. 2017, 77, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Nardelli, A.; Carbonez, A.; Ottoy, W.; Drieghe, J.; Goossens, A. Frequency of and trends in fragrance allergy over a 15-year period. Contact Dermat. 2008, 58, 134–141. [Google Scholar] [CrossRef] [PubMed]
- OECD. The Adverse Outcome Pathway for Skin Sensitisation Initiated by Covalent Binding to Proteins; OECD Series on Testing and Assessment, No. 168; OECD Publishing: Paris, France, 2014. [Google Scholar]
- Casati, S.; Aschberger, K.; Barroso, J.; Casey, W.; Delgado, I.; Kim, T.S.; Kleinstreuer, N.; Kojima, H.; Lee, J.K.; Lowit, A.; et al. Standardisation of defined approaches for skin sensitisation testing to support regulatory use and international adoption: Position of the International Cooperation on Alternative Test Methods. Arch. Toxicol. 2018, 92, 611–617. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, S.; Kleinstreuer, N.; Alépée, N.; Allen, D.; Api, A.M.; Ashikaga, T.; Clouet, E.; Cluzel, M.; Desprez, B.; Gellatly, N.; et al. Non-animal methods to predict skin sensitization (I): The Cosmetics Europe database. Crit. Rev. Toxicol. 2018, 48, 344–358. [Google Scholar] [CrossRef]
- Strickland, J.; Zang, Q.; Kleinstreuer, N.; Paris, M.; Lehmann, D.M.; Choksi, N.; Matheson, J.; Jacobs, A.; Lowit, A.; Allen, D.; et al. Integrated decision strategies for skin sensitization hazard. J. Appl. Toxicol. 2018, 38, 432. [Google Scholar] [CrossRef]
- Loveless, S.E.; Api, A.M.; Crevel, R.W.; Debruyne, E.; Gamer, A.; Jowsey, I.R.; Kern, P.; Kimber, I.; Lea, L.; Lloyd, P.; et al. Potency values from the local lymph node assay: Application to classification, labelling and risk assessment. Regul. Toxicol. Pharmacol. 2010, 56, 54–66. [Google Scholar] [CrossRef]
- Kimber, I.; Gerberick, G.F.; Basketter, D.A. Thresholds in contact sensitization: Theoretical and practical considerations. Food Chem. Toxicol. 1999, 37, 553–560. [Google Scholar] [CrossRef]
- Robinson, M.K.; Gerberick, G.F.; Ryan, C.A.; McNamee, P.; White, I.; Basketter, D.A. The importance of exposure in the assessment of skin sensitization risk. Contact Dermat. 2000, 42, 251–259. [Google Scholar] [CrossRef]
- White, S.J.; Friedmann, P.S.; Moss, C.; Simpson, J.M. The effect of altering area of application and dose per unit area on sensitization to DNCB. Br. J. Dermatol. 1986, 115, 663–668. [Google Scholar] [CrossRef] [PubMed]
- Kimber, I.; Pallardy, M. The use of T cells in hazard characterization of chemical and drug allergens and integration in testing strategies Foreword. EXS 2014, 104, 1–7. [Google Scholar]
- Corsini, E.; Roggen, E.L.; Galbiati, V.; Gibbs, S. Alternative Approach for Potency Assessment: In Vitro Methods. Cosmetics 2016, 3, 7. [Google Scholar] [CrossRef]
- Basketter, D.A.; Balikie, L.; Dearman, R.J.; Kimber, I.; Ryan, C.A.; Gerberick, G.F.; Harvey, P.; Evans, P.; White, I.R.; Rycroft, R.J. Use of the local lymph node assay for the estimation of relative contact allergenic potency. Contact Dermat. 2000, 42, 344–348. [Google Scholar] [CrossRef]
- Roberts, D.W.; Patlewicz, G. Non-animal assessment of skin sensitization hazard: Is an integrated testing strategy needed, and if so what should be integrated? J. Appl. Toxicol. 2018, 38, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Api, A.M.; Parakhia, R.; O’Brien, D.; Basketter, D.A. Fragrances Categorized According to Relative Human Skin Sensitization Potency. Dermatitis 2017, 28, 299–307. [Google Scholar] [CrossRef]
- Zang, Q.; Paris, M.; Lehmann, D.M.; Bell, S.; Kleinstreuer, N.; Allen, D.; Matheson, J.; Jacobs, A.; Casey, W.; Strickland, J. Prediction of skin sensitization potency using machine learning approaches. J. Appl. Toxicol. 2017, 37, 792–805. [Google Scholar] [CrossRef]
- Gibbs, S.; Corsini, E.; Spiekstra, S.W.; Galbiati, V.; Fuchs, H.W.; Degeorge, G.; Troese, M.; Hayden, P.; Deng, W.; Roggen, E. An epidermal equivalent assay for identification and ranking potency of contact sensitizers. Toxicol. Appl. Pharmacol. 2013, 272, 529–541. [Google Scholar] [CrossRef]
- Galbiati, V.; Papale, A.; Marinovich, M.; Gibbs, S.; Roggen, E.; Corsini, E. Development of an in vitro method to estimate the sensitization induction level of contact allergens. Toxicol. Lett. 2017, 271, 1–11. [Google Scholar] [CrossRef]
- Natsch, A.; Emter, R.; Haupt, T.; Ellis, G. Deriving a No Expected Sensitization Induction Level for Fragrance Ingredients Without Animal Testing: An Integrated Approach Applied to Specific Case Studies. Toxicol. Sci. 2018, 165, 170–185. [Google Scholar] [CrossRef] [PubMed]
- Van der Veen, J.W.; Rorije, E.; Emter, R.; Natsch, A.; van Loveren, H.; Ezendam, J. Evaluating the performance of integrated approaches for hazard identification of skin sensitizing chemicals. Regul. Toxicol. Pharmacol. 2014, 69, 371–379. [Google Scholar] [CrossRef] [PubMed]
- Jaworska, J.S.; Natsch, A.; Ryan, C.; Strickland, J.; Ashikaga, T.; Miyazawa, M. Bayesian integrated testing strategy (ITS) for skin sensitization potency assessment: A decision support system for quantitative weight of evidence and adaptive testing strategy. Arch. Toxicol. 2015, 89, 2355–2383. [Google Scholar] [CrossRef] [PubMed]
- Urbisch, D.; Mehling, A.; Guth, K.; Ramirez, T.; Honarvar, N.; Kolle, S.; Landsiedel, R.; Jaworska, J.; Kern, P.S.; Gerberick, F.; et al. Assessing skin sensitization hazard in mice and men using non-animal test methods. Regul. Toxicol. Pharmacol. 2015, 71, 337–351. [Google Scholar] [CrossRef] [PubMed]
- Karlberg, A.T.; Börje, A.; Duus Johansen, J.; Lidén, C.; Rastogi, S.; Roberts, D.; Uter, W.; White, I.R. Activation of non-sensitizing or low-sensitizing fragrance substances into potent sensitizers—Prehaptens and prohaptens. Contact Dermat. 2013, 69, 323–334. [Google Scholar] [CrossRef] [PubMed]
- Baron, J.M.; Holler, D.; Schiffer, R.; Frankenberg, S.; Neis, M.; Merk, H.F.; Jugert, F.K. Expression of multiple cytochrome P450 enzymes and multidrug resistance-associated transport proteins in human skin keratinocytes. J. Investig. Dermatol. 2001, 116, 541–548. [Google Scholar] [CrossRef]
- Swanson, H.I. Cytochrome P450 expression in human keratinocytes: An aryl hydrocarbon receptor perspective. Chem. Biol. Interact. 2004, 149, 69–79. [Google Scholar] [CrossRef]
- Bertrand, F.; Basketter, D.A.; Roberts, D.W.; Lepoittevin, J.P. Skin sensitization to eugenol and isoeugenol in mice: Possible metabolic pathways involving ortho-quinone and quinone methide intermediates. Chem. Res. Toxicol. 1997, 10, 335–343. [Google Scholar] [CrossRef]
- Smith, C.K.; Moore, C.A.; Elahi, E.N.; Smart, A.T.; Hotchkiss, S.A. Human skin absorption and metabolism of the contact allergens, cinnamic aldehyde, and cinnamic alcohol. Toxicol. Appl. Pharmacol. 2000, 168, 189–199. [Google Scholar] [CrossRef]
- Gerberick, G.F.; Vassallo, J.D.; Foertsch, L.M.; Price, B.B.; Chaney, J.G.; Lepoittevin, J.P. Quantification of chemical peptide reactivity for screening contact allergens: A classification tree model approach. Toxicol. Sci. 2007, 97, 417–427. [Google Scholar] [CrossRef]
- Natsch, A.; Bauch, C.; Foertsch, L.; Gerberick, F.; Norman, K.; Hilberer, A.; Inglis, H.; Landsiedel, R.; Onken, S.; Reuter, H.; et al. The intra- and inter-laboratory reproducibility and predictivity of the KeratinoSens assay to predict skin sensitizers in vitro: Results of a ring-study in five laboratories. Toxicol. In Vitro 2011, 25, 733–744. [Google Scholar]
- Corsini, E.; Galbiati, V.; Nikitovic, D.; Tsatsakis, A.M. Role of oxidative stress in chemical allergens induced skin cells activation. Food Chem. Toxicol. 2013, 61, 74–81. [Google Scholar] [CrossRef] [PubMed]
- McKim, J.M., Jr.; Keller, D.J., 3rd; Gorski, J.R. An in vitro method for detecting chemical sensitization using human reconstructed skin models and its applicability to cosmetic, pharmaceutical, and medical device safety testing. Cutan. Ocul. Toxicol. 2012, 31, 292–305. [Google Scholar] [CrossRef] [PubMed]
- Patlewicz, G.; Casati, S.; Basketter, D.A.; Asturiol, D.; Roberts, D.W.; Lepoittevin, J.P.; Worth, A.P.; Aschberger, K. Can currently available non-animal methods detect pre and pro-haptens relevant for skin sensitization? Regul. Toxicol. Pharmacol. 2016, 82, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Cottrez, F.; Boitel, E.; Ourlin, J.C.; Peiffer, J.L.; Fabre, I.; Henaoui, I.S.; Mari, B.; Vallauri, A.; Paquet, A.; Barbry, P.; et al. SENS-IS, a 3D reconstituted epidermis based model for quantifying chemical sensitization potency: Reproducibility and predictivity results from an inter-laboratory study. Toxicol. In Vitro 2016, 32, 248–260. [Google Scholar] [CrossRef] [PubMed]
- Saito, K.; Takenouchi, O.; Nukada, Y.; Miyazawa, M.; Sakaguchi, H. An in vitro skin sensitization assay termed EpiSensA for broad sets of chemicals including lipophilic chemicals and pre/pro-haptens. Toxicol. In Vitro 2017, 40, 11–25. [Google Scholar] [CrossRef] [PubMed]
| Fragrance | Human Evidences | LLNA | In Silico | KE1 | KE2 | KE3 |
|---|---|---|---|---|---|---|
| Acetanisole | − | − | no alert | − | + | − |
| Benzyl salicylate | − | + | alert | − | + | − |
| Citronellol | − | + | no alert | + | − | + |
| Hexyl salicylate | − | + | no alert | − | − | + |
| Linalool * | − | + | no alert | − | − | + |
| Methyl salicylate | − | − | no alert | − | − | − |
| Vanillin * | − | − | no alert | −/+ | −/+ | − |
| α-iso-Methylionone | − | + | alert | − | − | + |
| α-Amyl cinnamic aldehyde | −/+ | + | alert | − | + | + |
| 6-Methylcoumarin | −/+ | − | alert | − | + | − |
| Benzyl cinnamate | not classified | + | alert | − | + | − |
| Limonene * | − (not oxidized) | + | no alert | + | − | + |
| α-Hexyl cinnamic aldehyde | −/+ | + | alert | −/+ | borderline | − |
| 2-Phenylpropionaldehyde | + | + | alert | + | + | + |
| 3,4-Dihydrocoumarin | + | + | alert | + | − | + |
| Benzaldehyde | + | − | no alert | − | + | + |
| Cinnamic aldehyde | + | + | alert | + | + | + |
| Cinnamyl Alcohol * | + | + | no alert | + | + | + |
| Citral (geranial) | + | + | alert | + | + | + |
| Coumarin | + | − | alert | − | + | − |
| Eugenol * | + | + | no alert | + | +/− | + |
| Farnesol * | + | + | No alert | − | + | + |
| Geraniol * | + | + | no alert | − | + | + |
| Hydroxycitronellal | + | + | alert | + | + | + |
| Isoeugenol * | + | + | no alert | + | + | − |
| Lilial | + | + | alert | + | − | + |
| Lyral | + | + | alert | + | + | + |
| Methyl 2-nonynoate | + | + | alert | + | + | + |
| Methyl-2-octynoate/Methyl heptine carbonate | + | + | alert | + | + | + |
| Perillaldehyde | + | + | alert | + | + | + |
| Phenylacetaldehyde | + | + | alert | + | + | + |
| R-Carvone | + | + | alert | + | + | + |
| Safranal | + | + | alert | + | + | no data |
| trans-2-Hexenal | + | + | alert | + | + | + |
| 1-(p-Methoxyphenyl)-1-penten-3-one | no data | + | alert | + | + | + |
| 1-Phenyl-1,2-propanedione | no data | + | alert | + | + | + |
| 2-methoxy-4-methylphenol * | not classified | + | no alert | − | − | + |
| 2-Methylundecanal | not classified | + | alert | + | + | no data |
| 2,4-Heptadienal | no data | + | alert | + | + | + |
| 4-(N-Ethyl-N-2-methan-sulphonamido-ethyl)-2-methyl-1,4-phenylenediamine (CD3) * | no data | + | no alert | + | + | + |
| 4-Allylanisole | no data | + | no alert | + | +/− | + |
| 5-Methyl-2-phenyl-2-hexenal | no data | + | alert | no data | + | + |
| cis-6-Nonenal | no data | + | alert | − | + | no data |
| Cyclamen aldehyde | no data | + | alert | + | + | − |
| Diethyl acetaldehyde | no data | + | alert | + | + | + |
| Dihydroeugenol * | no data | + | no alert | − | + | no data |
| Ethyl vanillin * | no data | − | no alert | − | + | − |
| Farnesal | no data | + | alert | + | + | no data |
| Octanenitrile | no data | − | no alert | − | − | no data |
| trans-2-Decenal | no data | + | alert | + | + | no data |
| Undec-10-enal | no data | + | alert | − | + | − |
| α-methyl-trans-Cinnamaldehyde | not classified | + | alert | + | + | + |
| β-Damascone | not classified | + | no alert | no data | + | + |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Corsini, E.; Galbiati, V. Contact Allergy to Fragrances: In Vitro Opportunities for Safety Assessment. Cosmetics 2019, 6, 3. https://doi.org/10.3390/cosmetics6010003
Corsini E, Galbiati V. Contact Allergy to Fragrances: In Vitro Opportunities for Safety Assessment. Cosmetics. 2019; 6(1):3. https://doi.org/10.3390/cosmetics6010003
Chicago/Turabian StyleCorsini, Emanuela, and Valentina Galbiati. 2019. "Contact Allergy to Fragrances: In Vitro Opportunities for Safety Assessment" Cosmetics 6, no. 1: 3. https://doi.org/10.3390/cosmetics6010003
APA StyleCorsini, E., & Galbiati, V. (2019). Contact Allergy to Fragrances: In Vitro Opportunities for Safety Assessment. Cosmetics, 6(1), 3. https://doi.org/10.3390/cosmetics6010003






