Schisandra chinensis Protects the Skin from Global Pollution by Inflammatory and Redox Balance Pathway Modulations: An In Vitro Study
Abstract
1. Introduction
1.1. Antioxidants that Inhibit ROS
1.2. Plant-Derived Compounds to Limit ROS Production
2. Materials and Methods
2.1. Transcriptomic Analyses
2.2. The effect of the Extract from S.C. on the Protection of Human Keratinocytes (NHEK) Damages that are Caused by Pollution
2.2.1. Viability and Cytotoxicity Determination
2.2.2. Effect of Urban Pollution and the S.C. Extract on Cell Pathways
2.3. Statistical Analysis
3. Results
3.1. Transcriptomic Results
3.2. The Effect of Urban Dust and S.C. Extract on NF-kB Activation
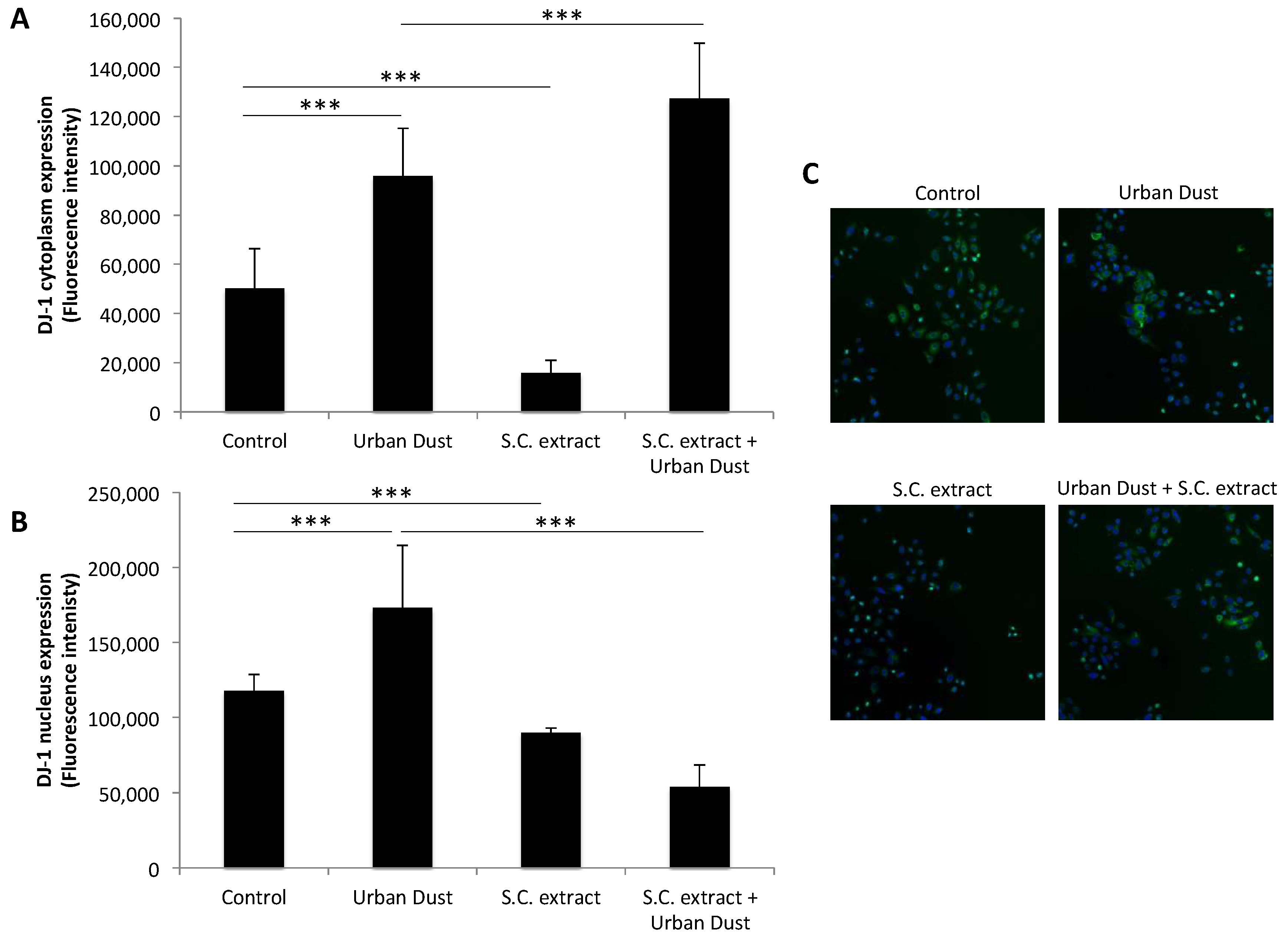
3.3. S.C. Extract Modulates Redox Pathways
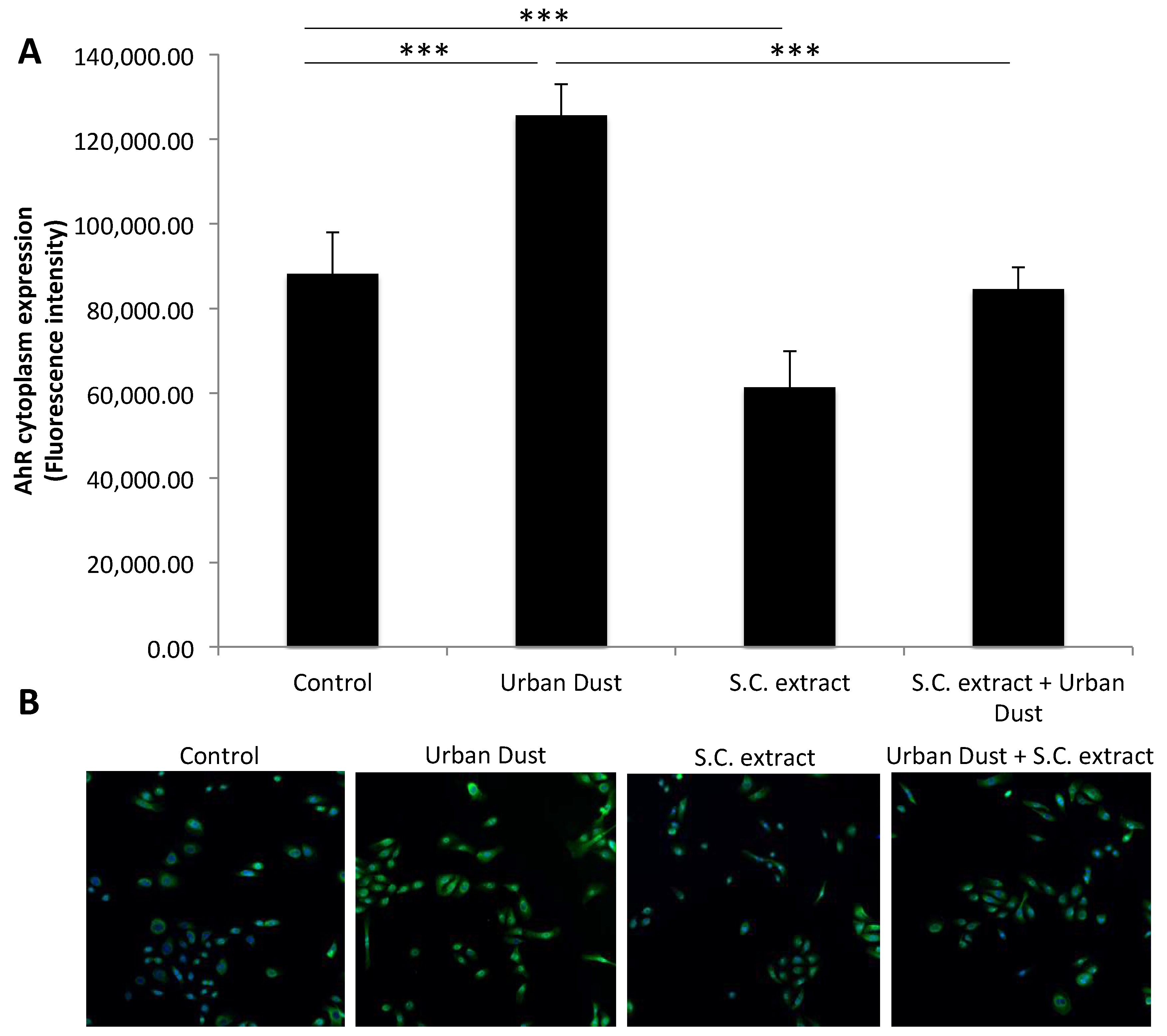
3.4. The Effect of Urban Dust and S.C. Extract on the Aryl Hydrocarbon Receptor (AhR)
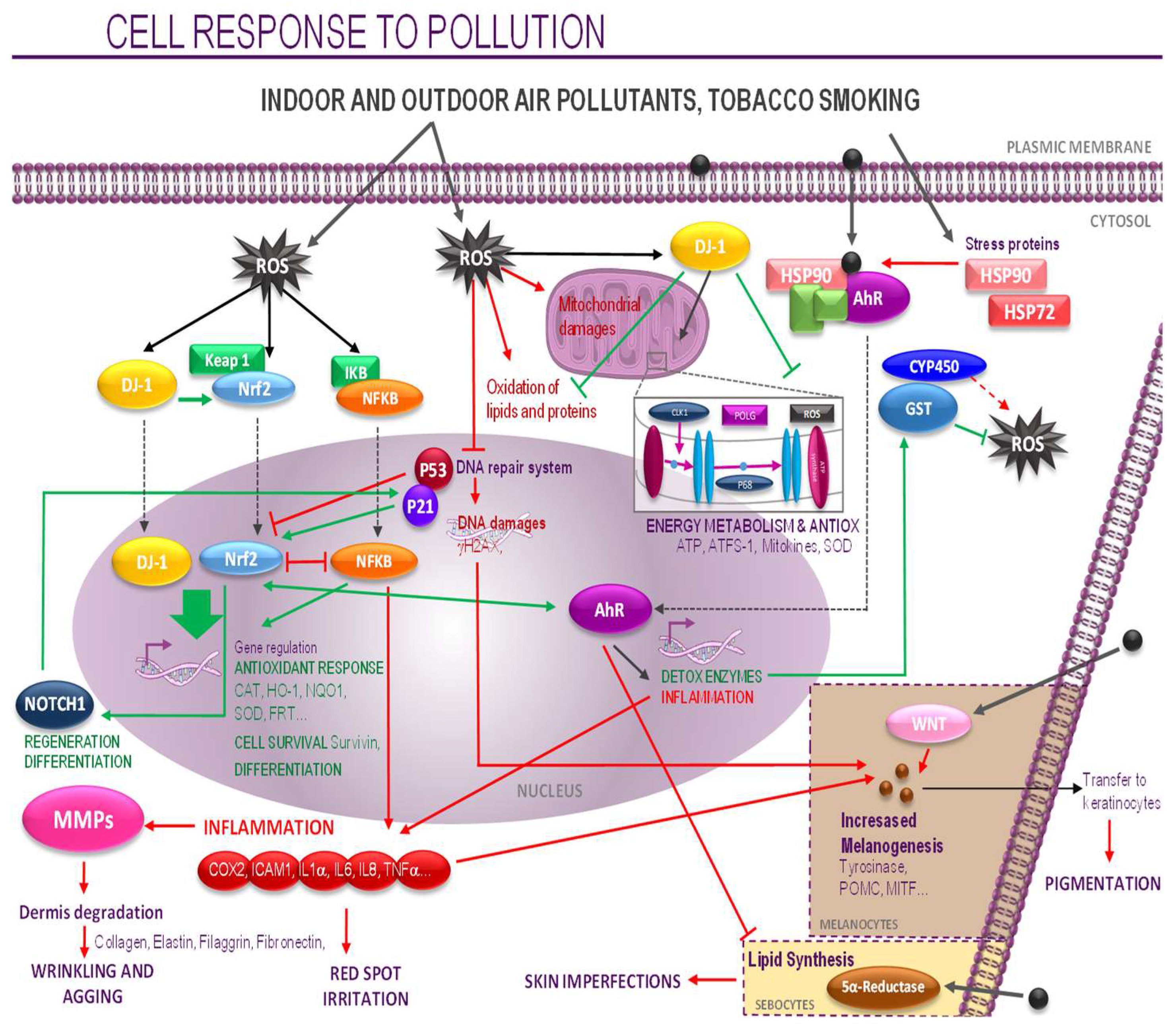
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Canova, C.; Heinrich, J.; Anto, J.M.; Leynaert, B.; Smith, M.; Kuenzli, N.; Zock, J.P.; Janson, C.; Cerveri, I.; De Marco, R.; et al. The influence of sensitization to pollens and moulds on seasonal variations in asthma attacks. Eur. Respir. J. 2013, 42, 935–945. [Google Scholar] [CrossRef] [PubMed]
- Rembiesa, J.; Ruzgas, T.; Engblom, J.; Holefors, A. The impact of pollution on skin and proper efficacy testing for anti-pollution claims. Cosmetics 2018, 5, 4. [Google Scholar] [CrossRef]
- Puri, P.; Nandar, S.K.; Kathuria, S.; Ramesh, V. Effects of air pollution on the skin: A review. Indian J. Dermatol. Venereol. Leprol. 2017, 83, 415–423. [Google Scholar] [PubMed]
- Lanuti, E.L.; Kirsner, R.S. Effects of pollution on skin aging. J. Investig. Dermatol. 2010, 130, 2696. [Google Scholar] [CrossRef] [PubMed]
- Vierkötter, A.; Schikowski, T.; Ranft, U.; Sugiri, D.; Matsui, M.; Krämer, U.; Krutmann, J. Airborne particle exposure and extrinsic skin aging. J. Investig. Dermatol. 2010, 130, 2719–2726. [Google Scholar] [CrossRef] [PubMed]
- Kampa, M.; Castanas, E. Human health effects of air pollution. Environ. Pollut. 2008, 151, 362–367. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.E.; Cho, D.; Park, H.J. Air pollution and skin diseases: Adverse effects of airborne particulate matter on various skin diseases. Life Sci. 2016, 152, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Krutmann, J.; Moyal, D.; Xiang, L.F.; Seité, S. Pollution and acne: Is there a link? Clin. Cosmet. Investig. Dermatol. 2017, 10, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Mc Daniel, D.; Farris, P.; Valacchi, G. Atmospheric skin aging: Contributors and inhibitors. J. Cosmet. Dermatol. 2017, 17, 124–137. [Google Scholar] [CrossRef] [PubMed]
- Im, J.Y.; Lee, K.W.; Woo, J.M.; Junn, E.; Mouradian, M.M. DJ-1 induces thioredoxin 1 expression through the Nrf2 pathway. Hum. Mol. Genet. 2012, 21, 3013–3024. [Google Scholar] [CrossRef] [PubMed]
- Lambert, J.D.; Elias, R.J. The antioxidant and pro-oxidant activities of green tea polyphenols: A role in cancer prevention. Arch. Biochem. Biophys. 2010, 501, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Juliano, C.; Magrini, G.A. Cosmetic functional ingredients from botanical sources for anti-pollution skincare products. Cosmetics 2018, 5, 19. [Google Scholar] [CrossRef]
- Mistry, N. Guidelines for formulating anti-pollution products. Cosmetics 2017, 4, 57. [Google Scholar] [CrossRef]
- Lephart, E.D. Equol’s anti-aging effects protect against environmental assaults by increasing skin antioxidant defense and ECM proteins while decreasing oxidative stress and inflamamtion. Cosmetics 2018, 5, 16. [Google Scholar] [CrossRef]
- Lephart, E.D. Skin aging and oxidative stress. Equol’s anti-aging effects via biochemical and molecular mechanisms. Ageing Res. Rev. 2016, 31, 36–54. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, Y.; Ide, F.; Kishi, R.; Akutagawa, T.; Sakai, S.; Nakamura, M.; Ishikawa, T.; Fuji-Kuriyama, Y.; Nakatsuru, Y. Aryl hydrocarbon receptor plays a significant role in mediating airborne particulate induced carcinogenesis in mice. Environ. Sci. Technol. 2017, 41, 3775–3780. [Google Scholar] [CrossRef]
- Casey, M.C.; McNally, R.S.; Conti, B.J.; Mak, T.W.; Ting, J.P.Y. DJ-1, a cancer- and Parkinson’s disease-associated protein, stabilizes the antioxidant transcriptional master regulator Nrf2. Proc. Natl. Acad. Sci. USA 2006, 103, 5091. [Google Scholar]
- Opletal, L.; Sovova, H.; Bartlova, M. Dibenzo[a,c]cyclooctadiene lignans of the genus Schisandra: Importance, isolation and determination. J. Chromatogr. B 2004, 812, 357–371. [Google Scholar] [CrossRef]
- Kim, J.; Kim, E.-H.; Oh, I.; Jung, K.; Han, Y.; Cheong, H.-K.; Ahn, K. Symptoms of atopic dermatitis are influenced by outdoor air pollution. J. Allergy Clin. Immunol. 2013, 132, 495–497. [Google Scholar] [CrossRef] [PubMed]
- Bosch, R.; Philips, N.; Suarez-Perez, J.A.; Juarranz, A.; Devmurari, A.; Chalensouk-Khaossat, J.; Gonzalez, M. Mechanisms of Photoaging and Cutaneous Photocarcinogenesis, and Photoprotective Strategies with Phytochemicals. Antioxidants 2015, 4, 248–268. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Cong, C.; Hue, N.J.; Ciao, B. Protective effect of Schizandrin B against damage of UVB irradiated skin cells depend on inhibition of inflammatory pathways. Bioengineered 2017, 8, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Jia, R.; Zhang, H.; Yang, Z.; Zhao, H.; Liu, F.; Wang, H.; Miao, M.; Wang, Q.; Liu, Y. Protective effects of Schisandrin B on cigarette smoke-induced airway injury in mice through Nrf2 pathway. Int. Immunopharmacol. 2017, 53, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Lin, Q.; Qin, X.; Shi, M.; Qin, Z.; Meng, Y.; Qin, Z.; Guo, S. Schisandrin B inhibits LPS-induced inflammatory response in human umbilical vein endothelial cells by activating Nrf2. Int. Immunopharmacol. 2017, 49, 142–147. [Google Scholar] [CrossRef] [PubMed]
- Lephart, E.D. Resveratrol, 4′Acetoxy Resveratrol, R-Equol, Racemic Equol or S-Equol as Cosmeceuticals to improve dermal health. Int. J. Mol. Sci. 2017, 18, 1193. [Google Scholar] [CrossRef] [PubMed]
- Lephart, E.D. Protective effects of equol and their polyphenolic isomers against dermal aging: Microarray/protein evidence with clinical implications and unique delivery into human skin. Pharm. Biol. 2013, 51, 1393–1400. [Google Scholar] [CrossRef] [PubMed]
- Lephart, E.D. Human Skin Gene Expression Doesn’t Correlate with Protein Expression? Unless Both Parameters Are quantified. J. Cosmet. Dermatol. 2018, 17, 244–245. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Liang, X.Y.; Shi, L.Y.; Wang, L.; Chen, J.L.; Kang, C.; Zhu, J.D.; Mi, M.T. Estrogen receptor and PI3K/Akt signaling pathway involvement in S-equol-induced activation of Nrf2/ARE in endothelia cells. PLoS ONE 2013, 8, e79075. [Google Scholar]
- Froyen, E.B.; Steinberg, F.M. Soy isoflavones increase quinone reductase in heap-1c1c7 cells via estrogen receptor beta and nuclear factor erythroid 2-related factor 2 binding to the antioxidant response element. J. Nutr. Biochem. 2011, 22, 843–848. [Google Scholar] [CrossRef] [PubMed]
- Schafer, M.; Werner, S. Nrf2-A regulator of keratinocyte redox signaling. Free Radic. Biol. Med. 2015, 88, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.; Cederbaum, A.I. Mitochondrial catalase and oxidative injury. Biol. Signals Recept. 2001, 10, 189–199. [Google Scholar] [CrossRef] [PubMed]
- Ishiwatari, S.; Takahashi, M.; Yasuda, C.; Nakagawa, M.; Saito, Y.; Noguchi, N.; Matsukuma, S. The protective role of DJ-1 in ultraviolet-induced damage of human skin: DJ-1 levels in the stratum corneum as an indicator of antioxidative defense. Arch. Dermatol. Res. 2015, 307, 925–935. [Google Scholar] [CrossRef] [PubMed]
- Nakahara, T.; Mitoma, C.; Hashimoto-Hachiya, A.; Takahara, M.; Tsuji, G.; Uchi, H.; Yan, X.; Hachisuka, J.; Chiba, T.; Esaki, H.; et al. Antioxidant Opuntia ficus-indica extract activates AhR-Nrf2 signaling and upregulates filaggrin and loricrin expression in human keratinocytes. J. Med. Food. 2015, 18, 1143–1149. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Uchi, H.; Morino-Koga, S.; Shi, S.; Furue, M.Z. Ligustilide ameliorated ultraviolet B-induced oxidative stress and inflammatory cytokine production in human keratinocytes through upregulation of Nrf2/HO-1 and suppression of NF-kB pathway. Exp. Dermatol. 2015, 24, 703–708. [Google Scholar] [CrossRef] [PubMed]
- Takei, K.; Hashimoto-Hachiya, A.; Takahara, M.; Tsuji, G.; Nakahara, T.; Furue, M. Cynaropicrin attenuates UVB-induced oxidative stress via the AhR-Nrf2-Nqo1 pathway. Toxicol. Lett. 2015, 234, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Li, C.Y.; Kong, A.N. Induction of phase I, II and III drug metabolism/transport by xenobiotics. Arch. Pharm. Res. 2005, 28, 249–268. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Ghio, A.J.; Cho, S.H.; Brinckerhoff, C.E.; Simon, S.A.; Liedtke, W. Diesel Exhaust Particles Activate the Matrix-Metalloproteinase-1 Gene in Human Bronchial Epithelia in a β-Arrestin—Dependent Manner via Activation of RAS. Environ. Health Perspect. 2009, 117, 400. [Google Scholar] [CrossRef] [PubMed]
- Woo, H.; Lee, J.; Park, D.; Jung, D. Protective Effect of Mulberry (Morus alba L.) Extract against Benzo[a]pyrene Induced Skin Damage through Inhibition of Aryl Hydrocarbon Receptor Signaling. J. Agric. Food Chem. 2017, 20, 10925–10932. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi-Bardbori, A.; Bengtsson, J.; Rannug, U.; Rannug, A.; Wincent, E. Quercetin resveratrol, and curcumin are indirect activators of the aryl hydrocarbon receptor (AhR). Chem. Res. Toxicol. 2012, 25, 1878–1884. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, G.; Takahara, M.; Uchi, H.; Matsuda, T.; Chiba, T.; Takeuchi, S. Identification of ketoconazole as an AhR-Nrf2 activator in cultured human keratinocytes: The basis of its anti-inflammatory effect. J. Investig. Dermatol. 2012, 132, 59–68. [Google Scholar] [CrossRef] [PubMed]


| Effect of Urban Dust (80 μg·mL−1) on Gene Expression | |||
|---|---|---|---|
| Gene Symbol | Target Name | Fold Change | p Value |
| CYP1A1 | cytochrome P450, family 1, subfamily A, polypeptide 1 | 23.186 | 0.001 |
| CYP1B1 | cytochrome P450, family 1, subfamily B, polypeptide 1 | 7.771 | 0.000 |
| MMP1 | matrix metallopeptidase 1 | 3.412 | 0.014 |
| ALDH3A1 | aldehyde dehydrogenase 3 family, member A1 | 2.364 | 0.011 |
| NQO1 | NAD(P)H dehydrogenase, quinone 1 | 2.339 | 0.000 |
| GPX2 | glutathione peroxidase 2 | 2.145 | 0.006 |
| IL1B | interleukin 1, beta | 2.053 | 0.046 |
| GSTM1 | glutathione S-transferase mu 1 | 1.784 | 0.037 |
| MMP9 | matrix mettalopeptidase 9 | 1.664 | 0.042 |
| HSPA5/BiP | heat shock 70kDa protein 5 | 1.562 | 0.003 |
| GADD45A | growth arrest and DNA-damage-inducible, alpha | 1.484 | 0.021 |
| FTH | ferritin, heavy polypeptide 1 | 1.183 | 0.035 |
| COL7A1 | collagen, type VII, alpha 1 | 1.337 | 0.029 |
| GSTP1 | glutathione S-transferase pi1 | 1.302 | 0.040 |
| SQSTM1 | sequestosome 1 | 1.203 | 0.034 |
| CLN3 | ceroid-lipofuscinosis, neuronal 3 | 1.172 | 0.013 |
| SPRR1A | small proline-rich protein 1A | 1.171 | 0.035 |
| GSR | glutathione reductase | 1.084 | 0.035 |
| FMO4 | flavin containing monooxygenase 4 | 0.810 | 0.012 |
| MT4 | metallothionin 4 | 0.434 | 0.040 |
| Effect of S.C extract (50 μg·mL−1) on gene expression | |||
| CYP1B1 | cytochrome P450, family 1, subfamily A, polypeptide 1 | 1.535 | 0.014 |
| HSPA5/Bip | heat shock 70kDa protein 5 | 1.415 | 0.014 |
| Effect of S.C extract (50 μg·mL−1) in presence of Urban Dust (80 μg·mL−1) on gene expression | |||
| GPX2 | glutathione peroxidase 2 | 1.365 | 0.007 |
| EPHX2 | epoxide hydrolase 2, cytoplasmic | 1.115 | 0.000 |
| FTL | ferritin, light polypeptide | 1.205 | 0.031 |
| SPRR1A | Small proline-rich protein 1A | 1.201 | 0.027 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ranouille, E.; Boutot, C.; Bony, E.; Bombarde, O.; Grosjean, S.; Lazewski, A.; Berthon, J.-Y.; Filaire, E. Schisandra chinensis Protects the Skin from Global Pollution by Inflammatory and Redox Balance Pathway Modulations: An In Vitro Study. Cosmetics 2018, 5, 36. https://doi.org/10.3390/cosmetics5020036
Ranouille E, Boutot C, Bony E, Bombarde O, Grosjean S, Lazewski A, Berthon J-Y, Filaire E. Schisandra chinensis Protects the Skin from Global Pollution by Inflammatory and Redox Balance Pathway Modulations: An In Vitro Study. Cosmetics. 2018; 5(2):36. https://doi.org/10.3390/cosmetics5020036
Chicago/Turabian StyleRanouille, Edwige, Carine Boutot, Emilie Bony, Oriane Bombarde, Sarah Grosjean, Antoine Lazewski, Jean-Yves Berthon, and Edith Filaire. 2018. "Schisandra chinensis Protects the Skin from Global Pollution by Inflammatory and Redox Balance Pathway Modulations: An In Vitro Study" Cosmetics 5, no. 2: 36. https://doi.org/10.3390/cosmetics5020036
APA StyleRanouille, E., Boutot, C., Bony, E., Bombarde, O., Grosjean, S., Lazewski, A., Berthon, J.-Y., & Filaire, E. (2018). Schisandra chinensis Protects the Skin from Global Pollution by Inflammatory and Redox Balance Pathway Modulations: An In Vitro Study. Cosmetics, 5(2), 36. https://doi.org/10.3390/cosmetics5020036





