Absorption and Photo-Stability of Substituted Dibenzoylmethanes and Chalcones as UVA Filters
Abstract
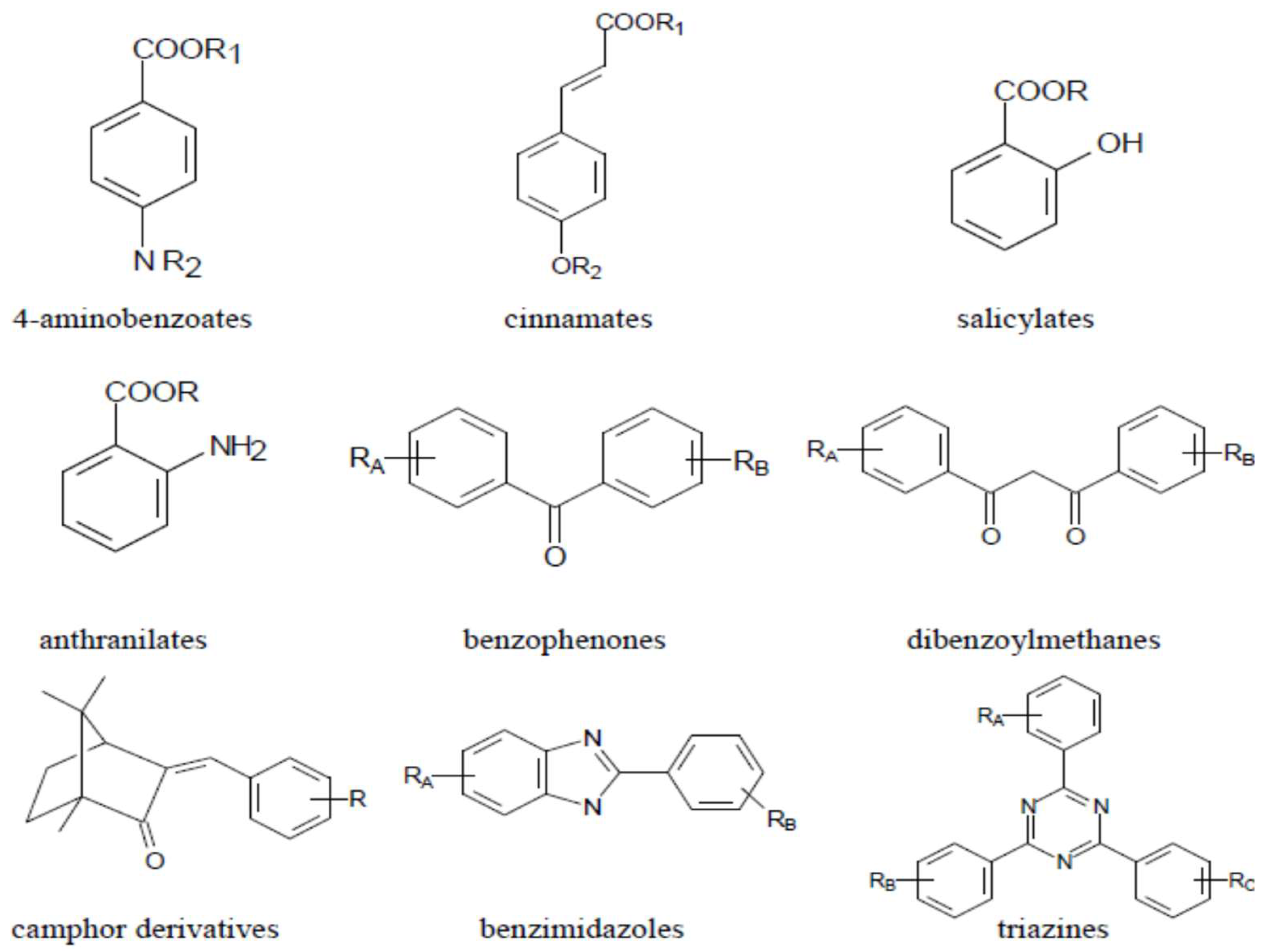
:1. Introduction
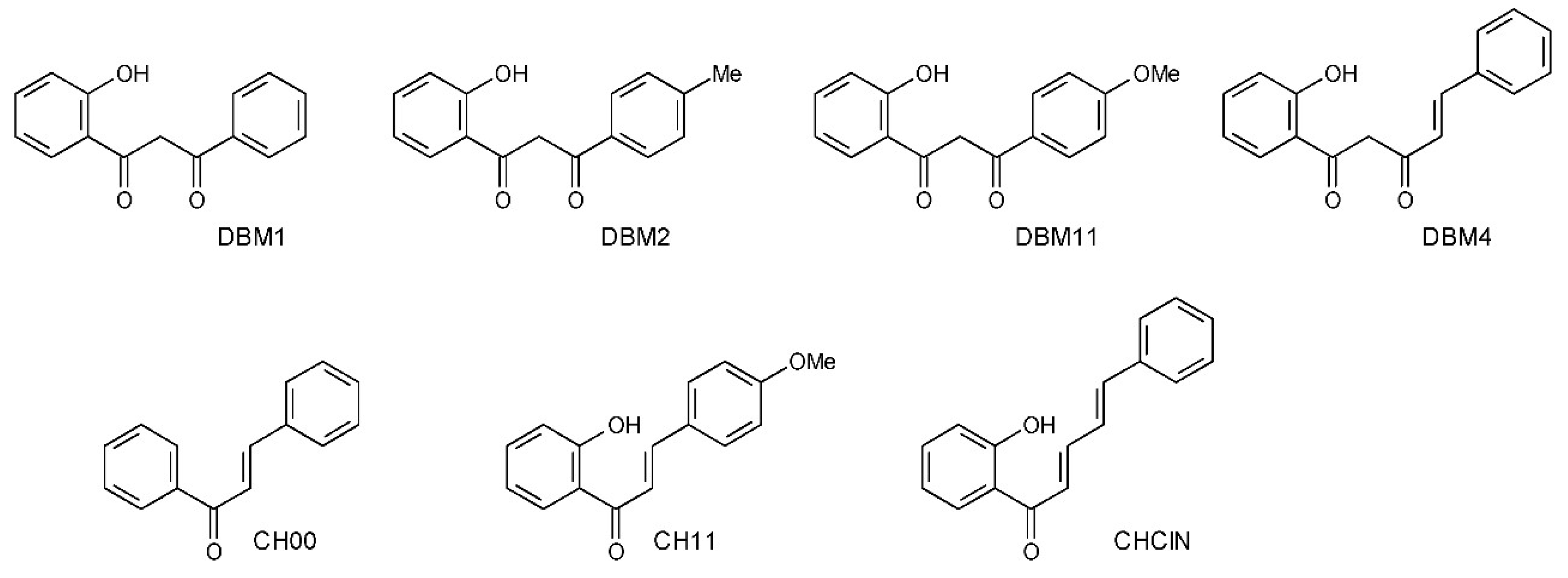
2. Materials and Methods
- (a)
- 1-(2-hydroxyphenyl)-3-phenyl-1,3-propanedione (DBM1)
- (b)
- 1-(2-hydroxyphenyl)-3-(4-methylphenyl)-1,3-propanedione (DBM2)
- (c)
- 1-(2-hydroxyphenyl)-3-(4-methoxyphenyl)-1,3-propanedione (DBM11)
- (d)
- (4E)-1-(2-hydroxyphenyl)-5-phenyl-4-penten-1,3-dione (DBM4)
- (e)
- (2E)-1,3-diphenyl-2-propen-1-one (CH00)
- (f)
- (2E)-1-(2-hydroxyphenyl)-3-(4-methoxyphenyl)-2-propen-1-one (CH11)
- (g)
- 1-(2-hydroxyphenyl)-5-phenyl-(2E,4E)-2,4-pentadien-1-one (CHClN)
3. Results
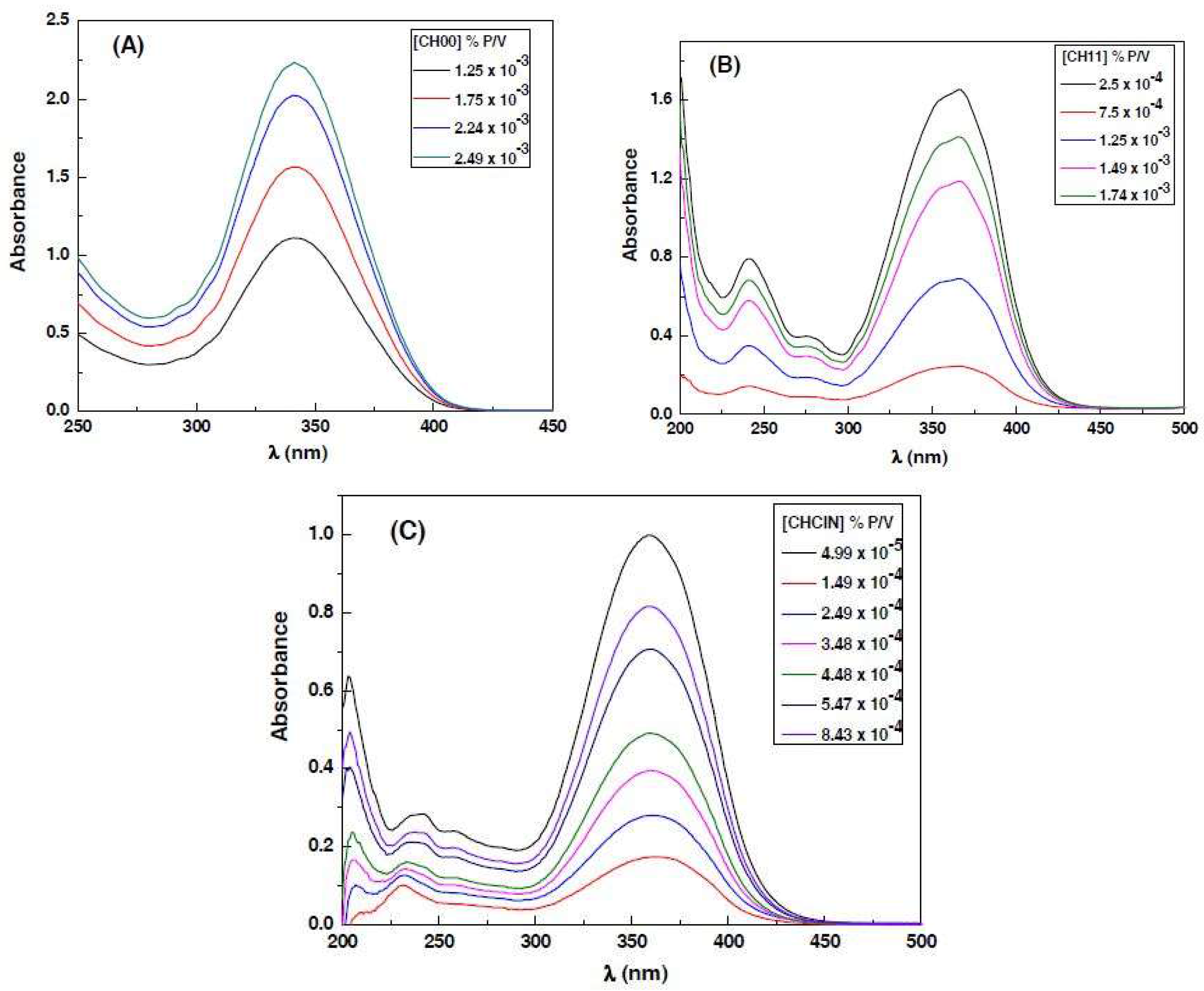
3.1. Absorption Spectra
3.2. Emission Spectra
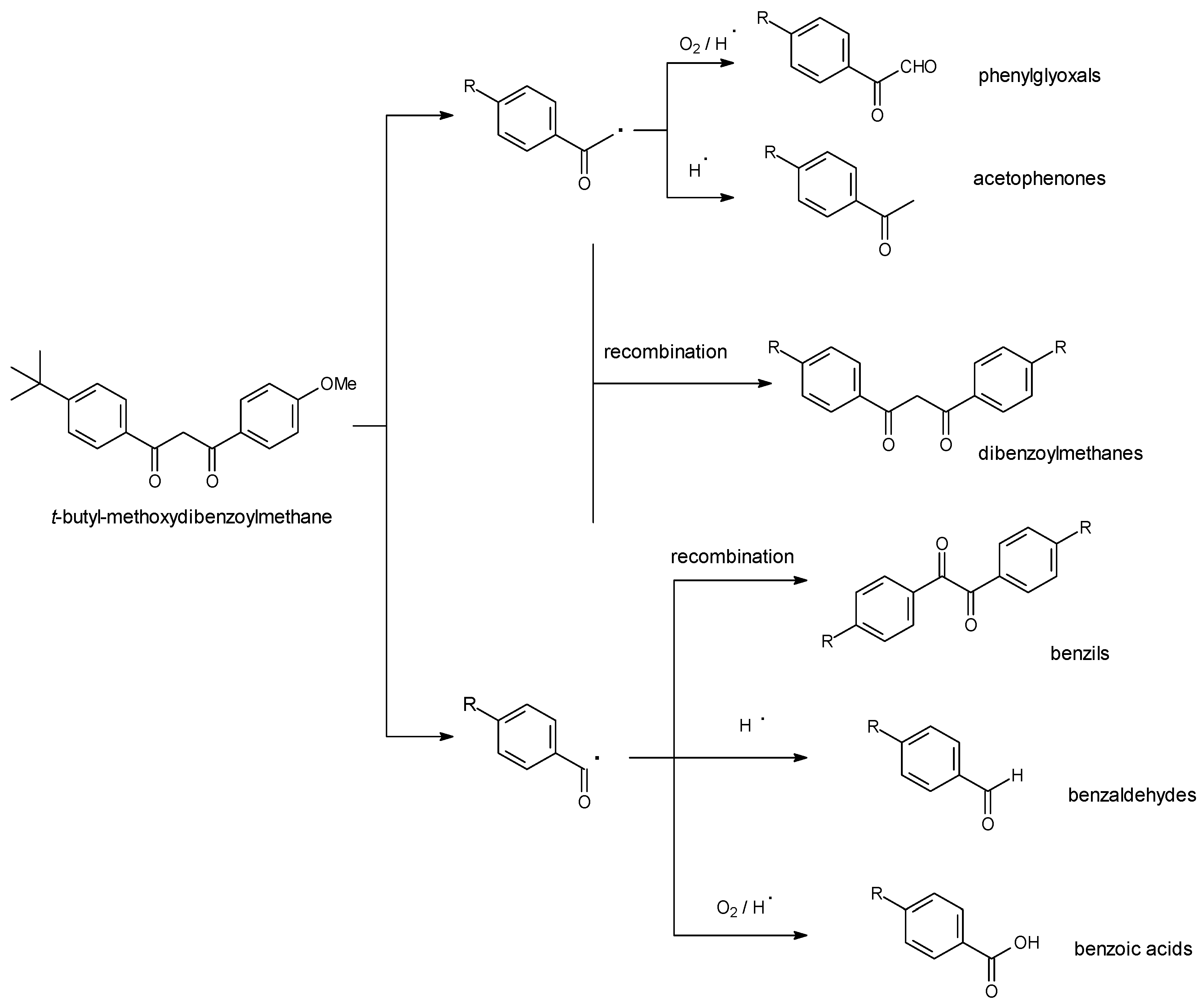
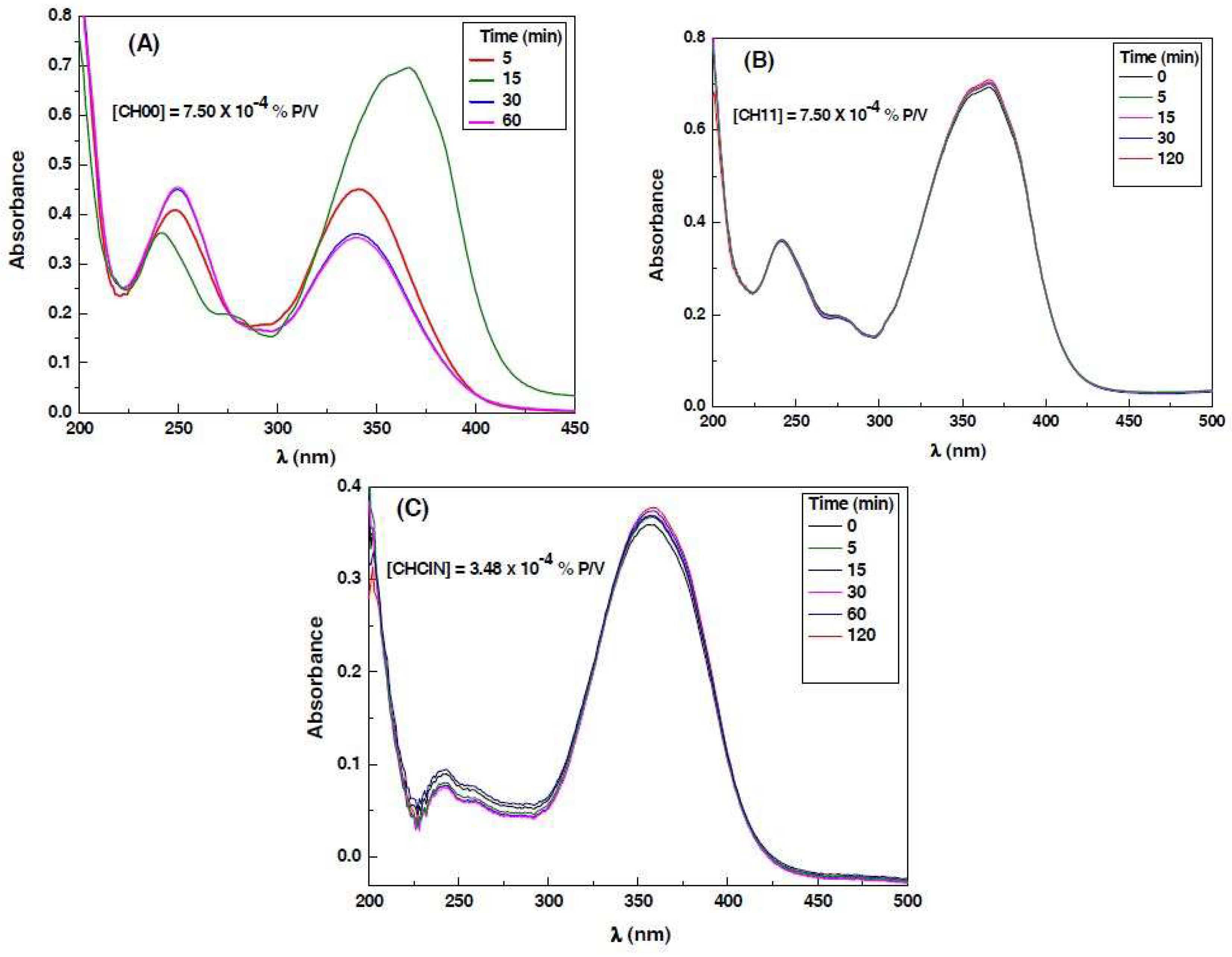
3.3. Decomposition Kinetics
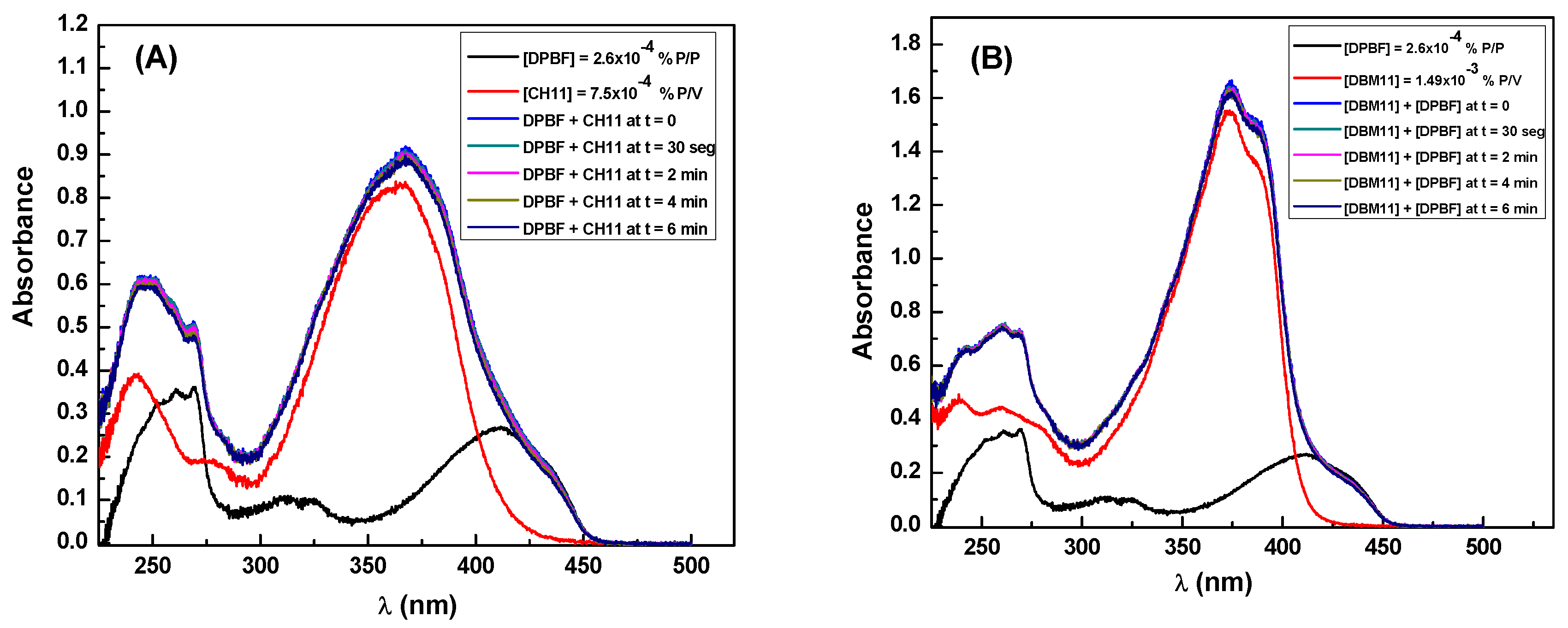
3.4. Singlet Oxygen and Free Radicals Studies
3.5. Decomposition Kinetics under More Stringent Conditions
4. Discussion
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References and Note
- Hossein-nezhad, A.; Holick, M.F. Vitamin D for health: A global perspective. Mayo Clin. Proc. 2013, 88, 720–755. [Google Scholar] [CrossRef] [PubMed]
- Papoutsaki, M.; Costanzo, A. Treatment of psoriasis and psoriatic arthritis. BioDrugs 2013, 27, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F. Vitamin D and sunlight: Strategies for cancer prevention and other health benefits. Clin. J. Am. Soc. Nephrol. 2008, 3, 1548–1554. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, D.L.; Saladi, R.N.; Fox, J.L. Ultraviolet radiation and skin cancer. Int. J. Dermatol. 2010, 49, 979–986. [Google Scholar] [CrossRef] [PubMed]
- Mason, R.S.; Reichrath, J. Sunlight, vitamin D and skin cancer. Anti-Cancer Agents Med. Chem. 2013, 13, 83–97. [Google Scholar] [CrossRef]
- Pfeifer, G.P.; Besaratinia, A. UV wavelength-dependent DNA damage and human non-melanoma and melanoma skin cancer. Photochem. Photobiol. Sci. 2012, 11, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Shaat, N.A. Sunscreen evolution. In Sunscreens, Regulations and Commercial Development; Shaat, N.A., Ed.; Taylor & Francis: Boca Raton, FL, USA, 2005; pp. 4–17. [Google Scholar]
- Shaath, N.A. Ultraviolet filters. Photochem. Photobiol. Sci. 2010, 9, 464–469. [Google Scholar] [CrossRef] [PubMed]
- Nash, J.F.; Tanner, P.R. Relevance of UV filter/sunscreen product photostability to human safety. Photodermatol. Photoimmunol. Photomed. 2014, 30, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Dondi, D.; Albini, A.; Serpone, N. Interactions between different solar UVB/UVA filters contained in commercial sunscreens and consequent loss of UV protection. Photochem. Photobiol. Sci. 2006, 5, 835–843. [Google Scholar] [CrossRef] [PubMed]
- Schwack, W.; Rudolph, T. Photochemistry of dibenzoylmethane UVA filters, Part I. J. Photochem. Photobiol. B 1995, 28, 229–234. [Google Scholar] [CrossRef]
- Kockler, J.; Oelgemöller, M.; Robertson, S.; Glass, B.D. Influence of titanium dioxide particle size on the photostability of the chemical UV-filters butyl methoxydibenzoylmethane and octocrylene in a microemulsion. Cosmetics 2014, 1, 128–139. [Google Scholar] [CrossRef]
- Svarc, F.E. A brief illustrated history on sunscreens and sun protection. Pure Appl. Chem. 2015, 87, 929–936. [Google Scholar] [CrossRef]
- Karlsson, I.; Hillerstrom, L.; Stenfeldt, A.L.; Martensson, J.; Borje, A. Photodegradation of dibenzoylmethanes: Potential cause of photocontact allergy to sunscreens. Chem. Res. Toxicol. 2009, 22, 1881–1892. [Google Scholar] [CrossRef] [PubMed]
- Sayre, R.M.; Dowdy, J.C.; Gerwig, A.J.; Shields, W.J.; Lloyd, R.V. Unexpected photolysis of the sunscreen octinoxate in the presence of the sunscreen avobenzone. Photochem. Photobiol. 2005, 81, 452–456. [Google Scholar] [CrossRef] [PubMed]
- Forestier, S.; Lang, G.; Richard, H. Cosmetic Use of Dibenzoylmethane Diorganopolysiloxanes and Novel Cosmetic Compositions Containing such Compounds for Protection of Skin and Hair. U.S. Patent 5,145,662, 8 September 1992. [Google Scholar]
- Bonda, C.A.; Marinelli, P.J.; Hessefort, Y.Z.; Trivedi, J.; Wentworth, G. Photostable Sunscreen Compositions Containing Dibenzoylmethane Derivative, e.g., Parsol® 1789, and Diesters or Polyesters of Naphthalene Dicarboxylic Acid Photostabilizers and Enhancers of the Sun Protection Factor (SPF). U.S. Patent 5,993,789, 30 November 1999. [Google Scholar]
- Hansenne, I.; Josso, M. Method for Providing Dibenzoylmethane Derivatives with Light Stability. U.S. Patent 6,569,409, 27 May 2003. [Google Scholar]
- Lang, G.; Malaval, A. Procedimiento de Preparación de una Composición Cosmética Que Contiene un Derivado del Dibenzoilmetano que Incluye un Grupo cuaternario. Spanish Patent ES 504.560, 5 August 1981. [Google Scholar]
- Asero, M.; Segal, A. Fotoestabilidad de avobenzona utilizando diferentes antioxidantes. In Proceedings of the 4th Argentine Congress of Cosmetic Chemistry (COARQC), Rosario, Argentina, 16–18 September 2016. [Google Scholar]
- Zawadiak, J.; Mrzyczek, M. Influence of substituent on UV absorption and keto-enol tautomerism equilibrium of dibenzoylmethane derivatives. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2012, 96, 815–819. [Google Scholar] [CrossRef] [PubMed]
- Wetz, F.; Routaboul, C.; Denis, A.; Ricco-Lattes, I. A new long-chain UV absorber derived from 4-tert-butyl-4′-methoxydibenzoilmethane: Absorbance stability under solar irradiation. J. Cosmet. Sci. 2005, 56, 135–148. [Google Scholar]
- Tarras-Wahlberg, N.; Stenhagen, G.; Larko, O.; Rosen, A.; Wenneberg, A.M.; Wennerström, O. Changes in ultraviolet absorption of sunscreens after ultraviolet irradiation. J. Investig. Dermatol. 1999, 113, 547–553. [Google Scholar] [CrossRef] [PubMed]
- Paris, C.; Lhiaubet-Vallet, V.; Jiménez, O.; Trullas, C.; Miranda, M.A. A blocked diketo form of avobenzone: Photostability, photosensitizing properties and triplet quenching by a triazine derived UVB-filter. Photochem. Photobiol. 2009, 85, 178–184. [Google Scholar] [CrossRef] [PubMed]
- Gonzenbach, H. Photostability of Cosmetic sunscreens. In Pharmaceutical Photostability and Stabilization Technology; Piechocki, J., Thoms, K., Eds.; Informa Healthcare: New York City, NY, USA, 2006; pp. 379–396. [Google Scholar]
- Deflandre, A.; Lang, G. Photostability assessment of sunscreens. Benzylidene camphor and dibenzoylmethane derivatives. Int. J. Cosm. Sci. 1988, 10, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Lhiaubet-Vallet, V.; Marin, M.; Jimenez, O.; Gorchs, O.; Trullas, C.; Miranda, M.A. Filter-filter interactions. Photostabilization, triplet quenching and reactivity with singlet oxygen. Photochem. Photobiol. Sci. 2010, 9, 552–558. [Google Scholar] [CrossRef] [PubMed]
- Tovar-Sanchez, A.; Sanchez-Quiles, D.; Basterretxea, G.; Benede, J.L.; Chisvert, A.; Salvador, A.; Moreno-Garrido, I.; Blasco, J. Sunscreen products as emerging pollutants to coastal waters. PLoS ONE 2013, 8, e65451. [Google Scholar] [CrossRef] [PubMed]
- Danovaro, R.; Bongiorni, L.; Corinaldesi, C.; Giovannelli, D.; Damiani, E.; Astolfi, P.; Greci, L.; Pusceddu, A. Sunscreens cause coral bleaching by promoting viral infections. Environ. Health Perspect. 2008, 116, 441–447. [Google Scholar] [CrossRef] [PubMed]
- Downs, C.A.; Kramarsky-Winter, E.; Segal, R.; Fauth, J.; Knutson, S.; Bronstein, O.; Ciner, F.R.; Jeger, R.; Lichtenfeld, Y.; Woodley, C.M.; et al. Toxicopathological effects of the sunscreen UV filter, oxybenzone (benzophenone-3), on coral planulae and cultured primary cells and its environmental contamination in Hawaii and the U.S. Virgin Islands. Arch. Environ. Contam. Toxicol. 2016, 70, 265–288. [Google Scholar] [CrossRef] [PubMed]
- Gago-Ferrero, P.; Diaz-Cruz, M.S.; Barcelo, D. An overview of UV-absorbing compounds (organic UV filters) in aquatic biota. Anal. Bioanal. Chem. 2012, 404, 2597–2610. [Google Scholar] [CrossRef] [PubMed]
- Fent, K.; Kunz, P. Occurrence, and effects of hormonally active UV filters in the aquatic environment. SOFW 2009, 135, 2–12. [Google Scholar]
- Poiger, T.; Buser, H.R.; Balmer, M.E.; Bergqvist, P.A.; Muller, M.D. Occurrence of UV filter compounds from sunscreens in surface waters: Regional mass balance in two Swiss lakes. Chemosphere 2004, 55, 951–963. [Google Scholar] [CrossRef] [PubMed]
- Giokas, D.L.; Salvador, A.; Chisvert, A. UV filters: From sunscreens to human body and the environment. Trends Anal. Chem. 2007, 26, 360–374. [Google Scholar] [CrossRef]
- Forestier, S.; Moire, C.; Lang, G. Preparation of Chalcone Derivatives as Sunscreens. Ger. Offen. DE 3742690 A1 19880630, 30 June 1988. [Google Scholar]
- Imokawa, G.; Takaishi, N.; Tejima, T.; Nakamura, K.; Hattori, M.; Masuda, S. Chalcone Derivatives and an Ultraviolet Absorber Containing Them. Ger. Offen. DE 3441636 A1 19850530, 20 October 1995. [Google Scholar]
- Fujii, A.; Sashita, Y.; Mimaki, Y.; Matsubara, K.; Hara, R.; Kitada, Y.; Nakajima, T.; Oosato, Y.; Oohata, S.; Myata, Y. 2,4,6-Trihydroxychalcone as a UV Absorbing Agent and Cosmetics Containing it. Jpn. KokaiTokkyoKoho. JP 08113521 A19960507, 7 May 1996. [Google Scholar]
- Okuya, F.; Nishio, H.; Kuwamura, S.; Deguchi, Y. ChalconeDerivatives and Copolymers Thereof as UV Absorbers. Jpn. KokaiTokkyoKoho. JP 05263067 A 19931012, 12 October 1993. [Google Scholar]
- Lahorkar, P.G.R.; Vaidya, A.A.; Chavan, M.V.; Gadgil, V.R. A Photoprotective Personal Care Composition. PCT Int. Appl. WO 2014191143-A1 20141204, 4 December 2014. [Google Scholar]
- Sagrera, G.; Bertucci, A.; Vazquez, A.; Seoane, G. Synthesis and antifungal activities of natural and synthetic biflavonoids. Bioorg. Med. Chem. 2011, 19, 3060–3073. [Google Scholar] [CrossRef] [PubMed]
- Sagrera, G. Síntesis de nuevos dibenzoilmetanos como potenciales filtros solares. In Proceedings of the XXII Latin American and Iberian Congress of Cosmetic Chemists, Punta del Este, Uruguay, 27–29 October 2015. [Google Scholar]
- Berger, D. Solar UV simulator skin testing. In Cosmetics and Toiletries Manufacture Worldwide; PA 19126-3331; Solar Light Co.: Philadelphia, PA, USA, 2003; pp. 255–257. [Google Scholar]
- Wilkinson, F.; Helman, W.P.; Ross, A.B. Rate constant for the decay and reactions of the lowest electronically excited singlet state of molecular oxygen in solution. J. Phys. Chem. 1995, 24, 663–1021. [Google Scholar] [CrossRef]
- Calzadilla, P.; Sapochnik, D.; Cosentino, S.; Diz, V.; Dicelio, L.E.; Calvo, J.C.; Guerra, L.N. N-acetylcysteine reduces markers of differentiation in 3T3-L1 adipocytes. Int. J. Mol. Sci. 2011, 12, 6936–6951. [Google Scholar] [CrossRef] [PubMed]
- Strassert, C.A.; Bilmes, G.M.; Awruch, J.; Dicelio, L.E. Comparative photophysical investigation of oxygen and sulfur as covalent linkers on octaalkylamino substituted zinc(II) phthalocyanines. Photochem. Photobiol. Sci. 2008, 7, 738–747. [Google Scholar] [CrossRef] [PubMed]
- Global Solar UV Index. Available online: http://www.who.int/uv/publications/globalindex/en/ (accessed on 9 February 2017).
- Amor, S.; Angeli, N.G.; Dicelio, L.E. Fotoestabilidad de Filtros Solares. In Proceedings of the XXVII Meeting of the Argentine Society of Cosmetic Chemists (AAQC), Pinamar, Argentina, 3–5 November 2000. [Google Scholar]
- Kavanaugh, E. Tentative Final Monograph for OTC Sunscreen Drug Products: Docket No. 78N0038; The Cosmetic, Toiletry, and Fragrance Association (CTFA): Washington, DC, USA, 1998. [Google Scholar]
- COLIPA Project Team IV. Guideline 2007a. Method for the in vitro Determination of UVA Protection Provided by Sunscreen Products. The European Cosmetic, Toiletry and Perfumery Association. Bruselles 2007.
- Margarida, S.M.; Pinto da Silva, L.; Esteves da Silva, J.C.G. UV Filter 2-ethylhexyl 4-methoxycinnamate: A structure, energetic and UV-vis spectral analysis based on density functional theory. J. Phys. Org. Chem. 2014, 27, 47–56. [Google Scholar] [CrossRef]






| Sample | Absorption at λ = 350 nm | Emission Area | Absorption Intensity | Quantum Yield (Fluorescence) |
|---|---|---|---|---|
| Rod 101 | 0.0134 | 528628676.92 | 0.03038 | 0.96 |
| DBM 1 | 0.1387 | 162205046.87 | 0.27339 | 0.033 |
| DBM 2 | 0.3064 | 99573752.80 | 0.50614 | 0.011 |
| DBM4 | 0.1788 | 169868850.73 | 0.33747 | 0.028 |
| DBM11 | 0.3223 | 168263751.04 | 0.52389 | 0.018 |
| CH00 | 0.1736 | 162546475.64 | 0.32949 | 0.027 |
| CH11 | 0.2316 | 225362192.66 | 0.41332 | 0.030 |
| CHClN | 0.3777 | 179803227.87 | 0.58092 | 0.017 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quintana Lazópulos, S.; Svarc, F.; Sagrera, G.; Dicelio, L. Absorption and Photo-Stability of Substituted Dibenzoylmethanes and Chalcones as UVA Filters. Cosmetics 2018, 5, 33. https://doi.org/10.3390/cosmetics5020033
Quintana Lazópulos S, Svarc F, Sagrera G, Dicelio L. Absorption and Photo-Stability of Substituted Dibenzoylmethanes and Chalcones as UVA Filters. Cosmetics. 2018; 5(2):33. https://doi.org/10.3390/cosmetics5020033
Chicago/Turabian StyleQuintana Lazópulos, Silvina, Federico Svarc, Gabriel Sagrera, and Lelia Dicelio. 2018. "Absorption and Photo-Stability of Substituted Dibenzoylmethanes and Chalcones as UVA Filters" Cosmetics 5, no. 2: 33. https://doi.org/10.3390/cosmetics5020033
APA StyleQuintana Lazópulos, S., Svarc, F., Sagrera, G., & Dicelio, L. (2018). Absorption and Photo-Stability of Substituted Dibenzoylmethanes and Chalcones as UVA Filters. Cosmetics, 5(2), 33. https://doi.org/10.3390/cosmetics5020033





