Secondary Plant Metabolites for Sun Protective Cosmetics: From Pre-Selection to Product Formulation
Abstract
1. Introduction
2. Photo-Chemical Barrier in Plants versus Human Skin
2.1. Photo-Active Secondary Metabolites in Terrestrial and Marine Plants: Biosynthesis and Physiological Effects
- -
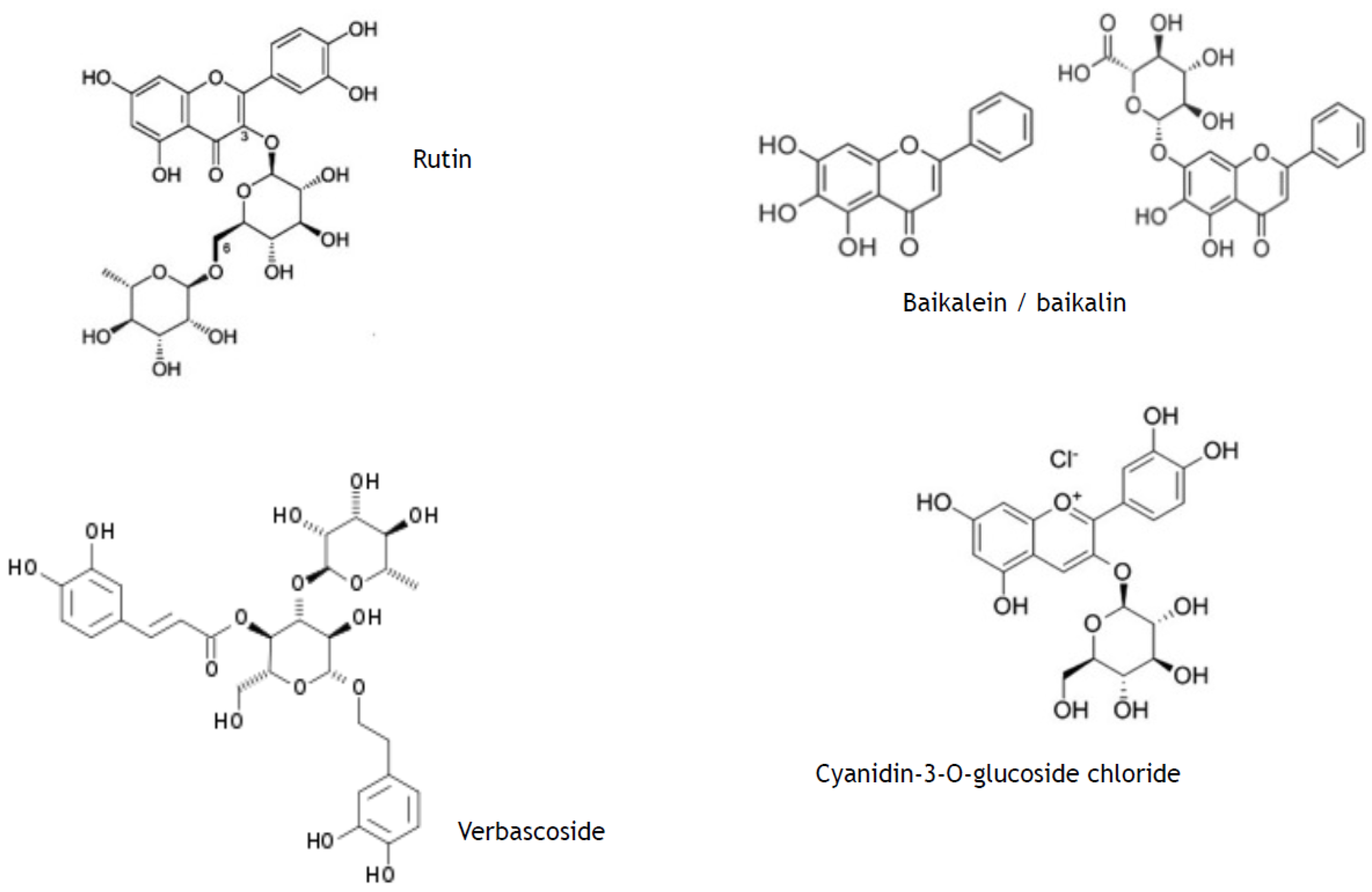
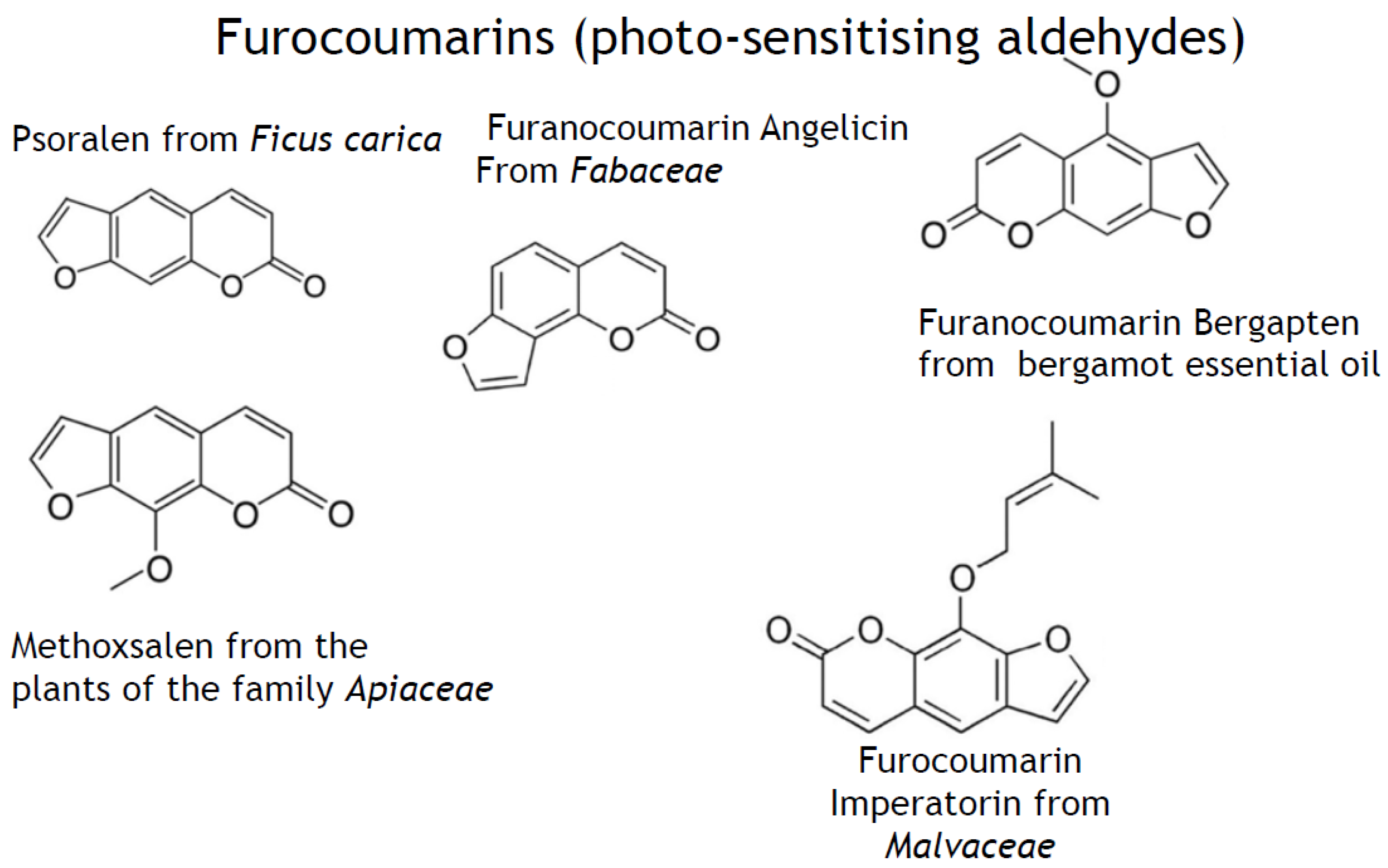
- phenylpropanoids (synonyms, ethylpropanoids) and their derivatives, such as simple polyphenolics (acids, alcohols, and aldehydes), aromatic/poly-aromatic polyphenols (flavonoids, stilbenes, curcuminoids, coumarins, etc.), and glycosides (glycoside moieties, are, mainly, rhamnose, mannose, rutinose, etc.). All these SPMs contain exclusively carbon, hydrogen, and oxygen atoms and multiple hydroxyl groups;
- -
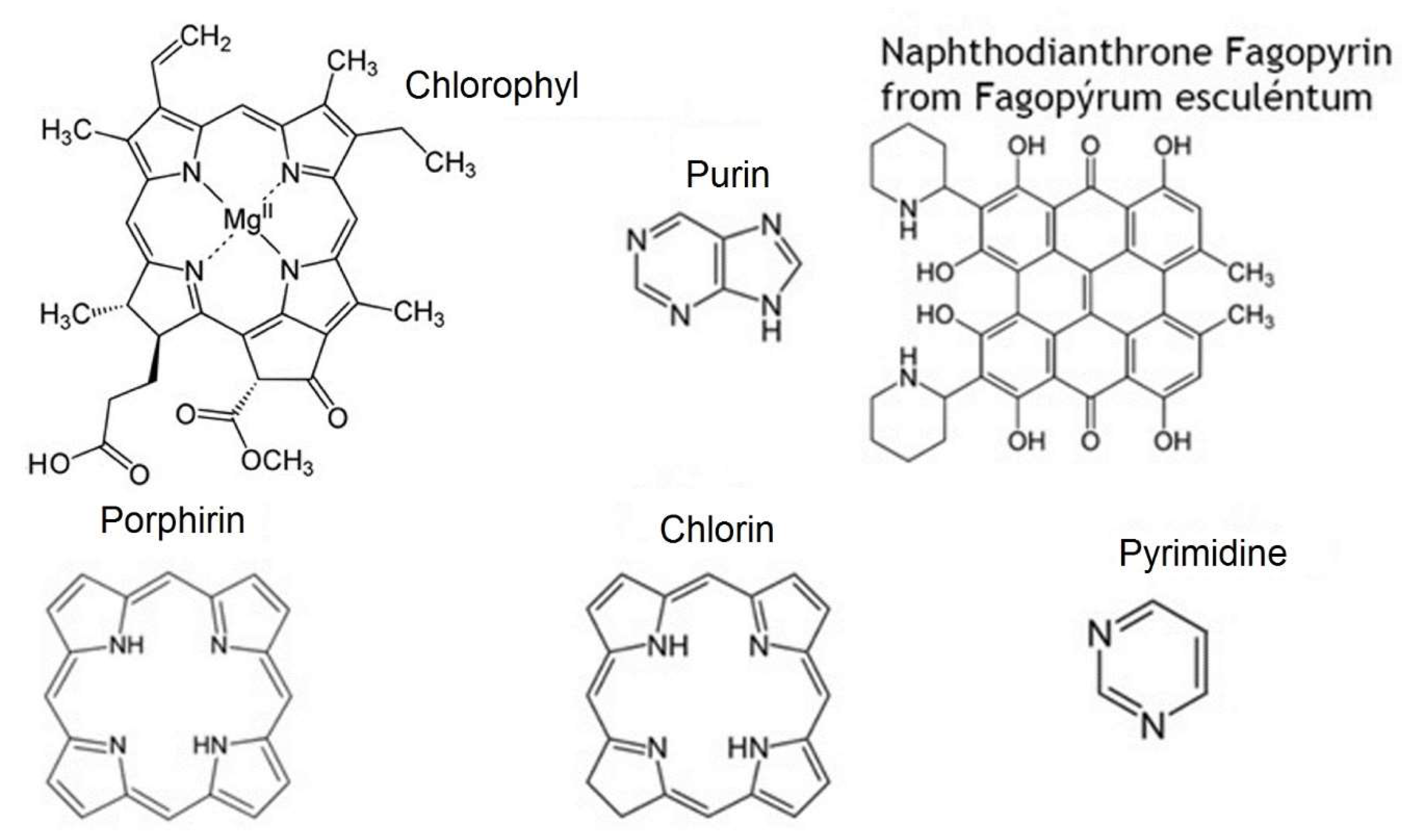
- terpenoids containing long, mainly, unsaturated C-H chain and nucleus of non aromatic C-H cycle;
- -
- nitrogen-containing heterocycles, such as alkaloids, purines, pyrimidines, porphyrins, chlorophylls, flavins, etc.
2.2. Photo-Chemical Barrier in Human Skin
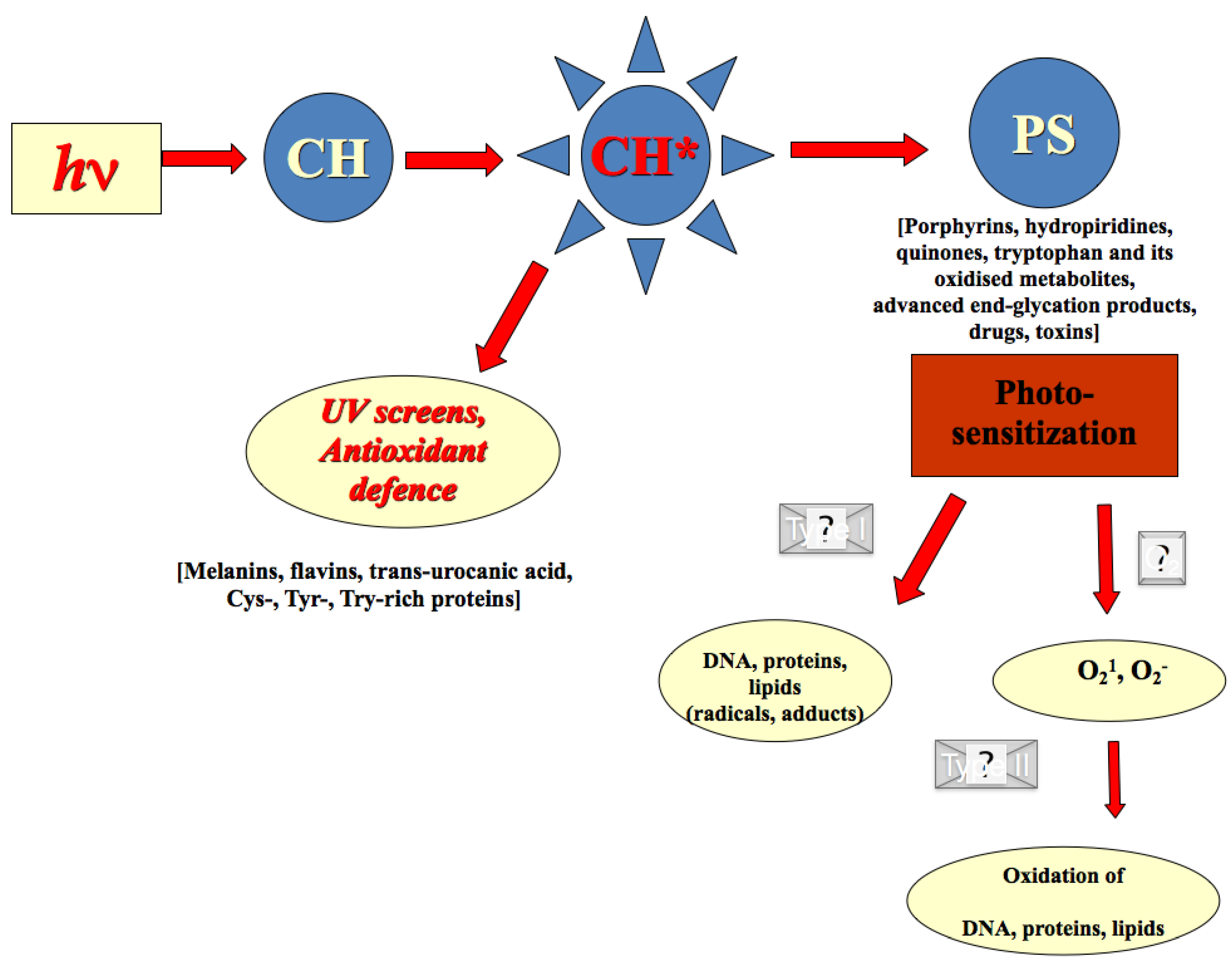
2.3. Redox Barrier of Human Skin
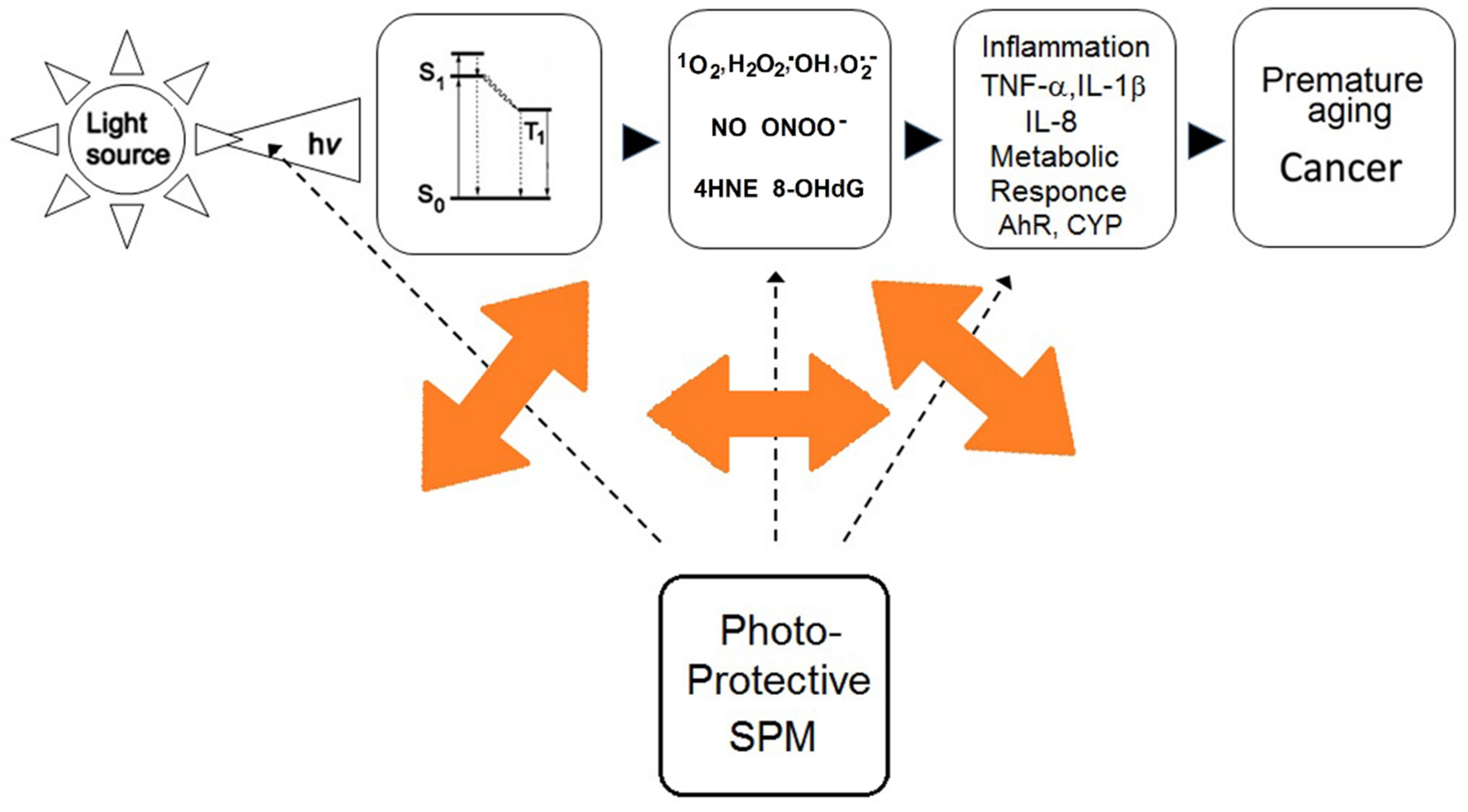
2.4. Effects of Secondary Plant Metabolites on Photo-Chemical and Redox Barriers of Human Skin
3. Metabolic Barrier of Human Skin
3.1. Metabolic Responses in Human Skin to Solar Irradiation
3.2. Effects of Secondary Plant Metabolites on Metabolic Responses to Solar Irradiation
4. Immune System in Human Skin
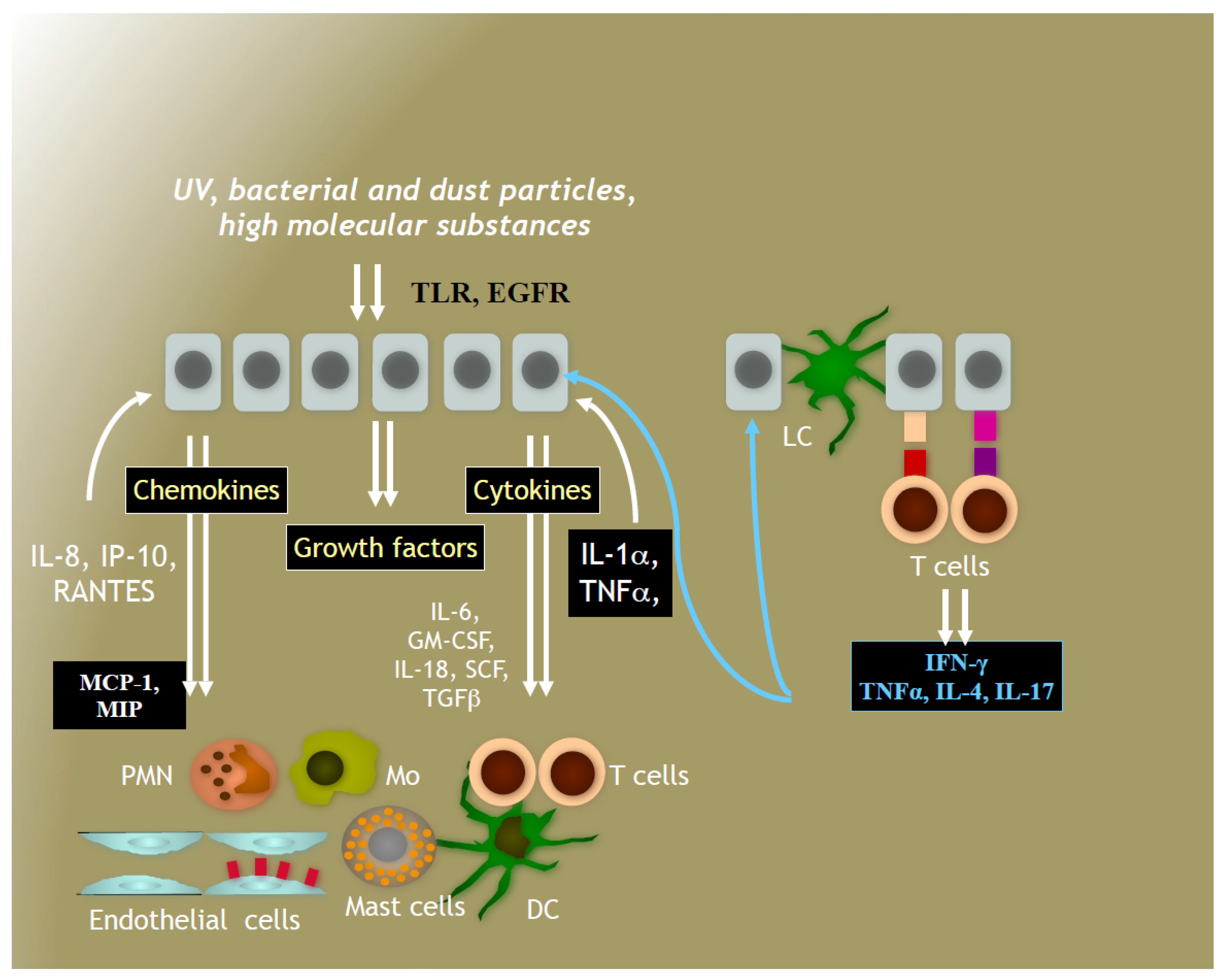
4.1. Immune Responses to Solar Irradiation
4.2. Effects of Secondary Plant Metabolites on Immune Responses to Solar Irradiation
5. Pre-Selection of Sun-Protective Secondary Plant Metabolites
6. Formulation of Sun Protective Cosmetics Based on Secondary Plant Metabolites
7. Conclusions: Advantages and Disadvantages of Fully Natural Sun Protective Cosmetics
Conflicts of Interest
References
- De Luca, C.; Valacchi, G. Surface lipids as multifunctional mediators of skin responses to environmental stimuli. Mediat. Inflamm. 2010, 2010, 321494. [Google Scholar] [CrossRef] [PubMed]
- Kostyuk, V.A.; Potapovich, A.I.; Lulli, D.; Stancato, A.; De Luca, C.; Pastore, S.; Korkina, L. Modulation of human keratinocyte responses to solar UV by plant polyphenols as a basis for chemoprevention of non-melanoma skin cancers. Curr. Med. Chem. 2013, 20, 869–879. [Google Scholar] [PubMed]
- Scharffetter-Kochanek, K.; Wlaschek, M.; Brenneisen, P.; Schauen, M.; Blaudschun, R.; Wenk, J. UV-induced reactive oxygen species in photocarcinogenesis and photoaging. Biol. Chem. 1997, 378, 1247–1257. [Google Scholar] [PubMed]
- Pallela, R.; Young, Y.N.; Kim, S.K. Anti-photoaging and photoprotective compounds derived from marine organisms. Mar. Drugs 2010, 8, 1189–1202. [Google Scholar] [CrossRef] [PubMed]
- Chanchal, D.; Swarnlata, S. Herbal photoprotective formulations and their evaluation. Open Nat. Prod. J. 2009, 2, 71–76. [Google Scholar] [CrossRef]
- Saewan, N.; Jimtaisong, A. Natural products as photoprotection. J. Cosmet. Dermatol. 2015, 14, 47–63. [Google Scholar] [CrossRef] [PubMed]
- Korkina, L.; Mayer, W.; De Luca, C. Meristem plant cells as a sustainable source of redox actives for skin rejuvenation. Biomolecules 2017, 7, 40. [Google Scholar] [CrossRef] [PubMed]
- Douglas, C.J. Phenylpropanoid metabolism and lignin biosynthesis: From weeds to trees. Trends Plant Sci. 1996, 1, 171–178. [Google Scholar] [CrossRef]
- Korkina, L.G. Phenylpropanoids as naturally occurring antioxidants: From plant defence to human health. Cell. Mol. Biol. 2007, 53, 15–25. [Google Scholar] [PubMed]
- Ramakrishna, A.; Ravishankar, G.A. Influence of abiotic stress signals on secondary metabolites in plants. Plant Signal. Behav. 2011, 6, 1720–1731. [Google Scholar] [PubMed]
- Groniger, A.; Sinha, R.P.; Klisch, M.; Häder, D.P. Photoprotective compounds in cyanobacteria, phytoplankton and macroalgae—A database. J. Photochem. Photobiol. B Biol. 2000, 58, 115–122. [Google Scholar] [CrossRef]
- Korkina, L.G.; Pastore, S.; De Luca, C.; Kostyuk, V.A. Metabolism of plant polyphenols in the skin: Beneficial versus deleterious effects. Curr. Drug Metab. 2008, 9, 710–729. [Google Scholar] [CrossRef] [PubMed]
- Perez-Sancez, A.; Barrajon-Catalan, E.; Caturla, N.; Castillo, J.; Benavente-Garcia, O.; Alcaraz, M.; Micol, V. Protective effects of citrus and rosemary extracts on UV-induced damage in skin cell model and human volunteers. J. Photochem. Photobiol. B 2014, 136, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Frohnmeyer, H.; Staiger, D. Ultraviolet-B radiation-mediated responses in plants. Balancing damage and protection. Plant Physiol. 2003, 133, 1420–1428. [Google Scholar] [CrossRef] [PubMed]
- Hahlbrock, K.; Scheel, D. Physiology and molecular biology of phenylpropanoid metabolism. Ann. Rev. Plant Physiol. Plant Mol. Biol. 1989, 4, 347–369. [Google Scholar] [CrossRef]
- Moyal, D.D.; Fourtanier, A.M. Broad-spectrum sunscreens provide better protection from solar ultraviolet-stimulated radiation and natural sunlight-induced immunosuppression in human beings. J. Am. Acad. Dermatol. 2008, 58, S149–S154. [Google Scholar] [CrossRef] [PubMed]
- Wolf, R.; Wolf, D.; Morganti, P.; Ruocco, V. Sunscreens. Clin. Dermatol. 2001, 9, 452–459. [Google Scholar] [CrossRef]
- Commission recommendation of 22 September 2006 on the efficacy of sunscreen products and the claims made relating thereto. Off. J. Eur. Union 2006, 265, 39–43.
- Dembitsky, V.M. Astonishing diversity of natural surfactants: 5. Biologically active glycosides of aromatic metabolites. Lipids 2005, 40, 869–900. [Google Scholar] [CrossRef] [PubMed]
- Ju, K.Y.; Kang, J.; Chang, J.H.; Lee, J.K. Clue to understanding the Janus behavior of eumelanin: Investigating the relationships between hierarchical assembly structure of eumelanin and its photo physical properties. Biomacromolecules 2016, 17, 2860–2872. [Google Scholar] [CrossRef] [PubMed]
- Bilkis, I.; Silman, I.; Weiner, L. Generation of reactive oxygen species by photosensitizers and their modes of action on proteins. Curr. Med. Chem. 2018. [Google Scholar] [CrossRef] [PubMed]
- Korkina, L. Metabolic and redox barriers in the skin exposed to drugs and xenobiotics. Exp. Opin. Drug Metab. Toxicol. 2016, 12, 377–388. [Google Scholar] [CrossRef] [PubMed]
- Kostyuk, V.; Potapovich, A.; Albuhaydar, A.R.; Mayer, W.; De Luca, C.; Korkina, L. Natural substances for prevention of skin photoageing: Screening systems in the development of sunscreen and rejuvenation cosmetics. Rejuvenation Res. 2017. [Google Scholar] [CrossRef]
- Herrmann, G.; Wlaschek, M.; Bolsen, K.; Prenzel, K.; Goerz, G.; Scharffetter-Kochanek, K. Photosensitisation of uroporphyrin augments the ultraviolet A-induced synthesis of matrix metalloproteinases in human dermal fibroblasts. J. Investig. Dermatol. 1996, 107, 398–403. [Google Scholar] [CrossRef] [PubMed]
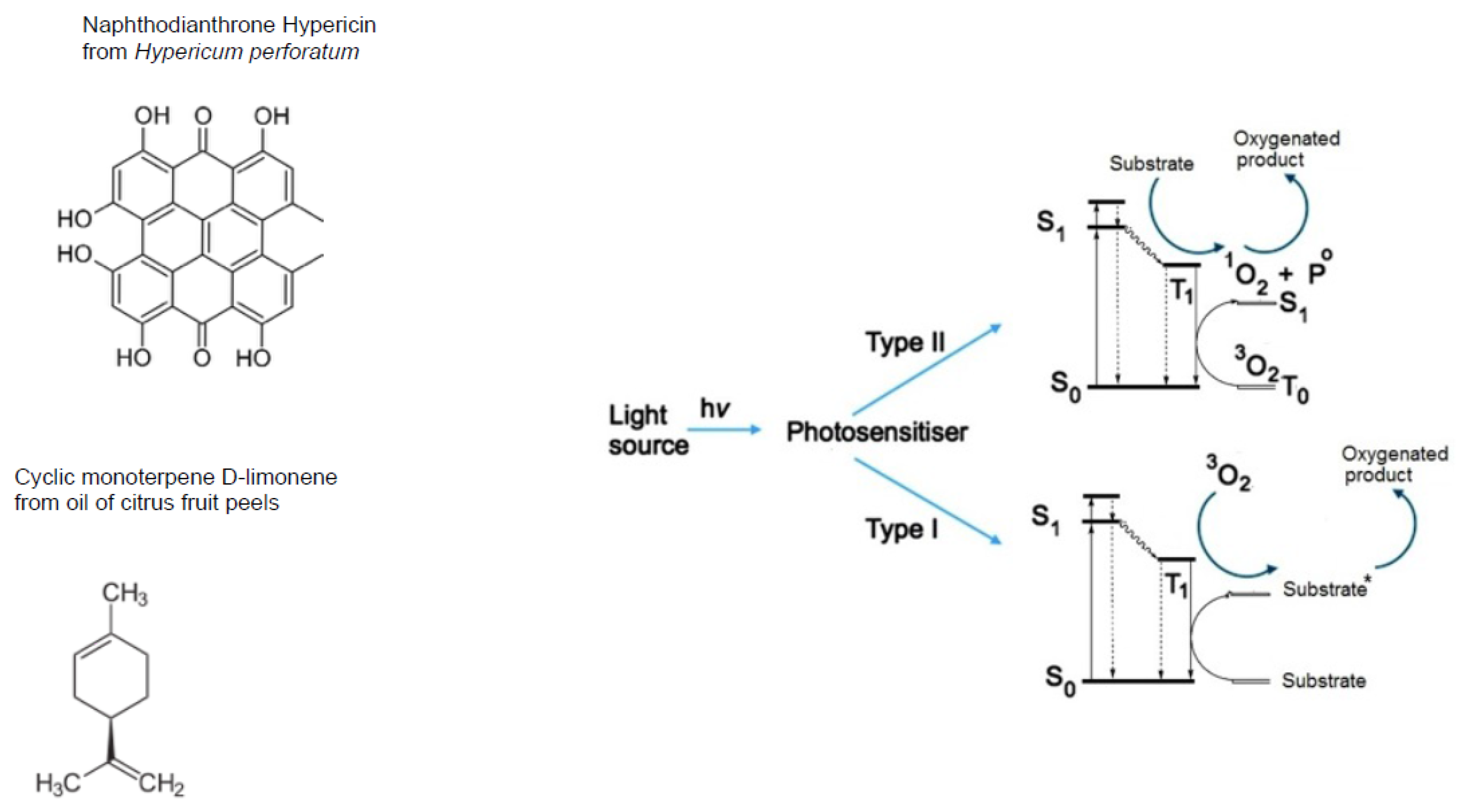
- Wondrak, G.T.; Jacobson, M.K.; Jacobson, E.L. Endogenous UVA-photosensitizers: Mediators of skin photodamage and novel targets for skin photoprotection. Photochem. Photobiol. Sci. 2006, 5, 215–237. [Google Scholar] [CrossRef] [PubMed]
- Cadet, J.; Douki, T.; Ravanat, J.L.; Di Mascio, P. Sensitised formation of oxidatively generated damage to cellular DNA by UVA radiation. Photochem. Photobiol. Sci. 2009, 8, 903–911. [Google Scholar] [CrossRef] [PubMed]
- Lamore, S.D.; Azimian, S.; Horn, D.; Anglin, B.L.; Uchida, K.; Cabello, C.M.; Wondrak, G.T. The malondialdehyde-derived fluorophore DPH-lysine is a potential sensitizer of UVA-induced photooxidative stress in human skin cells. J. Photochem. Photobiol. 2010, 101, 251–264. [Google Scholar] [CrossRef] [PubMed]
- Dickinson, S.E.; Wondrak, G.T. TLR4-directed molecular strategies targeting skin photodamage and carcinogenesis. Curr. Med. Chem. 2017. [Google Scholar] [CrossRef] [PubMed]
- De Luca, C.; Picardo, M.; Breathnach, A.; Passi, S. Lipoperoxidase activity of Pityrosporum: Characterisation of by-products and possible role in Pityriasis versicolor. Exp. Dermatol. 1996, 5, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Ekanayake Mudiyanselage, S.; Hamburger, M.; Elsner, P.; Thiele, J.J. Ultraviolet A induces generation of squalene monohydroperoxide isomers in human sebum and skin surface lipids in vitro and in vivo. J. Investig. Dermatol. 2003, 120, 915–922. [Google Scholar] [CrossRef] [PubMed]
- Picardo, M.; Mastrofrancesco, A.; Biro, T. Sebaceous gland—A major player in skin homeostasis. Exp. Dermatol. 2015, 24, 485–486. [Google Scholar] [CrossRef] [PubMed]
- Kostyuk, V.; Potapovich, A.; Stancato, A.; De Luca, C.; Lulli, D.; Pastore, S.; Korkina, L. Photo-oxidation products of skin surface squalene mediate metabolic and inflammatory responses to solar UV in human keratinocytes. PLoS ONE 2012, 7, e44472. [Google Scholar] [CrossRef] [PubMed]
- Auffray, B. Protection against singlet oxygen, the main actor of sebum squalene peroxidation during sun exposure, using Commiphora myrrha essential oil. Int. J. Cosmet. Sci. 2007, 29, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Rannug, A.; Fritsche, E. The aryl hydrocarbon receptor and light. Biol. Chem. 2006, 387, 1149–1157. [Google Scholar] [CrossRef] [PubMed]
- Uchida, K.; Shiraishi, M.; Naito, Y.; Torii, Y.; Nakamura, Y.; Osawa, T. Activation of stress signaling pathways by the end product of lipid peroxidation. J. Biol. Chem. 1999, 274, 2234–2242. [Google Scholar] [CrossRef] [PubMed]
- Hattori, T.; Obinata, H.; Ogawa, A.; Kishi, M.; Tatei, K.; Ishikawa, O.; Izumi, T. G2A plays proinflammatory roles in human keratinocytes under oxidative stress as a receptor for 9-hydroxyoctadecadenoic acid. J. Investig. Dermatol. 2008, 128, 1123–1133. [Google Scholar] [CrossRef] [PubMed]
- Hibbert, S.A.; Watson, R.E.B.; Gibbs, N.K.; Costello, P.; Baldock, C.; Weiss, A.S.; Griffiths, C.E.; Sherratt, M.J. A potential role for endogenous proteins as sacrificial sunscreens and antioxidants in human tissues. Redox Biol. 2015, 5, 101–113. [Google Scholar] [CrossRef] [PubMed]
- Kwon, M.J.; Han, J.; Kim, B.H.; Lee, Y.S.; Kim, T.Y. Superoxide dismutase 3 suppresses hyaluronic acid fragments mediated skin inflammation by inhibition of toll-like receptor 4 signaling pathway: Superoxide dismutase 3 inhibits reactive oxygen species-induced trafficking of toll-like receptor 4 to lipid rafts. Antioxid. Redox Signal. 2012, 16, 297–313. [Google Scholar] [PubMed]
- Thiele, J.; Barland, C.O.; Ghadially, R.; Elias, P. Permeability and antioxidant barriers in aged skin. In Skin Aging; Gilchrest, B., Krutmann, J., Eds.; Springer: Berlin, Germany, 2006. [Google Scholar]
- Packer, L.; Valacchi, G. Antioxidants and the response of skin to oxidative stress: Vitamin E as an indicator. Skin Pharmacol. Appl. Skin Physiol. 2002, 15, 282–290. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.T.; Zmijewski, M.A.; Semak, I.; Zbytek, B.; Pisarchik, A.; Li, W.; Zjawiony, J.; Tuckey, R.C. Cytochromes P450 and skin cancer: Role of local endocrine pathways. Anticancer Agents Med. Chem. 2014, 14, 77–96. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.T.; Kleszczynski, K.; Semak, I.; Janjetovic, Z.; Zmijewski, M.A.; Kim, T.K.; Slominski, R.M.; Reiter, R.J.; Fischer, T.W. Local melatoninergic system as the protector of skin integrity. Int. J. Mol. Sci. 2014, 15, 17705–17732. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.K.; Kleszczynski, K.; Janjetovic, Z.; Sweatman, T.; Lin, Z.; Li, W.; Reiter, R.J.; Fischer, T.W.; Slominski, A.T. Metabolism of melatonin and biological activity of intermediates of melatoninergic pathway in human skin cells. FASEB J. 2013, 27, 2742–2755. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Surh, Y.J. Nrf2 as a novel molecular target for chemoprevention. Cancer Lett. 2005, 224, 171–184. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q. Role of Nrf2 in oxidative stress and toxicity. Annu. Rev. Pharmacol. Toxicol. 2013, 53, 401–426. [Google Scholar] [CrossRef] [PubMed]
- Schafer, M.; Werner, S. Nrf2-A regulator of keratinocyte redox signaling. Free Radic. Biol. Med. 2015, 88, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Rojo de la Vega, M.; Krajisnik, A.; Zhang, D.D.; Wondrak, G.T. Targeting NRF2 for improved skin barrier function and photoprotection: Focus on the achiote-derived apocarotenoid bixin. Nutrients 2017, 9, 1371. [Google Scholar] [CrossRef] [PubMed]
- Hirota, A.; Kawachi, Y.; Yamamoto, M.; Koga, T.; Hamada, K.; Otsuka, F. Acceleration of UVB-induced photoageing in nrf2 gene-deficient mice. Exp. Dermatol. 2011, 20, 664–668. [Google Scholar] [CrossRef] [PubMed]
- Bosch, R.; Philips, N.; Suarez-Perez, J.A.; Juarranz, A.; Devmurari, A.; Chalensouk-Khaosaat, J.; Gonzalez, S. Mechanisms of Photoaging and Cutaneous Photocarcinogenesis, and Photoprotective Strategies with Phytochemicals. Antioxidants 2015, 4, 248–268. [Google Scholar] [CrossRef] [PubMed]
- Oesch, F.; Fabian, E.; Guth, K.; Landsiedel, R. Xenobiotic-metabolizing enzymes in the skin of rat, mouse, pig, guinea, and in human skin models. Arch. Toxicol. 2014, 88, 2135–2190. [Google Scholar] [CrossRef] [PubMed]
- Katiyar, S.K.; Matsui, M.S.; Mukhtar, H. Ultraviolet-B exposure of human skin induces cytochromes P450 1A1 and 1B1. J. Investig. Dermatol. 2000, 114, 328–333. [Google Scholar] [CrossRef] [PubMed]
- Pavek, P.; Dvorak, Z. Xenobiotic-induced transcriptional regulation of xenobiotic metabolizing enzymes of the cytochrome P450 superfamily in human extrahepatic tissues. Curr. Drug Metab. 2008, 9, 129–143. [Google Scholar] [CrossRef] [PubMed]
- Baron, J.M.; Holler, D.; Schiffer, R.; Frankenberg, S.; Neis, M.; Merk, H.F.; Jugert, F.K. Expression of multiple cytochrome P450 enzymes and multidrug resistance-associated transport proteins in human skin keratinocytes. J. Investig. Dermatol. 2001, 116, 541–548. [Google Scholar] [CrossRef] [PubMed]
- Gundert-Remy, U.; Bernauer, U.; Blomeke, B.; Döring, B.; Fabian, E.; Goebel, C.; Hessel, S.; Jäckh, C.; Lampen, A.; Oesch, F.; et al. Extrahepatic metabolism at the body’s internal-external interfaces. Drug Metab. Rev. 2014, 46, 291–324. [Google Scholar] [CrossRef] [PubMed]
- Chiaro, C.R.; Patel, R.D.; Perdew, G.H. 12(R)-hydroxy-5(Z),8(Z),10(E),14(Z)-eicosatetraenoic acid [12(R)-HETE], an arachidonic acid derivative, is an activator of the aryl hydrocarbon receptor. Mol. Pharmacol. 2008, 74, 1649–1656. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.P.; Bradfield, C.A. The search for endogenous activators of aryl hydrocarbon receptor. Chem. Res. Toxicol. 2008, 21, 102–116. [Google Scholar] [CrossRef] [PubMed]
- Stejskalova, L.; Dvorak, Z.; Pavek, P. Endogenous and exogenous ligands of aryl hydrocarbon receptor: Current state of art. Curr. Drug Metab. 2011, 12, 198–212. [Google Scholar] [CrossRef] [PubMed]
- Kalthoff, S.; Ehmer, U.; Freiberg, N.; Manns, M.P.; Strassburg, C.P. Interaction between oxidative stress sensor Nrf2 and xenobiotic-activated aryl hydrocarbon receptor in the regulation of the human phase II detoxifying UDP-glucuronosyltransferase 1A10. J. Biol. Chem. 2010, 285, 5993–6002. [Google Scholar] [CrossRef] [PubMed]
- Swanson, H.I. Cytochrome P450 expression in human keratinocytes: An aryl hydrocarbon receptor perspective. Chem. Biol. Interact. 2004, 149, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Puga, A.; Ma, C.; Marlowe, J.L. The aryl hydrocarbon receptor cross-talks with multiple signal transduction pathways. Biochem. Pharmacol. 1999, 77, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Ohtake, F.; Takeyama, K.; Matsumoto, T.; Kitagawa, H.; Yamamoto, Y.; Nohara, K.; Tohyama, C.; Krust, A.; Mimura, J.; Chambon, P.; et al. Modulation of estrogen receptor signaling by association with the activated dioxin receptor. Nature 2003, 423, 545–550. [Google Scholar] [CrossRef] [PubMed]
- Guyot, E.; Chevallier, A.; Barouki, R.; Coumoul, X. The AhR twist: Ligand-dependent AhR signaling and pharmaco-toxicological implications. Drug Discov. Today 2013, 18, 479–486. [Google Scholar] [CrossRef] [PubMed]
- Busbee, P.B.; Busbee, M.; Rouse, M.; Nagarkatti, M.; Nagarkatti, P.S. Use of natural AhR ligands as potential therapeutic modalities against inflammatory disorders. Nutr. Rev. 2013, 71, 353–369. [Google Scholar] [CrossRef] [PubMed]
- Pastore, S.; Lulli, D.; Potapovich, A.I.; Fidanza, P.; Kostyuk, V.A.; Dellambra, E.; De Luca, C.; Maurelli, R.; Korkina, L. Differential modulation of stress-inflammation responses by plant polyphenols in cultured normal human keratinocytes and immortalized HaCaT cells. J. Dermatol. Sci. 2011, 63, 104–114. [Google Scholar] [CrossRef] [PubMed]
- Katiyar, S.K. UV-induced immune suppression and photocarcinogenesis: Chemoprevention by dietary botanical agents. Cancer Lett. 2007, 255, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Katiyar, S.K.; Afaq, F.; Peres, A.; Mukhar, H. Green tea polyphenol (−)-epigallocatechin-3-gallate treatment of human skin inhibits ultraviolet radiation-induced oxidative stress. Carcinogenesis 2001, 22, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Afaq, F.; Adhami, V.M.; Ahmad, N.; Mukhtar, H. Inhibition of ultraviolet B-mediated activation of nuclear factor κB in normal human epidermal keratinocytes by green tea constituent (−)-epigallocatechin-3-gallate. Oncogene 2003, 22, 1035–1044. [Google Scholar] [CrossRef] [PubMed]
- Potapovich, A.I.; Lulli, D.; Fidanza, P.; Kostyuk, V.A.; De Luca, C.; Pastore, S.; Korkina, L. Plant polyphenols differentially modulate inflammatory responses of human keratinocytes by interfering with activation of transcriptional factors NFκB and AhR and EGFR-ERK pathways independently of their direct redox properties. Toxicol. Appl. Pharmacol. 2011, 255, 138–149. [Google Scholar] [CrossRef] [PubMed]
- Pastore, S.; Lulli, D.; Pascarella, A.; Maurelli, R.; Dellambra, E.; Potapovich, A.; Kostyuk, V.; De Luca, C.; Korkina, L. Resveratrol enhances solar UV induced responses in normal human epidermal keratinocytes. Photochem. Photobiol. 2012, 88, 1522–1530. [Google Scholar] [CrossRef] [PubMed]
- Wright, T.I.; Spencer, J.M.; Flowers, F.P. Chemoprevention of nonmelanoma skin cancer. J. Am. Acad. Dermatol. 2006, 54, 933–946. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.Y.; Huang, M.T.; Ferraro, T.; Wong, C.Q.; Lou, Y.R.; Reuhl, K.; Iatropulos, M.; Yang, C.S.; Connery, A.H. Inhibitory effect of green tea in the drinking water on tumorigenesis by ultraviolet light and 12-O-tetradecanoylphorbol-13-acetate in the skin of SKH-1 mice. Cancer Res. 1992, 52, 1162–1170. [Google Scholar] [PubMed]
- Wei, H.; Saladi, R.; Lu, Y.; Wang, Y.; Palep, S.R.; Moore, J.; Phelps, R.; Shyong, E.; Lebwohl, M.G. Isoflavone genistein: Photoprotection and clinical implications in dermatology. J. Nutr. 2003, 133, 3811S–3819S. [Google Scholar] [CrossRef] [PubMed]
- Picardo, M.; Zompetta, C.; De Luca, C.; Cirone, M.; Faggioni, A.; Nazzaro-Porro, M.; Passi, S.; Prota, G. Role of skin surface lipids in UV-induced epidermal cell changes. Arch. Dermatol. Res. 1991, 283, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Uchino, T.; Tokunaga, H.; Onodera, H.; Ando, M. Effect of squalene monohydroperoxide on cytotoxicity and cytokine release in a three-dimensional human skin model and human epidermal keratinocytes. Biol. Pharm. Bull. 2002, 25, 605–610. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhu, Q.; Zheng, Z.P.; Cheng, K.W.; Wu, J.J.; Zhang, S.; Tang, Y.S.; Sze, K.H.; Chen, J.; Chen, F.; Wang, M. Natural polyphenols as direct trapping agents of lipid peroxidation-derived acrolein and 4-hydroxy-trans-2-nonenal. Chem. Res. Toxicol. 2009, 22, 1721–1727. [Google Scholar] [CrossRef] [PubMed]
- Feng, Q.; Kumagai, T.; Torii, Y.; Nakamura, Y.; Osawa, T.; Uchida, K. Anticarcinogenic antioxidants as inhibitors against intracellular oxidative stress. Free Radic. Res. 2001, 35, 779–788. [Google Scholar] [CrossRef] [PubMed]
- Mejia-Giraldo, J.C.; Winkler, R.; Gallardo, C.; Sanchez-Zapata, A.M.; Puertas-Majia, M.A. Photoprotective potential of Baccharis antioquensis (Asteraceae) as natural sunscreens. Photochem. Photobiol. 2016, 92, 742–752. [Google Scholar] [CrossRef] [PubMed]
- Potapovich, A.I.; Kostyuk, V.A.; Kostyuk, T.V.; De Luca, C.; Korkina, L.G. Effects of pre- and post-treatment with plant polyphenols on human keratinocyte responses to solar UV. Inflamm. Res. 2013, 62, 773–780. [Google Scholar] [CrossRef] [PubMed]
- Lulli, D.; Patapovich, A.; Maurelli, L.; Dellambra, E.; Pressi, G.; Kostyuk, V.; Dal Toso, R.; De Luca, C.; Pastore, S.; Korkina, L. Anti-inflammatory effects of concentrated ethanol extracts of Edelweiss (Leontopodium alpinum Cass.) callus cultures towards human keratinocytes and endothelial cells. Mediat. Inflamm. 2012, 2012, 498373. [Google Scholar] [CrossRef]
- Pastore, S.; Lulli, D.; Maurelli, R.; Dellambra, E.; De Luca, C.; Korkina, L. Resveratrol induces long-lasting IL-8 expression and peculiar EGFR activation/distribution in human keratinocytes: Mechanisms and implications for skin administration. PLoS ONE 2013, 8, e59632. [Google Scholar] [CrossRef] [PubMed]
- Maier, H.; Schauberger, G.; Brunnhofer, K.; Honigsmann, H. Change in ultraviolet absorbance of sunscreens by exposure to solar-simulated radiation. J. Investig. Dermatol. 2001, 117, 256–262. [Google Scholar] [CrossRef] [PubMed]
- Marrot, L.; Belaidi, J.P.; Lejeune, F.; Maunier, J.R.; Asselineau, D.; Bernerd, F. Photostability of sunscreen products influences the efficiency of protection with regard to UV-induced genotoxic or photoageing-related endpoints. Br. J. Dermatol. 2004, 151, 1234–1244. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, A.; Marchetti, N.; Scalia, S. Photodegradation of (−)-epigallocatechin-3-gallate in topical cream formulations and its photostabilization. J. Pharm. Biomed. Anal. 2011, 56, 692–697. [Google Scholar] [CrossRef] [PubMed]
- Lademann, J.; Rudolph, A.; Jacobi, U.; Weigmann, H.J.; Schaefer, H.; Sterry, W.; Meinke, M. Influence of nonhomogeneous distribution of topically applied UV filters on sun protection factors. J. Biomed. Opt. 2004, 9, 1358–1362. [Google Scholar] [CrossRef] [PubMed]
- Sohn, M.; Hêche, A.; Herzog, B.; Imanidis, G. Film thickness frequency distribution of different vehicles determines sunscreen efficacy. Biomed. Opt. 2014, 19, 115005. [Google Scholar] [CrossRef] [PubMed]
- Durand, L.; Habran, N.; Henschel, V.; Amighi, K. In vitro evaluation of the cutaneous penetration of sprayable sunscreen emulsions with high concentrations of UV filters. Int. J. Cosmet. Sci. 2009, 31, 279–292. [Google Scholar] [CrossRef] [PubMed]
- Freitas, J.V.; Praça, F.S.; Bentley, M.V.; Gaspar, L.R. Trans-resveratrol and beta-carotene from sunscreens penetrate viable skin layers and reduce cutaneous penetration of UV-filters. Int. J. Pharm. 2015, 30, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Stiefel, C.; Schwack, W.; Nguyen, Y.-T.H. Photostability of Cosmetic UV Filters on Mammalian Skin under UV Exposure. Photochem. Photobiol. 2015, 91, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Roussel, L.; Gilbert, E.; Salmon, D.; Serre, C.; Gabard, B.; Haftek, H.; Maibach, H.I.; Pirot, F. Measurement, analysis and prediction of topical UV filter bioavailability. Int. J. Pharm. 2015, 478, 804–810. [Google Scholar] [CrossRef] [PubMed]
- Radice, M.; Manfredini, S.; Ziosi, P.; Dissette, V.; Buso, P.; Fallacara, A.; Vertuani, S. Herbal extracts, lichens and biomolecules as natural photo-protection alternatives to synthetic UV filters. A systematic review. Fitoterapia 2016, 114, 144–162. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, M.S.; Ozzetti, R.A.; Vergnanini, A.L.; de Brito-Gitirana, L.; Volpato, N.M.; de Freitas, Z.M.; Ricci-Júnior, E.; dos Santos, E.P. Evaluation of octyl p-methoxycinnamate included in liposomes and cyclodextrins in anti-solar preparations: Preparations, characterizations and in vitro penetration studies. Int. J. Nanomed. 2012, 7, 3045–3058. [Google Scholar]
- L’alloret, F.; Candau, D.; Seité, S.; Pygmalion, M.J.; Ruiz, L.; Josso, M.; Meaudre, H.; Gauchet, L.; Pena, A.M.; Colonna, A. New combination of ultraviolet absorbers in an oily emollient increases sunscreen efficacy and photostability. Dermatol. Ther. 2012, 2, 4. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kaur, C.D.; Saraf, S. In vitro sun protection factor determination of herbal oils used in cosmetics. Pharmacogn. Res. 2010, 2, 22–25. [Google Scholar]
- Cˇižinauskas, V.; Elie, N.; Brunelle, A.; Briedis, V. Skin Penetration Enhancement by Natural Oils for Dihydroquercetin Delivery. Molecules 2017, 22, 1536. [Google Scholar] [CrossRef] [PubMed]
- Niculae, G.; Lacatusu, I.; Badea, N.; Stan, R.; Vasile, B.S.; Meghea, A. Rice bran and raspberry seed oil-based nanocarriers with self-antioxidative properties as safe photoprotective formulations. Photochem. Photobiol. Sci. 2014, 13, 703–716. [Google Scholar] [CrossRef] [PubMed]
- Ramón, E.; Alonso, C.; Coderch, L.; De La Maza, A.; Lopez, O.; Parra, L.; Notario, J. Liposomes as Alternative Vehicles for Sun Filter Formulations. Drug Deliv. 2005, 12, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Ediriwickrema, A.; Yang, F.; Lewis, J.; Girardi, M.; Saltzman, W.M. A sunblock based on bioadhesive nanoparticles. Nat. Mater. 2015, 14, 1278–1285. [Google Scholar] [CrossRef] [PubMed]
- Luppi, B.; Cerchiara, T.; Bigucci, F.; Basile, R.; Zecchi, V. Polymeric nanoparticles composed of fatty acids and polyvinylalcohol for topical application of sunscreens. J. Pharm. Pharmacol. 2004, 56, 407–411. [Google Scholar] [CrossRef] [PubMed]
- Mitri, K.; Shegokar, R.; Gohla, S.; Anselmi, C.; Müller, R.H. Lipid nanocarriers for dermal delivery of lutein: Preparation, characterization, stability and performance. Int. J. Pharm. 2011, 414, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, C.A.; Peres, D.D.; Graziola, F.; Chacra, N.A.; Araújo, G.L.; Flórido, A.C.; Mota, J.; Rosado, C.; Velasco, M.V.; Rodrigues, L.M.; et al. Cutaneous biocompatible rutin-loaded gelatin-based nanoparticles increase the SPF of the association of UVA and UVB filters. Eur. J. Pharm. Sci. 2016, 81, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Brownlow, B.; Nagaraj, V.J.; Nayel, A.; Joshi, M.; Elbayoumi, T. Development and in vitro evaluation of vitamin E-enriched nanoemulsion vehicles loaded with genistein for chemoprevention against UVB-induced skin damage. J. Pharm. Sci. 2015, 104, 3510–3523. [Google Scholar] [CrossRef] [PubMed]
- Lopes, L.B.; Speretta, F.F.F.; Vitoria, M.; Bentley, L.B. Enhancement of skin penetration of vitamin K using monoolein-based liquid crystalline systems. Eur. J. Pharm. Sci. 2007, 32, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Fehér, P.; Ujhelyi, Z.; Váradi, J.; Fenyvesi, F.; Róka, E.; Juhász, B.; Varga, B.; Bombicz, M.; Priksz, D.; Bácskay, I.; Vecsernyés, M. Efficacy of Pre- and Post-Treatment by Topical Formulations Containing Dissolved and Suspended Silybum marianum against UVB-Induced Oxidative Stress in Guinea Pig and on HaCaT Keratinocytes. Molecules 2016, 21, 1269. [Google Scholar] [CrossRef] [PubMed]
- Steenvoorden, D.P.; van Henegouwen, G.M. The use of endogenous antioxidants to improve photoprotection. J. Photochem. Photobiol. B. 1997, 41, 1–10. [Google Scholar] [CrossRef]
- Sierra, A.F.; Ramírez, M.L.; Campmany, A.C.; Martínez, A.R.; Naveros, B.C. In vivo and in vitro evaluation of the use of a newly developed melatonin loaded emulsion combined with UV filters as a protective agent against skin irradiation. J. Dermatol. Sci. 2013, 69, 202–214. [Google Scholar] [CrossRef] [PubMed]
- De Villa, D.; da Silva Nagatomi, A.R.; Paese, K.; Guterres, S.; Ferreira Cestari, T. Reapplication Improves the Amount of Sunscreen, not its Regularity, under Real Life Conditions. Photochem. Photobiol. 2011, 87, 457–460. [Google Scholar] [CrossRef] [PubMed]
- Stiefel, C.; Schwack, W. Photoprotection in changing times—UV filter efficacy and safety, sensitization processes and regulatory aspects. Int. J. Cosmet. Sci. 2015, 37, 2–30. [Google Scholar] [CrossRef] [PubMed]
- Baldisserotto, A.; Buso, P.; Radice, M.; Dissette, V.; Lampronti, I.; Gambari, R.; Manfredini, S.; Vertuani, S. Moringa oleifera leaf extracts as mutifunctional ingredients for “natural and organic” sunscreens and photoprotective preparations. Molecules 2018, 23, 664. [Google Scholar] [CrossRef] [PubMed]
- Girotti, A.W. Photosensitized oxidation of membrane lipids: Reaction pathways, cytotoxic effects, and cytoprotective mechanisms. J. Photochem. Photobiol. B 2001, 63, 103–113. [Google Scholar] [CrossRef]
- Shen, T.; Chen, X.M.; Harder, B.; Long, M.; Wang, X.N.; Lou, H.X.; Wondrak, G.T.; Ren, D.M.; Zhang, D.D. Plant extracts of the family Lauraceae: A potential resource for chemopreventive agents that activate the nuclear factor-erythroid 2-related factor 2/antioxidant response element pathway. Planta Med. 2014, 80, 426–434. [Google Scholar] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Korkina, L.; Kostyuk, V.; Potapovich, A.; Mayer, W.; Talib, N.; De Luca, C. Secondary Plant Metabolites for Sun Protective Cosmetics: From Pre-Selection to Product Formulation. Cosmetics 2018, 5, 32. https://doi.org/10.3390/cosmetics5020032
Korkina L, Kostyuk V, Potapovich A, Mayer W, Talib N, De Luca C. Secondary Plant Metabolites for Sun Protective Cosmetics: From Pre-Selection to Product Formulation. Cosmetics. 2018; 5(2):32. https://doi.org/10.3390/cosmetics5020032
Chicago/Turabian StyleKorkina, Liudmila, Vladimir Kostyuk, Alla Potapovich, Wolfgang Mayer, Nigma Talib, and Chiara De Luca. 2018. "Secondary Plant Metabolites for Sun Protective Cosmetics: From Pre-Selection to Product Formulation" Cosmetics 5, no. 2: 32. https://doi.org/10.3390/cosmetics5020032
APA StyleKorkina, L., Kostyuk, V., Potapovich, A., Mayer, W., Talib, N., & De Luca, C. (2018). Secondary Plant Metabolites for Sun Protective Cosmetics: From Pre-Selection to Product Formulation. Cosmetics, 5(2), 32. https://doi.org/10.3390/cosmetics5020032




