Abstract
Hair is easily damaged by ultraviolet (UV) radiation, bleaching agents or permanent wave treatments, and as damage progresses, hair loses its gloss, develops split ends and breaks. However, the causes of hair damage due to UV radiation have not yet been clarified. We discovered that in one mechanism facilitating damage to wet hair by UV radiation, the unsaturated fatty acids in wet hair produce hydroxy radicals upon exposure to UV radiation, and these radicals produce cuticle holes between the cuticle layers. In wet hair exposed to UV radiation, cuticle holes were produced only between the cuticle layers, whereas when human hair was immersed in a solution containing hydroxy radicals produced by Fenton’s reaction, a random production of cuticle holes was noted. It is thought that hydroxy radicals are produced only between the cuticle layers by exposure to UV radiation, and cuticle holes are formed only in this region because one of the polyunsaturated fatty acids, linoleic acid, with a bis-allyl hydrogen, is found between the cuticle layers.
1. Introduction
Since ancient times, hair has been regarded as a symbol of beauty. Beautiful hair is glossy with a pleasant form and feel and is characterized by a high strength. The structure of hair is broadly classified into three layers; starting from the center, the layers are the medulla, the cortex, and the cuticle [1]. The cuticle present on the surface of hair contains 5–10 overlapping layers of plate-like cells with a thickness of approximately 500 nm. There is a directionality within the structural layer of the cuticle. The cuticle cells are slightly staggered with respect to one another, that is, sloping outwards from the root to the tip of the hair, like roofing tiles, and the angle of this slope is approximately 2–3°. The cuticle on the outer most surface of the hair protects the inner layers of hair and contributes to the pleasant feel and appearance of hair. However, the cuticle is subjected to day-to-day stimuli, such as hair washing, ultraviolet (UV) radiation, hot air drying and brushing. Thus, the structure is gradually damaged, successively detached from the outer most layer and lost. The principal factor regarding the appearance of hair is the reflection of light from the hair surface. The reflection of light from the surface of hair is considerably affected by the state of the cuticle present on the hair surface. If the structure of the cuticle is uneven, a decrease in the true reflection of light as well as an increase in the diffusion of light is caused by the unevenness of the surface structure, and as a result, the glossiness of the hair decreases [2].
The fatty acids squalene and 18-methyleicosanoic acid (18-MEA) have been confirmed in lipids on the surfaces of hair cuticles [3,4,5]. 18-MEA is linked by thioester bonds and is thought to be an important factor in protecting hair because it decreases wear [6]. Hair is easily damaged by UV radiation, chemical treatments, such as hair dyeing or permanent wave treatments, and heat from a dryer or curling or flattening iron; this type of damage leads to hair breakage and split ends [7,8,9,10]. UV radiation is known to cause a decrease in hair strength, cuticle edge peel-off, hair cavitation, protein loss, a decrease in 18-MEA content, and thiol formation [11,12].
Since the surface of the hair is exposed to higher energy UV radiation, for nearly every case of hair damaged by sunlight, the amino acids in the cuticle undergo greater changes than those in the cortex. The decrease in hair protein due to UV radiation has been shown to be mainly produced by radiation in the 254–400 nm wavelength region. Hair surface proteins are altered by UVB and UVA, and it has been reported that the cystine, proline and valine contents decrease more in brown hair than in black hair [13]. Oxidation of the carbon skeleton of hair peptides occurs upon exposure to UV radiation, and this produces carbonyls (α-keto amide intermediate), especially in the dry state rather than the wet state, which form crosslinked structures between and within cells [14,15]. The hair steadily becomes more brittle due to the photochemical breakdown of disulfide crosslinks in the structural units of the A layer and the formation of an intermolecular crosslinked structure due to the reactions between the carbonyl groups produced by UV exposure in the exocuticle and matrix of the cortex and the amino group of lysine, and the hair structure is gradually lost [14,15]. This reaction is similar to aging-related oxidative protein loss [16]. There has been a great deal of research on proteins and amino acids within the context of studies on hair damage due to UV radiation. Wertz and Downing reported that the covalently bonded fatty acids in human hair are 41% 18-MEA, 18% palmitic acid, 7% stearic acid, 4% oleic acid, small percentages of C16 through C20 fatty acids, and 9% uncharacterized [17]. In 1990, Kalkbrenner et al. demonstrated in cuticle cells isolated from sheep wool that all 18-MEA in hair is in the cuticle [18].
The damage caused by lipids in hair has yet to be clarified. In the present study, damage to the surface of human hair due to exposure to UV radiation was observed by an ultrahigh-resolution field emission scanning electron microscope (FE-SEM) and energy dispersive X-ray spectroscopy (EDS) with a focus on lipids to investigate the mechanism by which damage occurs.
2. Materials and Methods
2.1. Materials
Hydroxyphenyl fluorescein (HPF) was purchased from Sekisui Medical Co., Ltd. (Tokyo, Japan). Liperfluo was purchased from Dojindo Laboratories (Kumamoto, Japan). Dimethyl sulfoxide (DMSO), 1,3-butanediol, acetone, xylene, hexane, chloroform, diethyl ether, oleic acid, linoleic acid, triolein, methyl oleate cholesteryl oleate, cholesterol, methanol, chloroform, hexane, benzene, diethyl ether, acetic acid, copper(II) sulfate and iron(II) sulfate heptahydrate were purchased from Wako Pure Chemical Industries, Ltd. (Osaka, Japan). α-Linolenic acid was purchased from Nacalai Tesque Inc. (Kyoto, Japan), and squalene was purchased from Sigma-Aldrich Corporation (St. Louis, MO, USA). Ceramide TIC-001 (Takasago International Corporation, Tokyo, Japan) was used for ceramide 2. Phosphate buffered saline (PBS) tablets were purchased from Takara Bio Inc. (Siga, Japan). 9-Anthryldiazomethane (ADAM) was purchased from Funakoshi Co., Ltd. (Tokyo, Japan). Tissue-Tek® Cryomold No. 3 and Tissue-Tek® O.C.T. compounds were purchased from Sakura Finetek Japan Co., Ltd. (Tokyo, Japan).
2.2. Method of Exposure to UV Radiation
A xenon light source, Lightning Cure LC6 (150 W xenon lamp L8253; Hamamatsu Photonics K. K.; Hamamatsu, Japan), fitted with a UVC cut filter was used for UV-radiation exposure [19]. The spectrum of this xenon light source in the UV region closely resembled that of solar UV radiation, and the intensity of emission was 25 times greater than that of solar UV radiation [19]. The surface and cross-section of hair not exposed to UV radiation and similarly treated hair exposed to the xenon light source for 20 min were observed. The spectral irradiance was 15 μW/cm2/nm at 320 nm and 25 μW/cm2/nm at 350 nm.
2.3. Observation of the Surface and Cross-Section of Human Hair Exposed to UV Radiation Using the Ultrahigh-Resolution Field Emission Scanning Electron Microscope (FE-SEM))
Human hair not exposed to UV radiation in the wet state and human hair exposed to UV radiation for 20 min in the wet state were naturally dried for one day and left for another day in a desiccator, and then the surface of hair was observed with a field emission scanning electron microscope (FE-SEM) (JSM-7700F; JEOL Ltd., Tokyo, Japan). Similarly, treated hair was enveloped in an epoxy resin and cut into sections 5 µm thick using an ultramicrotome, and the hair cross-section was observed by FE-SEM. The observation conditions were as follows: voltage 10.0 kV, probe current 10 mA, emission 10. The number of cuticle holes was counted with the ImageJ cell counter function.
2.4. Detection of Hydroxy Radicals in the Surface and Cross-Section of Human Hair Exposed to UV Radiation
The development of hydroxy radicals in human hair was examined in the surface and cross-section of the hair using the hydroxy radical detecting reagent HPF. Human hair cut to 1 cm was immersed in PBS and exposed to UV radiation for 20 min using a xenon light source. After exposure, 20 μL of 1 μmol/L HPF was added, and the hair was observed with a fluorescence microscope (NIBA; excitation (Ex.) 470–490 nm, emission (Em.) 510–550 nm; Olympus Corporation, Tokyo, Japan). Then, 20 μL of 1 μmol/L HPF was added to hair not exposed to UV radiation, followed by observation under the fluorescence microscope. In addition, prepared samples were put lengthwise into Cryomolds containing the O.C.T. compound and frozen using liquid nitrogen. Cross-sections were cut from the frozen hair samples in a cryomicrotome (Cryostat; Leica Biosystems Nussloch GmbH, Eisfeld, Germany). The prepared cross-sections were observed under the fluorescence microscope.
2.5. Detection of Hydroxy Radicals in the Surface and Cross-Section of Defatted Human Hair Exposed to UV Radiation
To remove lipids from hair, approximately 20 hairs were put into a glass bottle, 2 mL of acetone, xylene or hexane was added, and the mixture was left to settle for 24 h. The hair was then removed and dried for 1–2 h. The dried hair was immersed in PBS, exposed to UV radiation for 20 min, and then placed on a glass slide; 20 μL of 1 μmol/L HPF was added, followed by observation under the fluorescence microscope. Then, 20 μL of 1 μmol/L HPF was added to hair not exposed to UV radiation, followed by observation under the fluorescence microscope. The prepared samples were put lengthwise into Cryomolds containing the O.C.T. compound and frozen using liquid nitrogen. Cross-sections were cut from the frozen hair samples in the cryomicrotome. The prepared cross-sections were observed under the fluorescence microscope.
2.6. Observation and Measurement of Fluorescence and Hydroxy Radicals in the Surface and Cross-Section of Human Hair Immersed in Fenton Reaction Solution
Hydroxy radicals were produced in a solution by Fenton’s reaction. The equation for Fenton’s reaction is presented below. Iron (II) sulfate heptahydrate and hydrogen peroxide were used; ferrous ions are oxidized to ferric ions by hydrogen peroxide, and hydroxy radicals and hydroxide ions are produced (1).
Fe2+ + H2O2 → Fe3+ + •OH + OH−
Hydroxy radicals were produced by a Fenton reaction solution using 1 mmol/L FeSO4·7H2O and 10 mmol/L hydrogen peroxide. Hair was washed with PBS and then added for 2 h to the Fenton reaction solution. After washing with a buffer solution, 1 μmol/mL HPF was added, followed by observation under the fluorescence microscope.
In addition, samples and reagents were added as follows to a 96-well plate and measured with a microplate reader (Multi-Detection Microplate POWERSAN HT; BioTek, Winooski, VT, USA) immediately after the addition and then after 30 min, 1 h, 2 h and 24 h. Measurements were carried out using the microplate reader with 485/20 nm (Ex.) and 528/20 nm (Em.).
The Fenton reaction solution contained PBS (70 μL), 100 mmol/L H2O2 (10 μL) and 10 mmol/L FeSO4·7H2O (10 μL), 7 mg of hair, and 10 μL of 10 μmol/L HPF.
2.7. Confirmation of Hydroxy Radical Production from Lipids Exposed to UV Radiation
The saturated fatty acids arachidic acid and 18-MEA, the unsaturated fatty acids oleic acid and linoleic acid, and the unsaturated hydrocarbon squalene were each prepared as 40 μmol/L solutions by employing ethanol. Each lipid solution was added (10 μL) to the wells of a 96-well plate, and PBS was added (80 µL, n = 3). Ten microliters of ethanol were added to the control, and then, 80 µL of PBS was added. The plate was then exposed to UV radiation for 20 min. A non-irradiated sample was also prepared.
Ten microliters of 10 μmol/L HPF was added to each well. The fluorescence intensity was measured with the microplate reader (Ex. 485 nm, Em. 528 nm).
2.8. Analysis of Human Hair Lipids and Analysis of Unsaturated Fatty Acids in the Surface of the Hair
Seven milligrams of cut hair (40 hairs) was put into each of three screw-top bottles; 2 mL of acetone, hexane or xylene was put into the screw-top bottles and agitated for 24 h in a shaker. The hair was removed from the screw-top bottles, and the extract solutions were concentrated, re-dissolved in 10 μL of solvent, and prepared as the extract samples. The extract samples and 2 µL of the reference lipid products were spotted onto TLC plates (TLC Silica gel 60 F254), and after drying, the plates were developed sequentially with developing solutions A to D and colored by heating with 3% copper (II) sulfate and 15% aqueous phosphoric acid solutions.
(liquid A, hexane 10 mL; liquid B, benzene 10 mL; liquid C, chloroform 9.5 mL and methanol 2 mL; liquid D, hexane 8 mL, diethyl ether 2 mL and acetic acid 0.1 mL)
The reference products used were α-linolenic acid, squalene, oleic acid, cholesteryl oleate, cholesterol, triolein, linoleic acid, methyl oleate and ceramide 2.
In addition, fatty acids close to the surface of the hair were labeled with ADAM and analyzed by high-performance liquid chromatography (HPLC; Shimadzu Corporation, Kyoto, Japan). The surface layer of the hair was removed by scratching (keratin removal), and 1 mg was extracted with 1 mL of acetone. After centrifugal separation, the supernatant was taken as the hair surface layer extract solution. The hair surface layer extract solution was diluted 200 times with methanol, and 25 μL of a 0.1% methanolic solution of ADAM was added to 1 mL of this solution. This solution reacted at room temperature for 60 min before the sample was measured. In addition, as reference products, 1 μL of a 1% methanolic solution of each of the reagents, that is, linoleic acid, α-linolenic acid, oleic acid, palmitoleic acid and 18-MEA, was diluted with 1 mL of methanol and further diluted 200 times, and 25 μL of a 0.1% methanolic solution of ADAM was added to 1 mL of this solution and reacted at room temperature for 60 min to measure the reference products.
HPLC measurement conditions:
Capcell PAK C18 UG120 column (4.6 mm × 250 mm, 5 μm diam.)
Injection volume, 5 μL
Flow rate, 1 mL/min
Pump program, 0 min 90% acetonitrile → 25 min 100% acetonitrile
→ 30 min 100% acetonitrile → 35 min 90% acetonitrile
Fluorescence detection wavelength, Ex./Em. = 365 nm/412 nm
In addition, the localization of unsaturated fatty acids was observed by investigating the localization of the peroxidized lipids produced when the hair cross-section was exposed to UV radiation using Liperfluo, which fluoresced in the presence of peroxidized lipids. First, the hair was cut into 25-µm cross-sections by the cryomicrotome. The O.T.C. compound adhering to the hair sections was washed with PBS. Then, a 1% DMSO solution of Liperfluo was added, and the solution was left at room temperature for 20 min. After observation with the fluorescence microscope and confirmation that there was no fluorescence, the sample was exposed to UV radiation for 30 min. The localization of unsaturated fatty acids was investigated by observing the differences under the fluorescence microscope with and without UV exposure.
2.9. FE-SEM Observations of Damage to Human Hair due to the Fenton Reaction
Ten hairs were cut into 1 cm pieces and placed into a screw-top tube. Then, 2 mL each of 1 mmol/L FeSO4·7H2O and 10 mmol/L H2O2 were added. After letting the solution settle for approximately 2 h, the hair was removed. The removed hair was observed by FE-SEM.
2.10. FE-SEM-EDS Analysis of Elemental Oxygen in the Surface of Human Hair Exposed to UV Radiation and FE-SEM-EDS Analysis of Elemental Oxygen of the Cross-Section of Human Hair Exposed to UV Radiation
FE-SEM-EDS is a device that measures the quantity of specific X-ray energy released when electrons are released by an electron beam incident on the sample being observed, and outer electrons move into the vacant space left behind. Elemental analysis was carried out using SEM-EDS to analyze whether the quantity of oxygen on the hair surface was altered by exposing the hair to UV radiation. An analysis of elemental oxygen was also carried out using FE-SEM-EDS when the cross-section of human hair was exposed to UV radiation.
2.11. Statistical Analysis
Microsoft Excel statistics were used, and the significance was tested by the unpaired t-test.
3. Results
3.1. FE-SEM Observations of the Surface and Cross-Section of Human Hair Not Exposed to UV Radiation and the Surface and Cross-Section of Human Hair Exposed to UV Radiation
Figure 1 presents the results of the FE-SEM observation of the surface and cross-section of hair not exposed to UV radiation and hair exposed to UV radiation in the wet state. The images show the surface of hair not exposed to UV radiation observed at ×500 (a) and ×2000 (b). The cuticle was observed in the form of scales overlapping on top of one another. On the other hand, in the images of the surface of hair exposed to UV radiation observed at ×500 (c) and ×2000 (d), the cuticle edge peeled-off, and numerous holes between the cuticle layers were observed on the surface of hair exposed to UV radiation. On counting the number of cuticle holes, there were no holes in the hair not exposed to UV radiation, whereas 221 cuticle holes/mm2 were counted in the images of the surface of hair exposed to UV radiation.
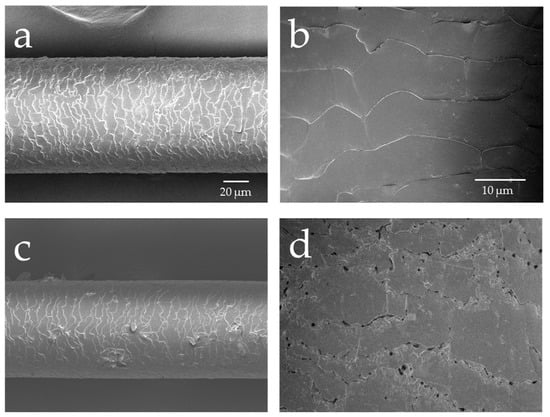
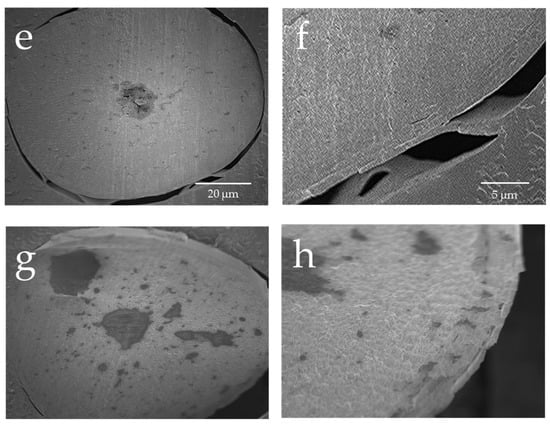
Figure 1.
Field emission scanning electron microscope (FE-SEM) images of the surface and cross-section of hair not exposed to ultraviolet (UV) radiation and hair exposed to UV radiation. Images of the surface of hair not exposed to UV radiation, ×500 (a) and ×2000 (b); images of the surface of hair exposed to UV radiation, ×500 (c) and ×2000 (d); images of the cross-section of hair not exposed to UV radiation, ×1000 (e) and ×3200 (f); images of the cross-section of hair exposed to UV radiation, ×1000 (g) and ×3200 (h).
Regarding the results of the observations at ×1000 (e), and ×3200 (f) for the cross-section of hair not exposed to UV radiation and the results of the observation at ×1000 (g) and ×3200 (h) of the cross-section of hair exposed to UV radiation, the cuticle was present in layers and there was no separation for the hair not exposed to UV radiation; however, for the hair exposed to UV radiation, there were voids in the cuticle layers, and vacuoles had formed in the cortex.
3.2. Detection of Hydroxy Radicals in the Surface and Cross-Section of Human Hair Exposed to UV Radiation
Figure 2a presents the results of the observations of the surface of hair not exposed to UV radiation and the surface of hair exposed to UV radiation using a reagent that detects hydroxy radicals. Hydroxy radicals were hardly detectable on the surface of hair not exposed to UV radiation, whereas the surface of hair exposed to UV radiation had clearly detectable hydroxy radicals between the cuticle layers. Figure 2b shows the results of the observations of the surface and cross-section of hair exposed to UV radiation using a reagent that detects hydroxy radicals. In the cross-section of hair exposed to UV radiation, hydroxy radicals were detected in the cuticle layers around the hair and within the cortex.
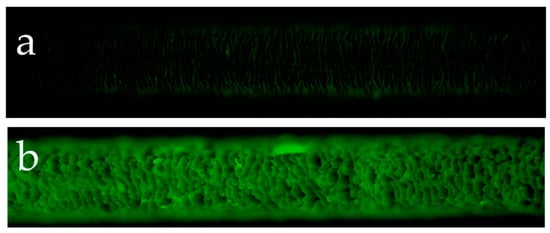
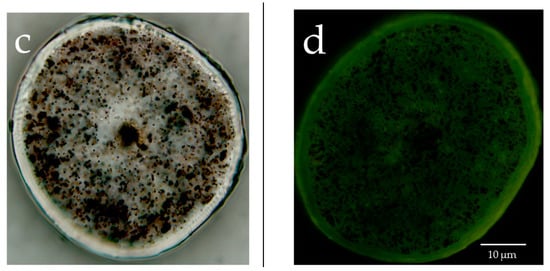
Figure 2.
Fluorescence microscope images of fluorescence due to hydroxy radicals in human hair exposed to UV radiation and not exposed to UV radiation. Fluorescence microscope image of hydroxy radicals on the surface of hair not exposed to UV radiation (a), fluorescence microscope image of hydroxy radicals on the surface of hair exposed to UV radiation (b). Images of the cross-section of hair exposed to UV radiation. A bright-field view image of the cross-section of hair exposed to UV radiation (c), fluorescence microscope image of hydroxy radicals in the cross-section of hair exposed to UV radiation (d).
3.3. Detection of Hydroxy Radicals in the Surface and Cross-Section of Hair When Defatted Human Hair Was Exposed to UV Radiation
Figure 3 shows the results of the fluorescence microscopic observation of hydroxy radicals in the surface and cross-section of hair when human hair defatted with acetone, xylene or hexane was exposed to UV radiation. In the results for both the surface and cross-section, the intensity of fluorescence from the hydroxy radicals in the cuticle diminished in the case of hair defatted by acetone and exposed to UV radiation. For defatting by xylene or hexane, the intensity of the fluorescence from the hydroxy radicals in the cuticle did not diminish.
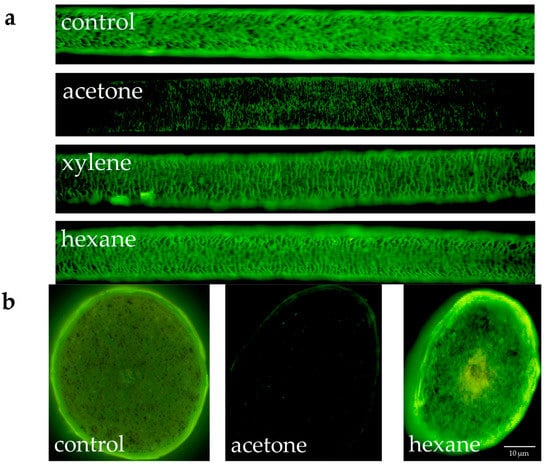
Figure 3.
Fluorescence microscope images of fluorescence due to hydroxy radicals in the cross-section of defatted human hair not exposed or exposed to UV radiation. (a) Images of the surface of hair when human hair was defatted with acetone, xylene or hexane and exposed to UV radiation; (b) Images of the cross-section of hair when human hair was defatted with acetone or hexane and exposed to UV radiation. PBS was used as a control instead of the organic solvents used for defatting.
3.4. Confirmation of Hydroxy Radical Production from Lipids Exposed to UV Radiation
Figure 4 presents the results of hydroxy radical production from oleic acid, linoleic acid, squalene, 18-MEA or arachidic acid exposed to UV radiation using a conversion of HPF to obtain a fluorescent product, and the results of adding HPF to oleic acid, linoleic acid, squalene, 18-MEA or arachidic acid not exposed to UV radiation. Hydroxy radicals were not produced from oleic acid, linoleic acid, squalene, 18-MEA or arachidic acid not exposed to UV radiation, whereas the production of hydroxy radicals was confirmed when linoleic acid was exposed to UV radiation.
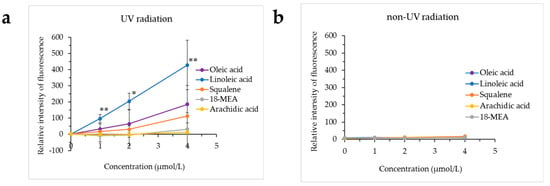
Figure 4.
Confirmation of the production of hydroxy radicals from lipids exposed to UV radiation. The production of hydroxy radicals from oleic acid, linoleic acid, squalene, 18-methyleicosanoic acid (18-MEA) or arachidic acid exposed to UV radiation was investigated using hydroxyphenyl fluorescein (HPF) fluorescence. (a) Exposed to UV radiation, (b) not exposed to UV radiation. * p < 0.05 vs. control (0 μmol/L), ** p < 0.01 vs. control (0 μmol/L).
The monounsaturated unsaturated fatty acid oleic acid, the unsaturated hydrocarbon squalene and the saturated fatty acids 18-MEA and arachidic acid did not produce hydroxy radicals even when exposed to UV radiation.
3.5. Analysis of Human Hair Lipids and Analysis of Unsaturated Fatty Acids in the Surface Layer of Hair
The lipids in human hair were extracted with acetone, hexane and xylene, and the respective extract solutions were analyzed by TLC. It was evident that more fatty acids were extracted using acetone than hexane or xylene (Figure 5a). Since we were able to determine that more fatty acids were extracted from hair using acetone, the acetone sample was used for the HPLC analysis of the free unsaturated fatty acids in the surface of the hair. The results showed that the hair surface layer contains oleic acid and linoleic acid (Figure 5b). When the hair was observed with a fluorescent reagent that reacts with peroxidized lipids (Liperfluo) to specify the distribution, it was evident that the cuticle layers included many unsaturated fatty acids (Figure 5c). In addition, the intensity of the fluorescence of the reagent that reacted with the peroxidized lipids (Liperfluo) increased from the root to the tip.
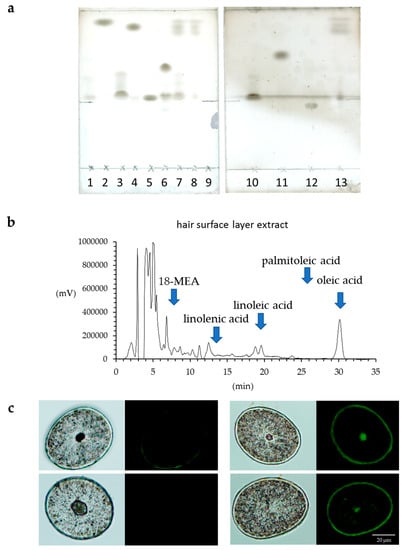
Figure 5.
Analysis of human hair lipids and unsaturated fatty acids in the surface layer of hair. (a) TLC analysis of hair lipids extracted with acetone, hexane or xylene. Lipids in the surface layer of human hair were eluted with acetone, hexane or xylene, and these eluates were analyzed by TLC. 1, α-linolenic acid; 2, squalene; 3, oleic acid; 4, cholesteryl oleate; 5, cholesterol; 6, triolein; 7, acetone extraction of hair; 8, hexane extraction of hair; 9, control; 10, linoleic acid; 11, methyl oleate; 12, ceramide 2; 13, xylene extraction of hair. (b) high-performance liquid chromatography (HPLC) analysis of unsaturated fatty acids in the surface layer of hair extracted with acetone. The chart of the fluorescence intensity of 9-anthryldiazomethane (ADAM) alone was subtracted from the chart of the fluorescence intensity of the ADAM-derivatized acetone extract from the hair surface layer, and the results are presented in the form of a graph. (c) Localization of peroxidized lipids in the cross-section of hair exposed to UV radiation. (left) Bright-field images and images stained with Liperfluo of the cross-section of hair not exposed to UV radiation. (right) Bright-field images and images stained with Liperfluo of the cross-section of hair exposed to UV radiation.
3.6. FE-SEM Observations of the Surface of Human Hair Immersed in a Fenton Reaction Solution
To investigate whether damage to hair due to exposure to UV radiation depends on hydroxy radicals, hair samples were immersed in a Fenton reaction system, and the damage due to UV radiation was compared with the damage caused by hydroxyl radicals. Figure 6 shows the images of the surface of hair not immersed in a Fenton reaction solution and immersed in a Fenton reaction solution. In the case of human hair immersed in a solution that produces hydroxy radicals, that is, Fenton reaction solution, the random production of cuticle holes was noted.
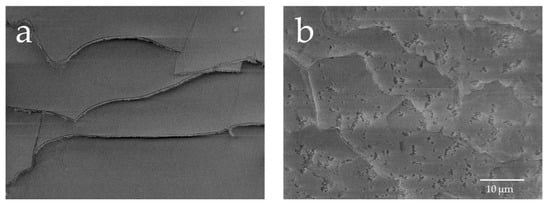
Figure 6.
FE-SEM observations of the surface layer of human hair immersed in a Fenton reaction solution. Image of the surface of hair immersed in water and image of the surface of human hair immersed in a Fenton reaction solution. (a) Image of the surface of hair immersed in water (×2000); (b) image of the surface of hair immersed in a Fenton reaction solution (×2000).
3.7. FE-SEM-EDS Analysis of Elemental Oxygen in the Surface and Cross-Section of Human Hair Exposed to UV Radiation
When a qualitative analysis was carried out on the surface of hair using FE-SEM-EDS, carbon, nitrogen, oxygen and sulfur were detected. Moreover, when elemental mapping was carried out, the oxygen distribution was comparatively uniform in hair not exposed to UV radiation, whereas on the surface of hair exposed to UV radiation, oxygen was detected with a skewed distribution around the cuticle layers, as shown by the arrow (Figure 7a). Similarly, on carrying out an analysis of elemental oxygen in a cross-section of human hair not exposed to UV radiation and a cross-section of human hair exposed to UV radiation, oxygen was confirmed to be localized in the cuticle layers (Figure 7b).
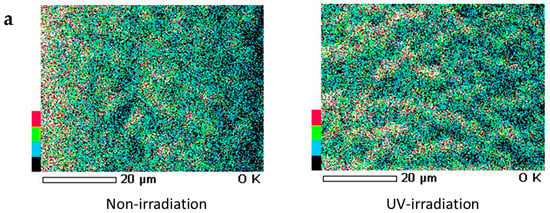
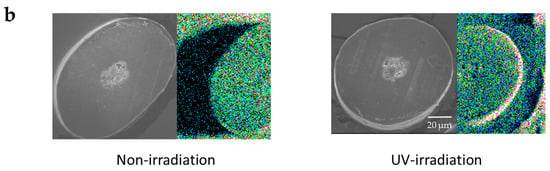
Figure 7.
Images of FE-SEM-EDS oxygen mapping of the surface and cross-section of hair. (a) Images of FE-SEM-EDS oxygen mapping of the surface of hair. (left) Surface of hair not exposed to UV radiation. (right) Surface of hair exposed to UV radiation. (b) Images of FE-SEM-EDS oxygen mapping of the cross-section of hair. (left) Analysis of elemental oxygen in the cross-section of human hair when not exposed to UV radiation. (right) Analysis of elemental oxygen in the cross-section of human hair when exposed to UV radiation. From left to right, FE-SEM images (×1000) and images of oxygen mapping by EDS (×1000).
4. Discussion
When the surface of hair was observed by FE-SEM, in hair not exposed to UV radiation, the cuticle was regularly arranged, but in hair exposed to UV radiation in the wet state, there was cuticle edge peel-off. Numerous cuticle holes (holes in the surface of the hair) were observed between the cuticle layers. This microdamage, which cannot be seen with the naked eye, is thought to be the cause of split ends and broken hairs. In the present study, it was discovered that numerous holes are produced between the cuticle layers, which is similar to the damage due to UV radiation, when hydroxy radicals act on hair. Cuticle edge peel-off and numerous cuticle holes between the cuticle layers were observed in hair exposed to UV radiation, whereas in hair immersed in the Fenton reaction solution the cuticle holes observed were randomly distributed in the cuticle surface. Thus, although damage is caused by hydroxy radicals, the cuticle holes produced by UV radiation are localized between the cuticle layers. These results suggest that in the production of cuticle holes by UV radiation, a constituent present between the cuticle layers causes damage by reacting to make the cuticle holes.
The fact that peroxidized lipids are produced between the cuticle layers by exposure to UV radiation suggests that unsaturated fatty acids contribute to the cuticle holes produced by UV radiation. From the results of the analysis of this component by TLC, the HPLC analysis of unsaturated fatty acids eluted from the surface layer of hair by acetone, the analysis of the production of peroxidized lipids from lipids exposed to UV radiation, and the FE-SEM-EDS analysis of elemental oxygen in the surface of hair exposed to UV radiation, it appears that at least one of the polyunsaturated fatty acids, linoleic acid, which has a bis-allyl hydrogen, contributes to the cuticle holes produced by UV radiation. In addition to the methylene hydrogen in fatty acids, unsaturated fatty acids have an allyl hydrogen as a removable hydrogen, and polyunsaturated fatty acids also have a bis-allyl hydrogen; these have bond dissociation energies (75 kcal/mol and 85 kcal/mol) lower than the bond dissociation energy for water (119 kcal/mol) [20]. In general, if the reactivity for lipid peroxy radicals (LOO•) is 1 for methylene hydrogen, it is 500 for an allyl hydrogen and 50,000 for a bis-allyl hydrogen. Linoleic acid has one bis-allyl hydrogen and two allyl hydrogens. Oleic acid does not have a bis-allyl hydrogen, but it has two allyl hydrogens. Squalene has an allyl hydrogen but does not have bis-allyl hydrogen; this is thought to be why oleic acid and squalene do not produce hydroxy radicals even when exposed to UV radiation. On the other hand, α-linolenic acid has two bis-allyl hydrogens and two allyl hydrogens, but α-linolenic acid was not detected in the hair used in these experiments. In addition, it has been reported that in systems such as micelles or emulsions in which water is present, linoleic acid has a lower oxidative stability [21]. In the case of linoleic acid, the hydrogen removal occurs from the bis-allyl hydrogen in C11, and as a result, two kinds of hydroperoxide isomers with a conjugated diene structure (9-monohydroperoxide and 13-monohydroperoxide) are produced [22]. The putative mechanism of hydroxy radical production from hair lipids due to UV exposure is thought to be as follows. Lipid radicals (L•) are produced by UV radiation [23]. The lipid radicals (L•) rapidly bind with oxygen to produce lipid peroxy radicals (LOO•). Since the bond dissociation energy of LOO-H (LOO•) (88 kcal/mol) is not adequate to remove an allyl hydrogen, the removal of a bis-allyl hydrogen is the only reaction that proceeds fully. Therefore, the LOO• removal of a hydrogen from other lipids (LH•; bis-allyl hydrogen) gives the lipid hydroperoxide (LOOH) and produces new lipid radicals (L•) via a chain reaction When a bis-allyl hydrogen is removed, another LOO• radical is produced, and the peroxidation proceeds as a chain reaction without a defined end point. For a chain peroxidation reaction to occur, the lipid being oxidized needs to have a bis-allyl hydrogen. Therefore, a radical chain reaction mediated by LOO• will only occur in linoleic acid or higher polyunsaturated fatty acids that include a bis-allyl hydrogen.
L• + O2 → LOO•
LOO• + LH → LOOH + L•
The bond dissociation energy of LOO-H (LOO•) (88 kcal/mol) is smaller than the bond dissociation energy of HO-H (•OH) (119 kcal/mol) and LO-H (LO•) (104 kcal/mol), and thus, ordinary •OH is not produced. However, UV radiation is reported to produce lipid alkoxy radicals (LO•) and hydroxy radicals (•OH) from lipid hydroperoxide (LOOH) [23].
LOOH → LO• + •OH
Therefore, the results of the analysis of lipids extracted from hair suggest that exposure to UV radiation causes the production of hydroxy radicals from a polyunsaturated fatty acid, linoleic acid, present between the cuticle layers, which causes damage to the hair due to UV exposure. Hydroxy radicals are a form of active oxygen, which is intensely toxic for cells; in hair and skin, they are known to be produced by the reaction systems of ferrous oxide (cuprous oxide) and hydrogen peroxide [24] and reactions between tyrosinase and phenol compounds [25]. It has been reported that morphological damage to hair is caused by UVB, and chemical changes in hair are caused by UVA [26]. As the result of investigating the production site of hydroxy radicals in hair, hydroxy radicals were detected between the cuticle layers on the surface of hair exposed to UV radiation, and it was evident that the radical concentration decreased by defatting. Moreover, the EDS analysis confirmed the presence of oxygen localized between the cuticle layers. Because the capacity of EDS to analyze oxygen is low, it is impossible to specify the state of the oxygen, but it is thought that in hair exposed to UV radiation, an oxide or some form of oxygen is produced at the boundary of the cuticle. From this result, it is inferred that hydroxy radicals are involved in hair damage due to UV radiation. Moreover, it is suggested that hydroxy radicals are produced from linoleic acid, which is the polyunsaturated fatty acid present between the cuticle layers of hair exposed to UV radiation, and cause damage to hair due to UV radiation exposure. This study did not investigate the wavelength that causes damage to hair due to UV radiation exposure, but if the wavelength of UV radiation that causes damage to hair is clarified in the future, this can be expected to lead to the development of methods for developing hair care sunscreen to protect hair from UV radiation.
5. Conclusions
In hair exposed to UV radiation in the wet state, the cuticle layers partially peeled off, and numerous cuticle holes were observed between the cuticle layers. A high oxygen content was evident between the cuticle layers in hair exposed to UV radiation. It is thought that linoleic acid, which is the unsaturated fatty acid between the cuticle layers in hair and has a bis-allyl hydrogen, is oxidized due to over-exposure to UV radiation. Due to this, a radical chain reaction occurs, producing a peroxidized lipid, and this peroxidized lipid is the source of production of highly reactive hydroxy radicals, which form cuticle holes and damage hair.
Acknowledgments
The authors gratefully acknowledge the technical assistance of Ms. Mayu Ogawa.
Author Contributions
J.Y., N.O., M.S., K.I. and T.S. performed the experiments. K.M. designed the study and performed the analysis. J.Y., N.O., M.S., K.I., T.S. and K.M. interpreted the data and drafted the manuscript. K.M. supervised the progress and critically revised the manuscript. All authors read and approved the final manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Robins, C.R. Chemical and Physical Behavior of Human Hair, 5th ed.; Springer: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Okamoto, M.; Yakawa, R.; Mamada, A.; Inoue, S.; Nagase, S.; Shibuichi, S.; Kariya, E.; Satoh, N. Influence of internal structures of hair fiber on hair appearance. III: Generation of lightscattering factors in hair cuticles and the influence on hair shine. J. Cosmet. Sci. 2003, 54, 353–366. [Google Scholar] [PubMed]
- Lshikawa, K.; Okamoto, M.; Aoyagi, S. Structural analysis of the outermost hair surface using TOF-SIMS with gas cluster ion beam sputtering. Biointerphases 2016, 11, 02A315. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Chen, G.; Ji, C.; Hoptroff, M.; Jones, A.; Collins, L.Z.; Janssen, H.G. Gas chromatography-mass spectrometry and Raman imaging measurement of squalene content and distribution in human hair. Anal. Bioanal. Chem. 2016, 408, 2357–2362. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, T.; Yoshida, S. Distribution of glycolipid and unsaturated fatty acids in human hair. Lipids 2014, 49, 905–917. [Google Scholar] [CrossRef] [PubMed]
- Jones, L.N.; Rivett, D.E. The role of 18-methyleicosanoic acid in the structure and formation of mammalian hair fibres. Micron 1997, 28, 469–485. [Google Scholar] [CrossRef]
- Chandrashekara, M.N.; Ranganathaiah, C. Chemical and photochemical degradation of human hair: A free-volume microprobe study. J. Photochem. Photobiol. B 2010, 101, 286–294. [Google Scholar] [CrossRef] [PubMed]
- Fedorkova, M.V.; Smolina, N.V.; Mikhalchik, E.V.; Balabushevich, N.G.; Ibragimova, G.A.; Gadzhigoroeva, A.G.; Dmitrieva, E.I.; Dobretsov, G.E. Effects of ultra violet radiation on the soluble proteins of human hair. J. Photochem. Photobiol. B 2014, 140, 390–395. [Google Scholar] [CrossRef] [PubMed]
- Richena, M.; Rezende, C.A. Effect of photodamage on the outermost cuticle layer of human hair. J. Photochem. Photobiol. B 2015, 153, 296–304. [Google Scholar] [CrossRef] [PubMed]
- Richena, M.; Rezende, C.A. Morphological degradation of human hair cuticle due to simulated sunlight irradiation and washing. J. Photochem. Photobiol. B 2016, 161, 430–440. [Google Scholar] [CrossRef] [PubMed]
- Sebetić, K.; Sjerobabski Masnec, I.; Cavka, V.; Biljan, D.; Krolo, I. UV damage of the hair. Coll. Antropol. 2008, 32 (Suppl. 2), 163–165. [Google Scholar] [PubMed]
- Fedorkova, M.V.; Brandt, N.N.; Chikishev, A.Y.; Smolina, N.V.; Balabushevich, N.G.; Gusev, S.A.; Lipatova, V.A.; Botchey, V.M.; Dobretsov, G.E.; Mikhalchik, E.V. Photoinduced formation of thiols in human hair. J. Photochem. Photobiol. B 2016, 164, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Hoting, E.; Zimmerman, M. Sunlight induced modification in bleached, permed or dyed human hair. J. Cosmet. Sci. 1997, 48, 79–91. [Google Scholar]
- Robbins, C.R.; Bahl, M. Analysis of hair by electron spectroscopy for chemical analysis. J. Soc. Cosmet. Chem. 1984, 35, 379–390. [Google Scholar]
- Dubief, C. Experiments with hair photodegradation. Cosmet. Toiletries 1992, 107, 95–102. [Google Scholar]
- Dean, R.T.; Fu, S.; Davies, M.J. Biochemistry and pathology of radical mediated protein oxidation. Biochem. J. 1997, 324, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Wertz, P.W.; Downing, D.T. Integral lipids of human hair. Lipids 1988, 23, 878–881. [Google Scholar] [CrossRef] [PubMed]
- Kalkbrenner, U.; Korner, A.; Hocker, H.; Rivett, D.E. Studies on the composition of the wool cuticle. In Proceedings of the 8th International Wool Textile Research Conference, Christchurch, New Zealand, 7–14 February 1990; pp. 398–407. [Google Scholar]
- Maeda, K. Analysis of ultraviolet radiation wavelengths causing hardening and reduced elasticity of collagen gels in vitro. Cosmetics 2018, 5, 14. [Google Scholar] [CrossRef]
- Terao, J. YUSHI NO KASANKA HANNOU RIRON. J. Cook. Sci. Jpn. 1995, 28, 190–195. (In Japanese) [Google Scholar]
- Miyashita, K.; Hirano, S.; Itabashi, Y.; Ota, T.; Nishikawa, M.; Nakayama, S. Oxidative stability of polyunsaturated monoacylglycerol and triacylglycerol in aqueous micelles. J. Jpn. Oil Chem. Soc. 1997, 46, 205–208. [Google Scholar] [CrossRef]
- Kobayashi, H.; Yoshida, M.; Miyashita, K. Comparative study of the product components of lipid oxidation in aqueous and organic systems. Chem. Phys. Lipids 2003, 126, 111–120. [Google Scholar] [CrossRef]
- Ivanova, I.M.; Piskarev, S.V. Trofimova. Initial stage of lipid peroxidation with HO2• radicals, Kinetic study. Am. J. Phys. Chem. 2013, 2, 44–51. [Google Scholar] [CrossRef]
- Marsh, J.M.; Davis, M.G.; Flagler, M.J.; Sun, Y.; Chaudhary, T.; Mamak, M.; McComb, D.W.; Williams, R.E.; Greis, K.D.; Rubio, L.; et al. Advanced hair damage model from ultra-violet radiation in the presence of copper. Int. J. Cosmet. Sci. 2015, 37, 532–541. [Google Scholar] [CrossRef] [PubMed]
- Gu, L.; Zeng, H.; Takahashi, T.; Kazuhisa Maeda, K. In Vitro methods for predicting chemical leukoderma caused by quasi-drug cosmetics. Cosmetics 2017, 4, 31. [Google Scholar] [CrossRef]
- Jeon, S.Y.; Pi, L.Q.; Lee, W.S. Comparison of hair shaft damage after UVA and UVB irradiation. J. Cosmet. Sci. 2008, 59, 151–156. [Google Scholar]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).