Chemical Stability Analysis of Hair Cleansing Conditioners under High-Heat Conditions Experienced during Hair Styling Processes
Abstract
:1. Introduction
2. Methods
2.1. Sample Preparation
2.2. HPLC-UV Analysis
2.3. GC-MS Analysis
2.4. FT-IR Analysis
3. Results
3.1. HPLC-UV
3.2. GC-MS
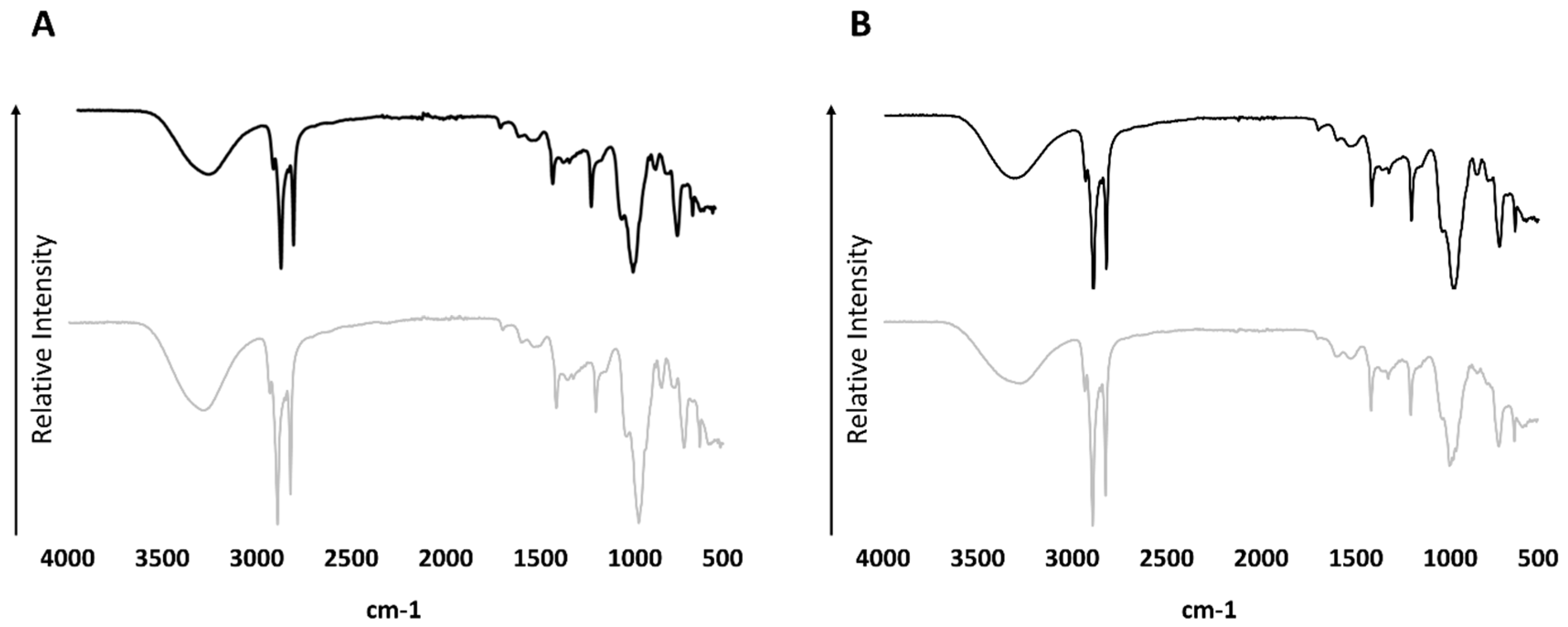
3.3. FT-IR
4. Discussion
5. Conclusions
Author Contributions
Conflicts of Interest
References
- Yeomans, M. Global Beauty Market to Reach $265 Billion in 2017 Due to an Increase in GDP. Available online: https://www.cosmeticsdesign.com/Article/2012/11/07/Global-beauty-market-to-reach-265-billion-in-2017-due-to-an-increase-in-GDP (accessed on 22 January 2018).
- Food and Drug Administration (FDA). FDA Authority over Cosmetics: How Cosmetics Are Not FDA-Approved, but Are FDA-Regulated. Available online: https://www.fda.gov/Cosmetics/GuidanceRegulation/LawsRegulations/ucm074162.htm (accessed on 27 November 2017).
- Regulation EU No. 1223/2009 of the European Parliament and of the Council of 30 November 2009 on Cosmetic Products (Recast) (Text with EEA Relevance). L 342/59. Available online: http://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:02009R1223-20160812&from=EN (accessed on 6 March 2018).
- Personal Care Products Council (PCPC). Guidelines for Industry: The Stability Testing of Cosmetics; Personal Care Products Council: Washington, DC, USA, 2011; p. 1. [Google Scholar]
- Cosmetic, Toiletry and Fragrance Association (CTFA); Colipa. Guidelines on Stability Testing of Cosmetic Products. 03/094—MC. March 2004; Cosmetics Europe, Personal Care Association: Brussels, Belgium, 2004. [Google Scholar]
- International Federation of Societies of Cosmetic Chemists (IFSCC). Monograph Number 2: The Fundamentals of Stability Testing; Micelle Press: Weymouth, UK, 1992. [Google Scholar]
- Lee, Y.; Kim, Y.D.; Hyun, H.J.; Pi, L.Q.; Jin, X.; Lee, W.S. Hair shaft damage from heat and drying time of hair dryer. Ann. Dermatol. 2011, 23, 455–462. [Google Scholar] [CrossRef] [PubMed]
- Breuning, E.E.; Papini, R.P. Hair straighteners: A significant burn risk. Burns 2008, 34, 703–706. [Google Scholar] [CrossRef] [PubMed]
- Dussaud, A.; Rana, B.; Lam, H.T. Progressive hair straightening using an automated flat iron: Function of silicones. J. Cosmet. Sci. 2013, 64, 119–131. [Google Scholar] [PubMed]
- Harding, E. How Much Time Do You Really Spend Doing Your Hair, Ladies? Answer: Ten Days a Year! Available online: http://www.dailymail.co.uk/femail/article-2250701/How-time-really-spend-doing-hair-ladies-Answer-Ten-days-year.html (accessed on 8 September 2016).
- Bickers, D.R.; Calow, P.; Greim, H.A.; Hanifin, J.M.; Rogers, A.E.; Saurat, J.H.; Sipes, I.G.; Smith, R.L.; Tagami, H. The safety assessment of fragrance materials. Regul. Toxicol. Pharmacol. 2003, 37, 218–273. [Google Scholar] [CrossRef]
- Scientific Committee on Consumer Safety (SCCS). Opinion on the Mixture of 5-Chloro-2-Methylisothiazolin-3(2H)-One and 2-Methylisothiazolin-3(2H)-One. COLIPA n° P56. SCCS/1238/09.
- Antignac, E.; Nohynek, G.J.; Re, T.; Clouzeau, J.; Toutain, H. Safety of botanical ingredients in personal care products/cosmetics. Food Chem. Toxicol. 2011, 49, 324–341. [Google Scholar] [CrossRef] [PubMed]
- Opdyke, D.L.J. Monographs on fragrance raw materials. Food Cosmet. Toxicol. 1975, 13, 683–923. [Google Scholar] [CrossRef]
- Skold, M.; Borje, A.; Matura, M.; Karlberg, A.T. Studies on the autoxidation and sensitizing capacity of the fragrance chemical linalool, identifying a linalool hydroperoxide. Contact Dermat. 2002, 46, 267–272. [Google Scholar] [CrossRef]
- Hagvall, L.; Backtorp, C.; Svensson, S.; Nyman, G.; Borje, A.; Karlberg, A.T. Fragrance compound geraniol forms contact allergens on air exposure. Identification and quantification of oxidation products and effect on skin sensitization. Chem. Res. Toxicol. 2007, 20, 807–814. [Google Scholar] [CrossRef] [PubMed]
- Skold, M.; Hagvall, L.; Karlberg, A.T. Autoxidation of linalyl acetate, the main component of lavender oil, creates potent contact allergens. Contact Dermat. 2008, 58, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Brared Christensson, J.; Forsstrom, P.; Wennberg, A.M.; Karlberg, A.T.; Matura, M. Air oxidation increases skin irritation from fragrance terpenes. Contact Dermat. 2009, 60, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Peleg, M.; Normand, M.D.; Corradini, M.G. The Arrhenius equation revisited. Crit. Rev. Food Sci. Nutr. 2012, 52, 830–851. [Google Scholar] [CrossRef] [PubMed]
- Schwarzenbach, R.P.; Gschwend, P.M.; Imboden, D.M. Environmental Organic Chemistry, 2nd ed.; J. Wiley: Hoboken, NJ, USA, 2003. [Google Scholar]
- Huang, Y.; Lenaghan, S.C.; Xia, L.; Burris, J.N.; Stewart, C.N., Jr.; Zhang, M. Characterization of physicochemical properties of ivy nanoparticles for cosmetic application. J. Nanobiotechnol. 2013, 11, 3. [Google Scholar] [CrossRef] [PubMed]
- Berthele, H.; Sella, O.; Lavarde, M.; Mielcarek, C.; Pense-Lheritier, A.M.; Pirnay, S. Determination of the influence of factors (ethanol, pH and a(w)) on the preservation of cosmetics using experimental design. Int. J. Cosmet. Sci. 2014, 36, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, F.; Sarmento, B.; Amaral, M.H.; Oliveira, M.B. Exploring the antioxidant potentiality of two food by-products into a topical cream: Stability, in vitro and in vivo evaluation. Drug Dev. Ind. Pharm. 2016, 42, 880–889. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, D.N.; Delafiori, J.; Ferreira, M.S.; Catharino, R.R. In vitro evaluation of Sun Protection Factor and stability of commercial sunscreens using mass spectrometry. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2015, 988, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Cattley, K.; Duracher, L.; Camattari, P.; Mavon, A.; Grooby, S. Pre-clinical formulation screening, development and stability of acetyl aspartic acid for cosmetic application. Int. J. Cosmet. Sci. 2015, 37 (Suppl. 1), 28–33. [Google Scholar] [CrossRef] [PubMed]
- Kern, S.; Dkhil, H.; Hendarsa, P.; Ellis, G.; Natsch, A. Detection of potentially skin sensitizing hydroperoxides of linalool in fragranced products. Anal. Bioanal. Chem. 2014, 406, 6165–6178. [Google Scholar] [CrossRef] [PubMed]
- Christensson, J.B.; Hagvall, L.; Karlberg, A.-T. Fragrance allergens, overview with a focus on recent developments and understanding of abiotic and biotic activation. Cosmetics 2016, 3, 19. [Google Scholar] [CrossRef]
- Loretz, L.; Api, A.M.; Barraj, L.; Burdick, J.; Davis, D.A.; Dressler, W.; Gilberti, E.; Jarrett, G.; Mann, S.; Pan, Y.L.; et al. Exposure data for personal care products: Hairspray, spray perfume, liquid foundation, shampoo, body wash and solid antiperspirant. Food. Chem. Toxicol. 2006, 44, 2008–2018. [Google Scholar] [CrossRef] [PubMed]
- Loretz, L.J.; Api, A.M.; Babcock, L.; Barraj, L.M.; Burdick, J.; Cater, K.C.; Jarrett, G.; Mann, S.; Pan, Y.H.L.; Re, T.A.; et al. Exposure data for cosmetic products: Facial cleanser, hair conditioner and eye shadow. Food Chem. Toxicol. 2008, 46, 1516–1524. [Google Scholar] [CrossRef] [PubMed]
- Scientific Committee on Consumer (SCCS). The SCCS Notes of Guidance for the Testing of Cosmetic Ingredients and Their Safety Evaluation, 9th Revision. SCCS/1564/15. Revised Version of 25 April 2016. Available online: http://ec.europa.eu/health/scientific_committees/consumer_safety/docs/sccs_o_190.pdf (accessed on 12 December 2017).
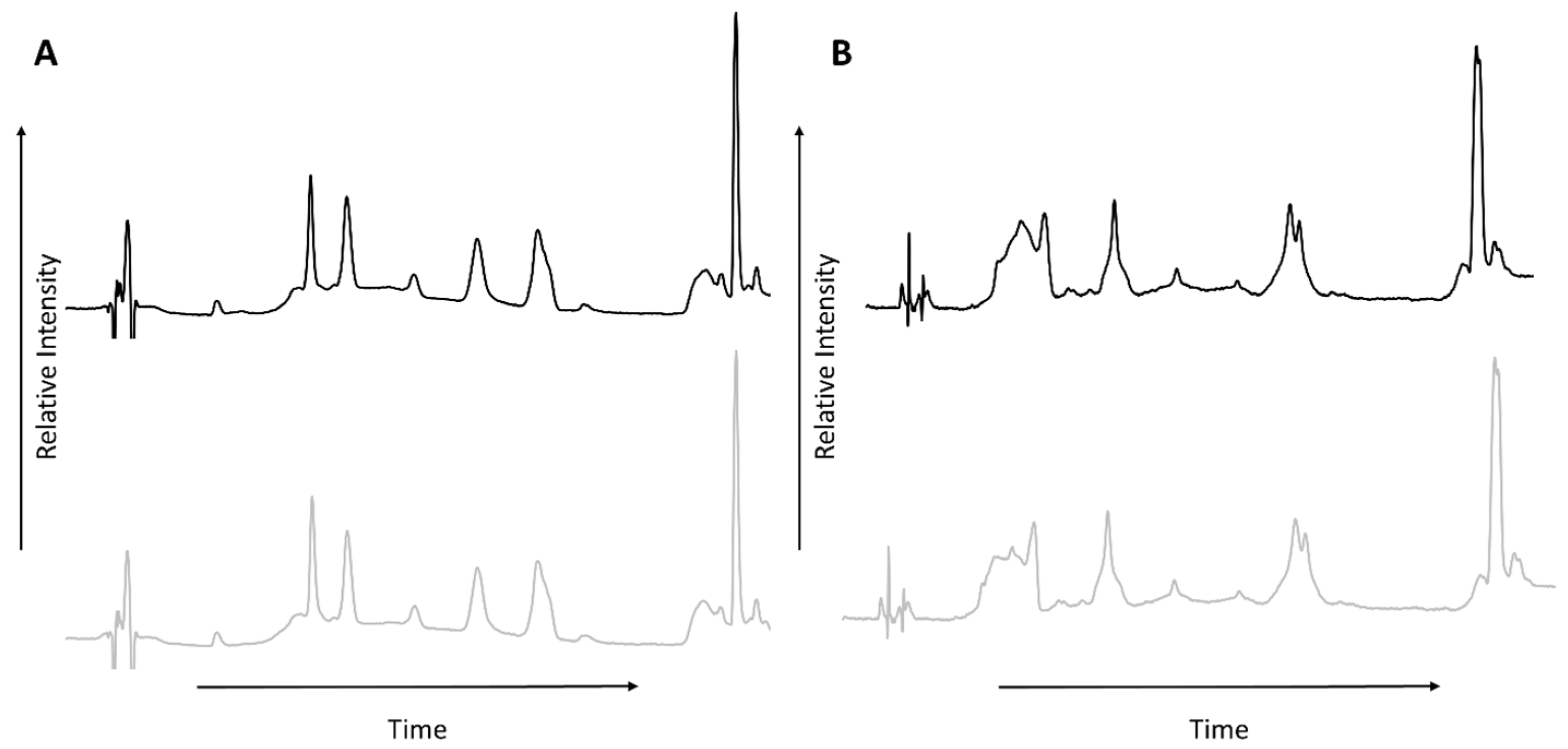

| Peak Number from Total Ion Chromatogram | Sample A (60 °C) | Sample B (185 °C) |
|---|---|---|
| 1 | d-Limonene | d-Limonene |
| 2 | Eucalyptol | Eucalyptol |
| 3 | 1-Methyl-4-(1-methylethyl)-cyclohexanol (Menthanol) | 1-Methyl-4-(1-methylethyl)-cyclohexanol (Menthanol) |
| 4 | Diethyl phthalate | Diethyl phthalate |
| 5 | Dexpanthenol | 1-Hexadecanol (Cetyl alcohol) |
| 6 | 1-Hexadecanol (Cetyl alcohol) | 1-Octadecanol (Stearyl alcohol) |
| 7 | 1-Octadecanol (Stearyl alcohol) | Dimantine |
| 8 | Dimantine | - |
| 9 | Stearyltrimethylammonium chloride | - |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Drechsel, D.A.; Towle, K.M.; Fung, E.S.; Novick, R.M.; Paustenbach, D.J.; Monnot, A.D. Chemical Stability Analysis of Hair Cleansing Conditioners under High-Heat Conditions Experienced during Hair Styling Processes. Cosmetics 2018, 5, 23. https://doi.org/10.3390/cosmetics5010023
Drechsel DA, Towle KM, Fung ES, Novick RM, Paustenbach DJ, Monnot AD. Chemical Stability Analysis of Hair Cleansing Conditioners under High-Heat Conditions Experienced during Hair Styling Processes. Cosmetics. 2018; 5(1):23. https://doi.org/10.3390/cosmetics5010023
Chicago/Turabian StyleDrechsel, Derek A., Kevin M. Towle, Ernest S. Fung, Rachel M. Novick, Dennis J. Paustenbach, and Andrew D. Monnot. 2018. "Chemical Stability Analysis of Hair Cleansing Conditioners under High-Heat Conditions Experienced during Hair Styling Processes" Cosmetics 5, no. 1: 23. https://doi.org/10.3390/cosmetics5010023
APA StyleDrechsel, D. A., Towle, K. M., Fung, E. S., Novick, R. M., Paustenbach, D. J., & Monnot, A. D. (2018). Chemical Stability Analysis of Hair Cleansing Conditioners under High-Heat Conditions Experienced during Hair Styling Processes. Cosmetics, 5(1), 23. https://doi.org/10.3390/cosmetics5010023





