Abstract
Exposure to pollution can cause oxidative stress, premature ageing, inflammation, and diseases. Since most of us are exposed to pollution, protection is important. This can be achieved through skin protection or through protection with respect to food and food supplements. There is a wide range of products on the market with anti-pollution claims. However, it is important that these claims are thoroughly validated by proper efficacy testing. When skin cells are exposed to pollution factors, changes in a number of skin properties can be observed, such as lipid composition, lipid and protein oxidation, pH, sebum secretion rate, oxidative stress, inflammation markers, and collagen and elastin levels. These can be measured and used as markers to verify anti-pollution claims. In the present review, we summarize some of the most important in vitro and in vivo tests that are used to determine if an ingredient or formulation has anti-pollution efficacy.
1. Introduction
The levels of air pollutants are constantly increasing worldwide, causing major concerns and health-related problems. The WHO report from 2016 states that over 3 million people die annually due to pollution, and about 90% of people live in an area that does not comply with the WHO Air Quality Guidelines []. The main sources of pollutions are particulate matter, polycyclic aromatic hydrocarbons (PAHs), volatile organic compounds (VOCs), nitrogen and sulfur oxides, carbon monoxide, ozone, and heavy metals [,]. These are generated mainly by industry and car exhaust. The quality of indoor air is crucial as well. North Americans spend about 90% of time indoors, where they are highly exposed to such pollutions as carbon monoxide, nitrogen dioxide, or VOCs coming from tobacco smoke, paints, varnishes, or air deodorizers. Dust, fungi or pets, and pest allergens also influence the quality of indoor air [,,].
It is well-known that environmental pollutants have a negative impact on human health. Excessive exposure to pollutants can lead to various health-related problems, including cardiovascular and pulmonary diseases and increased risk of microbial and viral infections []. Environmental contaminants also have a negative impact on the skin, which is the largest organ in the human body and one of the most important barriers against pollution. Exposure to contaminants can cause premature skin ageing, pigmentation spots, or acne. Exposure can also lead to more serious dermatological issues such as atopic dermatitis, psoriasis, and even skin cancer. Pollutants can also weaken the skin barrier function and penetrate through the skin, causing systemic toxicity in other organs [,,,].
In recent years, there has been an increasing interest in products that protect us from the negative impact of pollutants and that help to restore the skin barrier function. It is important to carry out proper efficacy testing for this kind of products, and most of the available methods have not yet been standardized. The aim of this review article is therefore to sum up the existing in vitro and in vivo tests for anti-pollution claims regarding the mechanisms of skin toxicity.
2. Effect of Pollutants on the Skin and Its Mechanism of Action
Pollution has a great impact on skin cells, as presented schematically in Figure 1. The negative impact can be observed at the skin surface, the stratum corneum, which is normally colonized with residual microorganisms. In the presence of pollutants, the skin microbiome changes for the benefit of pathogenic bacteria []. Moreover, environmental contamination enhances the production of reactive oxygen species (ROS), which depletes the content of antioxidants in the skin. This causes disturbance in the redox balance, causing stress to the cells. Some pollutants also tend to permeate through the stratum corneum into deeper skin layers. There, they act as a ligand for the Aryl hydrocarbon receptor (AhR), which takes part in mediating toxic effects of pollutants. The alterations of microflora, oxidative stress, and the activation of AhR cause the induction of an inflammatory cascade in the skin. The increased production of pro-inflammatory cytokines, such as interleukin1β or interleukin 8, greatly impacts the biological function of the cells, resulting in skin lesions and deterioration of skin appearance [].
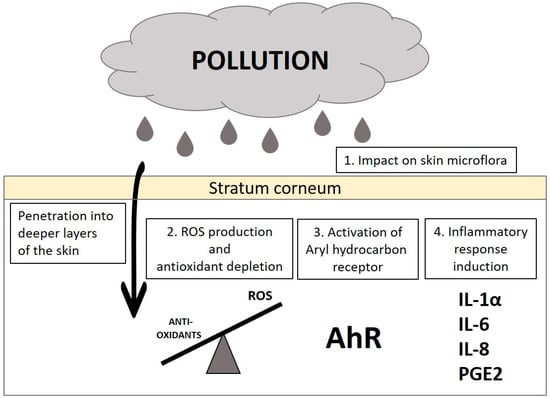
Figure 1.
Main mechanisms of pollutant action on the skin.
Pollution also causes a negative impact on other skin properties, which are necessary for proper skin function and health. Most importantly, the environmental contaminations influence composition of skin lipids: the ratio of lipids is disturbed, the cholesterol content is decreased, while the sebum secretion rate is higher. Oxidation of lipids and proteins in the skin also occurs. After exposure to pollution, the skin is characterized with increased pH and lactic acid content. These are only a few examples which, if properly monitored, could be regarded as chemical markers to determine anti-pollution efficacy [].
The impact of various pollutants on skin health and protection systems against environmental contaminants have been nicely reviewed by other authors [,,]. In the following paragraphs of this review, we will therefore focus on the main routes of action on the skin caused by pollutants.
2.1. Impact on Skin Microflora
The skin is colonialized by various types of microbiota, which form the residual skin microbiome. Its composition varies slightly between individuals and body parts, it is dependent on age, diet, lifestyle, and environment [,,]. It is known that the skin ecosystem is linked with the human immune system, supports the proper skin barrier function, and influences overall human health []. Air pollutants have a negative impact on skin microflora. He et al. observed a decrease in residual skin microflora of about 50% in the presence of ozone []. These alterations can cause colonization of the stratum corneum with pathogenic strains of bacteria, such as certain strains of Staphylococcus spp. and Streptococcus spp., which have been implicated to promote serious skin problems, such as cellulitis []. The relation between ambient air pollution and acne has also been confirmed. Pollution particles settle on the skin, blocking pores and therefore creating an anaerobic environment—ideal conditions for growth of Propionibacterium acnes, the main bacteria strain responsible for acne. Moreover, pollutions increase the sebum secretion rate, decrease the content of vitamin E in the skin, and promote inflammation, which worsens the skin condition [].
2.2. Generation of Reactive Oxygen Species (ROS)
Reactive oxygen species (ROSs) are formed in each living cell, mainly during mitochondrial reactions, but are also formed under the influence of exogenous factors such as UV-light, toxic chemicals, and other pollutants. At low concentrations, ROSs play an important role in regulating cellular signaling pathways related to cell proliferation and survival []. However, ROSs are unstable, so they can easily react with other molecules in the cell, causing damage. Each living cell also has defense mechanisms to neutralize the negative impact of ROS, such as antioxidant enzymes: catalase, superoxide dismutase, or glutathione peroxidase [,]. Air pollutants are known to deplete the antioxidant enzymes in epidermis. They also reduce the content of other antioxidants substances such as ascorbic acid, tocopherol, or glutathione. As a result, the redox balance is disturbed, causing oxidative stress and major damage to the skin cells. Accumulated ROSs react with the skin lipids, initiating the lipid peroxidation, due to which the permeability of the skin barrier is disrupted [,,,]. This can cause disorders in defense mechanisms against environmental toxins, allergens, pathogens, and UV radiation []. Isik et al. showed that pollutants contained in the cigarette smoke cause oxidative damage of lipids with production of malondialdehyde (MDA) in skin epithelial cells and inhibition of paraoxonase enzyme (PON1) []. PON1 has been shown to protect lipoproteins from oxidation. Thus, increased serum MDA and decreased activity of PON1 are possible markers of oxidative stress caused by pollutions.
2.3. Activation of the Aryl Hydrocarbon Receptor (AhR)
AhR is a ligand activated receptor, which is expressed in all types of cells in the skin and other tissues which are in contact with environmental factors, such as lungs or liver []. Its function is to support a cellular response for exogenous signals by regulation of cell homeostasis, activation of immune cells, and induction of xenobiotic metabolizing enzymes [,]. AhR is activated for example by environmental pollutants, mainly dioxins and PAHs, and takes part in the biochemical signaling cascade and toxic effects of these pollutants. It is also involved in processes such as cell growth, proliferation, and differentiation [,]. Tauchi et al (2005) showed that activation of the AhR in keratinocytes in transgenic mice caused inflammatory skin lesions and immunological imbalance, revealed as skin rashes and itches similar to those observed during atopic dermatitis []. The connection between inflammatory skin diseases and AhR transcript levels has also been confirmed on human volunteers []. Skin-biopsied samples were taken from patients diagnosed with atopic dermatitis and psoriasis and compared to healthy volunteers concerning the AhR transcript levels using quantitative real-time PCR analysis (qRT-PCR). Immunofluorescence staining was additionally performed to visualize the expression of AhR in the skin samples. Increased transcript levels of AhR were observed for affected skin samples as compared to healthy skin. Furthermore, an increased expression of inflammatory cytokines by the ELISA assay was found in epidermal keratinocytes of patients with psoriasis and atopic dermatitis, which shows the correlation between AhR activation and skin inflammation lesions []. Increased transcript levels of AhR were observed as well in normal human melanocytes in response to dioxins. The AhR activation caused an increase in tyrosinase activity and total melanin content, which are related to skin ageing, pigment spots, and skin cancer []. Moreover, the impact of AhR activation on matrix metalloproteinases (MMPs) was investigated after exposure to cigarette smoke. MMPs are responsible for the degradation of collagen and elastin fibers. They are as well engaged in the collagen biosynthesis. It was shown that AhR activation by cigarette smoke is related to increased expression of MMP-1 and MMP-3 in the skin. This caused an increased collagen degradation and reduced collagen biosynthesis, which resulted in premature skin ageing and formation of wrinkles [].
However, environmental pollutants are not the only ligands that bind to the AhR. Some natural components, for example, certain plant constituents, are known to interact with AhR, thereby inhibiting AhR signaling. Their binding will prevent the negative impact of AhR signaling in response to pollution. These AhR antagonist belongs mainly to polyphenol class such as flavonoids or catechins [,].
2.4. Induction of Inflammatory Cascade
Exposure of skin cells to pollution will cause inflammation. Several studies have shown that skin cell lines cultivated in vitro and treated with different pollutants are characterized by increased production of pro-inflammatory compounds [,,]. HaCaT (cultured human keratinocyte) cells in the presence of particulate matter released increased amounts of transforming growth factor (TNF-α) and interleukins (IL-1α and IL-8), which resulted in the accumulation of the inflammatory cells causing an inflammatory response []. Similar results were obtained for normal human epidermal keratinocytes (NHEKs) treated by pollution mixture consisting of heavy metals, particulate matter, and ozone. Here, an overproduction of two inflammatory markers, IL-1α and prostaglandin E2 (PGE2), was observed []. An increased IL-8 amount and, in the human keratinocytes during in vitro conditions, after application of benzo(a)pyrene, a compound present in cigarette smoke was detected. It was observed that benzo(a)pyrene binds to the AhR in the keratinocytes and increases CYP1A1 expression, which is a marker of oxidative stress. This cellular change was followed by the release of IL-8 []. Overproduction of TNFα, IL1-α, IL-6, IL-8, and other pro-inflammatory factors are related to inflammatory skin diseases, skin aging, and skin cancer [].
3. Testing of Anti-Pollution Claims
As mentioned above, our environment is excessively polluted, which has a great impact on human health. Since the skin is constantly exposed to external factors, such as pollutants, the development of new products that protect the skin from the negative impact of pollution is needed. There is as well a need for proper assays to evaluate their anti-pollution efficacy. It is very important that the study design is trustworthy, reproducible, and performed in conditions that are similar to real conditions. To confirm whether the product or ingredient has a significant anti-pollution effect, we need to know the nature of pollutants, how they act on the skin, and how to measure their effect. We then need to be sure that we are able to measure those changes and observe if they are significant or not. Several in vivo and in vitro anti-pollution study models have been proposed and tested []. In the present review, we are going to discuss some of these.
3.1. In Vivo Efficacy Testing
In vivo anti-pollution tests are performed directly on the skin of human volunteers. Therefore, the real conditions are mimicked well and the obtained results are reliable. Although, in this kind of study design, the mixture of pollutants needs to be applied to human skin, which can be an ethical consideration concerning health hazards. In many tests, volunteers are selected based on their living conditions and lifestyle, e.g., people who spend a high amount of time in highly polluted area or smokers—people who are already exposed to various types of pollutants. However, the study design often requires an artificial pollution mixture to be applied to the skin of volunteers.
A mixture of sebum, soil, and pollutants, called Sebollution, was designed to test the cleaning efficacy of cleansing formulation and devices. The Sebollution was applied to the cheeks of volunteers and followed by a washing process. The non-invasive evaluation platform was performed based on the photographs and image analysis before and after application of the pollution mixture and then again after washing []. The photo imaging technique with the use of pollutant mixture based on carbon particles is a fast and efficient way of evaluating the cleansing efficiency of products in vivo. However, to confirm the anti-pollution effect, it is important as well to measure the skin barrier integrity and protection against irritants. This can be done by monitoring the transepidermal water loss (TEWL) and chromametry analysis of skin after treatment with known irritants such as sodium dodecyl sulfate (SDS) or sodium hydroxide [].
In another in vivo test, the protective effect of different formulations on the skin treated with cigarette smoke was evaluated. Squalene monohydroperoxide (SQOOH) and malondialdehyde (MDA) were chosen as markers to measure the oxidative stress in the skin, since the amount of both of these substances tend to significantly increase during skin lipid oxidation. The skin of human volunteers was first treated with the tested product, followed by exposure to the cigarette smoke. Thereafter, lipid samples were collected from volunteers’ skin using a swabbing technique. The samples were further freeze-dried and analyzed with chromatographic techniques to evaluate SQOOH and MDA levels []. A similar study model was performed to assess the anti-pollution effect of baicaline []. There, the sebum samples were collected from volunteers’ skin prior the assay and the ex-vivo test was performed to study the impact of baicaline on lipid peroxidation. Levels of squalene and SQOOH in the sebum samples after exposure to the cigarette smoke were confirmed by chromatographic methods.
The in vivo methods for anti-pollution testing mimic real-life conditions, so some are perceived as more reliable as compared to in vitro studies. However, the data interpretation might be complicated because of the limited number of individuals in the examination group and high variability within the group. In vivo assays allow one to detect and measure the changes occurring in the skin cells after exposure to pollution. They also allow one to evaluate whether those changes are significant or not. However, it is not possible to observe the molecular routes responsible for those changes, which is necessary for proper prevention and treatment.
3.2. In Vitro Efficacy Testing
In vitro anti-pollution tests are based on cell models that are set up to reflect the in vivo state under laboratory conditions. Different types of skin cells can be used to observe the changes in a controlled environment. This gives a strong advantage, because in this kind of study design it is much easier to standardize the samples and conditions during treatments and measurements. It is also easier to understand the mechanism for action of pollutants and anti-pollution products on specific cells. However, cells grown in vitro are separated from their natural environment, which makes it difficult to predict if the cell behavior will reproduce these in the living organism [].
These in vitro studies are based on the cell lines grown under laboratory conditions. Such cell lines can be keratinocyte cell lines, such as HaCaT or NHEK [,,], or fibroblast cell lines, such as normal human foreskin-derived dermal fibroblasts (NHDF) [,]. These cells can be formed in a single layer or as a reconstructed skin model to create a more differentiated and accurate model of human skin characterized inner alia in the presence of the stratum corneum []. Some studies use skin fragments obtained from volunteers after treatments such as skin surgeries, followed by assays performed under in vitro conditions [,].
The impact of different pollutants can be tested using the in vitro platform. Usually the cells are treated with the pollutant mixture, which consists of, e.g., particulate matter, urban dust, and heavy metals or cigarette smoke [,,]. The impact of ozone or UV radiation can also be evaluated [,,].
The impact of pollution on skin cells and the effect of the anti-pollution treatment are assessed by the quantification of specific markers and cell parameters. The cell viability is often measured by a mitochondrial activity (MTT) assay [,], by an evaluation of lactate dehydrogenase (LDH) release, which is an indicator of cell damage [], or by cell counting using microscopic techniques []. The microscopic images allow one to observe morphological changes in the epidermal and dermal cells or the density and structure of the collagen network, after exposure to pollution [,]. Cell viability in response to stress factors can be also estimated by caspase 3 activity, which is a marker for cell apoptosis [].
Important indicators of cell response to pollution are the inflammation markers such as interleukins (IL-6, IL-8, IL-1α, and IL-1β), prostaglandins (PGE2), or transforming growth factor (TNF-α). Concentration of those markers in the culture medium is measured using ELISA assays [,,].
The negative impact of pollutants on cell culture can be observed by increased ROS accumulation. This is measured using a prefluorescent probe, which is oxidized by ROS and gives a fluorescent compound []. The effect of anti-pollution treatment is reflected in the antioxidant capacity of the skin and the ability of antioxidants to neutralize harmful substances. The antioxidant capacity can be assessed e.g., by the ferric reducing antioxidant power (FRAP) method [,].
The skin barrier integrity is crucial for proper function of the skin. Since pollutants highly influence this integrity, some in vitro assays have been designed to measure these changes. Western blot analysis is often implemented to determine the levels of proteins that support the skin barrier function []. Alternative assays are performed by measurement of transepidermal electrical resistance directly on epidermal skin cells [] or by estimating lipid peroxidation by MDA marker and heavy metal content in the ex vivo stratum corneum samples obtained via skin stripping []. In vitro anti-pollution tests enable one to study changes in gene expression by qRT-PCR to quantify transcript levels of relevant markers, such as interleukins and CYP1B1 []. CYP1B1 is linked to the AhR activation in response to pollution. By qRT-PCR-based analysis, it is also possible to determine changes in gene expression for genes related to skin firmness and integrity, such as those responsible for collagen, elastin, or matrix metalloproteinases production [].
Novel in vitro methods enable elucidation of different mechanisms related to cell response to pollution in one single study system, which is designed in a more sustainable way, e.g., using very low amounts of reagents. Microfluidic devices, integrated with a cell culture with a protein microarray chip allows for a fast and reliable evaluation of the cell cytotoxicity and apoptosis in response to pollution [].
In vitro anti-pollution tests provide a broad analysis platform, which focus on a deeper understanding of changes occurring in the skin cells, including the molecular mechanisms and modes of action. One of the biggest advantages of these assays is the control of specified conditions. However, it is important to remember that results provided by in vitro analysis can significantly differ from those observed in real situations. Therefore, it is recommended that such findings be supported by relevant in vivo studies.
4. Summary
Environmental pollutants have a tremendous impact on the life quality and health of individuals. There is no doubt that our protection from negative impact of pollutants is important. Research has offered various assays to determine the mechanism of action of the pollutants on living cells and to prove the protective efficacy of anti-pollution products. In vivo and in vitro assays have various advantages but also limitations, so combining these two approaches is the best option to provide reliable and trustworthy results.
Acknowledgments
We gratefully acknowledge support from the Knowledge Foundation, Synergy project 20170058.
Conflicts of Interest
The authors declare no conflict of interest.
References
- World Health Organization. Ambient Air Pollution: A Global Assessment of Exposure and Burden of Disease; World Health Organization: Geneva, Switzerland, 2016; pp. 1–131. [Google Scholar]
- Baudouin, C.; Charveron, M.; Tarroux, R.; Gall, Y. Environmental pollutants and skin cancer. Cell Biol. Toxicol. 2002, 18, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Drakaki, E.; Dessinioti, C.; Antoniou, C.V. Air pollution and the skin. Front. Environ. Sci. 2014, 2, 1–6. [Google Scholar] [CrossRef]
- Dales, R.; Liu, L.; Wheeler, A.J.; Gilbert, N.L. Public health: Quality of indoor residential air and health. Can. Med. Assoc. J. 2008, 179, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.H.; Kim, S.; Lee, J.H.; Kim, J.; Han, Y.; Kim, Y.M.; Kim, G.B.; Jung, K.; Cheong, H.K.; Ahn, K. Indoor air pollution aggravates symptoms of atopic dermatitis in children. PLoS ONE 2015, 10, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Misztal, P.K.; Nazaroff, W.W.; Goldstein, A.H. Volatile Organic Compound Emissions from Humans Indoors. Environ. Sci. Technol. 2016, 50, 12686–12694. [Google Scholar] [CrossRef] [PubMed]
- Folinsbee, L.J. Human Health Effects of Air Pollution. Environ. Health Perspect. 1992, 100, 45–56. [Google Scholar] [CrossRef]
- English, J.S.C.; Dawe, R.S.; Ferguson, J. Environmental effects and skin disease. Br. Med. Bull. 2003, 68, 129–142. [Google Scholar] [CrossRef] [PubMed]
- Freiman, A.; Bird, G.; Metelitsa, A.I.; Barankin, B.; Lauzon, G.J. Cutaneous effects of smoking. J. Cutan. Med. Surg. 2004, 8, 415–423. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.E.; Cho, D.; Park, H.J. Air pollution and skin diseases: Adverse effects of airborne particulate matter on various skin diseases. Life Sci. 2016, 152, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Koohgoli, R.; Hudson, L.; Naidoo, K.; Wilkinson, S.; Chavan, B.; Birch-Machin, M.A. Bad air gets under your skin. Exp. Dermatol. 2017, 26, 384–387. [Google Scholar] [CrossRef] [PubMed]
- Jo, J.H.; Kennedy, E.A.; Kong, H.H. Topographical and physiological differences of the skin mycobiome in health and disease. Virulence 2017, 8, 324–333. [Google Scholar] [CrossRef] [PubMed]
- Mancebo, S.E.; Wang, S.Q. Recognizing the impact of ambient air pollution on skin health. J. Eur. Acad. Dermatol. Venereol. 2015, 29, 2326–2332. [Google Scholar] [CrossRef] [PubMed]
- Lefebvre, M.A.; Pham, D.M.; Boussouira, B.; Bernard, D.; Camus, C.; Nguyen, Q.L. Evaluation of the impact of urban pollution on the quality of skin: A multicentre study in Mexico. Int. J. Cosmet. Sci. 2015, 37, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Puri, P.; Nandar, S.; Kathuria, S.; Ramesh, V. Effects of air pollution on the skin: A review. Indian J. Dermatol. Venerol. Leprol. 2017, 83, 415–423. [Google Scholar] [CrossRef]
- Grice, E.A.; Kong, H.H.; Conlan, S.; Deming, C.B.; Davis, J.; Young, A.C.; Bouffard, G.G.; Blakesley, R.W.; Murray, P.R.; Green, E.D.; et al. Topographical and Temporal Diversity of the Human Skin Microbiome. Science 2009, 324, 1190–1192. [Google Scholar] [CrossRef] [PubMed]
- Grice, E.A.; Segre, J.A. The skin microbiome. Nat. Rev. Microbiol. 2011, 9, 244–253. [Google Scholar] [CrossRef] [PubMed]
- Flores, G.E.; Caporaso, J.G.; Henley, J.B.; Rideout, J.R.; Domogala, D.; Chase, J.; Leff, J.W.; Vázquez-Baeza, Y.; Gonzalez, A.; Knight, R.; et al. Temporal variability is a personalized feature of the human microbiome. Genome Biol. 2014, 15, 531. [Google Scholar] [CrossRef] [PubMed]
- Prescott, S.L.; Larcombe, D.-L.; Logan, A.C.; West, C.; Burks, W.; Caraballo, L.; Levin, M.; Van Etten, E.; Horwitz, P.; Kozyrskyj, A.; et al. The skin microbiome: Impact of modern environments on skin ecology, barrier integrity, and systemic immune programming. World Allergy Organ. J. 2017, 10, 29. [Google Scholar] [CrossRef] [PubMed]
- He, Q.C.; Tavakkol, A.; Wietecha, K.; Begum-Gafur, R.; Ansari, S.A.; Polefka, T. Effects of environmentally realistic levels of ozone on stratum corneum function. Int. J. Cosmet. Sci. 2006, 28, 349–357. [Google Scholar] [CrossRef] [PubMed]
- Valacchi, G.; Porada, E.; Rowe, B. Ambient ozone and bacterium Streptococcus: A link between cellulitis and pharyngitis. Int. J. Occup. Med. Environ. Health 2015, 28, 771–774. [Google Scholar] [CrossRef] [PubMed]
- Krutmann, J.; Moyal, D.; Xiang, L.F.; Seité, S. Pollution and acne: Is there a link? Clin. Cosmet. Investig. Dermatol. 2017, 10, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Ray, P.D.; Huang, B.-W.; Tsuji, Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell Signal 2012, 24, 981–990. [Google Scholar] [CrossRef] [PubMed]
- Ghio, A.J.; Carraway, M.S.; Madden, M.C. Composition of air pollution particles and oxidative stress in cells, tissues, and living systems. J. Toxicol. Environ. Heal. Part B Crit. Rev. 2012, 15, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Birch-Machin, M.A.; Bowman, A. Oxidative stress and ageing. Br. J. Dermatol. 2016, 175, 26–29. [Google Scholar] [CrossRef] [PubMed]
- Thiele, J.J.; Traber, M.G.; Podda, M.; Tsang, K.; Cross, C.E.; Packer, L. Ozone depletes tocopherols and tocotrienols topically applied to murine skin. FEBS Lett. 1997, 401, 167–170. [Google Scholar] [CrossRef]
- Ahn, K. The role of air pollutants in atopic dermatitis. J. Allergy Clin. Immunol. 2014, 134, 993–999. [Google Scholar] [CrossRef] [PubMed]
- Del Rosso, J.Q.; Levin, J. The clinical relevance of maintaining the functional integrity of the stratum corneum in both healthy and disease-affected skin. J. Clin. Aesthet. Dermatol. 2011, 4, 22–42. [Google Scholar] [PubMed]
- Isik, B.; Ceylan, A.; Isik, R. Oxidative stress in smokers and non-smokers. Inhal. Toxicol. 2007, 19, 767–769. [Google Scholar] [CrossRef] [PubMed]
- Esser, C.; Bargen, I.; Weighardt, H.; Haarmann-Stemmann, T.; Krutmann, J. Functions of the aryl hydrocarbon receptor in the skin. Semin. Immunopathol. 2013, 35, 677–691. [Google Scholar] [CrossRef] [PubMed]
- Merches, K.; Haarmann-Stemmann, T.; Weighardt, H.; Krutmann, J.; Esser, C. AHR in the skin: From the mediator of chloracne to a therapeutic panacea? Curr. Opin. Toxicol. 2017, 2, 79–86. [Google Scholar] [CrossRef]
- Tauchi, M.; Hida, A.; Negishi, T.; Katsuoka, F.; Noda, S.; Mimura, J.; Hosoya, T.; Yanaka, A.; Aburatani, H.; Fujii-kuriyama, Y.; et al. Constitutive expression of aryl hydrocarbon receptor in keratinocytes causes in ammatory skin lesions. Society 2005, 25, 9360–9368. [Google Scholar] [CrossRef]
- Kim, H.O.; Kim, J.H.; Chung, B.Y.; Choi, M.G.; Park, C.W. Increased expression of the aryl hydrocarbon receptor in patients with chronic inflammatory skin diseases. Exp. Dermatol. 2014, 23, 278–281. [Google Scholar] [CrossRef] [PubMed]
- Luecke, S.; Backlund, M.; Jux, B.; Esser, C.; Krutmann, J.; Rannug, A. The aryl hydrocarbon receptor (AHR), a novel regulator of human melanogenesis. Pigment Cell Melanoma Res. 2010, 23, 828–833. [Google Scholar] [CrossRef] [PubMed]
- Morita, A.; Torii, K.; Maeda, A.; Yamaguchi, Y. Molecular Basis of Tobacco Smoke-Induced Premature Skin Aging. J. Investig. Dermatology Symp. Proc. 2009, 14, 53–55. [Google Scholar] [CrossRef] [PubMed]
- Abel, J.; Haarmann-Stemmann, T. An introduction to the molecular basics of aryl hydrocarbon receptor biology. Biol. Chem. 2010, 391, 1235–1248. [Google Scholar] [CrossRef] [PubMed]
- Palermo, C.M.; Hernando, J.I.M.; Dertinger, S.D.; Kende, A.S.; Gasiewicz, T.A. Identification of Potential Aryl Hydrocarbon Receptor Antagonists in Green Tea. Chem. Res. Toxicol. 2003, 16, 865–872. [Google Scholar] [CrossRef] [PubMed]
- Portugal-Cohen, M.; Soroka, Y.; Ma’or, Z.; Oron, M.; Zioni, T.; Brégégère, F.M.; Neuman, R.; Kohen, R.; Milner, Y. Protective effects of a cream containing Dead Sea minerals against UVB-induced stress in human skin. Exp. Dermatol. 2009, 18, 781–788. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Kang, Z.; Jiang, S.; Zhao, J.; Yan, S.; Xu, F.; Xu, J. Effects of ambient fine particles PM2.5 on human HaCaT cells. Int. J. Environ. Res. Public Health 2017, 14, 72. [Google Scholar] [CrossRef] [PubMed]
- Portugal-Cohen, M.; Oron, M.; Cohen, D.; Ma’or, Z. Antipollution skin protection—A new paradigm and its demonstration on two active compounds. Clin. Cosmet. Investig. Dermatol. 2017, 10, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, G.; Takahara, M.; Uchi, H.; Takeuchi, S.; Mitoma, C.; Moroi, Y.; Furue, M. An environmental contaminant, benzo(a)pyrene, induces oxidative stress-mediated interleukin-8 production in human keratinocytes via the aryl hydrocarbon receptor signaling pathway. J. Dermatol. Sci. 2011, 62, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Perin, F.; Blanché, C. Study design for anti-pollution claims. Personal Care Magazine, 16 May 2017; 23–25. [Google Scholar]
- Peterson, G.; Rapaka, S.; Koski, N.; Kearney, M.; Ortblad, K.; Tadlock, L. A robust sebum, oil, and particulate pollution model for assessing cleansing efficacy of human skin. Int. J. Cosmet. Sci. 2017, 39, 351–354. [Google Scholar] [CrossRef] [PubMed]
- Schlay, S.; Slotta, U. Efficient skin protection against negative environmental influences by breathable vegan silk polypeptides. Sofw. J. 2016, 142, 14–19. [Google Scholar]
- Bielfeldt, S.; Boehling, A.; Springmann, G.; Wilhelm, K. Pollution Protection and the Skin—Testing Strategies. Househ. Pers. Care Today, 2016, 11, 81–84. [Google Scholar]
- Levannier, K.L.; Planel, E.; Gu, W.; Yu, X.J. Baicalin Protects against Cigarette Smoke. In Proceedings of the 29th Congress of the International Federation of Societies of Cosmetic Chemists, Orlando, FL, USA, 31 October–2 November 2016. [Google Scholar]
- Valacchi, G.; Sticozzi, C.; Belmonte, G.; Chen, N.; Krol, Y.; Oresajo, C. Vitamin C compound mixtures prevent ozone-induced oxidative damage in human keratinocytes as initial assessment of pollution protection. PLoS ONE 2015, 10, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Costa, A.; Facchini, G.; Pinheiro, A.L.T.A.; da Silva, M.S.; Bonner, M.Y.; Arbiser, J.; Eberlin, S. Honokiol protects skin cells against inflammation, collagenolysis, apoptosis, and senescence caused by cigarette smoke damage. Int. J. Dermatol. 2017, 56, 754–761. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zheng, L.; Tuo, J.; Liu, Q.; Zhang, X.; Xu, Z.; Liu, S.; Sui, G. Analysis of PM2.5-induced cytotoxicity in human HaCaT cells based on a microfluidic system. Toxicol. In Vitro 2017, 43, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Hubaux, R.; Weisgerber, F.; Salmon, M. In vitro assays to study the effects of air pollutants on skin: Exposure to urban dust and cigarette smoke extract. In Proceedings of the 23rd IFSCC Conference, Montreux, Switzerland, 21–23 September 2015. [Google Scholar]
- Gimenez, A.; Davi, C.; Canadas, E.; Alminana, N.; Delgado, R. Finding new solutions against pollution. Sofw. J. 2016, 142, 20–25. [Google Scholar]
- Iddamalgoda, A.; Biswas, K.B.; Tanaka, K.; Takayama, S. Protection against pollution-induced skin ageing by a natural cosmetic ingredient. Sofw. J. 2016, 142, 8–13. [Google Scholar]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).