Development of a Natural Anti-Age Ingredient Based on Quercus pubescens Willd. Leaves Extract—A Case Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Plant Extraction
2.3. Fractionation of Q. pubescens Leaves Extract
2.4. High Performance Liquid Chromatography
2.4.1. HPLC Analyses
2.4.2. Semi-Preparative HPLC
2.5. Gas Chromatography-Mass Spectrometry (GC-MS)
2.6. Bioassays
2.6.1. Materials
2.6.2. Instrumentation
2.6.3. DPPH Radical Scavenging Assay
2.6.4. Tyrosinase Assays
2.6.5. Lipoxygenase Assay
2.6.6. Elastase Assay
2.6.7. Hyaluronidase Assay
2.6.8. Collagenase Assay
2.6.9. Total Polyphenol Analysis
2.6.10. Total Flavonoid Analysis
2.7. HPLC-ESI-MS Analyses
2.8. Discoloration of the Extract
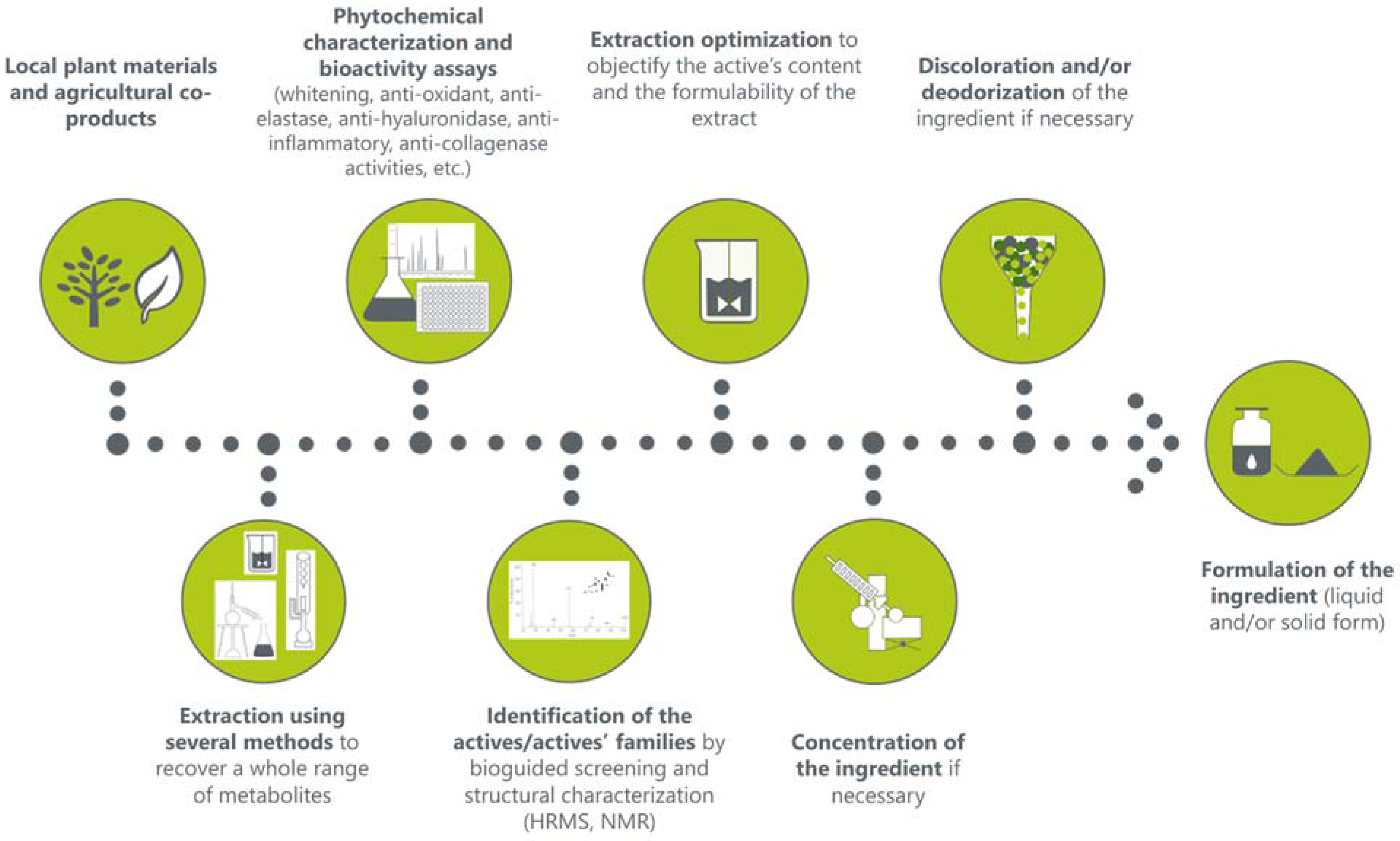
2.9. Development of Liquid and Solid Cosmetic Ingredients
2.10. Stability Testing
2.10.1. Solid Phase Extraction
2.10.2. Color Evaluation
3. Results
3.1. Crude Extract Oak1
3.1.1. In Vitro Bioassays
3.1.2. Fractionation Scheme 1
3.1.3. Fractionation Scheme 2
3.2. Development of Cosmetic Ingredients
3.2.1. Solid Cosmetic Ingredient
3.2.2. Liquid Cosmetic Ingredient
3.2.3. Stability Tests
3.3. Formulation
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lopaciuk, A.; Loboda, M. Global beauty industry trends in the 21st century. In Proceedings of the Management, Knowledge and Learning International Conference 2013, Zadar, Croatia, 19–21 June 2013; pp. 1079–1087. [Google Scholar]
- Cosmetics Market by Category (Skin & Sun Care Products, Hair Care Products, Deodorants, Makeup & Color Cosmetics, Fragrances) and by Distribution Channel (General Departmental Store, Supermarkets, Drug Stores, Brand Outlets)—Global Opportunity Analysis and Industry Forecast, 2014–2022. Available online: https://www.alliedmarketresearch.com/cosmetics-market (accessed on 17 November 2017).
- Beauty Survey 2017: Key Insights and System Update. Available online: http://go.euromonitor.com/WP-171024-BeautyandPersonalCareSurvey.html (accessed on 17 November 2017).
- Official Journal of the European Union. Commission Regulation (EU) No 655/2013 of 10 July 2013 Laying Down Common Criteria for the Justification of Claims Used in Relation to Cosmetic Products. 2013. Available online: http://ec.europa.eu/consumers/sectors/cosmetics/files/pdf/guide_reg_claims_en.pdf (accessed on 17 November 2017).
- Whitehouse, L. Proving Claims to Maximise on Beauty Trends. Available online: https://www.cosmeticsdesign-europe.com/Article/2017/06/30/Proving-claims-to-maximise-on-beauty-trends (accessed on 1 December 2017).
- Natural and Organic Beauty: Market Size Worldwide 2016–2024. Available online: https://www.statista.com/statistics/750779/natural-organic-beauty-market-worldwide/ (accessed on 17 November 2017).
- Cosmetic Ingredients: Global Market Size by Category 2015–2020. Available online: https://www.statista.com/statistics/627730/market-size-of-cosmetic-ingredients-worldwide-by-category/ (accessed on 17 November 2017).
- Cosmetic Ingredients: Global Market Size 2015–2020. Available online: https://www.statista.com/statistics/627786/market-size-of-cosmetic-ingredients-worldwide/ (accessed on 17 November 2017).
- Wynberg, R.P.; Laird, S.A. Access and benefit-sharing: Key points for policy-makers. The cosmetics industry. People Plants Int. 2015. Available online: https://www.researchgate.net/publication/303286830_Access_and_Benefit_Sharing_Key_Points_for_Policy-_Makers_-_The_Cosmetics_Industry (accessed on 22 November 2017).
- Chemat, F.; Strube, J. Green Extraction of Natural Products: Theory and Practice; John Wiley & Sons: Hoboken, NJ, USA, 2015; ISBN 978-3-527-33653-1. [Google Scholar]
- Mukherjee, P.K.; Maity, N.; Nema, N.K.; Sarkar, B.K. Bioactive compounds from natural resources against skin aging. Phytomedicine 2011, 19, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Krutmann, J.; Liu, W.; Li, L.; Pan, X.; Crawford, M.; Sore, G.; Seite, S. Pollution and skin: From epidemiological and mechanistic studies to clinical implications. J. Dermatol. Sci. 2014, 76, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Pan, T.-L.; Wang, P.-W.; Aljuffali, I.A.; Huang, C.-T.; Lee, C.-W.; Fang, J.-Y. The impact of urban particulate pollution on skin barrier function and the subsequent drug absorption. J. Dermatol. Sci. 2015, 78, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Plainfossé, H.; Burger, P.; Michel, T.; Landreau, A.; Fernandez, X. Actifs cosmétiques pour la réparation cutanée. Tech. Ing. 2017, J3002. Available online: https://www.techniques-ingenieur.fr/base-documentaire/procedes-chimie-bio-agro-th2/cosmetiques-42634210/actifs-cosmetiques-pour-la-reparation-cutanee-j3002/ (accessed on 25 November 2017).
- Ganceviciene, R.; Liakou, A.I.; Theodoridis, A.; Makrantonaki, E.; Zouboulis, C.C. Skin anti-aging strategies. Dermatoendocrinol 2012, 4, 308–319. [Google Scholar] [CrossRef] [PubMed]
- Life Expectancy. Available online: http://www.who.int/gho/mortality_burden_disease/life_tables/situation_trends_text/en/ (accessed on 22 November 2017).
- Osbourn, A.E.; Lanzotti, V. Plant-Derived Natural Products: Synthesis, Function, and Application; Springer Science & Business Media: Berlin, Germany, 2009; ISBN 978-0-387-85498-4. [Google Scholar]
- Médail, F.; Quézel, P. Biodiversity hotspots in the Mediterranean Basin: Setting global conservation priorities. Conserv. Biol. 1999, 13, 1510–1513. [Google Scholar] [CrossRef]
- Médail, F.; Quezel, P. Hot-spots analysis for conservation of plant biodiversity in the Mediterranean basin. Ann. Mo. Bot. Gard. 1997, 84, 112–127. [Google Scholar] [CrossRef]
- Quercus pubescens Willd. Available online: https://npgsweb.ars-grin.gov/gringlobal/taxonomydetail.aspx?id=30735 (accessed on 23 November 2017).
- Apostol, E.N.; Dinu, C.G.; Apostol, B.; Ciuvăț, A.L.; Lorent, A.; Pleșca, I.; Postolache, D.; Leca, S.; Enescu, C.M. Importance of pubescent oak (Quercus pubescens Willd.) for Romanian forests in the context of climate change. Rev. Silvic. Și Cinegetică 2016, 21, 29–33. [Google Scholar]
- Quercus pubescens—EUFORGEN European Forest Genetic Resources Program. Available online: http://www.euforgen.org/species/quercus-pubescens/ (accessed on 15 November 2017).
- Gallé, A.; Haldimann, P.; Feller, U. Photosynthetic performance and water relations in young pubescent oak (Quercus pubescens) trees during drought stress and recovery. New Phytol. 2007, 174, 799–810. [Google Scholar] [CrossRef] [PubMed]
- CosIng—Cosmetics—GROWTH—European Commission. Available online: http://ec.europa.eu/growth/tools-databases/cosing/index.cfm?fuseaction=search.advanced (accessed on 23 November 2017).
- Lee, M.W.; Kim, H.H.; Kim, D.H. Composition for anti-wrinkling comprising fractions from leaves of Quercus mongolica. International Patent Application No. KR101483872, 19 January 2015. Available online: https://worldwide.espacenet.com/publicationDetails/biblio?FT=D&date=20150119&DB=EPODOC&locale=fr_EP&CC=KR&NR=101483872B1&KC=B1&ND=6 (accessed on 27 November 2017).
- Yang, K.W.; Kim, J.M.; Kim, K.M.; Ko, H.N.; Lee, M.Y.; Lee, Y.B. Composition for improving wrinkles using Quercus glauca branch extracts. International Patent Application No. KR20140137837, 3 December 2014. Available online: https://worldwide.espacenet.com/publicationDetails/biblio?FT=D&date=20141203&DB=EPODOC&locale=fr_EP&CC=KR&NR=20140137837A&KC=A&ND=5 (accessed on 27 November 2017).
- Charlois, S.; Riboulet, J.-M.; Bregou, M. Active Ingredient Based on an Oak Plant Extract with Anti-Skin Aging Effect and Cosmetic Composition Comprising Same. International Patent Application No. PCT/FR2014/052962, 28 May 2015. Available online: https://worldwide.espacenet.com/publicationDetails/biblio?FT=D&date=20150528&DB=EPODOC&locale=fr_EP&CC=WO&NR=2015075377A1&KC=A1&ND=5 (accessed on 27 November 2017).
- Favre, E. Cosmetic Composition Containing an Anti-Oxidant Agent Containing Oak and Aniseed Extracts. International Patent Application No. PCT/FR2008/051742, 23 April 2009. Available online: http://www.google.ca/patents/WO2009050398A2 (accessed on 30 November 2017).
- McLafferty, F.W.; Stauffer, D.B. The Wiley/NBS Registry of Mass Spectral Data; Wiley: New York, NY, USA, 1989; ISBN 978-0-471-62886-6. [Google Scholar]
- Boelens Aroma Chemical Information Service. BACIS ESO 2000, the Complete Database of Essential Oils; Leffingwell & Associates: Canton, GA, USA, 1999. [Google Scholar]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Koleva, I.I.; van Beek, T.A.; Linssen, J.P.H.; de Groot, A.; Evstatieva, L.N. Screening of plant extracts for antioxidant activity: A comparative study on three testing methods. Phytochem. Anal. PCA 2002, 13, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Burger, P.; Landreau, A.; Azoulay, S.; Michel, T.; Fernandez, X. Skin whitening cosmetics: Feedback and challenges in the development of natural skin lighteners. Cosmetics 2016, 3, 36. [Google Scholar] [CrossRef]
- Lu, W.; Zhao, X.; Xu, Z.; Dong, N.; Zou, S.; Shen, X.; Huang, J. Development of a new colorimetric assay for lipoxygenase activity. Anal. Biochem. 2013, 441, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Debelle, L.; Tamburro, A.M. Elastin: Molecular description and function. Int. J. Biochem. Cell Biol. 1999, 31, 261–272. [Google Scholar] [CrossRef]
- Papakonstantinou, E.; Roth, M.; Karakiulakis, G. Hyaluronic acid: A key molecule in skin aging. Dermatoendocrinol 2012, 4, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Baumann, L. Skin ageing and its treatment. J. Pathol. 2007, 211, 241–251. [Google Scholar] [CrossRef] [PubMed]
- Roy, A.; Sahu, R.K.; Matlam, M.; Deshmukh, V.K.; Dwivedi, J.; Jha, A.K. In vitro techniques to assess the proficiency of skin care cosmetic formulations. Pharmacogn. Rev. 2013, 7, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- CIE. Colorimetry—Part 4: CIE 1976 L*A*B* Colour Space; CIE DS 014-4.3/E:2007; CIE Central Bureau: Vienna, Austria, 2007. [Google Scholar]
- Rhein, L.D.; Schlossman, M.; O’Lenick, A.; Somasundaran, P. Surfactants in Personal Care Products and Decorative Cosmetics, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2006; ISBN 978-1-4200-1612-3. [Google Scholar]
- ViewSonic. Technical Introduction. Delta E ≤ 2 Colour Accuracy. Available online: https://www.viewsonic.com/uk/products/lcd/pdf/ti_delta_E.pdf (accessed on 12 December 2017).
- Engel, R.; Gülz, P.-G.; Herrmann, T.; Nahrstedt, A. Glandular trichomes and the volatiles obtained by steam distillation of Quercus robur leaves. Z. Für Naturforschung C 1993, 48, 736–744. [Google Scholar]
- Jordão, A.M.; Ricardo-da-Silva, J.M.; Laureano, O.; Adams, A.; Demyttenaere, J.; Verhé, R.; De Kimpe, N. Volatile composition analysis by solid-phase microextraction applied to oak wood used in cooperage (Quercus pyrenaica and Quercus petraea): Effect of botanical species and toasting process. J. Wood Sci. 2006, 52, 514–521. [Google Scholar] [CrossRef]
- Palma-Fleming, H.A.; Kepner, R.E. Volatile components of California live oak, Quercus agrifolia. Phytochemistry 1983, 22, 1503–1505. [Google Scholar] [CrossRef]
- Kameoka, H.; Aso, Y.; Tsujino, H. Constituents of essential oil of leaves of Quercus dentata Thunb. J. Agric. Chem. Soc. Jpn. 1983, 57, 135–138. [Google Scholar]
- Mosedale, J.R.; Feuillat, F.; Baumes, R.; Dupouey, J.-L.; Puech, J.-L. Variability of wood extractives among Quercus robur and Quercus petraea trees from mixed stands and their relation to wood anatomy and leaf morphology. Can. J. For. Res. 1998, 28, 994–1006. [Google Scholar] [CrossRef]
- Fengel, D.; Wegener, G. Wood: Chemistry, Ultrastructure, Reactions; Walter de Gruyter: Berlin, Germany, 1983. [Google Scholar]
- Rocha, S.M.; Coimbra, M.A.; Delgadillo, I. Occurrence of furfuraldehydes during the processing of Quercus suber L. cork. Simultaneous determination of furfural, 5-hydroxymethylfurfural and 5-methylfurfural and their relation with cork polysaccharides. Carbohydr. Polym. 2004, 56, 287–293. [Google Scholar] [CrossRef]
- Plazonić, A.; Mornar, A.; Maleš, Ž.; Kujundžić, N. Phenolic content and antioxidant activities of Burr Parsley (Caucalis platycarpos L.). Molecules 2013, 18, 8666–8681. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, J.F.M.; de Souza, M.C.; Matta, S.R.; de Andrade, M.R.; Vidal, F.V.N. Correlation analysis between phenolic levels of Brazilian propolis extracts and their antimicrobial and antioxidant activities. Food Chem. 2006, 99, 431–435. [Google Scholar] [CrossRef]
- Pientaweeratch, S.; Panapisal, V.; Tansirikongkol, A. Antioxidant, anti-collagenase and anti-elastase activities of Phyllanthus emblica, Manilkara zapota and silymarin: An in vitro comparative study for anti-aging applications. Pharm. Biol. 2016, 54, 1865–1872. [Google Scholar] [CrossRef] [PubMed]
- Justino, G.C.; Correia, C.F.; Mira, L.; Borges Dos Santos, R.M.; Martinho Simões, J.A.; Silva, A.M.; Santos, C.; Gigante, B. Antioxidant activity of a catechol derived from abietic acid. J. Agric. Food Chem. 2006, 54, 342–348. [Google Scholar] [CrossRef] [PubMed]
- Kolayli, S.; Sahin, H.; Can, Z.; Yildiz, O.; Sahin, K. Honey shows potent inhibitory activity against the bovine testes hyaluronidase. J. Enzyme Inhib. Med. Chem. 2016, 31, 599–602. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, X.; Merck, F.; Kerdudo, A. Conservateurs pour les cosmétiques—Généralités et conservateursantimicrobiens. Tech. Ing. 2012, J2284. Available online: https://www.techniques-ingenieur.fr/base-documentaire/procedes-chimie-bio-agro-th2/cosmetiques-42634210/conservateurs-pour-cosmetiques-j2284/ (accessed on 23 November 2017).
- Kerdudo, A.; Burger, P.; Merck, F.; Dingas, A.; Rolland, Y.; Michel, T.; Fernandez, X. Development of a natural ingredient—Natural preservative: A case study. Comptes Rendus Chim. 2016, 19, 1077–1089. [Google Scholar] [CrossRef]
- d’Enfert, V. The Nagoya Protocol imposes new rules. Expr. Cosmétique 2013, 24, 40–42. [Google Scholar]
- Salminen, W.F. Integrating toxicology into cosmetic ingredient research and development. Int. J. Cosmet. Sci. 2002, 24, 217–224. [Google Scholar] [CrossRef] [PubMed]
- SCCS. The SCCS Notes of Guidance for the Testing of Cosmetic Ingredients and Their Safety Evaluation, 9th Revision; SCCS: Brussels, Belgium, 2016. [Google Scholar]

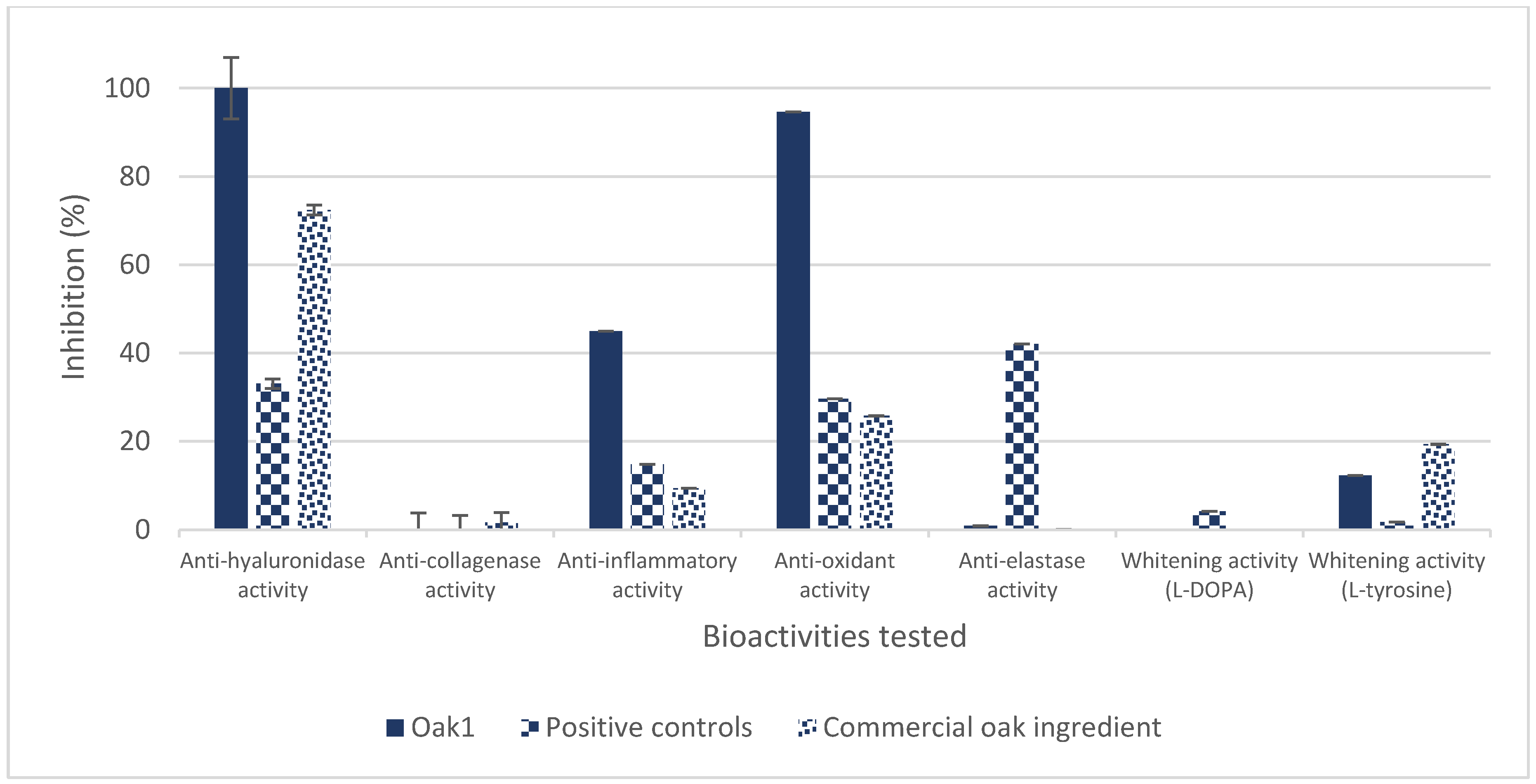
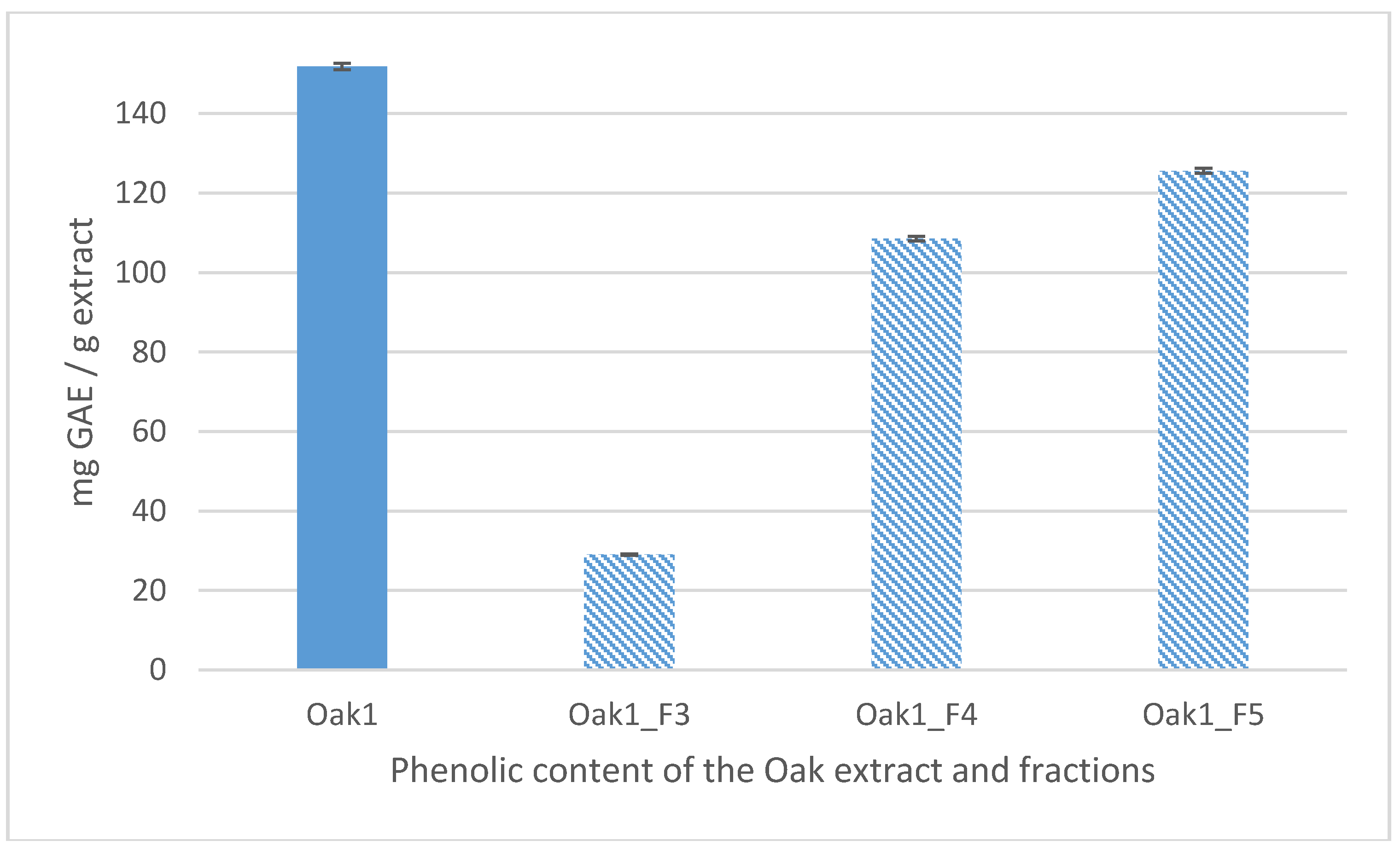
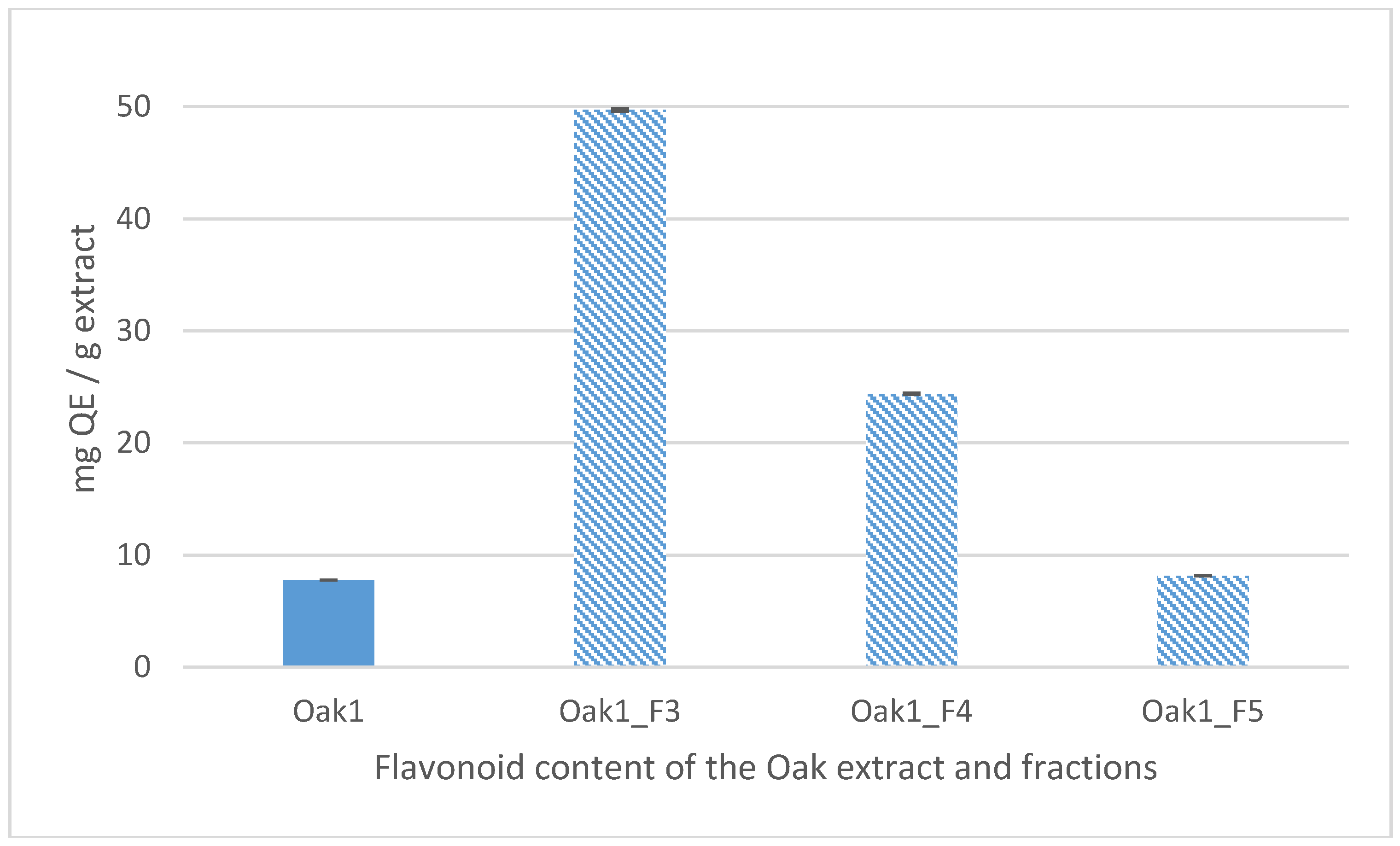
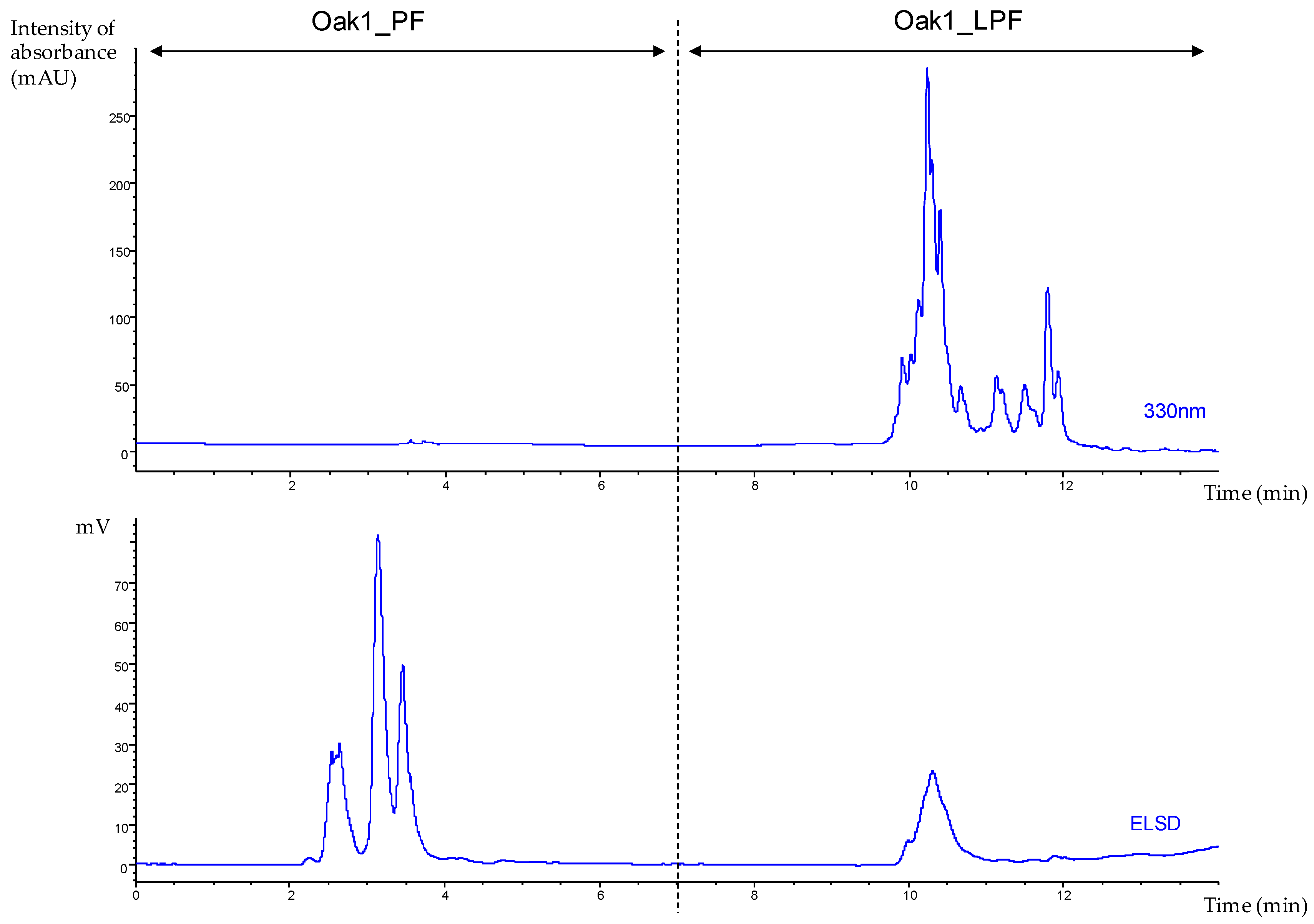
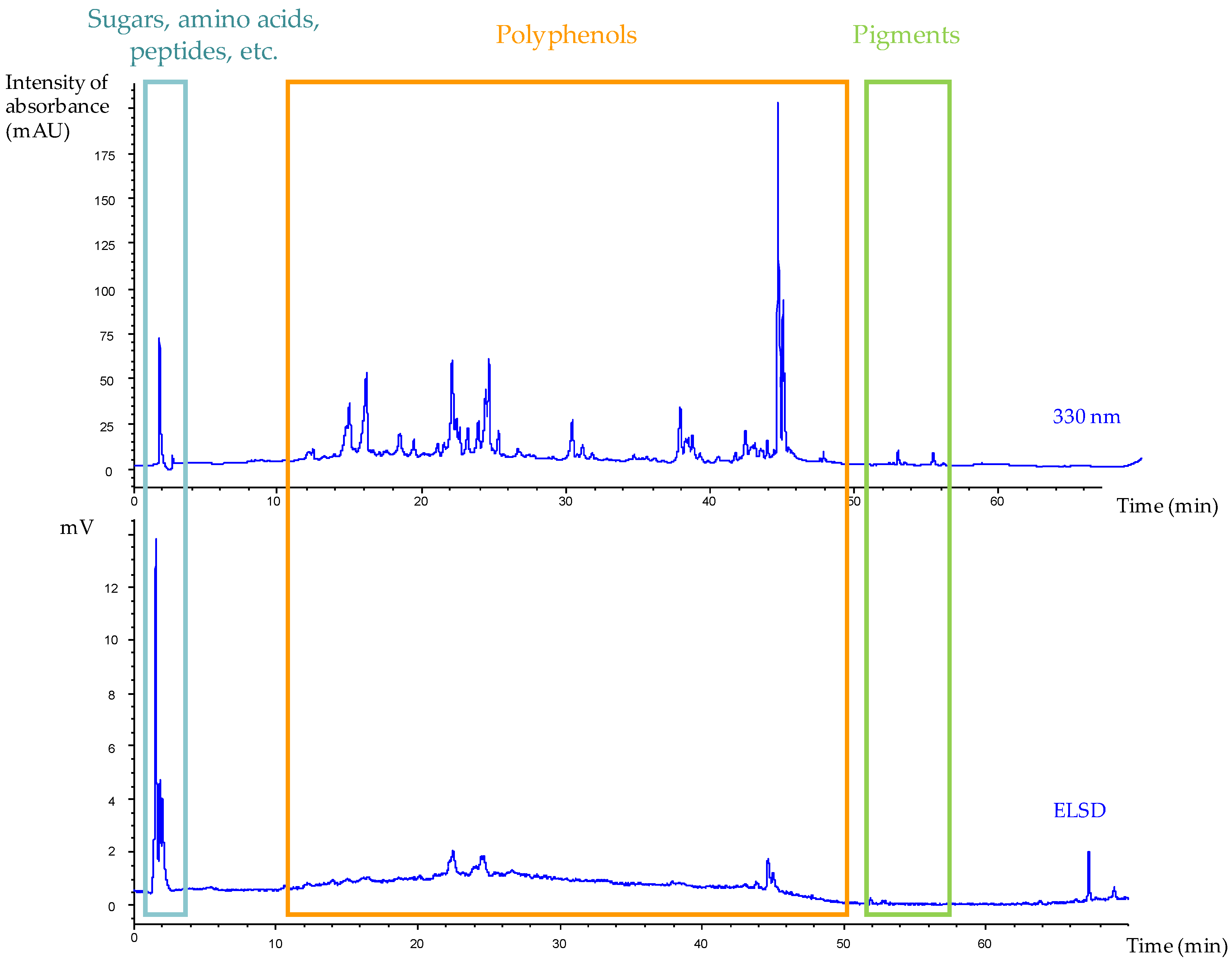

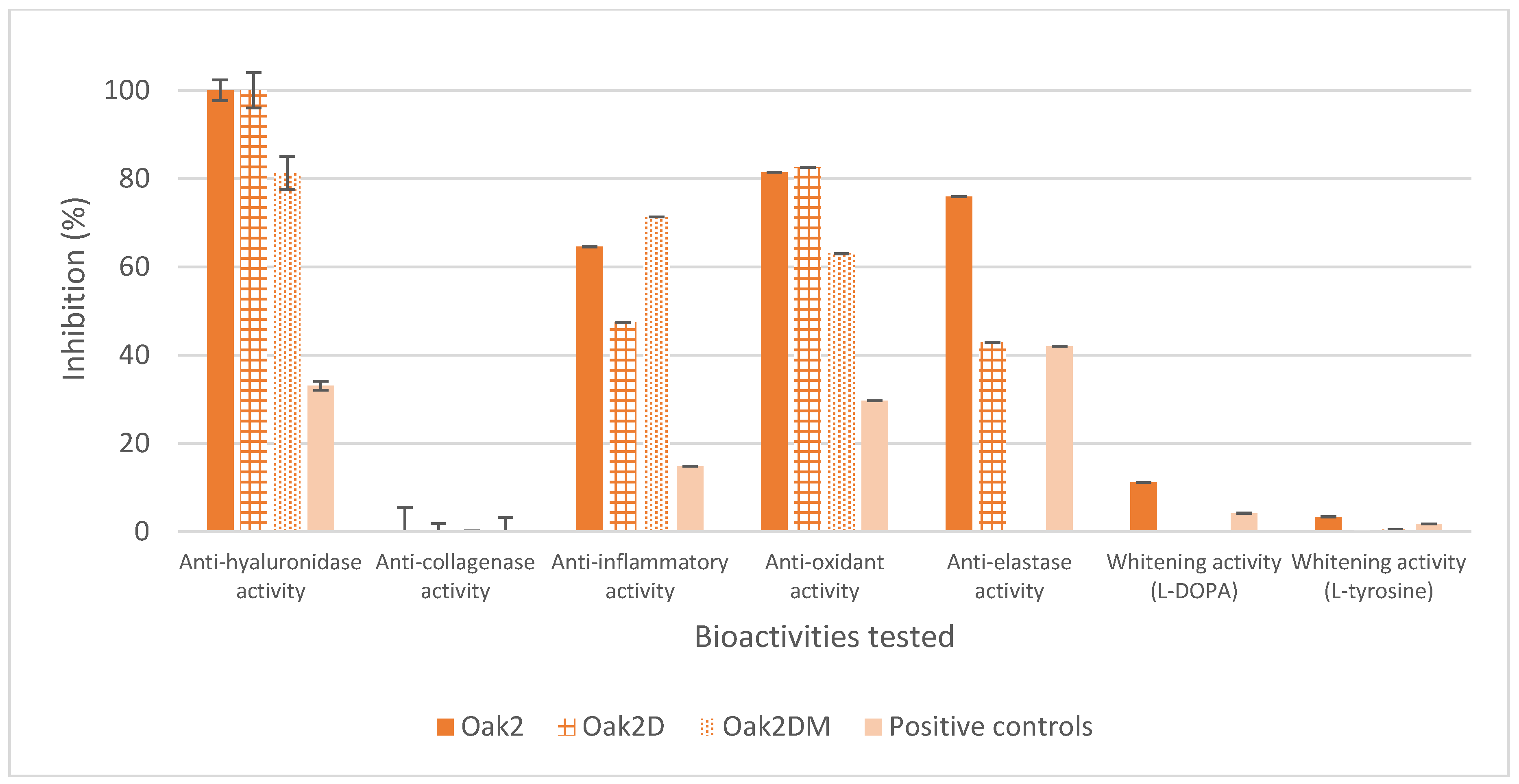
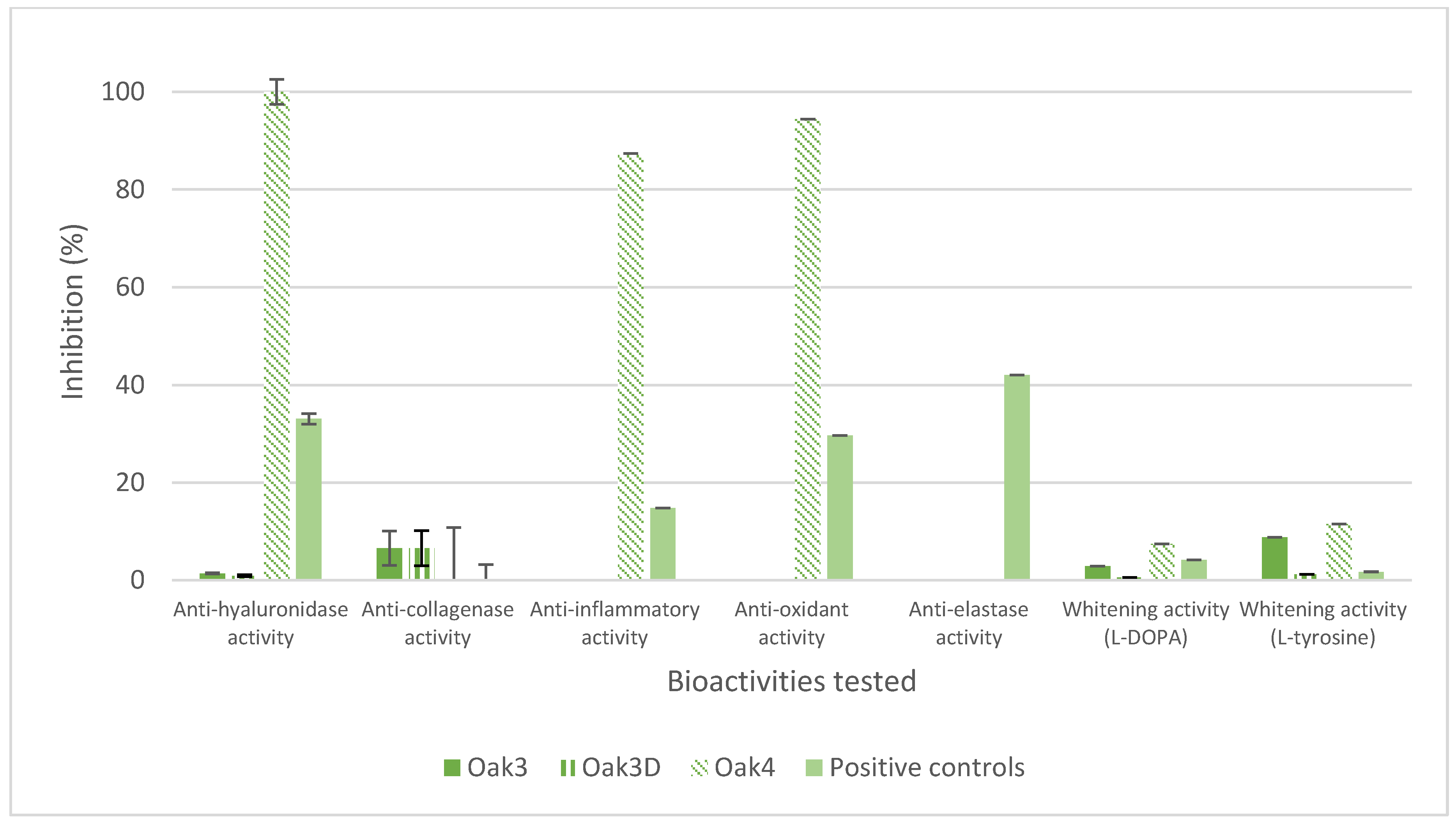
| Extraction Parameters/Bioactivities | Oak1_F1 | Oak1_F2 | Oak1_F3 | Oak1_F4 | Oak1_F5 | Positive Control | |
|---|---|---|---|---|---|---|---|
| Extraction (250 mL solvent) | cyclohexane | cyclohexane /diethyl ether 1/1 | diethyl ether | methanol | methanol/water 1/1 | ||
| Fractions’ mass | 0.60 mg | 34.00 mg | 150.50 mg | 304.00 mg | 2.34 g | ||
| Yield | 0.02% | 0.96% | 4.24% | 28.53% | 65.98% | ||
| Whitening activity | L-DOPA | n.d. | − | − | − | − | ++++ |
| L-Tyrosine | n.d. | − | − | − | − | ++++ | |
| Anti-inflammatory activity | n.d. | − | − | − | − | +++ | |
| Anti-collagenase activity | n.d. | − | − | − | − | − | |
| Anti-elastase activity | n.d. | + | +++ | ++ | + | ++++ | |
| Anti-oxidant activity | n.d. | − | − | ++++ | ++++ | − | |
| Anti-hyaluronidase activity | n.d. | − | − | ++++ | ++++ | ++ | |
| Extract/Fraction | Constituant | RI a,b HP-1/RI Lit | References |
|---|---|---|---|
| Oak1 | 6,10,14-trimethyl-2-pentadecanone | 1827/1830 | identified in Q. robur leaves essential oil [43] |
| Oak1_F3 | nonanal | 1081/1082 | identified in Q. pyrenaica and Q. petraea wood extracts [44], as well as in Q. agrifolia and Q. robur leaves essential oils [43,45] |
| dihydroactinidiolide | 1481/1475 | identified in Q. dentata leaves essential oil [46] | |
| dodecanoic acid | 1543/1547 | identified in Q. robur and Q. petraea wood extracts [47] | |
| tetradecanoic acid | 1743/1750 | identified in Q. robur and Q. petraea wood extracts [47], as well as in Q. robur leaves essential oil [43] | |
| methyl palmitate | 1903/1909 | - | |
| hexadecanoic acid | 1945/1940 | identified in Q. robur, Q. petraea and Q. alba wood extracts [47,48], as well as in Q. robur leaves essential oil [43] | |
| linoleic acid | 2105/2109 | identified in Q. robur, Q. petraea and Q. alba wood extracts [47,48] | |
| Oak1_F4 | dihydro-2.3-benzofuran | 1197/1188 | |
| 6,10,14-trimethyl-2-pentadecanone | 1827/1830 | identified in Q. robur leaves essential oil [43] | |
| Oak1_F5 | furfural | 805/805 | identified in Q. suber wood [49] |
| furfuryl acohol | 838/835 | - |
| Oak Ingredients | Color Characteristics | ||||
|---|---|---|---|---|---|
| L *(Lightness) ± SD | a *(Redness) ± SD | b *(Yellowness) ± SD | ΔE *ab ± SD | ||
| Solid ingredient | Oak2 | −32.16 ± 0.03 | 26.71 ± 0.03 | −12.76 ± 0.04 | 8.18 ± 1.79 |
| Oak2D | −26.73 ± 0.96 | 28.42 ± 0.09 | −6.89 ± 1.56 | ||
| Liquid ingredient | Oak3 | −29.92 ± 0.19 | 27.06 ± 0.03 | −13.84 ± 0.04 | 0.58 ± 0.06 |
| Oak3D | −30.42 ± 0.13 | 27.01 ± 0.03 | −13.55 ± 0.05 | ||
| Oak4 | −29.92 ± 0.19 | 27.06 ± 0.03 | −13.84 ± 0.04 | 1.22 ± 0.14 | |
| Oak4D | −30.99 ± 0.05 | 27.17 ± 0.04 | −13.27 ± 0.06 | ||
| INCI a Name | Quantity (%) |
|---|---|
| Aqua | q.s. b 100 |
| Glycerin | 1.00 |
| Stearic acid (and) Palmitic acid | 3.00 |
| Argania spinosa seed oil | 5.00 |
| Helianthus annuus hybrid oil | 5.00 |
| Sorbitan stearate | 2.90 |
| Polyglyceryl-4 laurate/Succinate (and) Aqua | 2.65 |
| Q. pubescens extract | 0.10–1.00 |
| Sodium benzoate (and) Potassium sorbate | 0.80 |
| Citric acid | 0.40 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Plainfossé, H.; Burger, P.; Azoulay, S.; Landreau, A.; Verger-Dubois, G.; Fernandez, X. Development of a Natural Anti-Age Ingredient Based on Quercus pubescens Willd. Leaves Extract—A Case Study. Cosmetics 2018, 5, 15. https://doi.org/10.3390/cosmetics5010015
Plainfossé H, Burger P, Azoulay S, Landreau A, Verger-Dubois G, Fernandez X. Development of a Natural Anti-Age Ingredient Based on Quercus pubescens Willd. Leaves Extract—A Case Study. Cosmetics. 2018; 5(1):15. https://doi.org/10.3390/cosmetics5010015
Chicago/Turabian StylePlainfossé, Hortense, Pauline Burger, Stéphane Azoulay, Anne Landreau, Grégory Verger-Dubois, and Xavier Fernandez. 2018. "Development of a Natural Anti-Age Ingredient Based on Quercus pubescens Willd. Leaves Extract—A Case Study" Cosmetics 5, no. 1: 15. https://doi.org/10.3390/cosmetics5010015
APA StylePlainfossé, H., Burger, P., Azoulay, S., Landreau, A., Verger-Dubois, G., & Fernandez, X. (2018). Development of a Natural Anti-Age Ingredient Based on Quercus pubescens Willd. Leaves Extract—A Case Study. Cosmetics, 5(1), 15. https://doi.org/10.3390/cosmetics5010015





