1. Introduction
The skin is the largest organ in the body, located at the interphase between the external and internal environment, requiring development of efficient sensory and effector capabilities to differentially react to changes in the environment [
1]. The dermis is a 1.0–3.0-mm-thick layer of the skin on the inner side of the epidermis. The dermis is partly composed of extracellular matrix components, such as collagenous fibers, elastic fibers, and proteoglycans (e.g., hyaluronic acid and chondroitin sulfate), that contribute to the elasticity and strength of the skin. The dry weight of the dermis is approximately 70% collagenous fibers and 1–2% elastic fibers, that form a 3D structural network that maintains the elasticity of the dermis. Long-term exposure to UVB and UVA radiation in sunlight denatures the collagenous fibers in the dermis decreasing skin elasticity and causing wrinkles. Research into the effects of UVB and UVA rays on the skin of hairless mice has shown that daily exposure of hairless mice to UVB and UVA rays for two months led to a permanent decrease in skin elasticity [
2,
3,
4]. However, long-term exposure to sunlight is needed for UVB and UVA rays to reduce skin elasticity in this way, which makes the evaluation of anti-photoaging agents in vivo problematic.
Currently, two standard test methods for efficacy of UVA protection are used internationally: the observation of persistent pigment darkening (PPD), which presents as immediate pigment darkening (IPD) 2–24 h after exposure in humans with skin type II to IV (in vivo) [
5,
6,
7,
8]; and the creation of an absorption curve of thin film or Transpore™ tape that has had the test material applied to it (in vitro) [
9,
10,
11]. However, there is currently no standard evaluation method for the determination of the efficacy of UVA protection that considers the reduction in skin elasticity, which is the main damaging effect caused by UVB and UVA rays. Additionally, there is currently no research that has directly elucidated the correlation between the elasticity and the effect of UV radiation on the structure and function of collagenous fibers.
As it is not possible to employ traditional experimental methods using cell cultures or diluted collagen solutions to investigate the mechanism of elasticity reduction as a result of sunlight, new methods are needed to discover the actual mechanism of the reduction in elasticity caused by UVA rays. Dr. Bissett reported that increased skin thickness and wrinkles were induced by UVB radiation from 300 to 310 nm; however, skin sagging was induced by UVA radiation from 320 to 380 nm in hairless mice [
12]. Previously studies of protective efficacy against skin damage caused by UVA rays were investigated [
13,
14,
15]. IPD and PPD induced by UVA rays were clarified that their peaked at 340 nm and decreased gradually as the wavelength increased to 400 nm [
16,
17,
18]. However, whether the wavelengths that damage collagen are the same as the wavelengths that cause IPD or PPD is not clarified yet. Therefore, current standard in vivo test methods for UVA protective efficacy that use skin PPD as an indicator, do not tell us how effective the UV protection products are for the prevention of elasticity reduction in the skin. Thus, analysis of the UV wavelengths that induce elasticity reduction in collagen fibers is of great academic significance. The collagen fiber model was exposed to ultraviolet (UV) rays, following which (1) hardness and elasticity were evaluated, (2) the spectra of wavelengths that caused an increase in hardness and decrease in elasticity were analyzed, and (3) biochemical mechanisms responsible for the increase in hardness and the decrease in elasticity were investigated.
2. Materials and Methods
2.1. Materials
An acidic collagen solution (native collagen bovine dermis; I-AC 3 mg/mL) from Koken Co., Ltd. (Tokyo, Japan) was used. Phosphate buffered saline (PBS) was prepared from PBS tablets (Takara Bio Inc., Shiga, Japan). Frozen Caucasian human breast skin disks 20 mm in diameter were purchased from KAC Co., Ltd. (Kyoto, Japan). A Capcell Pak C18 UG120 column (4.6 mm × 250 mm, C18 column; Shiseido Co., Ltd., Tokyo, Japan) was used for high-performance liquid chromatography (HPLC). All other materials were purchased from Wako Pure Pharmaceutical Industries Ltd. (Osaka, Japan).
2.2. Equipment
Dermaray 200 (Terumo Clinical Supply Co., Ltd., Tokyo, Japan) and Lightning Cure LC6 (150 W xenon lamp L8253; Hamamatsu Photonics K. K.; Hamamatsu, Japan) lamps were used as the UV sources. The long pass filter with 280 nm cutoff (A9616-03; Hamamatsu Photonics K. K.) was used in the Lightning Cure LC6. Elasticity was measured by a Cutometer MPA580 (Courage and Khazaka Electronic GmbH, Köln, Germany), and hardness was measured using a Rheo TEX SD-700 (Sun Scientific Co., Ltd., Tokyo, Japan). Edmund Optics 10-nm band-pass filters (300 nm, 310 nm, 320 nm, 330 nm, 340 nm, 350 nm, 360 nm, 370 nm, 380 nm), a high-performance liquid chromatography system (Shimadzu Corporation, Kyoto, Japan), and a high-speed centrifuge (Thermo Fisher Scientific, Waltham, MA, USA) were also used in the experiments.
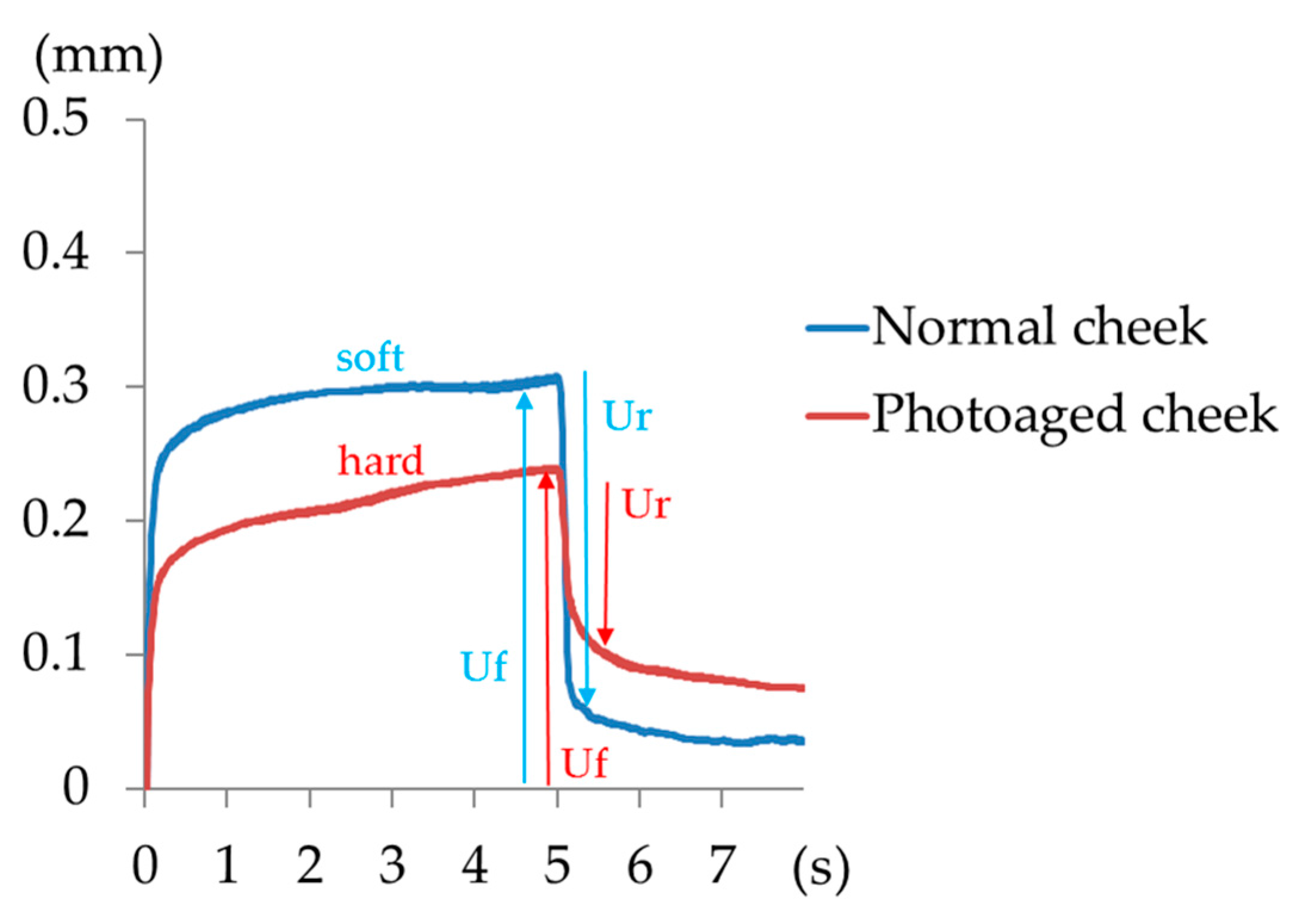
2.3. Use of the Cutometer MPA580 and Rheo TEX SD-700
The probe of the Cutometer MPA580 was pressed to the skin surface, and using negative pressure suction the decrease in the internal pressure caused a skin bulge. The change in height of the bulge was accurately measured within 0.01 mm. This device measures displacement by repeated sudden decreases in pressure that are followed by sudden releases. Skin hardness and elasticity can be calculated from the maximal extension (Uf), which is the height of the skin that is induced by a constant negative pressure, and the immediate retraction (Ur) of the skin when the pressure is released. The smaller the ratio of the immediate retraction to the maximal extension (Ur/Uf) of the sample, the lower is the biological elasticity. Typical curves of normal skin and photoaged skin, as measured by the Cutometer MPA580, are shown in
Figure 1. A difference between the normal skin and photoaged skin is observed (
Figure 1). Cheek elasticity (Ur/Uf) decreases with age. Hardness can be measured using the Rheo TEX SD-700 by increasing the load on the measurement probe, once it reaches a fixed depth.
2.4. Exposure to UVB and UVA Lamps
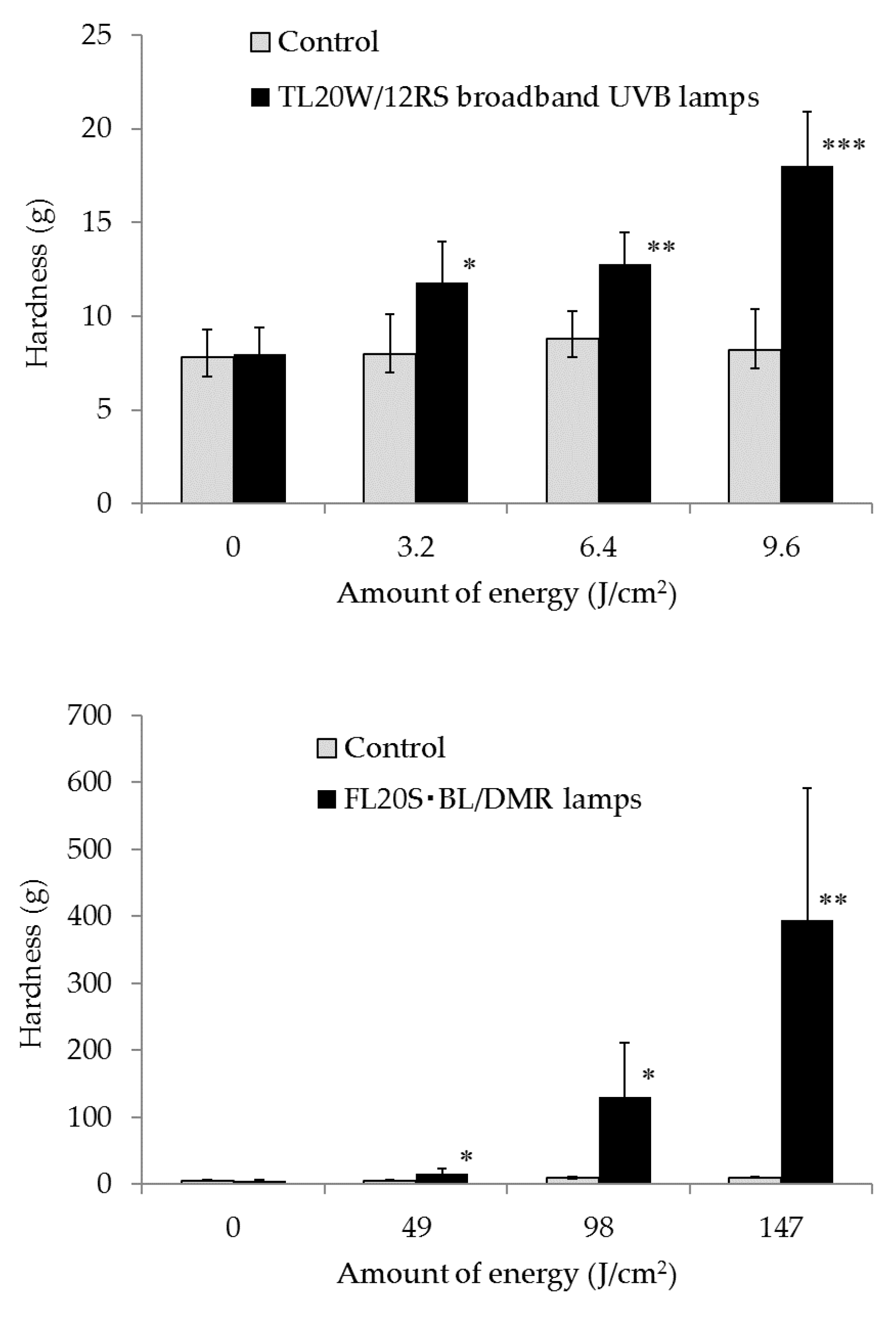
Five different TL20W/12RS broadband UVB lamps (290–320 nm; peak, 305 nm; strength at 305 nm: 0.53 mW/cm
2) and 10 FL20S BL/DMR lamps (320–400 nm; peak, 365 nm; strength at 365 nm: 8.20 mW/cm
2) were used in the Dermaray 200. The collagen gels were exposed to UVB and UVA radiation. Unexposed collagen gels were covered with aluminum foil, and the foil-wrapped samples were then irradiated with UV rays for the same amount of time. The energy quantities in the experiments are detailed in
Table 1. These exposures approximate 2 h of midsummer sun exposure, where solar UV rays consist of 0.75 J/cm
2 UVB and 15 J/cm
2 UVA (UVA-II 2.3 J/cm
2, UVA-I 12.7 J/cm
2). Given that only 10% of the absorbed UV rays penetrate to the dermis, the dermis is estimated to be exposed to 0.075 J/cm
2 UVB and 1.5 J/cm
2 UVA (UVA-II 0.23 J/cm
2, UVA-I 1.27 J/cm
2) radiation over this period. On the assumption that a year’s worth of exposure comprises 2 h per day for 30 days in the summer, the dermis is estimated to be exposed to 2.25 J/cm
2 UVB and 45 J/cm
2 UVA radiation (UVA-II 6.9 J/cm
2, UVA-I 38.1 J/cm
2) per year. Based on the same level of exposure over 2 and 3 years, the dermis is estimated to be exposed to 4.5 J/cm
2 UVB and 90 J/cm
2 UVA radiation (UVA-II 13.8 J/cm
2, UVA-I 76.2 J/cm
2), and 6.75 J/cm
2 UVB and 135 J/cm
2 UVA radiation (UVA-II 20.7 J/cm
2, UVA-I 114.3 J/cm
2), respectively. These estimated values were used as the exposure doses.
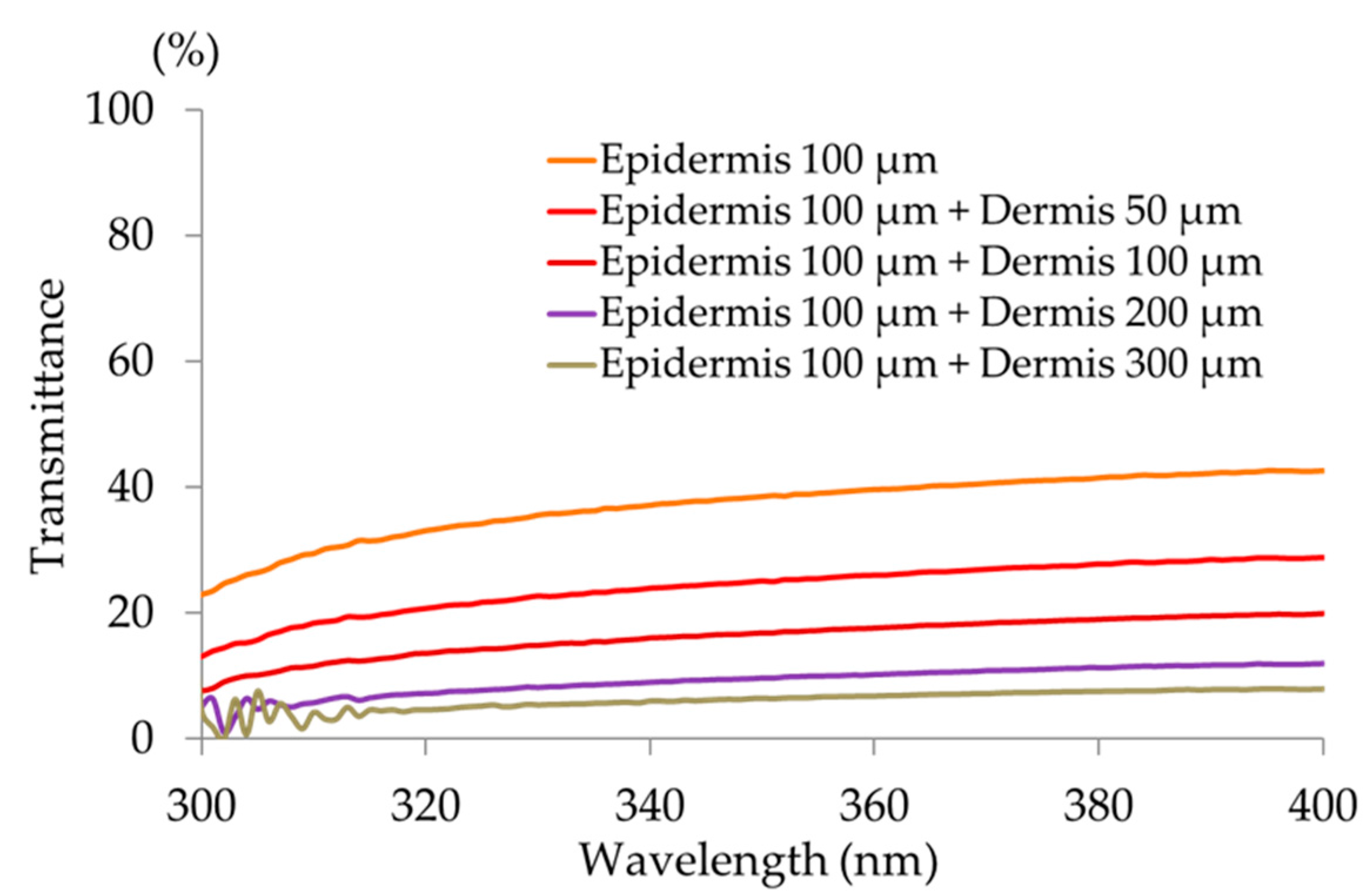
2.5. Measurement of Skin Transmittance for Different Wavelengths
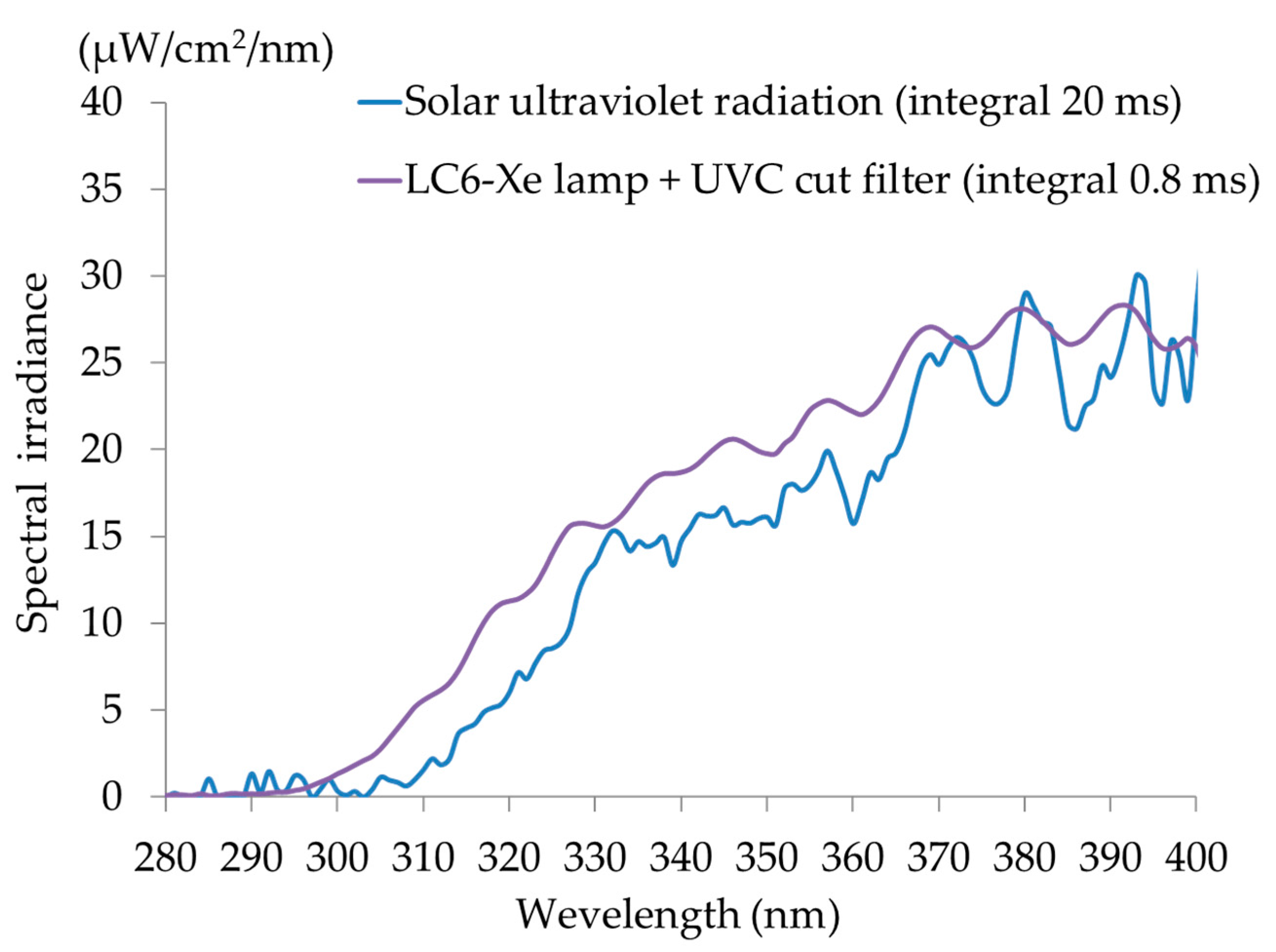
Frozen 50-μm sections of Caucasian skin were sliced from the stratum corneum side, and sections of the stratum corneum side overlapping every 50 μm were exposed to UV rays from a xenon light source (Xe lamp L8253, 150 W, Lightning Cure LC6,
Figure 2) that emits a UV spectrum similar to that of solar UV rays. The light detector of a spectroradiometer (USR-45, Ushio Co., Tokyo, Japan) contacted the opposite side of the tissue and measured the light output.
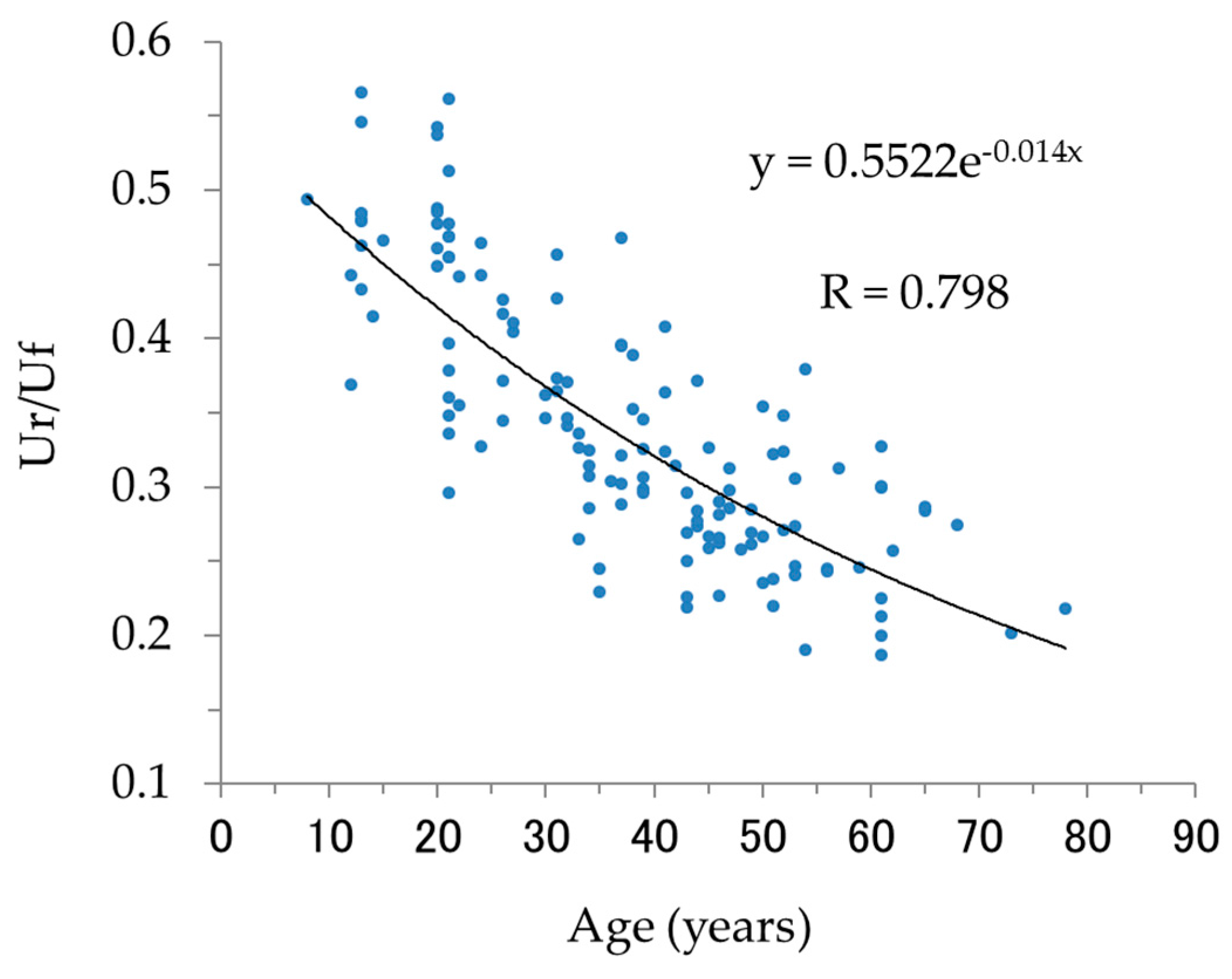
2.6. Measurement of Elasticity (Ur/Uf) in Humans of Various Ages
The biological elasticity (Ur/Uf) of the cheeks of 136 Japanese females (skin phototype II–IV, [
19], average age: 37 years), ranging in age between 8 and 78, was measured under the following conditions. After face washing, the subjects were kept at constant temperature and humidity (22 °C ± 1.1 °C, 40% ± 2%) for 20 min and biological elasticity (Ur/Uf) was measured using the Cutometer MPA580 (pressure, 380 mbar; probe aperture, 2 mm; on-time, 2 s; off-time, 3 s; repetitions, 3; pre-time, 5 s). All volunteers had given their informed consent to participate, and the study protocol was approved by the ethics committee.
2.7. Measurement of Hardness and Elasticity of Collagen Gels
Sodium bicarbonate (NaHCO3), purified water, and acidic collagen solution (0.3%) were added to 10× PBS in 35-mm dishes to produce collagen gels. The gels were exposed to 3.2 J/cm2, 6.4 J/cm2, and 9.6 J/cm2 UVB by the Dermaray 200 with the TL20W/12RS broadband UVB lamps. They were also exposed to 49 J/cm2, 98 J/cm2, and 147 J/cm2 UVA from the FL20S BL/DMR lamps. Moreover, hardness of the collagen gels was measured using the Rheo TEX SD-700 (measurement parameters: distance, 1.0 mm; weight, 15 g), and elasticity (Ur/Uf) of the collagen gels was measured using the Cutometer MPA580.
2.8. Changes in the Hardness and Elasticity of Collagen Gels at Specific UV Wavelengths
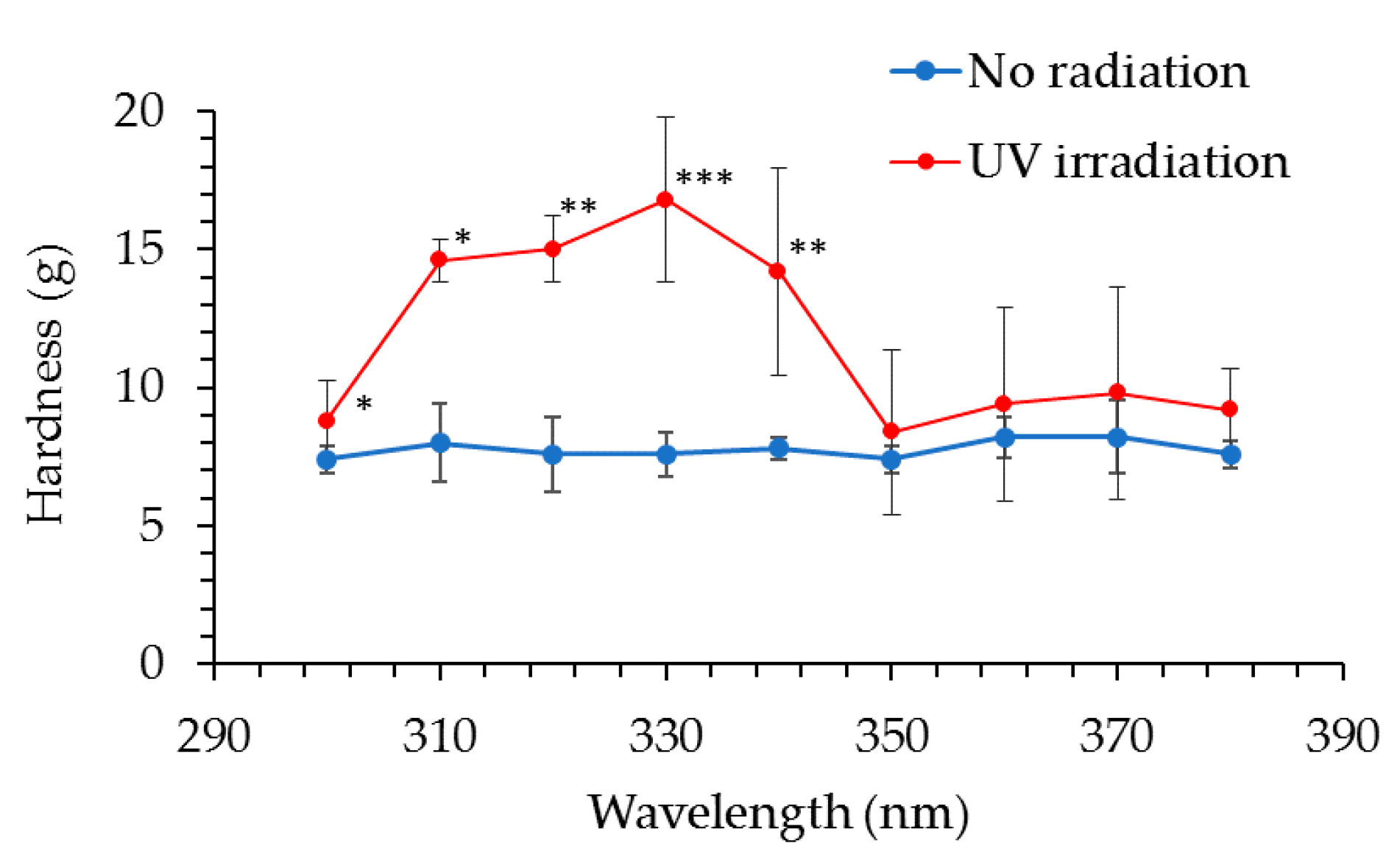
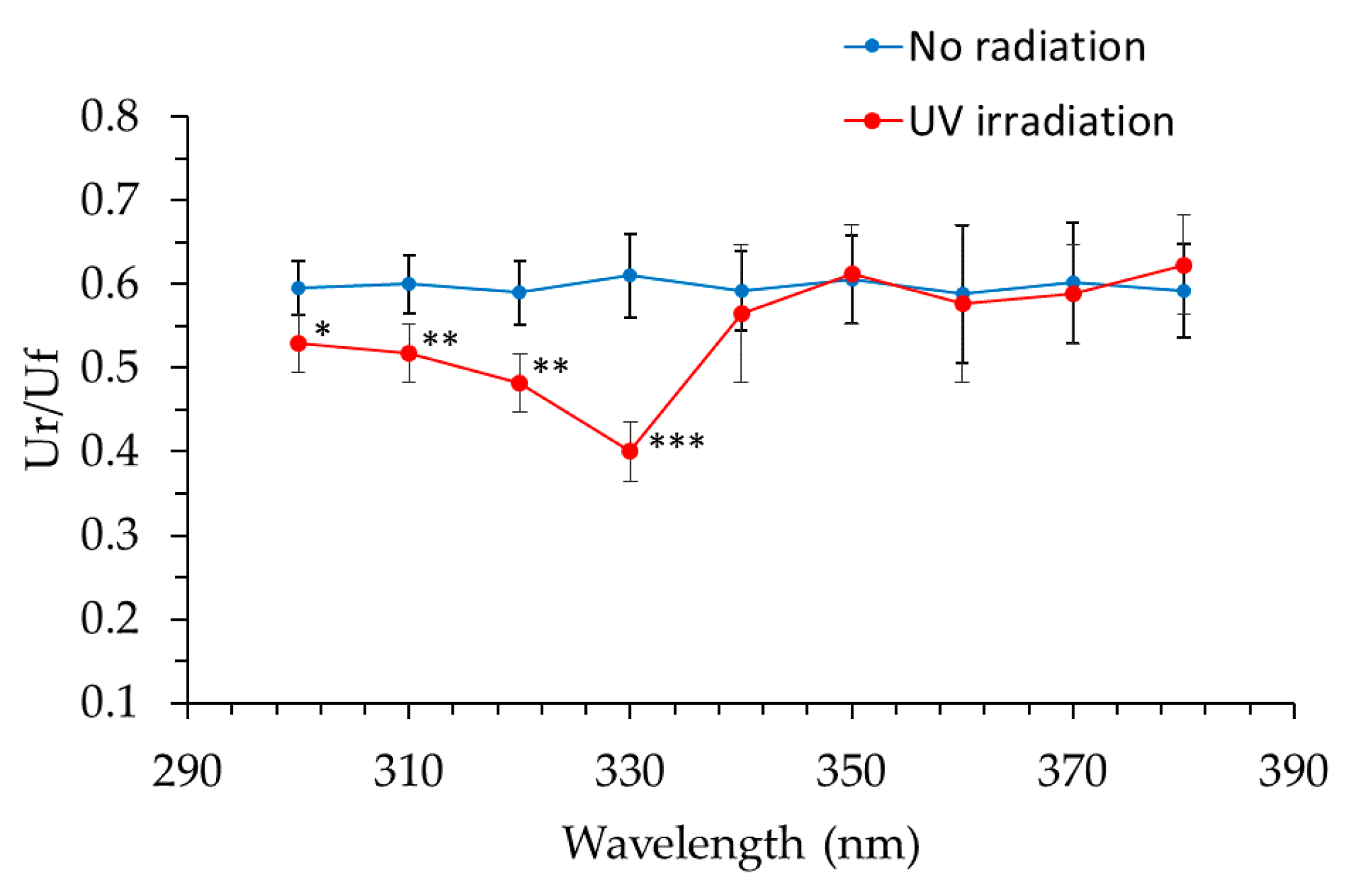
Collagen gels were exposed to UV radiation at 10-nm intervals from a xenon lamp (Lightning Cure LC6) as a light source and a 10-nm band pass filter (300 nm, 310 nm, 320 nm, 330 nm, 340 nm, 350 nm, 360 nm, 370 nm and 380 nm) at the energies shown in
Table 2. Exposure energies were determined based on the intensity of each wavelength in solar radiation. Furthermore, the exposure intensity of each band-pass filter was measured with a spectroradiometer. Hardness of the collagen gels was measured using the Rheo TEX SD-700, and biological elasticity (Ur/Uf) was measured using the Cutometer MPA580.
2.9. Measurement of Tyrosine Dimers in Collagen Gels
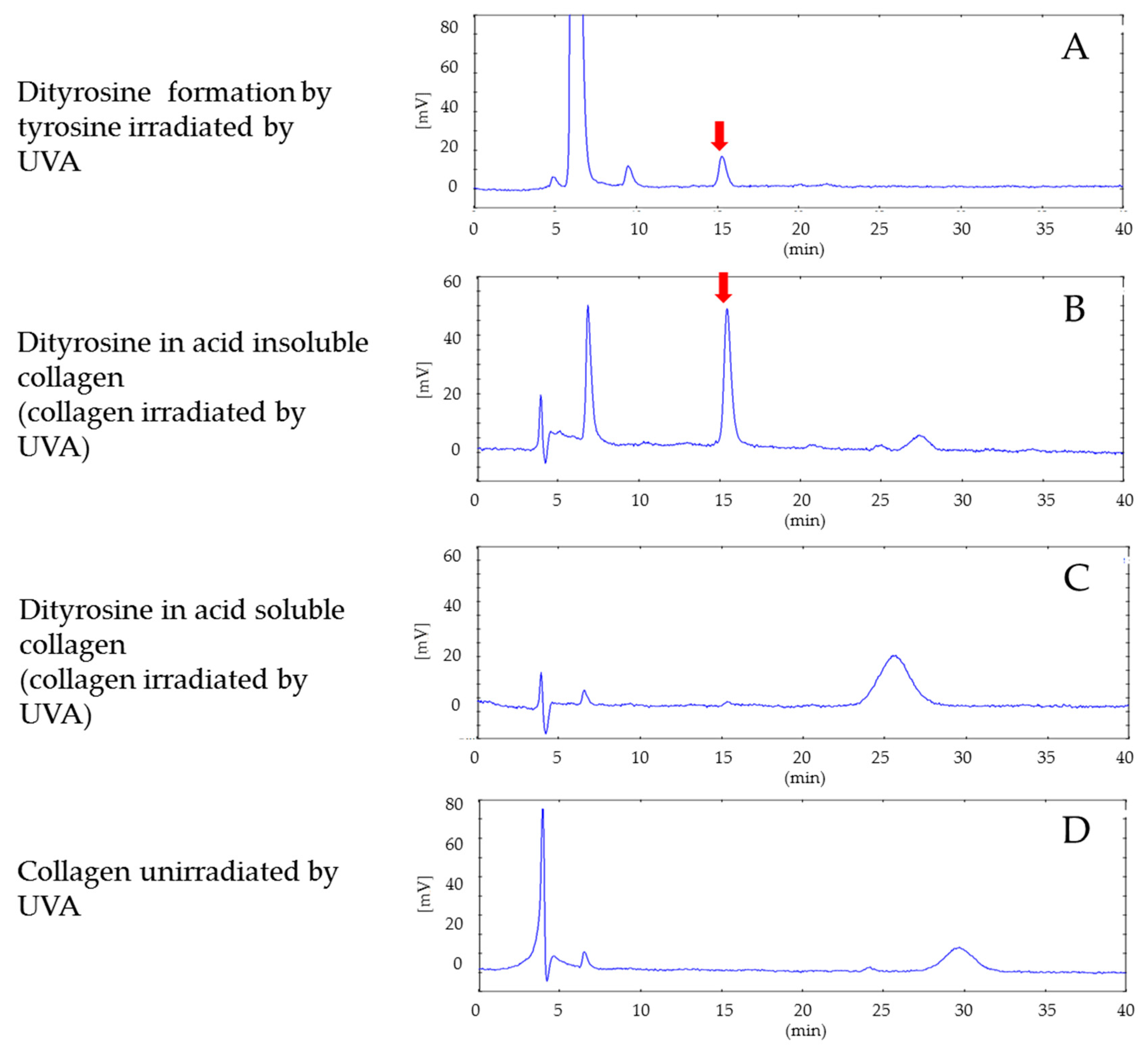
An aqueous solution of tyrosine made with L-tyrosine diluted in purified water to a concentration of 0.01 mg/mL was exposed to 330-nm UVA rays (26 J/cm
2). Tyrosine reacted with horseradish peroxidase and hydrogen peroxide solution to form dityrosines [
20]. Horseradish peroxidase (15 μg/mL), 5.25 mmol/L hydrogen peroxide, and 5 mmol/L of L-tyrosine were dissolved in 50 mL of 0.2 mol/L borate buffer (pH 9.5) and allowed to react at 37 °C. After neutralization with hydrochloric acid, dityrosine was collected by filtration through a C18 cartridge. Unreacted tyrosine, horseradish peroxidase, and hydrogen peroxide were washed away with 2 mL of purified water, and dityrosine was eluted with 5 mL of 20% methanol.
Five 330 nm UVA-exposed (26 J/cm2) collagen gel samples and five control non-exposed collagen gel samples were prepared using a 5 mm Dermapunch. The collagen samples were placed in 0.5 mol/L acetic acid for separation into acetic acid-soluble collagen and acetic acid-insoluble collagen. After the samples were concentrated by centrifugation, water was discarded and the samples were hydrolyzed with 6 mol/L hydrochloric acid. Two hundred microliters of the HCl-treated liquid were adjusted to pH 2 by the addition of 300 μL of 2 mol/L NaOH. Fluorescence intensities (Ex: 283 nm, Em: 415 nm) of the HCl hydrolysate of the UVA-exposed acetic acid-insoluble fraction of the collagen gels, the HCl hydrolysate of the UVA-exposed acetic acid-soluble fraction of the collagen gels, and the HCl hydrolysate of the non-exposed acetic acid-soluble fraction collagen gels were measured by HPLC.
HPLC was conducted on the Capcell PAK C18 UG120 column (4.6 mm × 250 mm, φ5 μm) with a flow rate of 0.8 mL/min and an injection volume of 5 μL, and fluorescence intensity of the eluents was detected (Ex: 283 nm, Em: 415 nm). The mobile phase was composed of (A) 0.5% acetic acid and (B) methanol (A:B = 24:1).
2.10. Statistical Analysis
An independent t-test for significance of the measured values was computed by Microsoft Excel.
4. Discussion
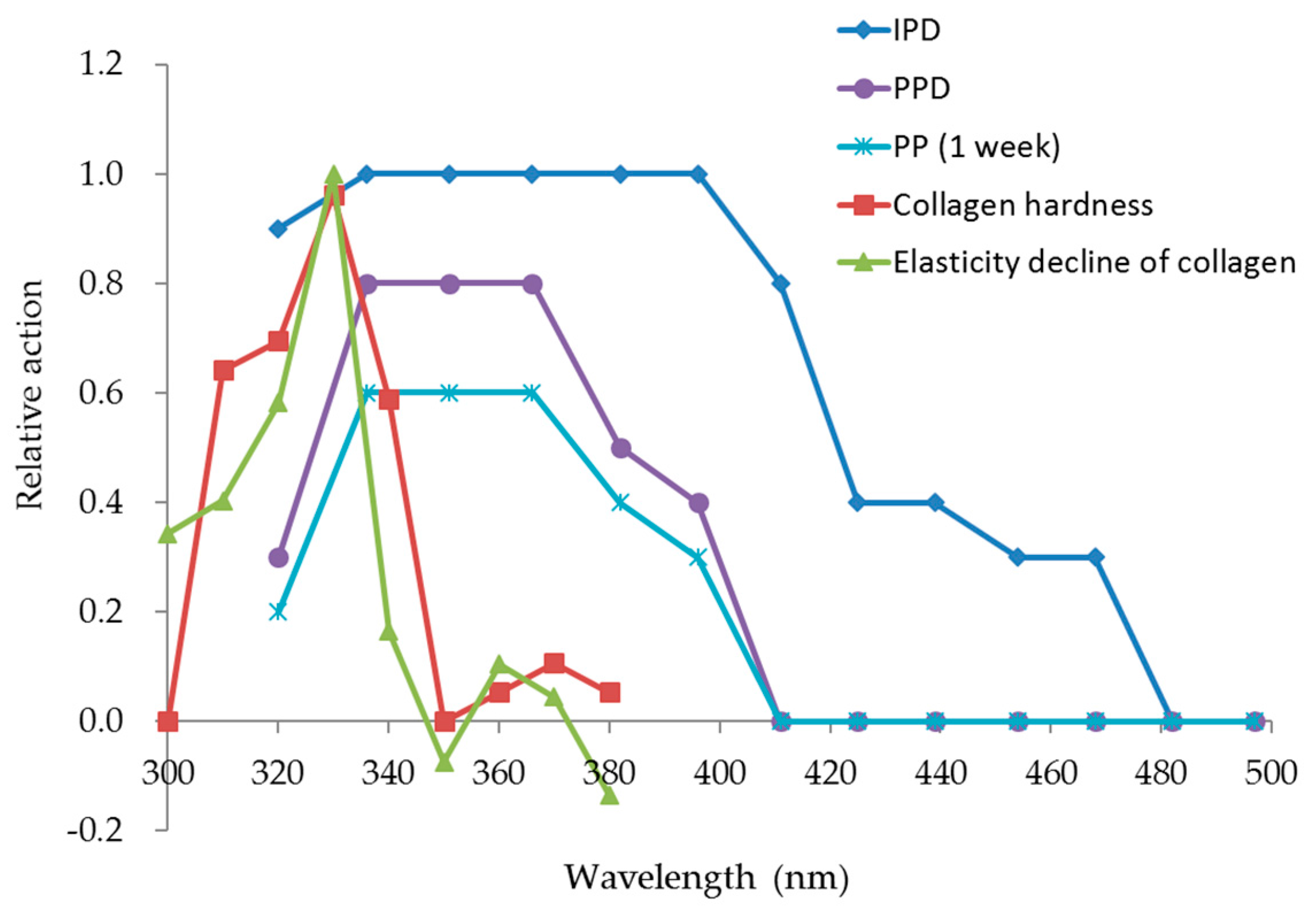
This study clearly showed that decreased cheek elasticity (Ur/Uf) was correlated with increased age in Japanese females as previously reported for Caucasian [
21]. As cheeks receive significant exposure to solar UV radiation, solar UV radiation may contribute to this correlation; in addition, the natural aging process may contribute to this correlation. The results of our investigation into the transmittance of different wavelengths through the skin specimen showed that both UVB and UVA radiation reached the papillary layer and subpapillary layer of dermis. UVA (330 nm) transmittance was calculated to be 1.60 times higher than that of UVB (310 nm) in the dermis at a depth of 200 μm, and 1.67 times higher in the dermis at a depth of 300 μm; longer wavelengths penetrate more effectively to the dermis. It is thought that the ultraviolet ray transmissivity of the dermis changes according to the difference in the thickness of stratum corneum layers. Skin transmittance of different wavelengths of UV radiation is the result of carrying out by frozen skin specimen. By comparing with the experimental result in fresh human skin, skin transmittance of UV radiation will become clearer. Our collagen fiber model was exposed to 10 times the daily exposure of UVB and UVA rays using UVB lamps (290–320 nm; peak, 305 nm) and UVA lamps (320–400 nm; peak, 365 nm), which led to hardening of the collagen and a decrease in its elasticity. We found that UV radiation between 300–340 nm (UVB and UVA-II rays) caused these effects (with the maximum effect observed at 330 nm), while radiation at the wavelengths from 350–380 nm (UVA-I rays) did not have an effect. This system of evaluation considered one portion of the skin hardening and the elasticity reduction mechanism from continued exposure to UVB and UVA-II rays in vivo. In order to validate the data obtained from collagen gels, the evaluation is going to study ex vivo skin samples.
In addition to physiological damage that accompanies aging, skin is aged by the damage caused by long-term exposure to sunlight, especially UV rays, which is called photoaging. With aging, the collagen producing ability of dermal fibroblasts decreases, which results in decreased collagen in the dermis [
22]. Conversely, in skin inflammation that arises from excessive UVB exposure, there is a transient increase in the inactivation of collagenases that degrade collagen that decreases the amount of collagen in the skin. However, it is not clear whether collagenases contribute to the breakdown of collagen in skin aging that results from chronic UVA exposure (i.e., photoaged skin that does not accompany inflammation). Instead, UVA rays are thought to exert a direct influence on the structure of collagen. This photoaging element has been shown to qualitatively alter collagenous fibers in the dermis. Solar UV rays that reach the Earth’s surface include wavelengths of 320–400 nm (UVA) and 290–320 nm (UVB). UVA rays have a longer wavelength than UVB rays, so UVA rays penetrate more deeply, as reflected by the changes observed in the collagen gels. Similarly, photoaged skin is harder and our collagen gel experiments suggested that regular exposure of the face to solar rays leads to skin hardening and reduced elasticity as a result of UV ray exposure, a process that promotes wrinkle formation. Metabolic turnover of collagen protein is slow; and the half-life of collagen in the skin is 14.8 years (95% confidence interval; 9.4–22.3 years), as calculated from the rate of racemization of aspartic acid [
23]. Collagen damage accumulates from long-term exposure to solar rays, which reduce skin elasticity. Our model indicated that photoaging may be partially responsible for this cumulative damage. UV radiation at 330 nm hardens collagen and reduces its elasticity, suggesting that solar UV rays at this wavelength is likely to cause photoaging.
The International Commission on Illumination defined the standard doses of different wavelengths of UV radiation needed to cause erythema (skin reddening). According to the commission, the UVB range (280–300 nm) has a relatively large influence on erythema. Longer wavelengths (UVA rays; 300–320 nm) become rapidly less important, and wavelengths over 320 nm have virtually no effect on erythema. If the influence of the wavelengths in the 280–300 nm range is considered to be one, the relative influence of wavelengths in the UVA-II range, 320 nm and 340 nm, is 0.01 and 0.001, respectively. However, approximately five times more solar UV radiation in the UVA-II (320–340 nm) range reaches the Earth’s surface than UV radiation in the UVB (280–320 nm) range, so the influence of UVA-II rays cannot be ignored. Consequently, the most effective way to prevent photoaging is to avoid exposure to solar UV radiation of wavelengths shorter than 340 nm. It is also reported that UVA-I and UVA-II show a difference in the regulation of the immune function of the skin. Although UVA-II causes immune suppression like UVB, UVA-I may offer partial protection against UVB-induced immune suppression in human skin [
24].
Dr. Kligman, a professor in the dermatology department of the University of Pennsylvania, used the term “photoaging skin” in 1986 to describe skin on the face, neck, and back of the hands that is repeatedly exposed to sunlight for long periods; this popularized the use of the word “photoaging” [
25]. Skin photoaging is different from skin aging that occurs naturally with the passage of time. The skin loses its texture, becomes yellowed, loses its glossiness, multiple spots appear, and there is an increase in thick and deep wrinkles due to epidermal hyperplasia. Our results suggest that this increase in thick, deep wrinkles is caused by UVB and UVA radiation of wavelengths shorter than 340 nm. UVB and UVA are the only terms used to expediently differentiate between certain wavelengths; in practice, all wavelengths of solar UV radiation shorter than 340 nm are likely to cause photodamage to collagen. Consequently, the best way to prevent photodamage to collagen is to avoid exposure to solar UV rays of wavelengths lower than 340 nm.
Intermolecular crosslinking is necessary for collagenous fibers to maintain elasticity [
26]. It is important to note that qualitative and quantitative changes in collagen fibril crosslinks occur naturally with age [
27]. Through the measurement of fluorescence, researchers have found that the amount of collagen that can be digested by pepsin increases with age but is decreased by photoaging [
28]. As collagen does not contain tryptophan, its fluorescence upon excitation by UVA irradiation mainly arises from tyrosine. Dityrosine crosslinking of collagen fibrils is known to increase with long-term exposure to UVA rays [
29]. Our measurements of tyrosine dimers in collagen gels supported this, as dityrosines were formed in collagen gels upon exposure to UVA rays [
30]. Dityrosines are not present in normal collagen; they may be rogue crosslinks formed by UVA exposure. Our experiments on UVA exposure of acetic acid-soluble and acid-insoluble collagen gels showed that tyrosine dimers (dityrosines) were formed in UVA-exposed acetic acid-insoluble gels.
Persistent pigment darkening (PPD) or persistent pigmentation (PP) as a result of UVA radiation peaked at 340 nm and gradually decreased at longer wavelengths up to 400 nm [
7,
15,
17]. However, the wavelengths that damaged collagen gels were different from the spectrum of wavelengths that cause PPD or PP, so it remains unclear how much wrinkle protection is provided by UV protection products that have been evaluated by the current standard in vivo test methods for UVA protective efficacy (which use skin PPD as an indicator). Also, the PA (Protection Grade of UVA), which is listed on cosmetic UV-protection products in Japan, is based on the PFA (Protection Factor of UVA). PFA uses PPD as an indicator. The spectra of UV rays that caused hardening and reduced elasticity in collagen gels differed from the PPD spectrum. Thus, the efficacy of PA-rated products for protection against UVA radiation cannot be used to estimate the damage to collagen, which is representative of wrinkles, caused by UV radiation in the skin, and a new method to determine the protective efficacy of sunscreen products is required. Skin sagging is mainly caused by aging, and it is reported to be also caused by UVA radiation in the 320 to 380 nm range [
12]. Elastin is a long-lasting protein like the collagen [
31]. Accumulation of abnormal elastin is thought to be involved in sagging skin. Since elastase is participating in a rejuvenation of abnormal elastin just like collagenase is participating in a rejuvenation of abnormal collagen, development of an in vitro evaluation system containing collagen, elastin, and fibroblasts will be needed in future.