Essential Oils and Their Single Compounds in Cosmetics—A Critical Review
Abstract
:1. Introduction
2. Allergy Contact Dermatitis
3. Essential Oils
- Adding of single raw materials,
- Adding cheaper essential oils of the same plant but from another country—adjuncts,
- Adding of cheap synthetic compounds (identical to natural) isolated from other oils,
- Adding of individual synthetic substances to oils and aromatic raw ingredients,
- Labelling one essential oil as another,
- Blending with less expensive essential oils of the same plant taken from a different part of the plant.
3.1. Selected Essential Oils in Cosmetic Products
3.1.1. Immortelle Essential Oil
3.1.2. Lavender Essential Oil
- Lavender essential oil, the classic lavender, is distilled from the flowers of L. angustifolia. It has a sweet floral aroma and contains a high percentage of esters, mostly linalyl acetate. It does not contain camphor which distinguishes it from other lavender varieties. The oil is often used for its anti-inflammatory, calming, headache relieving, sedative and skin healing properties. One of the rarely known effects and qualities of L. angustifolia oil is its ability to relieve menstrual pain [29,30].
- Spike lavender essential oil is obtained from flowers of L. latifolia Medik. This species of lavender contains high percentage of 1,8-cineol and camphor and therefore has a strong camphoraceous odour. Due to these components, spike lavender oil is recommended for skin damages (cuts, burns, stings), as a pain reliever, for headache treatment and for its antimicrobial properties. It has anti-bacterial, antiviral, antimycotic, anti-inflammatory and nourishing properties and is one of most common used and best investigated oil in aromatherapy. It is used for relaxing and stress relief, for nose and throat infections, for skin care, in wound treatments and for stomach problems. It has a pleasant aroma. Lavender oil is also commonly used in pharmaceutical products and as a fragrance ingredient in soaps, cosmetics and perfumes [31].
3.1.3. German Chamomile Oil
3.1.4. Neroli Essential Oil
3.1.5. Peppermint Essential Oil
3.1.6. Rosemary Essential Oil
3.1.7. Rose Oil
3.1.8. Tea Tree Oil
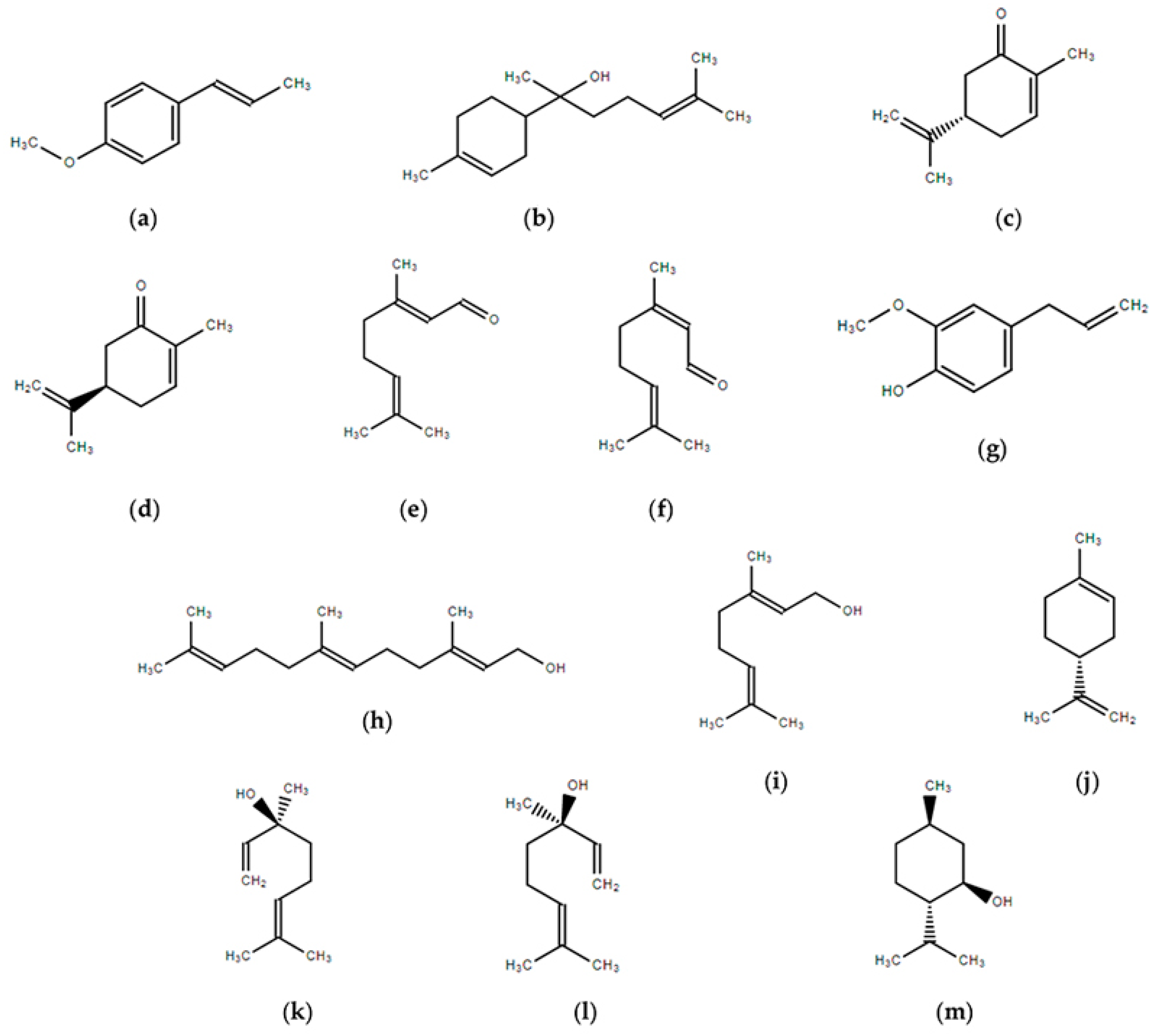
4. Individual Fragrances
4.1. Anethole
4.2. Bisabolol
4.3. Carvone
4.4. Citral
4.5. Eugenol
4.6. Farnesol
4.7. Geraniol
4.8. Limonene
4.9. Linalool
4.10. Menthol
5. Conclusions
Author Contributions
Conflicts of Interest
References
- De Groot, A.C.; Schmidt, E. Essential Oils: Contact Allergy and Chemical Composition; CRC Press: Boca Raton, FL, USA, 2016; p. 1058. [Google Scholar]
- Available online: http://eurlex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2009:342:0059:0209:en:PDF (accessed on 20 October 2017).
- Sticher, O.; Heilmann, J.; Zündorf, I. Hänsel & Sticher Pharmakognosie-Phytopharmazie, 10th ed.; Wissenschaftliche Verlagsgesellschaft Press: Stuttgart, Germany, 2015; p. 673. [Google Scholar]
- Friedl, S.M.; Heuberger, E.; Oedendorfer, K.; Kitzer, S.; Jaganjac, L.; Stappen, I.; Reznicek, G. Quantifiaction of 1,8-cineole in human blood and plasma and the impact of liner choice in head-space chromatography. Curr. Bioact. Comp. 2015, 11, 49–55. [Google Scholar] [CrossRef]
- Montenegro, L.; Pasquinucci, L.; Zappala, A.; Chiechio, S.; Turnaturi, R.; Parenti, C. Rosemary essential oil-loaded lipid nanoparticles: In vivo topical activity from gel vehicles. Pharmaceutics 2017, 9, 48. [Google Scholar] [CrossRef] [PubMed]
- Orchard, A.; Sandasi, M.; Kamatou, G.; Viljoen, N.; van Vuuren, S. The in vitro antimicrobial activity and chemometric modelling of 59 commercial essential oils against pathogens of dermatological relevance. Chem. Biodivers. 2017, 14, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Available online: http://www.ifraorg.org (accessed on 14 October 2017).
- Oakley, A.; Post, R. Allergic Contact Dermatitis. Available online: https://www.dermnetnz.org/topics/allergic-contact-dermatitis (accessed on 12 September 2017).
- Kaddu, S.; Kerl, H.; Wolf, P. Accidental bullous phototoxic reactions to Bergamot aromatherapy oil. J. Am. Acad. Dermatol. 2001, 45, 458–461. [Google Scholar] [CrossRef] [PubMed]
- Sticher, O.; Heilmann, J.; Zündorf, I. Hänsel/Sticher Pharmakognosie-Phytopharmazie, 10th ed.; Wissenschaftliche Verlagsgesellschaft Press: Stuttgart, Germany, 2015; pp. 666–667. [Google Scholar]
- Sticher, O.; Heilmann, J.; Zündorf, I. Hänsel/Sticher Pharmakognosie-Phytopharmazie, 10th ed.; Wissenschaftliche Verlagsgesellschaft Press: Stuttgart, Germany, 2015; p. 664. ISBN 978-3-8047-3144-8. [Google Scholar]
- De Groot, A.C.; Schmidt, E. Part II: General Aspects. Dermatitis 2016, 27, 43–49. [Google Scholar] [CrossRef] [PubMed]
- De Groot, A.C.; Schmidt, E. Essential Oils: Contact Allergy and Chemical Composition; CRC Press: Boca Raton, FL, USA, 2016; p. 17. [Google Scholar]
- Mestri, S. Adulteration of Essential Oils and Detection Techniques. Available online: https://www.linkedin.com/pulse/adulteration-essential-oils-detection-techniques-dr-sudhi-mestri (accessed on 28 August 2017).
- European Pharmacopoeia. Essential Oils-Aetherolea, 9th ed.; Verlag Österreich, Press: Vienna, Austria, 2016; p. 1241. [Google Scholar]
- European Directorate for the Quality of Medicines & HealthCare of the Council of Europe. Guidance on Essential Oils in Cosmetic Products. Available online: https://www.edqm.eu/en/guidance-essential-oils-cosmetic-products (accessed on 5 September 2017).
- The European Communities. Labelling of ingredients in cosmetics Directive 76/768/EEC Update February 2008. Available online: http://ec.europa.eu/consumers/sectors/cosmetics/files/doc/guide_labelling200802_en.pf (accessed on 10 September 2017).
- Bennike, N.; Oturai, N.; Müller, S.; Kirkeby, C.; Jørgensen, C.; Christensen, A.; Zachariae, C.; Johansen, J. Fragrance contact allergens in 5588 cosmetic products identified through a novel smartphone application. J. Eur. Acad. Dermatol. Venereol. 2017. [Google Scholar] [CrossRef]
- Carvalho, R.; Maio, P.; Amaro, C.; Santos, R.; Cardoso, J. Hydroxyisohexyl 3-cyclohexene carboxaldehyde (Lyral®) as allergen: Experience from a contact dermatitis unit. Cutan. Ocul. Toxicol. 2011, 30, 249–250. [Google Scholar] [CrossRef] [PubMed]
- Heisterberg, M.V.; Torkil, M.; Johansen, D.J. Contact allergy to the 26 specific fragrance ingredients to be declared on cosmetic products in accordance with the EU Cosmetics Directive. Contact Dermat. 2011, 65, 266–275. [Google Scholar] [CrossRef] [PubMed]
- Lysdal, S.H.; Johansen, J.D. Fragrance contact allergic patients. Strategies for use of cosmetic products and perceived impact on life situation. Contact Dermat. 2009, 61, 320–324. [Google Scholar] [CrossRef] [PubMed]
- Kladar, N.V.; Anačkov, G.T.; Rat, M.M.; Srđenović, B.U.; Grujić, N.N.; Šefer, E.I.; Božin, B.N. Biochemical characterization of Helichrysum italicum (Roth) G.Don subsp. italicum (Asteraceae) from Montenegro: Phytochemical screening, chemotaxonomy and antioxidant properties. Chem. Biodivers. 2015, 12, 419–431. [Google Scholar] [PubMed]
- Chinou, I.B.; Roussis, V.; Perdetzolou, D.; Loukis, A. Chemical and biological studies on two Helichrysum species of Greek origin. Planta Med. 1996, 62, 377–379. [Google Scholar] [CrossRef] [PubMed]
- Morone-Fortunato, I.; Montemurro, C.; Ruta, C.; Perrini, R.; Sabetta, W.; Blanco, A.; Lorusso, E.; Avato, P. Essential oils, genetic relationships and in vitro establishment of Helichrysum italicum (Roth) G. Don ssp. italicum from wild Mediterranean germplasm. Ind. Crops Prod. 2010, 32, 639–649. [Google Scholar] [CrossRef]
- Viegas, D.A.; Palmeira-de-Oliveira, A.; Salgueiro, L.; Martinez-de-Oliveira, J.; Palmeira-de-Oliveira, R. Helichrysum italicum: From traditional use to scientific data. J. Ethnopharmacol. 2014, 151, 54–65. [Google Scholar] [CrossRef] [PubMed]
- Nostro, A.; Bisignano, G.; Angela, C.M.; Crisafi, G.; Paola, G.M.; Alonzo, V. Effects of Helichrysum italicum extract on growth and enzymatic activity of Staphylococcus aureus. Int. J. Antimicrob. Agents 2001, 17, 517–520. [Google Scholar] [CrossRef]
- Bertoli, A.; Conti, B.; Mazzoni, V.; Meini, L.; Pistelli, L. Volatile chemical composition and bioactivity of six essential oils against the stored food insect Sitophilus zeamais Motsch. (Coleoptera Dryophthoridae). Nat. Prod. Res. 2012, 26, 2063–2071. [Google Scholar] [PubMed]
- ISO 3515 Essential Oil of Lavander. Available online: http://www.iso.org (accessed on 13 August 2017).
- Bakhtshirin, F.; Abedi, S.; Yusefi Zoj, P.; Razmjooee, D. The effect of aromatherapy massage with lavender oil on severity of primary dysmenorrhea in Arsanjan students. Iran. J. Nurs. Midwifery 2015, 20, 156–160. [Google Scholar]
- Nikjou, R.; Kazemzadeh, R.; Rostamnegad, M.; Moshfegi, S.; Karimollahi, M.; Salehi, H. The effect of lavender aromatherapy on the pain severity of primary dysmenorrhea: A triple-blind randomized clinical trial. Ann. Med. Health Sci. Res. 2016, 6, 211–215. [Google Scholar] [PubMed]
- Steflitsch, W.; Wolz, D.; Buchbauer, G. Aromatherapie in Wissenschaft und Praxis, 1st ed.; Stadelmann Verlag Press: Wiggensbach, Germany, 2013; pp. 204–207. [Google Scholar]
- Sugiura, M.; Hayakawa, R.; Kato, Y.; Sugiura, K.; Hashimoto, R. Results of patch testing with lavender oil in Japan. Contact Dermat. 2000, 43, 157–160. [Google Scholar] [CrossRef]
- Goiriz, R.; Delgado, J.Y.; Sánchez, P.J.; García, D.A. Photoallergic contact dermatitis from lavender oil in topical ketoprofen. Contact Dermat. 2007, 57, 381–382. [Google Scholar] [CrossRef] [PubMed]
- De Groot, A.C.; Schmidt, E. Essential Oils, Part V: Peppermint Oil, Lavender Oil and Lemongrass Oil. Dermatitis 2016, 27, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Hagvall, L.; Bråred, C.J. Patch testing with main sensitizers does not detect all cases of contact allergy to oxidized lavender oil. Acta Dermato Venereol. 2016, 96, 679–683. [Google Scholar] [CrossRef] [PubMed]
- De Groot, A.C.; Schmidt, E. Essential Oils: Contact Allergy and Chemical Composition; CRC Press: Boca Raton, FL, USA, 2016; pp. 193–201. [Google Scholar]
- Sticher, O.; Hänsel, R. Hänsel/Sticher Pharmakognosie-Phytopharmazie, 9th ed.; Springer Medizin Verlag Press: Heidelberg, Germany, 2010; p. 1064. [Google Scholar]
- Lee, S.H.; Heo, Y.; Kim, Y.C. Effect of German chamomile oil application on alleviating atopic dermatitis-like immune alterations in mice. J. Vet. Sci. 2010, 11, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Charousaei, F.; Dabirian, A.; Mojab, F. Using chamomile solution or a 1% topical hydrocortisone ointment in the management of peristomal skin lesions in colostomy patients: Results of a controlled clinical study. Ostomy Wound Manag. 2011, 57, 28–36. [Google Scholar]
- Shoara, R.; Hashempur, M.H.; Ashraf, A.; Salehi, A.; Dehshahri, S.; Habibagahi, Z. Efficacy and safety of topical Matricaria chamomilla L. (chamomile) oil for knee osteoarthritis: A randomized controlled clinical trial. Complement. Ther. Clin. Pract. 2015, 21, 181–187. [Google Scholar] [PubMed]
- Khan, A.I.; Abourashed, A.E. Leung’s Encyclopedia of Common Natural Ingredients: Used in Food, Drugs and Cosmetics, 3rd ed.; John Wiley & Sons Press: New York, NY, USA, 2010; pp. 477–481. [Google Scholar]
- Ammar, A.H.; Bouajila, J.; Lebrihi, A.; Mathieu, F.; Romdhane, M.; Zagrouba, F. Chemical composition and in vitro antimicrobial and antioxidant activities of Citrus aurantium L. flowers essential oil (neroli oil). Pak. J. Biol. Sci. 2012, 21, 1034–1040. [Google Scholar]
- De Groot, A.C.; Schmidt, E. Essential Oils: Contact Allergy and Chemical Composition; CRC Press: Boca Raton, FL, USA, 2016; pp. 575–581. [Google Scholar]
- Wang, K.; Zhu, R.Z.; Rong, F.Q.; Li, Z.Y. Comprehensive two-dimensional gas chromatography–time-of-flight mass spectrometry for the analysis of volatile components in neroli essential oil. Mendeleev Commun. 2012, 22, 45–46. [Google Scholar] [CrossRef]
- Available online: https://nhrorganicoils.com/uploads/Allegens%20essential%20oils.pdf (accessed on 25 August 2017).
- Newsham, J.; Rai, S.; Williams, J.D.L. Two cases of allergic contact dermatitis to neroli oil. In Proceedings of the 91th Annual Meeting of the British Associations of the Dermatologist, London, UK, 5–7 July 2011. [Google Scholar]
- Matthieu, L.; Meuleman, L.; Van Hecke, E.; Blondeel, A.; Dezfoulian, B.; Constandt, L.; Goossens, A. Contact and photocontact allergy to ketoprofen. The Belgian experience. Contact Dermat. 2004, 50, 238–241. [Google Scholar] [CrossRef] [PubMed]
- WHO Monographs on Selected Medicinal Plants. Available online: http://apps.who.int/medicinedocs/en/d/Js4927e/ (accessed on 20 August 2017).
- Sugiura, T.; Uchida, S.; Namiki, N. Taste-masking effect of physical and organoleptic methods on peppermint-scented orally disintegrating tablet of famotidine based on suspension spray-coating method. Chem. Pharm. Bull. 2012, 60, 315–319. [Google Scholar] [CrossRef] [PubMed]
- Tran, A.; Pratt, M.; DeKoven, J. Acute allergic contact dermatitis of the lips from peppermint oil in a lip balm. Dermatitis 2010, 21, 111–115. [Google Scholar] [PubMed]
- Bourgeois, P.; Goossens, A. Allergic contact cheilitis caused by menthol in toothpaste and throat medication: A case report. Contact Dermat. 2016, 75, 113–115. [Google Scholar] [CrossRef] [PubMed]
- Nardelli, A.; Drieghe, J.; Claes, L.; Boey, L.; Goossens, A. Fragrance allergens in ‘specific’ cosmetic products. Contact Dermat. 2011, 64, 212–219. [Google Scholar] [CrossRef] [PubMed]
- Travassos, A.R.; Claes, L.; Boey, L.; Drieghe, J.; Goossens, A. Non-fragrance allergens in specific cosmetic products. Contact Dermat. 2011, 65, 276–285. [Google Scholar] [CrossRef] [PubMed]
- Tomi, K.; Kitao, M.; Konishi, N.; Murakami, H.; Matsumura, Y.; Hayashi, T. Enantioselective GC–MS analysis of volatile components from rosemary (Rosmarinus officinalis L.) essential oils and hydrosols. Biosci. Biotechnol. Biochem. 2016, 80, 840–847. [Google Scholar] [CrossRef] [PubMed]
- Abdelaziz, E.; Saadia, Z.; Mohamed, B.; Bachir, B. A study of Moroccan rosemary oils. J. Essent. Oil Res. 2000, 12, 487–495. [Google Scholar]
- Panahi, Y.; Taghizadeh, M.; Marzony, E.T.; Sahebkar, A. Rosemary oil vs Minoxidil 2% for the treatment of androgenetic alopecia: A randomized comparative trial. Skinmed 2015, 13, 15–21. [Google Scholar] [PubMed]
- De Groot, A.C.; Schmidt, E. Essential Oils: Contact Allergy and Chemical Composition; CRC Press: Boca Raton, FL, USA, 2016; p. 706. [Google Scholar]
- De Groot, A.C.; Schmidt, E. Essential Oils: Contact Allergy and Chemical Composition; CRC Press: Boca Raton, FL, USA, 2016; pp. 711–722. [Google Scholar]
- Mohamadi, M.; Shamspur, T.; Mostafavi, A. Comparison of microwave-assisted distillation and conventional hydrodistillation in the essential oil extraction of flowers Rosa damascena Mill. J. Essent. Oil Res. 2013, 25, 55–61. [Google Scholar] [CrossRef]
- Jalali-Heravi, M.; Parastar, H.; Sereshti, H. Development of a method for analysis of Iranian damask rose oil: Combination of gas chromatography-mass spectrometry with chemometric techniques. Anal. Chim. Acta 2008, 623, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Verma, R.S.; Padalia, R.C.; Chauhan, A.; Singh, A.; Yadav, A.K. Volatile constituents of essential oil and rose water of damask rose (Rosa damascena Mill.) cultivars from North Indian hills. Nat. Prod. Res. 2011, 25, 1557–1584. [Google Scholar] [CrossRef] [PubMed]
- Mohamadi, M.; Mostafavi, A.; Shamspur, T. Effect of storage on essential oil content and composition of Rosa damascena Mill. petals under different conditions. J. Essent. Oil Bear. Plant. 2013, 14, 430–441. [Google Scholar] [CrossRef]
- Boskabady, M.H.; Shafei, M.N.; Saberi, Z.; Amini, S. Pharmacological effects of Rosa damascena. Iran. J. Basic Med. Sci. 2011, 14, 295–307. [Google Scholar] [PubMed]
- Bleasel, N.; Tate, B.; Rademaker, M. Allergic contact dermatitis following exposure to essential oils. Australas. J. Dermatol. 2002, 43, 211–213. [Google Scholar]
- De Groot, A.C.; Schmidt, E. Essential Oils: Contact Allergy and Chemical Composition; CRC Press: Boca Raton, FL, USA, 2016; p. 722. [Google Scholar]
- De Groot, A.C.; Schmidt, E. Essential Oils: Contact Allergy and Chemical Composition; CRC Press: Boca Raton, FL, USA, 2016; pp. 810–816. [Google Scholar]
- Available online: https://ec.europa.eu/health/ph_risk/committees/04_sccp/docs/sccp_o_160.pdf (accessed on 17 August 2017).
- Enshaieh, S.; Jooya, A.; Siadat, A.H.; Iraji, F. The efficacy of 5% topical tea tree oil gel in mild to moderate acne vulgaris: A randomized, double-blind placebo-controlled study. Indian J. Dermatol. Venereol. Leprol. 2007, 73, 22–25. [Google Scholar] [PubMed]
- Chandrdas, D.; Jayakumar, H.L.; Chandra, M.; Katodia, L.; Sreedevi, A. Evaluation of antimicrobial efficacy of garlic, tea tree oil, cetylpyridinium chloride, chlorhexidine and ultraviolet sanitizing device in the decontamination of toothbrush. Indian J. Dent. 2014, 5, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Forrer, M.; Kulik, E.M.; Filippi, A.; Waltimo, T. The antimicrobial activity of alpha-bisabolol and tea tree oil against Solo bacterium moorei, a gram-positive bacterium associated with halitosis. Arch. Oral Biol. 2013, 58, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Richards, D.B.; Wang, G.S.; Buchanan, J.A. Pediatric tea tree oil aspiration treated with surfactant in the emergency department. Pediatr. Emerg. Care 2015, 31, 279–280. [Google Scholar] [CrossRef] [PubMed]
- Rutherford, T.; Nixon, R.; Tam, M.; Tate, B. Allergy to tea tree oil: Retrospective review of 41 cases with positive patch tests over 4.5 years. Australas. J. Dermatol. 2007, 48, 83–87. [Google Scholar] [CrossRef] [PubMed]
- Santesteban Muruzábal, R.; Hervella Garcés, M.; Larrea García, M.; Loidi Pascual, L.; Agulló Pérez, A.; Yanguas Bayona, I. Secondary effects of topical application of an essential oil. Allergic contact dermatitis due to tea tree oil. An. Sist. Saint. Navar. 2015, 38, 163–167. [Google Scholar] [CrossRef]
- De Groot, A.C.; Schmidt, E. Tea tree oil: Contact allergy and chemical composition. Contact Dermat. 2016, 75, 129–143. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.A.; McLean, M.K.; Slater, M.R. Concentrated tea tree oil toxicosis in dogs and cats: 443 cases (2002–2012). J. Am. Vet. Med. Assoc. 2014, 244, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Available online: http://pubchem.ncbi.nlm.nih.gov/compound/trans-Anethole (accessed on 15 September 2017).
- Chemotechnique Diagnostics. Available online: http://www.chemotechnique.se/get_pdf.php?l=en&p=18 (accessed on 16 September 2017).
- Poon, T.S.C.; Freeman, S. Cheilitis caused by contact allergy to anethole in spearmint flavoured toothpaste. Australas. J. Dermatol. 2006, 47, 300–301. [Google Scholar] [CrossRef] [PubMed]
- Horst, N.; Leysen, J.; Mellaerts, T.; Lambert, J.; Aerts, O. Allergic contact cheilitis from anethole-containing toothpastes: A practical solution. J. Eur. Acad. Dermatol. Venereol. 2017, 31, 374–375. [Google Scholar] [CrossRef] [PubMed]
- Aschenbeck, K.A.; Hylwa, S.A. Brushing your way to allergic contact dermatitis: Anethole Allergy. Dermatitis 2017, 28, 219–220. [Google Scholar] [CrossRef] [PubMed]
- Kamatou, G.P.; Viljoen, A. A review of the application and pharmacological properties of α-bisabolol and α-bisabolol-rich oils. J. Am. Oil Chem. Soc. 2009, 87, 1–7. [Google Scholar] [CrossRef]
- Jacob, S.E.; Matiz, C.; Herro, E.M. Compositae-associated allergic Contact Dermatitis from bisabolol. Dermatitis 2011, 22, 102–105. [Google Scholar] [PubMed]
- Sarre, M.E.; Guérin-Moreau, M.; Lepoittevin, J.P.; Martin, L.; Avenel-Audran, M. Allergic contact cheilitis caused by polysilicone-15 (Parsol® SLX) in a lipcare balm. Contact Dermat. 2014, 70, 119–121. [Google Scholar] [CrossRef] [PubMed]
- Schaller, C.P. Available online: https://www.employees.csbsju.edu/cschaller/Principles%20Chem/stereochem/stereo_enantiomerB.htm (accessed on 27 August 2017).
- Available online: http://www.pubchem.ncbi.nlm.nih.gov/compound/carvone (accessed on 25 August 2017).
- Hansson, C.; Bergendorff, O.; Wallengren, J. Contact urticaria caused by carvone in toothpaste. Contact Dermat. 2011, 65, 362–364. [Google Scholar] [CrossRef] [PubMed]
- Hausen, B.M. Toothpaste allergy. Dtsch. Med. Wochenschr. 1984, 109, 300–302. [Google Scholar] [CrossRef] [PubMed]
- Corazza, M.; Levratti, A.; Virgili, A. Allergic contact cheilitis due to carvone in toothpastes. Contact Dermat. 2002, 46, 366–367. [Google Scholar] [CrossRef]
- Kroona, L.; Warfvinge, G.; Isaksson, M.; Ahlgren, C.; Dahlin, J.; Sörensen, Ö.; Bruze, M. Quantification of l-carvone in toothpastes available on the Swedish market. Contact Dermat. 2017, 77, 224–230. [Google Scholar] [CrossRef] [PubMed]
- Ahlgren, C.; Axéll, T.; Möller, H.; Isaksson, M.; Liedholm, R.; Bruze, M. Contact allergies to potential allergens in patients with oral lichen lesions. Clin. Oral. Investig. 2014, 18, 227–237. [Google Scholar] [CrossRef] [PubMed]
- Brown, W.H. Available online: http://www.britannica.com/science/citral (accessed on 20 September 2017).
- De Mozzi, P.; Johnston, G.A. An outbreak of allergic contact dermatitis caused by citral in beauticians working in a health spa. Contact Dermat. 2014, 70, 377–379. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://pubchem.ncbi.nlm.nih.gov/compound/eugenol (accessed on 26 July 2017).
- Schmidt, E.; Jirovetz, L.; Wlcek, K.; Buchbauer, G.; Gochev, V.; Girova, T.; Stoyanova, A.; Geissler, M. Antifungal activity of eugenol and various eugenol-containing essential oils against 38 clinical isolates of Candida albicans. J. Essent. Oil Bear. Plant. 2013, 10, 421–429. [Google Scholar] [CrossRef]
- López-Sáez, M.P.; Carrillo, P.; Huertas, A.J.; Fernández-Nieto, M.; López, J.D. Occupational asthma and dermatitis induced by eugenol in a cleaner. J. Investig. Allergol. Clin. Immunol. 2015, 25, 64–65. [Google Scholar] [PubMed]
- Behzad, M.; Michl, C.; Arweiler, N.; Pfützner, W. Lichenoid contact reaction to eugenol presenting as oral lichen planus. Allergo J. Int. 2014, 23, 242–245. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://pubchem.ncbi.nlm.nih.gov/compound/farnesol (accessed on 2 August 2017).
- Pónyai, G.; Németh, I.; Altmayer, A.; Nagy, G.; Irinyi, B.; Battyáni, Z.; Temesvári, E. Patch tests with Fragrance Mix II and its components. Dermatitis 2012, 23, 71–74. [Google Scholar] [CrossRef] [PubMed]
- Krautheim, A.; Uter, W.; Frosch, P.; Schnuch, A.; Geier, J. Patch testing with Fragrance Mix II: Results of the IVDK 2005–2008. Contact Dermat. 2010, 63, 262–269. [Google Scholar] [CrossRef] [PubMed]
- Mowitz, M.; Svedman, C.; Zimerson, E.; Isaksson, M.; Pontén, A.; Bruze, M. Simultaneous patch testing with fragrance mix I, fragrance mix II and their ingredients in southern Sweden between 2009 and 2015. Contact Dermat. 2017, 77, 280–287. [Google Scholar] [CrossRef] [PubMed]
- Available online: http://pubchem.ncbi.nlm.nih.gov/compound/geraniol (accessed on 20 September 2017).
- Hagvall, L.; Karlberg, A.T.; Christensson, J.B. Contact allergy to air-exposed geraniol: Clinical observations and report of 14 cases. Contact Dermat. 2012, 67, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Hagvall, L.; Christensson, J.B. Cross-reactivity between citral and geraniol-can it be attributed to oxidized geraniol. Contact Dermat. 2014, 71, 280–288. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://pubchem.ncbi.nlm.nih.gov/compound/limonene (accessed on 16 August 2017).
- Swerdlin, A.; Rainey, D.; Storrs, F.J. Fragrance mix reactions and lime allergic contact dermatitis. Dermatitis 2010, 21, 214–216. [Google Scholar] [PubMed]
- Deza, G.; García-Bravo, B.; Silvestre, J.F.; Pastor-Nieto, M.A.; González-Pérez, R.; Heras-Mendaza, F.; Mercader, P.; Fernández-Redondo, V.; Niklasson, B.; Giménez-Arnau, A.M.; et al. Contact sensitization to limonene and linalool hydroperoxides in Spain: A GEIDAC prospective study. Contact Dermat. 2017, 76, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Pesonen, M.; Suomela, S.; Kuuliala, O.; Henriks-Eckerman, M.L.; Aalto-Korte, K. Occupational contact dermatitis caused by d-Limonene. Contact Dermat. 2014, 71, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Christensson, J.B.; Andersen, K.E.; Bruze, M.; Johansen, J.D.; Garcia-Bravo, B.; Gimenez-Arnau, A.; Goh, C.L.; Nixon, R.; White, I.R. Positive patch test reactions to oxidized limonene: Exposure and relevance. Contact Dermat. 2014, 71, 264–272. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://pubchem.ncbi.nlm.nih.gov/compound/linalool (accessed on 18 August 2017).
- Bennike, N.H.; Zachariae, C.; Johansen, J.D. Non-mix fragrances are top sensitizers in consecutive dermatitis patients—A cross-sectional study of the 26 EU-labelled fragrance allergens. Contact Dermat. 2017, 77, 270–279. [Google Scholar] [CrossRef] [PubMed]
- Millelid, R.; Isaksson, M. Allergic contact dermatitis caused by exfoliating socks. Contact Dermat. 2017, 76, 59–60. [Google Scholar] [CrossRef] [PubMed]
- Sticher, O.; Heilmann, J.; Zündorf, I. Hänsel/Sticher Pharmakognosie-Phytopharmazie, 10th ed.; Wissenschaftliche Verlagsgesellschaft Press: Stuttgart, Germany, 2015; pp. 730–731. [Google Scholar]
- Bourgeois, P.; Goossens, A. Allergic contact cheilitis caused by menthol in toothpaste and throat medication: A case report. Contact Dermat. 2016, 75, 113–115. [Google Scholar] [CrossRef] [PubMed]
- Bayat, R.; Borici-Mazi, R. A case of anaphylaxis to peppermint. Allergy Asthma Clin. Immunol. 2014, 10, 6. [Google Scholar] [CrossRef] [PubMed]
- Arikan-Ayyildiz, Z.; Akgül, F.; Yilmaz, S.; Ozdemir, D.; Uzuner, N. Anaphylaxis in an infant caused by menthol-containing cologne. Allergol. Immunopathol. 2012, 40, 198. [Google Scholar] [CrossRef] [PubMed]
- De Groot, A.C.; Schmidt, E. Essential Oils: Contact Allergy and Chemical Composition; CRC Press: Boca Raton, FL, USA, 2016; p. 2. [Google Scholar]
| Fragrances |
|---|
| Amylcinnamal |
| Amylcinnamyl alcohol |
| Anisyl alcohol |
| Benzyl alcohol |
| Benzyl benzoate |
| Benzyl cinnamate |
| Benzyl salicylate |
| Cinnamyl alcohol |
| Cinnamal |
| Citral |
| Citronellol |
| Coumarin |
| Eugenol |
| Farnesol |
| Geraniol |
| Hexyl cinnamicaldehyde |
| Hydroxy-citronellal |
| Hydroxy-methylpentylcyclohexenecarboxaldehyde |
| Isoeugenol |
| d-Limonene |
| Linalool |
| Methyl heptin carbonate |
| 3-Methyl-4-(2,6,6-tri-methyl-2-cyclohexen-1-yl)-3-buten-2-one |
| Oak moss and treemoss extract |
| Treemoss extract |
| 2-(4-tert-Butylbenzyl) propionaldehyde |
| Component | Concentration |
|---|---|
| Terpinen-4-ol | 30–48% |
| γ-Terpinene | 10–28% |
| 1,8-Cineol | traces-15% |
| α-Terpinene | 5–13% |
| α-Terpineol | 1.5–8% |
| p-Cymene | 0.5–8% |
| α-Pinene | 1–6% |
| Terpinolene | 1.5–5% |
| Sabinene | traces-3.5% |
| Aromadendrene | traces-3% |
| δ-Cadinene | traces-3% |
| Viridiflorene | traces-3% |
| Limonene | 0.5–1.5% |
| Globulol | traces-1% |
| Viridiflorol | traces-1% |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sarkic, A.; Stappen, I. Essential Oils and Their Single Compounds in Cosmetics—A Critical Review. Cosmetics 2018, 5, 11. https://doi.org/10.3390/cosmetics5010011
Sarkic A, Stappen I. Essential Oils and Their Single Compounds in Cosmetics—A Critical Review. Cosmetics. 2018; 5(1):11. https://doi.org/10.3390/cosmetics5010011
Chicago/Turabian StyleSarkic, Asja, and Iris Stappen. 2018. "Essential Oils and Their Single Compounds in Cosmetics—A Critical Review" Cosmetics 5, no. 1: 11. https://doi.org/10.3390/cosmetics5010011
APA StyleSarkic, A., & Stappen, I. (2018). Essential Oils and Their Single Compounds in Cosmetics—A Critical Review. Cosmetics, 5(1), 11. https://doi.org/10.3390/cosmetics5010011




