The Bioactivity Study of Active Compounds in Wolffia globosa Extract for an Alternative Source of Bioactive Substances
Abstract
:1. Introduction
2. Materials and Methods
2.1. Key Chemicals, Reagents and Cell Culture
2.2. Plant Materials
2.3. Analytical Apparatus
2.4. Preparation of Ethanolic Extract
2.5. Chromatographic Separation
2.6. Spectroscopic Characterization
2.7. Nitric Oxide Assay
2.8. Cytotoxicity Against Human Dermal Fibroblast (HDFn)
2.9. DPPH Free Radical Scavenging Activity
3. Results and Discussion
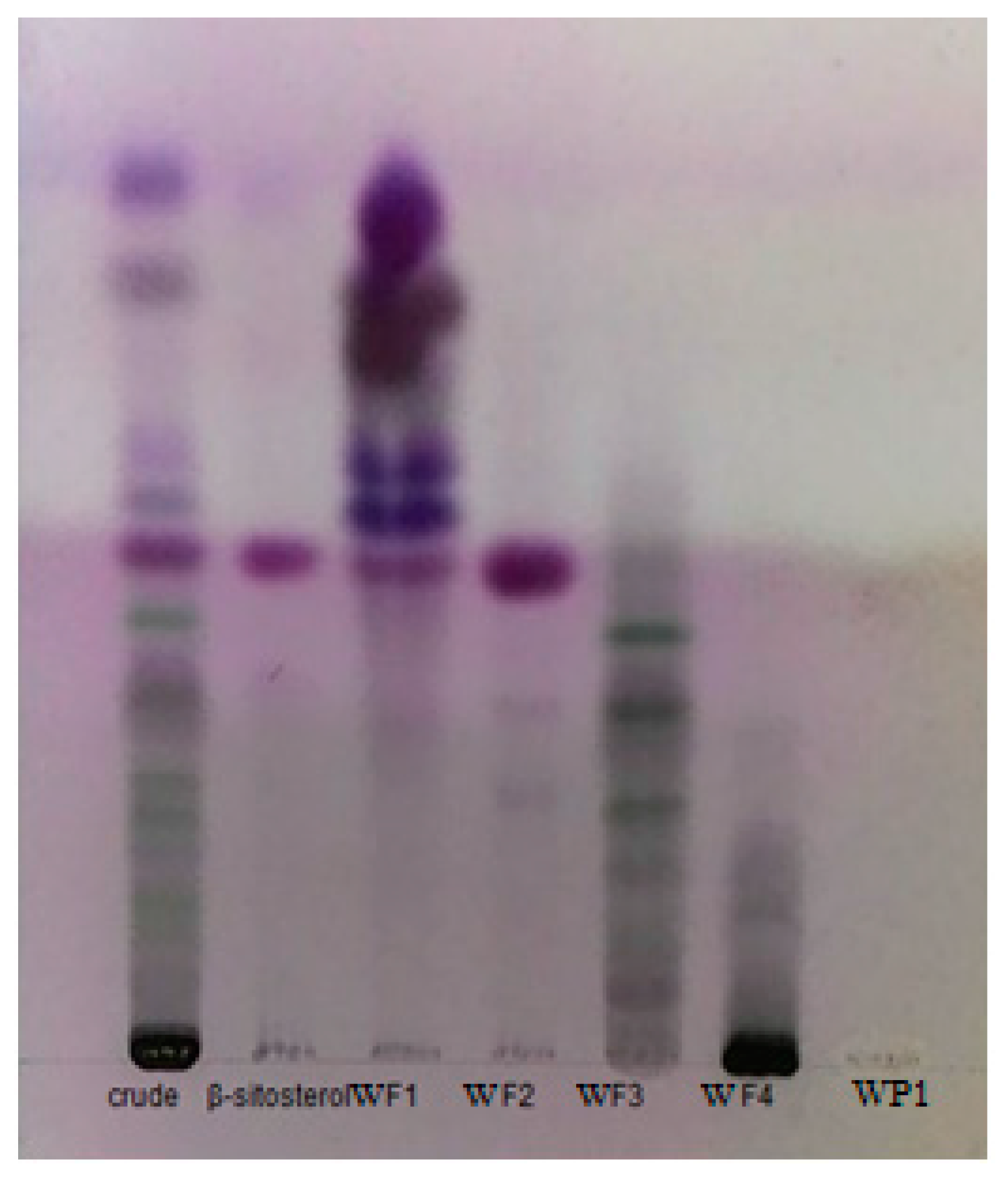
3.1. Extraction and Chromatographic Separation by Thin Layer and Column Chromatography
3.2. Spectroscopic Characterization by FT-IR and 1H-NMR
3.3. Nitric Oxide Assay
3.4. Cytotoxicity Against Human Dermal Fibroblast (HDFn)
3.5. DPPH Radical Scavenging Assay
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Chantiratikul, A.; Pooponpan, P.; Santhaweesuk, S.; Chantiratikul, P.; Sangdee, A.; Maneechote, U.; Bunchasak, C.; Chinrasri, O. Effect of Wolffia meal [Wolffia globosa (L). Wimm.] as a dietary protein replacement on performance and carcass characteristics in broilers. Int. J. Poult. Sci. 2010, 9, 664–668. [Google Scholar]
- Kotowska, U.; Piotrowska, A.; Isidorova, A.; Bajguz, A.; Isidorov, V. Gas chromatographic-mass spectrometric investigation of the chemical composition of the aquatic plant Wolffia arrhiza (Lemnaceae). Oceanol. Hydrobiol. Stud. 2013, 42, 181–187. [Google Scholar] [CrossRef]
- Landolt, E. Taxonomy and Ecology of the Section Wolffia of the genus Wolffia (Lemnaceae); Veröffentlichungen des Geobotanischen Institutes der ETH, Stiftung Rübel, Zürich: Zürich, Switzerland, 1994; Volume 60, pp. 137–151. [Google Scholar]
- Ruekaewma, N. Optimal conditions for Production of Khai-Nam Wolffia globosa. Ph.D. Thesis, Chulalongkorn University, Bangkok, Thailand, 2011. [Google Scholar]
- Bhanthumnavin, K.; McGarry, M.G. Wolffia arrhiza as a possible source of inexpensive protein. Nature 1971, 232, 495. [Google Scholar] [CrossRef] [PubMed]
- Kirjakov, I.; Katya, V. Wolffia globosa (Roxburgh) Hartog Et Plas (Lemnaceae): A new species in Bulgarian flora. J. Biolobical. Sci. Opin. 2013, 1, 356–357. [Google Scholar]
- Szamrej, I.K.; Czerpak, R. The effect of sex steroids and corticosteroids on the content of soluble proteins, nucleic acids and reducing sugars in Wolffia arrhiza (L.) Wimm. (Lemnaceae). Pol. J. Environ. Stud. 2004, 13, 565–571. [Google Scholar]
- Klaus, J.; Appenroth, K.; Sree, S. Duckweed for human nutrition. Newsl. Community Duckweed Res. Appl. 2016, 4, 313–314. [Google Scholar]
- Woyengo, T.A.; Ramprasath, V.R.; Jones, P.J.H. Anticancer effects of phytosterols. Eur. J. Clin. Nutr. 2009, 63, 813–820. [Google Scholar] [CrossRef] [PubMed]
- Nini, W.; Yang, F.; Tao, Y.; Guoyou, L.; Hai, Z. Chemical constituents of Wolffia globosa. Chin. J. Appl. Environ. Biol. 2014, 20, 1016–1019. [Google Scholar]
- Mittal, S. Thin layer chromatography and high pressure liquid chromatography profiling of plant extracts of Viola odorata Linn. Int. J. Pharma Bio Sci. 2013, 4, 542–549. [Google Scholar]
- Boukes, G.J.; van de Venter, M.; Oosthuizen, V. Quantitative and qualitative analysis of sterols/sterolins and hypoxoside contents of three Hypoxis (African potato) spp. Afr. J. Biotechnol. 2008, 7, 1624–1629. [Google Scholar]
- Torres-Rodríguez, M.L.; García-Chávez, E.; Berhow, M.; de Mejia, E.G. Anti-inflammatory and anti-oxidant effect of Calea urticifolia lyophilized aqueous extract on lipopolysaccharide-stimulated RAW 264.7 macrophages. J. Ethnopharmacol. 2016, 188, 266–274. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, J.; Wilson, I.; Orton, T.; Pognan, F. Investigation of the Alamar Blue (resazurin) fluorescent dye for the assessment of mammalian cell cytotoxicity. Eur. J. Biochem. 2000, 267, 5421–5426. [Google Scholar] [CrossRef] [PubMed]
- Gülçin, İ.; Kirecci, E.; Akkemik, E.; Fevzi, T.; Hisar, O. Antioxidant, antibacterial, and anticandidal activities of an aquatic plant: Duckweed (Lemna minor L. Lemnaceae). Turkish J. Biol. 2010, 34, 175–188. [Google Scholar]
- Pierre, L.L.; Moses, M.N. Isolation and characterisation of stigmasterol and β-sitosterol from Odontonema strictum (Acanthaceae). J. Innov. Pharm. Biol. Sci. 2015, 2, 88–95. [Google Scholar]
- Komboj, A.; Saluja, A.K. Isolation of stigmasterol and β-sitosterol from petroleum ether extract of aerial parts of Ageratum conyzoides (Asteraceae). Int. J. Pharm. Pharm. Sci. 2011, 3, 94–96. [Google Scholar]
- Sen, A.; Dhavan, P.; Shukla, K.K.; Singh, S.; Tejovathi, G. Analysis of IR, NMR and antimicrobial activity of β-sitosterol isolated from Momordica charantia. Sci. Secur. J. Biotechnol. 2012, 1, 9–13. [Google Scholar]
- Joo, T.; Sowndhararajan, K.; Hong, S.; Lee, J.; Park, S.Y.; Kim, S.; Jhoo, J.W. Inhibition of nitric oxide production in LPS-stimulated RAW 264.7 cells by stem bark of Ulmus pumila L. Saudi J. Biol. Sci. 2014, 21, 427–435. [Google Scholar] [CrossRef] [PubMed]
- Sudsai, T.; Wattanapiromsakul, C.; Tewtrakul, S. Inhibition of nitric oxide production by compounds from Boesenbergia longiflora using lipopolysaccharide-stimulated RAW264.7 macrophage cells. Songklanakarin J. Sci. Technol. 2013, 35, 317–323. [Google Scholar]
- Ifeoma, O.; Oluwakanyinsola, S. Screening of herbal medicines for potential toxicities. In New Insights into Toxicity and Drug Testing; InTech: London, UK, 2013. [Google Scholar]
- Intarat, A.; Houghton, P.J.; Eno-Amooquaye, E.; Burke, P.J.; Sampson, J.H.; Raman, A. In vitro cytotoxic activity of Thai medicinal plants used traditionally to treat cancer. J. Ethnopharmacol. 2004, 90, 33–38. [Google Scholar]
- Alam, N.; Bristi, N.J.; Rafiquzzaman, M.D. Review on in vivo and in vitro methods evaluation of antioxidant activity. Saudi Pharm. J. 2013, 21, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Ou, B.; Prior, R.L. The chemistry behind antioxidant capacity assays. J. Agric. Food chem. 2005, 53, 1841–1856. [Google Scholar] [CrossRef] [PubMed]
- Seo, C.S.; Lee, M.Y.; Shin, I.S.; Lee, J.A.; Ha, H.; Shin, H.K. Spirodela polyrhiza (L.) Sch. ethanolic extract inhibits LPS-induced inflammation in RAW264.7 cells. Immunopharmacol. Immunotoxicol. 2012, 34, 794–802. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Kim, M.H.; Choi, Y.Y.; Kim, E.H.; Hong, J.; Kim, K.; Yang, W.M. Improvement of a topic dermatitis with topical application of Spirodela polyrhiza. J. Ethnopharmacol. 2016, 180, 12–17. [Google Scholar] [CrossRef] [PubMed]
- Daduang, J.; Vichitphan, S.; Daduang, S.; Hongsprabhas, P.; Boonsiri, P. High phenolics and antioxidants of some tropical vegetables related to antibacterial and anticancer activities. Afr. J. Pharm. Pharmacol. 2011, 5, 608–615. [Google Scholar] [CrossRef]
- Appenroth, K.J.; Sree, K.S.; Böhm, V.; Hammann, S.; Vetter, W.; Leiterer, M.; Jahreis, G. Nutritional value of duckweeds (Lemnaceae) as human food. Food Chem. 2017, 217, 266–273. [Google Scholar] [CrossRef] [PubMed]

| Sample | % Nitric Oxide Inhibition at Various Concentrations (µg/mL) | ||||
|---|---|---|---|---|---|
| 0.1 | 1 | 10 | 100 | 1000 | |
| BSS | −17.70 ± 3.84 | 13.01 ± 1.48 | 16.84 ± 1.81 | 5.97 ± 2.22 | 5.65 ± 3.52 |
| TA | 9.73 ± 2.81 | 14.75 ± 2.02 | 15.93 ± 5.13 | 16.52 ± 4.45 | 18.88 ± 3.90 |
| Sample | Concentration (µg/mL) | Fluorescence Unit | Cytotoxicity (%) | |
|---|---|---|---|---|
| Average | SD | |||
| Ellipticine | 0.00 | 6091 | 295 | 0.00 |
| 0.313 | 5564 | 515 | 8.65 | |
| 0.625 | 4875 | 392 | 19.97 | |
| 1.25 | 4330 | 568 | 28.91 | |
| 2.50 | 3875 | 142 | 36.38 | |
| 5.00 | 1961 | 270 | 67.81 | |
| 10.00 | 36 | 16 | 99.41 | |
| W. globosa extract | 0.00 | 6091 | 295 | 0.00 |
| 3.13 | 5621 | 362 | 7.72 | |
| 6.25 | 4351 | 449 | 28.57 | |
| 12.50 | 4499 | 171 | 26.14 | |
| 25.00 | 4023 | 324 | 33.94 | |
| 50.00 | 3759 | 385 | 38.28 | |
| 100.00 | 3368 | 172 | 44.71 | |
| Concentration mg/mL | Radical Scavenging Activity (%) | |||||
|---|---|---|---|---|---|---|
| Crude Extract | WP1 | WF1 | WF2 | WF3 | WF4 | |
| 0.06 | 3.47 ± 0.10 | 2.12 ± 0.04 | 0.13 ± 0.03 | 0.26 ± 0.04 | 2.56 ± 0.05 | 0.96 ± 0.05 |
| 0.13 | 5.53 ± 0.10 | 2.75 ± 0.01 | 1.07 ± 0.02 | 1.31 ± 0.08 | 3.52 ± 0.06 | 1.48 ± 0.05 |
| 0.20 | 8.72 ± 0.08 | 3.87 ± 0.05 | 1.15 ± 0.06 | 1.28 ± 0.02 | 5.48 ± 0.03 | 2.30 ± 0.05 |
| 0.27 | 10.70 ± 0.73 | 4.90 ± 0.08 | 1.17 ± 0.05 | 1.45 ± 0.03 | 6.72 ± 0.60 | 3.71 ± 0.01 |
| 0.34 | 13.94 ± 0.06 | 7.26 ± 0.04 | 0.88 ± 0.02 | 2.12 ± 0.03 | 7.38 ± 0.07 | 5.06 ± 0.04 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tipnee, S.; Jutiviboonsuk, A.; Wongtrakul, P. The Bioactivity Study of Active Compounds in Wolffia globosa Extract for an Alternative Source of Bioactive Substances. Cosmetics 2017, 4, 53. https://doi.org/10.3390/cosmetics4040053
Tipnee S, Jutiviboonsuk A, Wongtrakul P. The Bioactivity Study of Active Compounds in Wolffia globosa Extract for an Alternative Source of Bioactive Substances. Cosmetics. 2017; 4(4):53. https://doi.org/10.3390/cosmetics4040053
Chicago/Turabian StyleTipnee, Supannee, Aranya Jutiviboonsuk, and Paveena Wongtrakul. 2017. "The Bioactivity Study of Active Compounds in Wolffia globosa Extract for an Alternative Source of Bioactive Substances" Cosmetics 4, no. 4: 53. https://doi.org/10.3390/cosmetics4040053
APA StyleTipnee, S., Jutiviboonsuk, A., & Wongtrakul, P. (2017). The Bioactivity Study of Active Compounds in Wolffia globosa Extract for an Alternative Source of Bioactive Substances. Cosmetics, 4(4), 53. https://doi.org/10.3390/cosmetics4040053




