An Overview of Trials´Accreditation and Recognition of Brazilian Tests Used for the Safety Evaluation of Cosmetic Products
Abstract
:1. Introduction
1.1. Brief History of the Use of Cosmetic Products
1.2. The Brazilian Cosmetics Scenery
1.3. A Brief History of Adverse Reactions to Cosmetics
1.4. INMETRO’s Function in Ensuring Product Safety
2. Experimental Section
2.1. Materials
- (i).
- ANVISA documents, which is the Brazilian regulatory authority for cosmetic products. In this context are included technical statements and guidelines, as well as RDC.
- (ii).
- Information of the INMETRO website, a division of the CGCRE site, which is the Brazilian accreditation body. This information is divided into two groups: accreditation and recognition programs provided by CGCRE; as well as the contents of the scopes of the accredited Conformity Assessment Bodies (CAB) and test facilities recognized by CGCRE.
2.2. Development of the Work
3. Results and Discussion
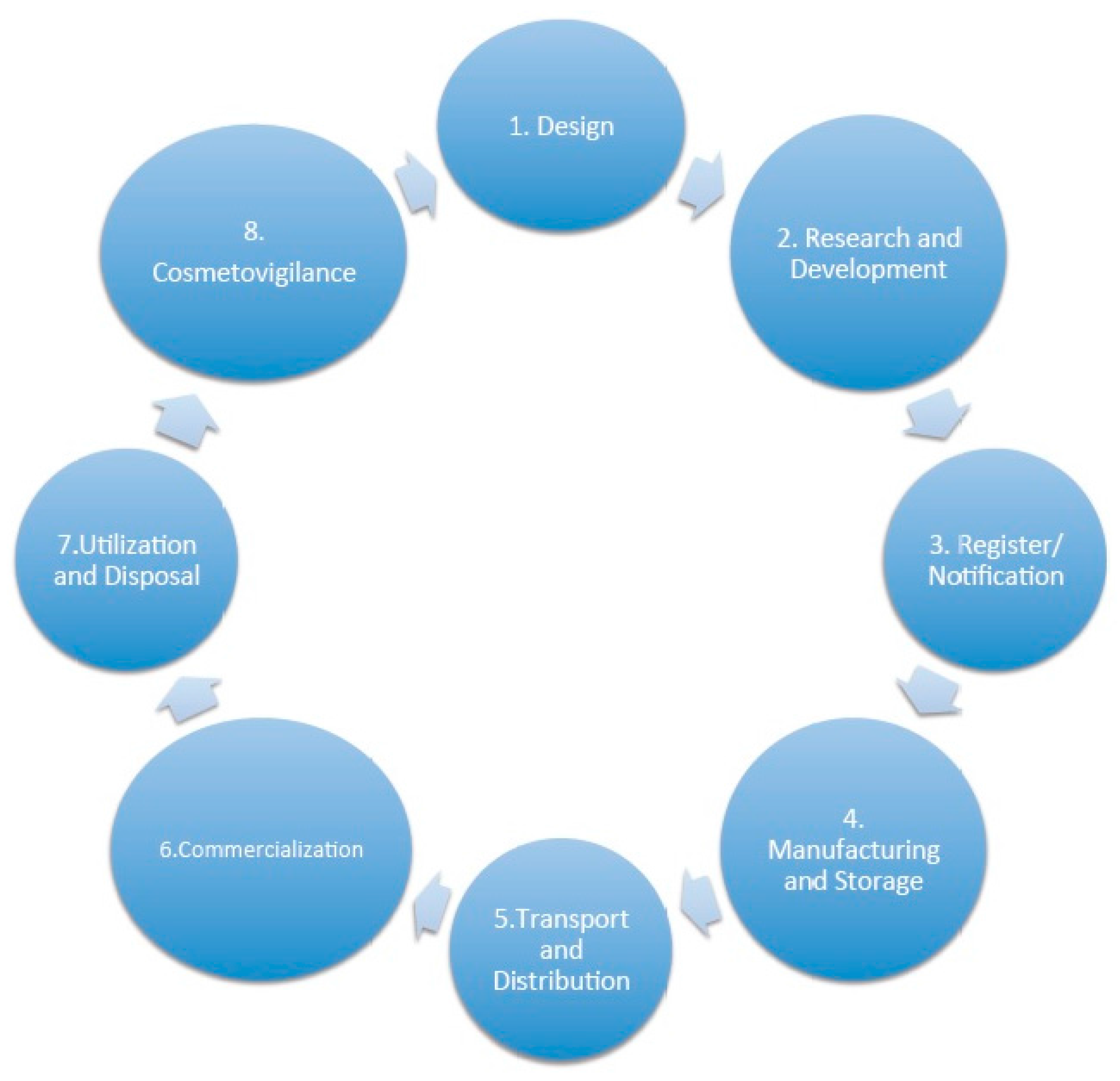
3.1. Lifecycles of a Cosmetic Product
3.1.1. Phase 2: Research and Development
3.1.2. Phase 3: Register and Notification
3.1.3. Phase 4: Manufacturing and Storage
3.2. Toxicological Evaluation of the Ingredients and Cosmetic Formula Per Se
- (1)
- Acute systemic toxicity;
- (2)
- Corrosiveness and dermal irritation;
- (3)
- Skin sensitization;
- (4)
- Skin absorption/penetration;
- (5)
- Repeated-dose;
- (6)
- Mutagenicity/genotoxicity;
- (7)
- Sub-acute and sub-chronic toxicity;
- (8)
- Eye irritation;
- (9)
- Mucosa irritation;
- (10)
- Toxic effects induced by UV radiation (phototoxicity, genotoxicity, photoallergy);
- (11)
- Carcinogenicity;
- (12)
- Reproductive and development toxicity (teratogenicity);
- (13)
- Toxicokinetics and toxicodynamics.
- (i)
- Seven test facilities are recognized for the realization of physicochemical tests;
- (ii)
- Five test facilities are recognized for the realization of toxicological tests;
- (iii)
- Five test facilities are recognized for the realization of mutagenicity tests;
- (iv)
- Two test facilities are recognized for the realization of cytotoxicity;
- (v)
- Two test facilities are recognized for the realization of analytic and clinical chemistry.
3.3. Clinical Trials
- (i)
- if clinical trials are liable for recognition;
- (ii)
- if nowadays there already exists a recognition program for the clinical trials by an accreditation body and;
- (iii)
- if the recognition activity has been practiced in Brazil and in other countries by non-accreditation bodies.
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Hall, B.; Tozer, S.; Safford, B.; Coroama, M.; Steiling, W.; Leneveu-Duchemin, M.C.; McNamara, C.; Gibney, M. European consumer exposure to cosmetic products, a framework for conducting population exposure assessments. Food Chem. Toxicol. 2007, 45, 2097–2108. [Google Scholar] [CrossRef] [PubMed]
- Brown, V.J. Metals in lip products a cause for concern? Environ. Health Perspect. 2013, 121, A196. [Google Scholar] [CrossRef] [PubMed]
- Barata, E.A.F. Cosmetology—Basic Principles. In Basic Concepts; Tecnopress Editoring and Publicity Ltda: São Paulo, Brazil, 1995. [Google Scholar]
- Gonçalves, J.P.R.V. Pharmacy and Cosmetics in 19th Century in Portugal. Master’s Thesis, Lusophone University of Humanities and Technologies, Lisbon, Portugal, 2013; p. 95. [Google Scholar]
- Klingman, A.M. What are cosmeceuticals? In Procedures in Cosmetic Dermatology: Cosmeceuticals; Draelos, Z.D., Ed.; Elsevier: Philadelphia, PA, USA, 2005; pp. 1–2. [Google Scholar]
- Anunciato, T.P.; da Rocha Filho, P.A. Carotenoids and polyphenols in nutricosmetics, nutraceuticals, and cosmeceuticals. J. Cosmet. Dermatol. 2012, 11, 51–54. [Google Scholar] [CrossRef] [PubMed]
- ANVISA. RDC No 7, of 10 February 2015. About Technical Requirements for the Regularization of Personal Hygiene Products, Cosmetics and Perfumes; ANVISA: Brasilia, Brazil, 2015. [Google Scholar]
- Abihpec. Brazilian Cosmetics Market Grows 11% in 2014. Available online: http://brazilbeautynews.com/mercado-brasileiro-de-cosmeticos-cresceu-de-11-em,630 (accessed on 26 September 2015). (In Portuguese)
- Use of Repellents Skyrockets in Brazil Due to Dengue and Zika Outbreaks. Available online: http://cbn.globoradio.globo.com/editorias/economia/2016/02/07/VENDAS-DE-REPELENTES-NO-BRASIL-DISPARAM-COM-SURTO-DE-DENGUE-E-ZIKA.htm (accessed on 10 February 2016). (In Portuguese)
- Costa, A. The International Cosmeceutical Treaty. In Foundations for the Cosmeceutical World; Guanabara Koogan: Rio de Janeiro, Brazil, 2012. [Google Scholar]
- Bad Reaction to Cosmetics? Tell FDA, March 2011. Available online: http://www.fda.gov/downloads/For-Consumers/ConsumerUpdates/UCM248686.pdf (accessed on 27 September 2015). (In Portuguese)
- SCCS—Scientific Committee on Consumer Safety. Opinion on p-Phenylenediamine, COLIPA No A7. Available online: http://ec.europa.eu/health/scientific_committees/consumer_safety/docs/sccs_o_094.pdf (accessed on 26 May 2016).
- Farquharson, A.A.; Stoopler, E.T.; Houston, A.M.; Brown, R.S. Erythema multiforme major secondary to a cosmetic facial cream: First case report. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2016, 121, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Natural Repellent Causes Allergic Reactions in Many Consumers. Available online: http://www.clickguarulhos.com.br/repelente-natural-causa-reacao-alergica-em-varios-usuarios (accessed on 10 February 2016). (In Portuguese)
- ANVISA. RDC No 48, 16 March 2006. Approves the Technical Regulation about the List of Substances That Can Not Be Used in Personal Care Products, Cosmetics and Perfumes; ANVISA: Brasilia, Brazil, 2006. [Google Scholar]
- EU (European Union). Annex II of Regulation (EC) No. 1223/2009 of the European Parliament and of the Council of 30 November 2009 on Cosmetic Products. Available online: http://eur-lex.europa.eu/legal-content/EN/ALL/?uri=CELEX%3A32009R1223 (accessed on 26 May 2016).
- INMETRO. What is Inmetro? Rio de Janeiro, Brazil, 2016. Available online: http://www.inmetro.gov.br/inmetro/oque.asp (accessed on 26 May 2016). (In Portuguese)
- Ministry of Development, Industry and Foreign Trade. Imetro´s Bylaws. Ordinance No 165, of 02 April 2013; Ministry of Development, Industry and Foreign Trade: Brasilia, Brazil, 2013. [Google Scholar]
- INMETRO. Accreditation of Laboratories (ABNT NBR ISO/IEC 17025:2005). Rio de Janeiro, Brazil, 2016. Available online: http://inmetro.gov.br/credenciamento/acre_lab.asp (accessed on 26 May 2016). (In Portuguese)
- INMETRO. GLP Monitoring. Available online: http://www.inmetro.gov.br/monitoramento_BPL/reconhecimento_BPL.asp (accessed on 16 January 2016). (In Portuguese)
- ABNT. Conformity Assessment—Vocabulary and General Principles; ABNT NBR ISO/IEC 17.000:2005; ABNT: Rio de Janeiro, Brazil, 2005. [Google Scholar]
- ANVISA. ANVISA RDC No 12, of 16 February 2012. Order About the Brazilian Network of Analytical Laboratories in Health (REBLAS); ANVISA: Brasilia, Brazil, 2012. [Google Scholar]
- ANVISA. RDC No 332, of 1 December 2005. The Manufacturers and/or Importers of Personal Care Products and Cosmetics Perfumes, Installed in the National Territory Shall Implement a Cosmetovigilance System from 31 December 2005; ANVISA: Brasilia, Brazil, 2005. [Google Scholar]
- Zweers, P.G.M.A.; Gilmour, N.J.; Hepburn, P.A.; Gerritsen, R.F.; van Puijenbroek, E.P. Causality methods in Cosmetovigilance: Comparison of Colipa and PLM versus global introspection. Regul. Toxicol. Pharmacol. 2012, 63, 409–417. [Google Scholar] [CrossRef] [PubMed]
- Giovanni, C.D.; Arcoraci, V.; Gambardella, L.; Sautebin, L. Cosmetovigilance survey: Are cosmetics considered safe by consumers? Pharmacol. Res. 2006, 53, 16–21. [Google Scholar] [CrossRef] [PubMed]
- ANVISA. Guidelines for the Evaluation of Cosmetic Products Safety, 2nd ed.; Brazilian Health Surveillance Agency (ANVISA): Brasilia, Brazil, 2012. [Google Scholar]
- Nohynek, G.; Antignac, E.; Re, T.; Toutain, H. Review Safety assessment of personal care products/cosmetics and their ingredients. Toxicol. Appl. Pharmacol. 2010, 243, 239–259. [Google Scholar] [CrossRef] [PubMed]
- SCCS—Scientific Committee on Consumer Safety. The SCCS Notes of Guidance for the Testing of Cosmetic Ingredients and Their Safety Evaluation, 9th Edition, Adopted on 29 September 2015. Available online: http://ec.europa.eu/health/scientific_committees/consumer_safety/docs/sccs_o_190.pdf (accessed on 26 May 2016).
- ANVISA. RDC No 15, of 24 April 2015. About Technical Requirements for the Concession of Registry of Personal Hygiene Products, Cosmetics and Children Perfumes; ANVISA: Brasilia, Brazil, 2015. [Google Scholar]
- ANVISA. RDC No 44, of 9 August 2012. Approves Mercosul Technical Regulation about “List of Pigments Permitted for Personal Hygiene Products, Cosmetics and Perfumes” and Other Arrangements; ANVISA: Brasilia, Brazil, 2012. [Google Scholar]
- ANVISA. RDC No 29, of 1 June 2012. Approves Mercosul Technical Regulation about “List of Conservative Substances Permitted for Personal Hygiene Products, Cosmetics and Perfumes” and Other Arrangements; ANVISA: Brasilia, Brazil, 2012. [Google Scholar]
- ANVISA. RDC No 03, of 18 January 2012. Mercosul Technical Regulation about “List of Substances That Personal Hygiene Products, Cosmetics and Perfumes Should not Have, Except in Established Conditions and Restrictions”; ANVISA: Brasilia, Brazil, 2012. [Google Scholar]
- ANVISA. RDC No 47, of 16 March 2006. Technical Regulation “List of Ultraviolet Filters Permitted for Personal Hygiene Products, Cosmetics and Perfumes”; ANVISA: Brasilia, Brazil, 2006. [Google Scholar]
- ANVISA. Guidelines of Quality Control of Cosmetic Products—An Approach about Physical and Chemical Trials, 2nd ed.; ANVISA: Brasilia, Brazil, 2008. [Google Scholar]
- ANVISA. Theme Series—Cosmetics, Quality 1: Guidelines of the Cosmetics Stability; ANVISA: Brasilia, Brazil, 2004; Volume 1. [Google Scholar]
- ANVISA. RDC No 481, 23 September 1999. Establishes Parameters for the Microbiological Control of Personal Hygiene, Cosmetics and Perfumes; ANVISA: Brasilia, Brazil, 1999. [Google Scholar]
- ANVISA. RDC No 48, 25 October 2013. Approves the Technical Regulation of Good Practice in Manufacturing Personal Hygiene Products, Cosmetics and Perfumes; ANVISA: Brasilia, Brazil, 2013. [Google Scholar]
- ANVISA. Catec Technical Report, No 1, 15 December 2008. Use of Lead Acetate in Hair Dyes; ANVISA: Brasilia, Brazil, 2008. [Google Scholar]
- ANVISA. Catec Technical Report, No 7, 28 September 2001 (updated in 16 February 2006). Use of Alpha-Hydroxiacids in Cosmetics; ANVISA: Brasilia, Brazil, 2001. [Google Scholar]
- ANVISA. Catec Technical Statements, No 1, 29 January 2002. Use of Methylxantines in Cosmetic Preparations; ANVISA: Brasilia, Brazil, 2002. [Google Scholar]
- ANVISA. Catec Technical Report, No 6, 21 December 2010. Use of Camphor in Cosmetic Products; ANVISA: Brasilia, Brazil, 2010. [Google Scholar]
- ANVISA. Catec Technical Report, No 5, 22 March 2002 (updated in 07/05/2011). Cosmetic Products for Hair Blanching; ANVISA: Brasilia, Brazil, 2002. [Google Scholar]
- ANVISA. Resolution, No 19, 10 April 2013. About Technical Requirements for the Concession of Registries of Cosmetic Products and Repellents; ANVISA: Brasilia, Brazil, 2013. [Google Scholar]
- ANVISA. Catec Technical Report, No 2, 23 December 2009. Use of DEET and Other Repellent Substances; ANVISA: Brasilia, Brazil, 2009. [Google Scholar]
- Carcinogenic Potential of Sodium Lauryl Sulphate. Available online: http://www.anvisa.gov.br/cosmeticos/informa/parecer_lauril.htm (accessed on 20 September 2015). (In Portuguese)
- ANVISA. Catec Technical Report, No 8, 1 November 2005. Menthol in Cosmetic Products; ANVISA: Brasilia, Brazil, 2005. [Google Scholar]
- ANVISA. Catec Technical Report, No 1, 26 July 2006. Inclusion of “Methylisothiazolinone” in the Approved Conservatives List; ANVISA: Brasilia, Brazil, 2006. [Google Scholar]
- ANVISA. Catec Technical Report, No 10, 25 October 2002. Oral Hygiene Products Indicated for Dentine Hypersensitization; ANVISA: Brasilia, Brazil, 2002. [Google Scholar]
- ANVISA. Catec Technical Report, No 4, 6 July 2005. Use of Potassium Salts (Nitrate, Citrate e Chloride) in Products for Dentine Hypersensitization; ANVISA: Brasilia, Brazil, 2005. [Google Scholar]
- ANVISA. Catec Technical Report, No 6, 23 August 2005. Toxicological Evaluation of Methyl Salicylate; ANVISA: Brasilia, Brazil, 2005. [Google Scholar]
- ANVISA. Catec Technical Report, No 2, 13 January 2010. Use of Turpentine in Cosmetics; ANVISA: Brasilia, Brazil, 2010. [Google Scholar]
- ANVISA. Catec Technical Report, No 5, 21 December 2010. Use of Urea in Cosmetic Products; ANVISA: Brasilia, Brazil, 2010. [Google Scholar]
- ANVISA. Catec Technical Report, No 4, 21 December 2010. Use of Retinoids in Cosmetic Products; ANVISA: Brasilia, Brazil, 2010. [Google Scholar]
- The Organisation for Economic Co-operation and Development (OECD). Available online: http://www.oecd.org/about/ (accessed on 17 January 2016).
- OECD. Good Laboratory Practice (GLP). Available online: http://www.oecd.org/chemicalsafety/testing/goodlaboratorypracticeglp.htm (accessed on 17 January 2016).
- GLP Monitoring. Available online: http://www.inmetro.gov.br/monitoramento_BPL/historico.asp?iacao=imprimir (accessed on 16 January 2016). (In Portuguese)
- Almeida, L.S. Proposal of Scope of Clinical Assays of Safety and Effectiveness of Cosmetics Products. J. Phys. Conf. Ser. 2015, 575. [Google Scholar] [CrossRef]
- ANVISA. Catec Technical Report, No 6, de 28 September 2001. Use of the Term “for Sensitive Skin” in Cosmetic Products; ANVISA: Brasilia, Brazil, 2001. [Google Scholar]
- ANVISA. Catec Technical Report, No 5, 23 August 2005. Toxicological Evaluation of Methyl Nicotinate; ANVISA: Brasilia, Brazil, 2005. [Google Scholar]
- ANVISA. Catec Technical Report, No 3, 29 June 2001 (updated in 28 June 2004). Use of Vitamin C in Cosmetic Products; ANVISA: Brasilia, Brazil, 2001. [Google Scholar]
- ANVISA. Catec Technical Report, No 5, 28 September 2001. Use of the “Hypoallergenic” in Personal Hygiene Products, Cosmetics and Perfumes; ANVISA: Brasilia, Brazil, 2001. [Google Scholar]
- ANVISA. Catec Technical Report, No 1, 28 May 2004 (updated in 20 May 2005). Intimate Hygiene Products; ANVISA: Brasilia, Brazil, 2004. [Google Scholar]
| Technical Requirements | Location in This Article of the Results and Discussions Related to the Accreditation/Recognition | Legislation, Guidelines and Applicable Reference |
|---|---|---|
| Toxicological evaluation of the ingredients in cosmetic formulations | Section 3.2 | [26] |
| Evaluation of the cosmetic formula per se (pre-clinical trial) | Section 3.2 | [10,26,27] |
| Clinical trials | Section 3.3 | [26] |
| Safety information of the product: proof of safety (Addendum III of RDC No 7 of 10 February 2015) | Section 3.2 and Section 3.3 | [7,26] |
| Technical Requirements | Location in This Article of the Results and Discussions Related to the Accreditation/Recognition | Applicable Legislation and Guidelines |
|---|---|---|
| Technical organoleptic and physicochemical specifications for finished products (Addendum III of RDC No 7 of 10 February 2015) | Table 4 and Table 5 | [7,29,30,31,32,33,34], Technical Reports of Technical Group on Cosmetics (CATEC) |
| Stability Data (Addendum III of RDC No 7 of 10 February 2015) | Table 6 and Table 7 | [7,35] |
| Stability Data (Addendum III of RDC No 7 of 10 February 2015) | Table 5 and Table 6 | [7,35] |
| Microbiological finished product specifications (Addendum III of RDC No 7 of 10 February 2015) | Table 8 | [7,36] |
| Product safety data: proof of safety (Addendum III of RDC No 7 of 10 February 2015) | Section 3.2 and Section 3.3 | [7,26] |
| Technical Requirements | Location in This Article of the Results Related to Accreditation | Applicable Guidelines and Legislations |
|---|---|---|
| The quality control laboratory should carry out all of the necessary trials to confirm that the finished product meets the acceptance criteria (Requirement 18.16 of RDC No 48 of 25 October 2013) | Table 4 and Table 5 | [29,30,31,32,33,34,37], CATEC Technical Reports |
| For a finished product with a microbiological specification, the acceptance limits for the total counts of microorganisms and pathogens should be in conformity with the current legislation (Requirement 18.23.6 of RDC No 48 of 25 October 2013) | Table 8 | [36,37] |
| Organoleptic Characteristics/Physicochemical Trials | Accredited Laboratories | Products of Laboratory Scope |
|---|---|---|
| Aspect | Lab 1, Lab 3, Lab 5, Lab 9, Lab 10 and Lab 13 | Cosmetic products, oral hygiene products |
| Color | Lab 1, Lab 3, Lab 5, Lab 9, Lab 10 and Lab 13 | Cosmetic products, oral hygiene products |
| Odor and/or flavor | Lab 1, Lab 3, Lab 5, Lab 9, Lab 10 and Lab 13 | Cosmetic products, oral hygiene products |
| pH | Lab 1, Lab 2, Lab 3, Lab 4, Lab 5, Lab 7, Lab 8, Lab 9 and Lab 10 | Cosmetic products, shampoos, conditioners, liquid soaps, hair creams, lotions, roll-on deodorants and creams |
| Density | Lab 1, Lab 3, Lab 6 and Lab 9 | Cosmetic products |
| Relative density * | Lab 1, Lab 2, Lab 3, Lab 4, Lab 6, Lab 9 and Lab 10 | Cosmetic products, shampoos, conditioners, liquid soaps, hair creams, lotions, roll-on deodorants and creams |
| Apparent density | Lab 5 and Lab 7 | Cosmetic products |
| Viscosity | Lab 1, Lab 2, Lab 3, Lab 5, Lab 6, Lab 7, Lab 9 and Lab 10 | Cosmetic products, shampoos, conditioners, liquid soaps, hair creams, lotions, roll-on deodorants and creams |
| Melting point | Lab 1 and Lab 9 | Cosmetic products |
| Alcohol content | Lab 1 | Cosmetic products |
| Free alkalinity **/free fatty acid | Lab 1, Lab 2, Lab 9 and Lab 12 | Cosmetic products, soaps |
| Humidity | Lab 1, Lab 3, Lab 7, Lab 9 and Lab 12 | Cosmetic products and bar soaps |
| Active content | See Table 5 | See Table 5 |
| Actives and Other Ingredients of the Formulation | Suggested Products by the Regulator | Regulatory Reference | Accredited Laboratories | Products of Laboratory Scope |
|---|---|---|---|---|
| Lead acetate | Hair dye | [29,38] | Lab 1 and Lab 9 | Cosmetic product |
| Boric acid | Talcum | [34] | Lab 1 | Cosmetic product |
| Glycolic acid | Bleacher/chemical scrub | [34] | Lab 7 | Cosmetic product |
| Thioglycolic acid, its salts and esters | Smoothers/curler, depilatory products | [34] | Lab 7 | Cosmetic product |
| Alpha-hydroxy acids | Cosmetic product | [39] | None | None |
| Ammonia | Hair dye | [34] | Lab 1 and Lab 7 | Cosmetic product |
| Arsenic * | Hair dye | [38] | Lab 1 | Cosmetic product and raw materials used in cosmetics |
| Caffeine | Cosmetic product | [40] | Lab 1 | Cosmetic product |
| Camphor | Cosmetic product, including nail polish and children products | [41] | Lab 1 | Cosmetic product |
| Carbamide | Hair bleacher | [42] | None | None |
| Guanidine carbonate ** | Smoothers/curlers | [34] | None | None |
| Preservatives | Personal hygiene products, cosmetics and perfumes | [31] | Lab 1 (benzoic acid, salicylic acid, sorbic acid, methyl and propylparaben, formic acid, benzylic alcohol, triclosan); Lab 4 (chlorhexidine); Lab 7 (salicylic acid) | Cosmetic products |
| Pigments, metals in artificial organic pigments (barium, arsenic and lead), among other impurities in pigments | Personal hygiene products, cosmetics and perfumes | [30] | Lab 1 (metals, fluorescein, among others) | Cosmetic products and raw materials used in cosmetics |
| N,N-diethyl-meta-toluamide (DEET) and other repellent substances | Mosquito repellents | [43,44] | Lab 4 (DEET and Insect Repellent 3535) | Cosmetic products |
| Eucalyptol | Cosmetic products | [41] | Lab 7 | Cosmetic products |
| Ultra-violet filters | Sunscreens and other products with sun protection | [33,34] | Lab 7 | Cosmetic products |
| Fluorine | Toothpastes | [34] | Lab 1, Lab 7 and Lab 11 | Mouth rinses, cosmetic products |
| Formaldehyde and/or paraformaldehyde | Nail hardener and other products *** | [29,34] | Lab 7 and Lab 9 | Cosmetic products |
| Hydroquinone | Skin bleacher and hair dye | [34] | Lab 7 | Cosmetic products |
| Sodium, potassium, lithium, calcium and ammonic hydroxides | Smoothers and curlers | [34] | Lab 7 (potassium, sodium and calcium hydroxides) | Cosmetic products |
| Lauryl sodium and ammonia sulfate and other surfactants | Cosmetic products | [45] | Lab 2 (anionic and cationic surfactants), Lab 4 and Lab 7 | Cosmetic products; conditioner and hair cream; shampoos and liquid soaps |
| Menthol | Cosmetic products | [41,46] | Lab 1 | Cosmetic products |
| Mercury * | Hair dye | [38] | Lab 1 | Cosmetic products and raw materials used in cosmetics |
| Methylisothiazolinone *** | Cosmetic products | [47] | None | None |
| Potassium nitrate | Oral hygiene products | [48] | None | None |
| Hydrogen peroxide | Hydrogen peroxide, neutralizer, hair bleacher and oral hygiene products | [34,42] | Lab 1, Lab 7 and Lab 9 | Cosmetic products |
| Sodium, potassium and ammonic persulfates | Hair bleaching powder | [34,42] | None | None |
| Zinc pyrithione | Antidandruff products | [34] | None | None |
| Pyrogallol | Hair smoothers and dyers | [34] | None | None |
| Quinine and its salts | Scalp lotion and shampoos | [34] | None | None |
| Resorcinol | Hair dye, shampoo, hair lotion and acne products | [34] | None | None |
| Aluminum, chlorine and zirconium salts | Antitranspirant deodorants | [34] | Lab 7 (aluminum, chlorine and zirconium salts) | Antitranspirant deodorants |
| Potassium salts (nitrate, citrate and chloride) | Dentine sensitivity products | [49] | Lab 1 (potassium nitrate) | Cosmetic products |
| Methyl salicylate | Cosmetic products | [50] | None | None |
| Substances with established conditions and restrictions of usage | Personal hygiene products, cosmetics and perfumes | Addendum II of RDC No 3 of 20 January 2012 | Lab 1 (borate, ammonia, hydrogen peroxide, potassium hydroxide, nitromethane, chlorides, undelycenic acid, ketoconazole, salicylic acid, methanol, fluorines, calcium hydroxide and toluene) | Cosmetic products |
| Sulfide/selenium disulfide | Anti-acne products | [34] | Lab 1 (selenium sulfide) | Cosmetic products |
| Alkaline-earth sulfides | Depilatory products | [34] | None | None |
| Turpentine | Fragrances | [51] | None | None |
| Tetraborates | Talcum powders, bath products and hair curlers | [34] | None | None |
| Urea | Moisturizer | [34,52] | Lab 1 | Cosmetic products |
| Vitamin A in retinol formulations, retinol esters and retinaldehyde | Cosmetic products | [53] | Lab 1 | Cosmetic products |
| Xanthines, except caffeine | Cosmetic products | [40] | None | None |
| Zinc | Hair bleacher | [42] | Lab 1 | Cosmetic products |
| Type of Stability Study | Parameters | Regulatory Reference | Coded Accredited Laboratories |
|---|---|---|---|
| Preliminary stability (sorting test, accelerated or short-run stability) | Organoleptic characteristics (aspect, color, odor and flavor) and physicochemical characteristics (pH, viscosity, density or others) | [35] | Lab 4 and Lab 10 |
| Accelerated stability (normal or exploratory) | Organoleptic characteristics. Physicochemical characteristics. Microbiological characteristics: study of the preservative system of the product by the challenge test done before or after the period of accelerated study. | [35] | Lab 4 and Lab 10 |
| Shelf test (long-term or shelf life) | Organoleptic characteristics. Physicochemical characteristics. Microbiological characteristics: study of the preservation system of the product by the challenge test done before or after the period of accelerated study | [35] | None |
| Type of Stability Study | Parameters | Regulatory Reference | Accredited Laboratories Codes |
|---|---|---|---|
| Evaluation of the efficiency of the preservation system | Challenge of the preservation system facing purposeful contamination of determined microorganisms | [35] | Lab 1, Lab 3, Lab 4, Lab 5, Lab 7, Lab 9, Lab 10, Lab 11, Lab 14, Lab 15, Lab 19, Lab 20, Lab 21, Lab 22 and Lab 25 |
| Product/Application Area and Age Group | Acceptance Limits [36] | Accredited Laboratories Codes |
|---|---|---|
Type I Cosmetic Products:
|
| Lab 1 *, Lab 3, Lab 4, Lab 5, Lab 7 **, Lab 8 *, Lab 9 *, Lab 10 *, Lab 11 *, Lab 12 *, Lab 14, Lab 15, Lab 16, Lab 17 *, Lab 18, Lab 19, Lab 20, Lab 21, Lab 23, Lab 24, Lab 25 * |
| Type II Cosmetic Products: (a) All other products subject to microbiological contamination |
| Lab 1 *, Lab 3, Lab 4, Lab 5, Lab 7 **, Lab 8 *, Lab 9 *, Lab 10 *, Lab 11 *, Lab 12 *, Lab 14, Lab 15, Lab 16, Lab 17 *, Lab 18, Lab 19, Lab 20, Lab 21, Lab 23, Lab 24, Lab 25 * |
| Clinical Trial Classification | Type of Study | Objective | Examples of the Application of the Study to: the Attributes of Product Safety and/or the Target Audience and/or the Specific Products * |
|---|---|---|---|
| Compatibility | Evaluation of primary and accumulated skin irritation | Prove the absence of skin irritation reactions | Dermatologically tested (SA). Products indicated for sensitive skin (TA) [58]. Products indicated for children (TA) [29]. Repellents (SP) [43,44]. Products with retinoids (SP) [53]. Products with urea in concentrations between 3% and 10% (SP) [52]. Products with methyl salicylate (SP) [50]. Products with methyl nicotinate (SP) [59]. Products with ascorbic acid (vitamin C) and its derivatives (SP) [60]. |
| Evaluation of patch comedogenicity | Prove the absence of comedogenicity | Non-comedogenic (SA) | |
| Evaluation of skin sensitization | Prove the absence of allergic reactions (delayed hypersensitivity immune reaction or Type IV) | Hypoallergenic (SA) [61]. Products indicated for people with sensitive skin (TA) [58]. Products indicated for children (TA) [29]. Repellents (SP) [43,44]. Products with retinoids (SP) [53]. Products with urea in concentrations between 3% and 10% (SP) [52]. Products with methyl salicylate (SP) [50]. Products with methyl nicotinate (SP) [59]. | |
| Evaluation of phototoxicity | Prove the absence of a potential irritant of a product when applied to the skin exposed to UV radiation | Applicable to sunscreens (SP). Products with retinoids (SP) [53]. | |
| Evaluation of photosensitization (or photoallergy) | Prove the absence of a potential allergenic of a product when applied to the skin exposed to UV radiation | Products indicated for people with sensitive skin (TA) [58]. Some products indicated for children (TA) [29]. Hypoallergenic (SA) [61]. Repellents (SP) [43,44]. | |
| Acceptability | Evaluation of skin irritation | Prove the absence of irritations analyzing the particularities of use spots; example: oral mucosa and teeth, by a dentist; genital mucosa and skin, in intimate care products, by a gynecologist/urologist | Evaluated by pediatricians/gynecologists/urologists/dentists/others (SA). Clinically testes (SA). Products indicated for people with sensitive skin (TA). Products indicated for children (TA) [29]. Products indicated for pregnant women ** (TA). Products for intimate hygiene (SP) [62]. |
| Evaluation of acnegenicity and comedogenicity in use | Prove the absence of acnegenicity and comedogenicity | Non-acnegenic (SA). Non-comedogenic (SA). | |
| Evaluation of a product for sensitive skin | Prove the absence of a potential irritant in sensitive skin | Sensitive skin (TA). Clinically tested (SA) | |
| Verification of eye acceptability | Prove product safety in the periocular area | Ophthalmologically tested (SA). Clinically tested (SA). |
© 2016 by the author; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dos Santos Almeida, L. An Overview of Trials´Accreditation and Recognition of Brazilian Tests Used for the Safety Evaluation of Cosmetic Products. Cosmetics 2016, 3, 20. https://doi.org/10.3390/cosmetics3020020
Dos Santos Almeida L. An Overview of Trials´Accreditation and Recognition of Brazilian Tests Used for the Safety Evaluation of Cosmetic Products. Cosmetics. 2016; 3(2):20. https://doi.org/10.3390/cosmetics3020020
Chicago/Turabian StyleDos Santos Almeida, Luciana. 2016. "An Overview of Trials´Accreditation and Recognition of Brazilian Tests Used for the Safety Evaluation of Cosmetic Products" Cosmetics 3, no. 2: 20. https://doi.org/10.3390/cosmetics3020020
APA StyleDos Santos Almeida, L. (2016). An Overview of Trials´Accreditation and Recognition of Brazilian Tests Used for the Safety Evaluation of Cosmetic Products. Cosmetics, 3(2), 20. https://doi.org/10.3390/cosmetics3020020





