Fragrance Allergens, Overview with a Focus on Recent Developments and Understanding of Abiotic and Biotic Activation
Abstract
:1. Introduction
2. Fragrance Markers in Baseline Series
3. Activation of Fragrance Chemicals to Strong Sensitizers
4. Oxidation and Detection
5. Exposure
6. Multiple Reactions to Fragrances Are Common
7. Quality of Life
8. Conclusions
Conflicts of Interest
References
- Frosch, P.J.; Duus Johansen, J.; Schuttelaar, M.L.; Silvestre, J.F.; Sanchez-Perez, J.; Weisshaar, E.; Uter, W. Patch test results with fragrance markers of the baseline series—Analysis of the European Surveillance System on Contact Allergies (ESSCA) network 2009–2012. Contact Dermat. 2015, 73, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Nardelli, A.; Carbonez, A.; Drieghe, J.; Goossens, A. Results of patch testing with fragrance mix 1, fragrance mix 2, and their ingredients, and myroxylon pereirae and colophonium, over a 21-year period. Contact Dermat. 2013, 68, 307–313. [Google Scholar] [CrossRef] [PubMed]
- Uter, W.; Johansen, J.D.; Borje, A.; Karlberg, A.T.; Liden, C.; Rastogi, S.; Roberts, D.; White, I.R. Categorization of fragrance contact allergens for prioritization of preventive measures: Clinical and experimental data and consideration of structure–activity relationships. Contact Dermat. 2013, 69, 196–230. [Google Scholar] [CrossRef] [PubMed]
- Smith, V.M.; Clark, S.M.; Wilkinson, M. Allergic contact dermatitis in children: Trends in allergens, 10 years on. A retrospective study of 500 children tested between 2005 and 2014 in one UK centre. Contact Dermat. 2016, 74, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Belloni Fortina, A.; Cooper, S.M.; Spiewak, R.; Fontana, E.; Schnuch, A.; Uter, W. Patch test results in children and adolescents across Europe analysis of the ESSCA network 2002–2010. Pediatr. Allergy Immunol. 2015, 26, 446–455. [Google Scholar] [CrossRef] [PubMed]
- Diepgen, T.L.; Ofenloch, R.F.; Bruze, M.; Bertuccio, P.; Cazzaniga, S.; Coenraads, P.J.; Elsner, P.; Goncalo, M.; Svensson, A.; Naldi, L. Prevalence of contact allergy in the general population in different European regions. Br. J. Dermatol. 2016, 174, 319–329. [Google Scholar] [CrossRef] [PubMed]
- Diepgen, T.L.; Ofenloch, R.; Bruze, M.; Cazzaniga, S.; Coenraads, P.J.; Elsner, P.; Goncalo, M.; Svensson, A.; Naldi, L. Prevalence of fragrance contact allergy in the general population of five European countries: A cross-sectional study. Br. J. Dermatol. 2015, 173, 1411–1419. [Google Scholar] [CrossRef] [PubMed]
- Lagrelius, M.; Wahlgren, C.F.; Matura, M.; Kull, I.; Liden, C. High prevalence of contact allergy in adolescence: Results from the population-based bamse birth cohort. Contact Dermat. 2016, 74, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Heisterberg, M.V.; Andersen, K.E.; Avnstorp, C.; Kristensen, B.; Kristensen, O.; Kaaber, K.; Laurberg, G.; Menne, T.; Nielsen, N.H.; Sommerlund, M.; et al. Fragrance mix II in the baseline series contributes significantly to detection of fragrance allergy. Contact Dermat. 2010, 63, 270–276. [Google Scholar] [CrossRef] [PubMed]
- Uter, W.; Geier, J.; Schnuch, A.; Gefeller, O. Risk factors associated with sensitization to hydroxyisohexyl 3-cyclohexene carboxaldehyde. Contact Dermat. 2013, 69, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Scheman, A.; Rakowski, E.M.; Chou, V.; Chhatriwala, A.; Ross, J.; Jacob, S.E. Balsam of Peru: Past and future. Dermatitis 2013, 24, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Karlberg, A.T. Colophony: Rosin in unmodified and modified form. In Occupational Skin Diseases, 2nd ed.; Rustemeyer, T., Elsner, P., John, S.M., Maibach, H.I., Eds.; Springer: Berlin/Heidelberg, Germany, 2012; Volume 1, pp. 467–479. [Google Scholar]
- Nardelli, A.; Carbonez, A.; Ottoy, W.; Drieghe, J.; Goossens, A. Frequency of and trends in fragrance allergy over a 15-year period. Contact Dermat. 2008, 58, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Bruze, M.; Svedman, C.; Andersen, K.E.; Bruynzeel, D.; Goossens, A.; Johansen, J.D.; Matura, M.; Orton, D.; Vigan, M. Patch test concentrations (doses in mg/cm2) for the 12 non-mix fragrance substances regulated by European legislation. Contact Dermat. 2012, 66, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Europe Union. Directive 2003/15/EC of the European parliament and of the Council of 27 Febuary 2003 amending Council Directive 76/768/EEC in the approximation of the Laws of the member states relating to cosmetic products. Off. J. Eur. Union 2003, L66, 26–35. [Google Scholar]
- Heisterberg, M.V.; Menne, T.; Johansen, J.D. Contact allergy to the 26 specific fragrance ingredients to be declared on cosmetic products in accordance with the EU cosmetics directive. Contact Dermat. 2011, 65, 266–275. [Google Scholar] [CrossRef] [PubMed]
- Mann, J.; McFadden, J.P.; White, J.M.; White, I.R.; Banerjee, P. Baseline series fragrance markers fail to predict contact allergy. Contact Dermat. 2014, 70, 276–281. [Google Scholar] [CrossRef] [PubMed]
- Van Oosten, E.J.; Schuttelaar, M.L.; Coenraads, P.J. Clinical relevance of positive patch test reactions to the 26 EU-labelled fragrances. Contact Dermat. 2009, 61, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Public Consultation on Fragrance Allergens in the Framework of Regulation (EC) No. 1223/2009 of the European Parliament and of the Council on Cosmetic Products. Available online: http://ec.europa.eu/dgs/health_food-safety/dgs_consultations/ca/consultation_cosmetic-products_fragrance-allergens_201402_en.htm (accessed on 27 May 2016).
- Baron, J.M.; Holler, D.; Schiffer, R.; Frankenberg, S.; Neis, M.; Merk, H.F.; Jugert, F.K. Expression of multiple cytochrome p450 enzymes and multidrug resistance-associated transport proteins in human skin keratinocytes. J. Investig. Dermatol. 2001, 116, 541–548. [Google Scholar] [CrossRef] [PubMed]
- Bergstrom, M.A.; Ott, H.; Carlsson, A.; Neis, M.; Zwadlo-Klarwasser, G.; Jonsson, C.A.M.; Merk, H.F.; Karlberg, A.T.; Baron, J.M. A skin-like cytochrome p450 cocktail activates prohaptens to contact allergenic metabolites. J. Investig. Dermatol. 2007, 127, 1145–1153. [Google Scholar] [CrossRef] [PubMed]
- Merk, H.F.; Abel, J.; Baron, J.M.; Krutmann, J. Molecular pathways in dermatotoxicology. Toxicol. Appl. Pharmacol. 2004, 195, 267–277. [Google Scholar] [CrossRef] [PubMed]
- Guengerich, F.P. Common and uncommon cytochrome p450 reactions related to metabolism and chemical toxicity. Chem. Res. Toxicol. 2001, 14, 611–650. [Google Scholar] [CrossRef] [PubMed]
- Wiegand, C.; Hewitt, N.J.; Merk, H.F.; Reisinger, K. Dermal xenobiotic metabolism: A comparison between native human skin, four in vitro skin test systems and a liver system. Skin Pharmacol. Physiol. 2014, 27, 263–275. [Google Scholar] [PubMed]
- Karlberg, A.T.; Bergstrom, M.A.; Borje, A.; Luthman, K.; Nilsson, J.L.G. Allergic contact dermatitis —Formation, structural requirements, and reactivity of skin sensitizers. Chem. Res. Toxicol. 2008, 21, 53–69. [Google Scholar] [CrossRef] [PubMed]
- Karlberg, A.T.; Borje, A.; Duus Johansen, J.; Liden, C.; Rastogi, S.; Roberts, D.; Uter, W.; White, I.R. Activation of non-sensitizing or low-sensitizing fragrance substances into potent sensitizers—Prehaptens and prohaptens. Contact Dermat. 2013, 69, 323–334. [Google Scholar] [CrossRef] [PubMed]
- Gerberick, G.F.; Ryan, C.A.; Dearman, R.J.; Kimber, I. Local lymph node assay (LLNA) for detection of sensitization capacity of chemicals. Methods 2007, 41, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Kimber, I.; Hilton, J.; Dearman, R.J.; Gerberick, G.F.; Ryan, C.A.; Basketter, D.A.; Scholes, E.W.; Ladics, G.S.; Loveless, S.E.; House, R.V.; et al. An international evaluation of the murine local lymph node assay and comparison of modified procedures. Toxicology 1995, 103, 63–73. [Google Scholar] [CrossRef]
- Sköld, M.; Karlberg, A.T.; Matura, M.; Börje, A. The fragrance chemical beta-caryophyllene—Air oxidation and skin sensitization. Food Chem. Toxicol. 2006, 44, 538–545. [Google Scholar] [CrossRef] [PubMed]
- Niklasson, I.B.; Delaine, T.; Islam, M.N.; Karlsson, R.; Luthman, K.; Karlberg, A.T. Cinnamyl alcohol oxidizes rapidly upon air exposure. Contact Dermat. 2013, 68, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Gerberick, G.F.; Ryan, C.A.; Kern, P.S.; Schlatter, H.; Dearman, R.J.; Kimber, I.; Patlewicz, G.Y.; Basketter, D.A. Compilation of historical local lymph node data for evaluation of skin sensitization alternative methods. Dermatitis 2005, 16, 157–202. [Google Scholar] [PubMed]
- Hagvall, L.; Backtorp, C.; Norrby, P.O.; Karlberg, A.T.; Borje, A. Experimental and theoretical investigations of the autoxidation of geranial: A dioxolane hydroperoxide identified as a skin sensitizer. Chem. Res. Toxicol. 2011, 24, 1507–1515. [Google Scholar] [CrossRef] [PubMed]
- Hagvall, L.; Backtorp, C.; Svensson, S.; Nyman, G.; Borje, A.; Karlberg, A.T. Fragrance compound geraniol forms contact allergens on air exposure. Identification and quantification of oxidation products and effect on skin sensitization. Chem. Res. Toxicol. 2007, 20, 807–814. [Google Scholar] [CrossRef] [PubMed]
- Bråred Christensson, J.; Johansson, S.; Hagvall, L.; Jonsson, C.; Borje, A.; Karlberg, A.T. Limonene hydroperoxide analogues differ in allergenic activity. Contact Dermat. 2008, 59, 344–352. [Google Scholar] [CrossRef] [PubMed]
- Sköld, M.; Börje, A.; Harambasic, E.; Karlberg, A.T. Contact allergens formed on air exposure of linalool. Identification and quantification of primary and secondary oxidation products and the effect on skin sensitization. Chem. Res. Toxicol. 2004, 17, 1697–1705. [Google Scholar] [CrossRef] [PubMed]
- Sköld, M.; Börje, A.; Matura, M.; Karlberg, A.T. Studies on the autoxidation and sensitizing capacity of the fragrance chemical linalool, identifying a linalool hydroperoxide. Contact Dermat. 2002, 46, 267–272. [Google Scholar] [CrossRef]
- Sköld, M.; Hagvall, L.; Karlberg, A.T. Autoxidation of linalyl acetate, the main component of lavender oil, creates potent contact allergens. Contact Dermat. 2008, 58, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Bergstrom, M.A.; Luthman, K.; Nilsson, J.L.G.; Karlberg, A.T. Conjugated dienes as prohaptens in contact allergy: In vivo and in vitro studies of structure—Activity relationships, sensitizing capacity, and metabolic activation. Chem. Res. Toxicol. 2006, 19, 760–769. [Google Scholar] [CrossRef] [PubMed]
- Rudbäck, J.; Bergström, M.A.; Börje, A.; Nilsson, U.; Karlberg, A.T. Alpha-terpinene, an antioxidant in tea tree oil, autoxidizes rapidly to skin allergens on air exposure. Chem. Res. Toxicol. 2012, 25, 713–721. [Google Scholar] [CrossRef] [PubMed]
- Hagvall, L.; Sköld, M.; Bråred-Christensson, J.; Börje, A.; Karlberg, A.T. Lavender oil lacks natural protection against autoxidation, forming strong contact allergens on air exposure. Contact Dermat. 2008, 59, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Hagvall, L.; Baron, J.M.; Borje, A.; Weidolf, L.; Merk, H.F.; Karlberg, A.T. Cytochrome p450-mediated activation of the fragrance compound geraniol forms potent contact allergens. Toxicol. Appl. Pharmacol. 2008, 233, 308–313. [Google Scholar] [CrossRef] [PubMed]
- Schnuch, A.; Uter, W.; Geier, J.; Lessmann, H.; Frosch, P.J. Sensitization to 26 fragrances to be labelled according to current european regulation. Results of the IVDK and review of the literature. Contact Dermat. 2007, 57, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Hagvall, L.; Karlberg, A.T.; Bråred Christensson, J. Finding the optimal patch test material and test concentration to detect contact allergy to geraniol. Contact Dermat. 2013, 68, 224–231. [Google Scholar] [CrossRef] [PubMed]
- Hagvall, L.; Brared Christensson, J. Cross-reactivity between citral and geraniol—Can it be attributed to oxidized geraniol? Contact Dermat. 2014, 71, 280–288. [Google Scholar] [CrossRef] [PubMed]
- Hagvall, L.; Karlberg, A.T.; Brared Christensson, J. Contact allergy to air-exposed geraniol: Clinical observations and report of 14 cases. Contact Dermat. 2012, 67, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Santucci, B.; Cristaudo, A.; Cannistraci, C.; Picardo, M. Contact dermatitis to fragrances. Contact Dermat. 1987, 16, 93–95. [Google Scholar] [CrossRef]
- Matura, M.; Goossens, A.; Bordalo, O.; Garcia-Bravo, B.; Magnusson, K.; Wrangsjo, K.; Karlberg, A.T. Oxidized citrus oil (R-limonene): A frequent skin sensitizer in Europe. J. Am. Acad. Dermatol. 2002, 47, 709–714. [Google Scholar] [CrossRef] [PubMed]
- Matura, M.; Skold, M.; Borje, A.; Andersen, K.E.; Bruze, M.; Frosch, P.; Goossens, A.; Johansen, J.D.; Svedman, C.; White, I.R.; et al. Not only oxidized R-(+)- but also S-(−)-limonene is a common cause of contact allergy in dermatitis patients in Europe. Contact Dermat. 2006, 55, 274–279. [Google Scholar] [CrossRef] [PubMed]
- Uter, W.; Geier, J.; Frosch, P.; Schnuch, A. Contact allergy to fragrances: Current patch test results (2005–2008) from the information network of departments of dermatology. Contact Dermat. 2010, 63, 254–261. [Google Scholar] [CrossRef] [PubMed]
- Bråred Christensson, J.; Andersen, K.E.; Bruze, M.; Johansen, J.D.; Garcia-Bravo, B.; Gimenez-Arnau, A.; Goh, C.L.; Nixon, R.; White, I.R. An international multicentre study on the allergenic activity of air-oxidized R-limonene. Contact Dermat. 2013, 68, 214–223. [Google Scholar] [CrossRef] [PubMed]
- Audrain, H.; Kenward, C.; Lovell, C.R.; Green, C.; Ormerod, A.D.; Sansom, J.; Chowdhury, M.M.; Cooper, S.M.; Johnston, G.A.; Wilkinson, M.; et al. Allergy to oxidized limonene and linalool is frequent in the UK. Br. J. Dermatol. 2014, 171, 292–297. [Google Scholar] [CrossRef] [PubMed]
- Hagvall, L.; Brared Christensson, J. Patch-testing with main sensitizers does not detect all cases of contact allergy to oxidized lavender oil. Acta Derm. Venereol. 2015, 28, 559–564. [Google Scholar] [CrossRef] [PubMed]
- De Groot, A.C.; Coenraads, P.J.; Bruynzeel, D.P.; Jagtman, B.A.; van Ginkel, C.J.; Noz, K.; van der Valk, P.G.; Pavel, S.; Vink, J.; Weyland, J.W. Routine patch testing with fragrance chemicals in the Netherlands. Contact Dermat. 2000, 42, 184–185. [Google Scholar]
- Bråred Christensson, J.; Matura, M.; Gruvberger, B.; Bruze, M.; Karlberg, A.T. Linalool—A significant contact sensitizer after air exposure. Contact Dermat. 2010, 62, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Fregert, S.; Hjorth, N. Results of standard patch tests with substances abandoned. Contact Dermat. 1969, 5, 85–86. [Google Scholar]
- Frosch, P.J.; Pilz, B.; Andersen, K.E.; Burrows, D.; Camarasa, J.G.; Dooms-Goossens, A.; Ducombs, G.; Fuchs, T.; Hannuksela, M.; Lachapelle, J.M.; et al. Patch testing with fragrances: Results of a multicenter study of the European Environmental and Contact Dermatitis Research Group with 48 frequently used constituents of perfumes. Contact Dermat. 1995, 33, 333–342. [Google Scholar] [CrossRef]
- Buckley, D.A. Allergy to oxidized linalool in the UK. Contact Dermat. 2011, 64, 240–241. [Google Scholar] [CrossRef] [PubMed]
- Bråred Christensson, J.; Andersen, K.; Bruze, M.; Johansen, J.; Garcia Bravo, B.; Arnau, A.G.; Goh, C.L.; Nixon, R.; White, I.R. Air-oxidized linalool—A frequent cause of fragrance allergy. Contact Dermat. 2012, 67, 247–259. [Google Scholar] [CrossRef] [PubMed]
- De Groot, A.C.; Liem, D.H.; Nater, J.P.; van Ketel, W.G. Patch tests with fragrance materials and preservatives. Contact Dermat. 1985, 12, 87–92. [Google Scholar] [CrossRef]
- Matura, M.; Skold, M.; Borje, A.; Andersen, K.E.; Bruze, M.; Frosch, P.; Goossens, A.; Johansen, J.D.; Svedman, C.; White, I.R.; et al. Selected oxidized fragrance terpenes are common contact allergens. Contact Dermat. 2005, 52, 320–328. [Google Scholar] [CrossRef] [PubMed]
- Frosch, P.J.; Johansen, J.D.; Menné, T.; Pirker, C.; Rastogi, S.C.; Andersen, K.E.; Bruze, M.; Goossens, A.; Lepoittevin, J.P.; White, I.R. Further important sensitizers in patients sensitive to fragrances. II. Reactivity to essential oils. Contact Dermat. 2002, 47, 279–287. [Google Scholar] [CrossRef]
- Hagvall, L.; Berglund, V.; Bråred Christensson, J. Air-oxidized linalyl acetate—An emerging fragrance allergen? Contact Dermat. 2015, 72, 216–223. [Google Scholar] [CrossRef] [PubMed]
- Frosch, P.J.; Johansen, J.D.; Menne, T.; Pirker, C.; Rastogi, S.C.; Andersen, K.E.; Bruze, M.; Goossens, A.; Lepoittevin, J.P.; White, I.R. Further important sensitizers in patients sensitive to fragrances. I. Reactivity to 14 frequently used chemicals. Contact Dermat. 2002, 47, 78–85. [Google Scholar] [CrossRef]
- Meesters, R.J.W.; Duisken, M.; Hollender, J. Study on the cytochrome p450-mediated oxidative metabolism of the terpene alcohol linalool: Indication of biological epoxidation. Xenobiotica 2007, 37, 604–617. [Google Scholar] [CrossRef] [PubMed]
- Karlberg, A.T.; Shao, L.P.; Nilsson, U.; Gafvert, E.; Nilsson, J.L. Hydroperoxides in oxidized d-limonene identified as potent contact allergens. Arch. Dermatol. Res. 1994, 286, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Karlberg, A.T.; Magnusson, K.; Nilsson, U. Air oxidation of d-limonene (the citrus solvent) creates potent allergens. Contact Dermat. 1992, 26, 332–340. [Google Scholar] [CrossRef]
- Nilsson, U.; Bergh, M.; Shao, L.P.; Karlberg, A.-T. Analysis of contact allergenic compounds in oxidized d-limonene. Chromatographia 1996, 42, 199–205. [Google Scholar] [CrossRef]
- Karlberg, A.T.; Boman, A.; Melin, B. Animal experiments on the allergenicity of d-limonene—The citrus solvent. Ann. Occup. Hyg. 1992, 35, 419–426. [Google Scholar] [CrossRef]
- Bråred Christensson, J.; Matura, M.; Backtorp, C.; Borje, A.; Nilsson, J.L.; Karlberg, A.T. Hydroperoxides form specific antigens in contact allergy. Contact Dermat. 2006, 55, 230–237. [Google Scholar] [CrossRef] [PubMed]
- Matura, M.; Goossens, A.; Bordalo, O.; Garcia-Bravo, B.; Magnusson, K.; Wrangsjö, K.; Karlberg, A.T. Patch testing with oxidized R-(+)-limonene and its hydroperoxide fraction. Contact Dermat. 2003, 49, 15–21. [Google Scholar] [CrossRef]
- Bråred Christensson, J.; Hellsén, S.; Börje, A.; Karlberg, A.T. Limonene hydroperoxide analogues show specific patch test reactions. Contact Dermat. 2014, 70, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Johansen, J.D.; Menne, T. The fragrance mix and its constituents: A 14-year material. Contact Dermat. 1995, 32, 18–23. [Google Scholar] [CrossRef]
- Schnuch, A.; Lessmann, H.; Geier, J.; Frosch, P.J.; Uter, W. Contact allergy to fragrances: Frequencies of sensitization from 1996 to 2002. Results of the IVDK*. Contact Dermat. 2004, 50, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Loveless, S.E.; Ladics, G.S.; Gerberick, G.F.; Ryan, C.A.; Basketter, D.A.; Scholes, E.W.; House, R.V.; Hilton, J.; Dearman, R.J.; Kimber, I. Further evaluation of the local lymph node assay in the final phase of an international collaborative trial. Toxicology 1996, 108, 141–152. [Google Scholar] [CrossRef]
- Bertrand, F.; Basketter, D.A.; Roberts, D.W.; Lepoittevin, J.P. Skin sensitization to eugenol and isoeugenol in mice: Possible metabolic pathways involving ortho-quinone and quinone methide intermediates. Chem. Res. Toxicol. 1997, 10, 335–343. [Google Scholar] [CrossRef] [PubMed]
- Gerberick, G.F.; Troutman, J.A.; Foertsch, L.M.; Vassallo, J.D.; Quijano, M.; Dobson, R.L.; Goebel, C.; Lepoittevin, J.P. Investigation of peptide reactivity of pro-hapten skin sensitizers using a peroxidase-peroxide oxidation system. Toxicol. Sci. 2009, 112, 164–174. [Google Scholar] [CrossRef] [PubMed]
- Niklasson, I.B.; Ponting, D.J.; Luthman, K.; Karlberg, A.T. Bioactivation of cinnamic alcohol forms several strong skin sensitizers. Chem. Res. Toxicol. 2014, 27, 568–575. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.K.; Moore, C.A.; Elahi, E.N.; Smart, A.T.; Hotchkiss, S.A. Human skin absorption and metabolism of the contact allergens, cinnamic aldehyde, and cinnamic alcohol. Toxicol. Appl. Pharmacol. 2000, 168, 189–199. [Google Scholar] [CrossRef] [PubMed]
- Kern, S.; Dkhil, H.; Hendarsa, P.; Ellis, G.; Natsch, A. Detection of potentially skin sensitizing hydroperoxides of linalool in fragranced products. Anal. Bioanal. Chem. 2014, 406, 6165–6178. [Google Scholar] [CrossRef] [PubMed]
- Rudbäck, J.; Islam, N.; Nilsson, U.; Karlberg, A.T. A sensitive method for determination of allergenic fragrance terpene hydroperoxides using liquid chromatography coupled with tandem mass spectrometry. J. Sep. Sci. 2013, 36, 1370–1378. [Google Scholar] [CrossRef] [PubMed]
- Rudbäck, J.; Ramzy, A.; Karlberg, A.T.; Nilsson, U. Determination of allergenic hydroperoxides in essential oils using gas chromatography with electron ionization mass spectrometry. J. Sep. Sci. 2014, 37, 982–989. [Google Scholar] [CrossRef] [PubMed]
- Kern, S.; Granier, T.; Dkhil, H.; Haupt, T.; Ellis, G.; Natsch, A. Stability of limonene and monitoring of a hydroperoxide in fragranced products. Flavour Fragr. J. 2014, 29, 277–286. [Google Scholar] [CrossRef]
- Rudback, J.; Islam, M.N.; Borje, A.; Nilsson, U.; Karlberg, A.T. Essential oils can contain allergenic hydroperoxides at eliciting levels, regardless of handling and storage. Contact Dermat. 2015, 73, 253–254. [Google Scholar] [CrossRef] [PubMed]
- Work Plan: Develop A Shared Common Understanding of Risk Assessment Methodologies, Processes and Criteria to Identify Fragrance Allergens of Concern. Available online: http://www.ideaproject.info/topics (accessed on 27 May 2016).
- Karlberg, A.T.; Magnusson, K.; Nilsson, U. Influence of an anti-oxidant on the formation of allergenic compounds during auto-oxidation of d-limonene. Ann. Occup. Hyg. 1994, 38, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Foti, M.C.; Ingold, K.U. Mechanism of inhibition of lipid peroxidation by gamma-terpinene, an unusual and potentially useful hydrocarbon antioxidant. J. Agric. Food Chem. 2003, 51, 2758–2765. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Chen, F.; Wu, C.; Wang, X.; Chung, H.Y.; Jin, Z. Evaluation of antioxidant activity of australian tea tree (Melaleuca alternifolia) oil and its components. J. Agric. Food Chem. 2004, 52, 2849–2854. [Google Scholar] [CrossRef] [PubMed]
- Ruberto, G.; Baratta, M.T.; Deans, S.G.; Dorman, H.J. Antioxidant and antimicrobial activity of foeniculum vulgare and crithmum maritimum essential oils. Planta Med. 2000, 66, 687–693. [Google Scholar] [CrossRef] [PubMed]
- Johansen, J. Fragrance contact allergy: Clinical and experimental investigations of the fragrance mix and its ingredients. Contact Dermat. 2002, 46, 1–31. [Google Scholar] [CrossRef]
- Buckley, D.A. Fragrance ingredient labelling in products on sale in the UK. Br. J. Dermatol. 2007, 157, 295–300. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, S.C.; Heydorn, S.; Johansen, J.D.; Basketter, D.A. Fragrance chemicals in domestic and occupational products. Contact Dermat. 2001, 45, 221–225. [Google Scholar] [CrossRef]
- Rastogi, S.C.; Menne, T.; Johansen, J.D. The composition of fine fragrances is changing. Contact Dermat. 2003, 48, 130–132. [Google Scholar] [CrossRef]
- Rastogi, S.C.; Johansen, J.D.; Bossi, R. Selected important fragrance sensitizers in perfumes—Current exposures. Contact Dermat. 2007, 56, 201–204. [Google Scholar] [CrossRef] [PubMed]
- Yazar, K.; Johnsson, S.; Lind, M.L.; Boman, A.; Liden, C. Preservatives and fragrances in selected consumer-available cosmetics and detergents. Contact Dermat. 2011, 64, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Uter, W.; Yazar, K.; Kratz, E.M.; Mildau, G.; Liden, C. Coupled exposure to ingredients of cosmetic products: I. Fragrances. Contact Dermat. 2013, 69, 335–341. [Google Scholar] [CrossRef] [PubMed]
- Belsito, D.; Bickers, D.; Bruze, M.; Calow, P.; Greim, H.; Hanifin, J.M.; Rogers, A.E.; Saurat, J.H.; Sipes, I.G.; Tagami, H.; et al. A toxicologic and dermatologic assessment of cyclic and non-cyclic terpene alcohols when used as fragrance ingredients. Food Chem. Toxicol. 2008, 46, S1–S71. [Google Scholar] [CrossRef] [PubMed]
- Schnuch, A.; Uter, W.; Lessmann, H.; Geier, J. Risk of sensitization to fragrances estimated on the basis of patch test data and exposure, according to volume used and a sample of 5451 cosmetic products. Flavour Fragr. J. 2015, 30, 208–217. [Google Scholar] [CrossRef]
- Bråred Christensson, J.; Andersen, K.E.; Bruze, M.; Johansen, J.D.; Garcia-Bravo, B.; Gimenez Arnau, A.; Goh, C.L.; Nixon, R.; White, I.R. Positive patch test reactions to oxidized limonene: Exposure and relevance. Contact Dermat. 2014, 71, 264–272. [Google Scholar] [CrossRef] [PubMed]
- De Mozzi, P.; Johnston, G.A. An outbreak of allergic contact dermatitis caused by citral in beauticians working in a health spa. Contact Dermat. 2014, 70, 377–379. [Google Scholar] [CrossRef] [PubMed]
- Pesonen, M.; Suomela, S.; Kuuliala, O.; Henriks-Eckerman, M.L.; Aalto-Korte, K. Occupational contact dermatitis caused by d-limonene. Contact Dermat. 2014, 71, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Jensen, P.; Menne, T.; Johansen, J.D.; Thyssen, J.P. Facial allergic contact dermatitis caused by fragrance ingredients released by an electric shaver. Contact Dermat. 2012, 67, 380–381. [Google Scholar] [CrossRef] [PubMed]
- Nardelli, A.; Drieghe, J.; Claes, L.; Boey, L.; Goossens, A. Fragrance allergens in ‘specific’ cosmetic products. Contact Dermat. 2011, 64, 212–219. [Google Scholar] [CrossRef] [PubMed]
- Fischer, L.A.; Menne, T.; Avnstorp, C.; Kasting, G.B.; Johansen, J.D. Hydroxyisohexyl 3-cyclohexene carboxaldehyde allergy: Relationship between patch test and repeated open application test thresholds. Br. J. Dermatol. 2009, 161, 560–567. [Google Scholar] [CrossRef] [PubMed]
- Schnuch, A.; Uter, W.; Dickel, H.; Szliska, C.; Schliemann, S.; Eben, R.; Rueff, F.; Gimenez-Arnau, A.; Loffler, H.; Aberer, W.; et al. Quantitative patch and repeated open application testing in hydroxyisohexyl 3-cyclohexene carboxaldehyde sensitive-patients. Contact Dermat. 2009, 61, 152–162. [Google Scholar] [CrossRef] [PubMed]
- Svedman, C.; Engfeldt, M.; Api, A.M.; Politano, V.T.; Belsito, D.V.; Isaksson, M.; Bruze, M. A pilot study aimed at finding a suitable eugenol concentration for a leave-on product for use in a repeated open application test. Contact Dermat. 2012, 66, 137–139. [Google Scholar] [CrossRef] [PubMed]
- Andersen, K.E.; Johansen, J.D.; Bruze, M.; Frosch, P.J.; Goossens, A.; Lepoittevin, J.P.; Rastogi, S.; White, I.; Menne, T. The time-dose-response relationship for elicitation of contact dermatitis in isoeugenol allergic individuals. Toxicol. Appl. Pharmacol. 2001, 170, 166–171. [Google Scholar] [CrossRef] [PubMed]
- Johansen, J.D.; Andersen, K.E.; Menne, T. Quantitative aspects of isoeugenol contact allergy assessed by use and patch tests. Contact Dermat. 1996, 34, 414–418. [Google Scholar] [CrossRef]
- Heisterberg, M.V.; Menne, T.; Andersen, K.E.; Avnstorp, C.; Kristensen, B.; Kristensen, O.; Kaaber, K.; Laurberg, G.; Henrik Nielsen, N.; Sommerlund, M.; et al. Deodorants are the leading cause of allergic contact dermatitis to fragrance ingredients. Contact Dermat. 2011, 64, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Bruze, M.; Johansen, J.D.; Andersen, K.E.; Frosch, P.; Goossens, A.; Lepoittevin, J.P.; Rastogi, S.C.; White, I.; Menne, T. Deodorants: An experimental provocation study with isoeugenol. Contact Dermat. 2005, 52, 260–267. [Google Scholar] [CrossRef] [PubMed]
- Jorgensen, P.H.; Jensen, C.D.; Rastogi, S.; Andersen, K.E.; Johansen, J.D. Experimental elicitation with hydroxyisohexyl-3-cyclohexene carboxaldehyde-containing deodorants. Contact Dermat. 2007, 56, 146–150. [Google Scholar] [CrossRef] [PubMed]
- Andersch Björkman, Y.; Hagvall, L.; Siwmark, C.; Niklasson, B.; Karlberg, A.T.; Bråred Christensson, J. Air-oxidized linalool elicits eczema in allergic patients—A repeated open application test study. Contact Dermat. 2014, 70, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Madsen, J.T.; Vogel, S.; Johansen, J.D.; Andersen, K.E. Encapsulating contact allergens in liposomes, ethosomes, and polycaprolactone may affect their sensitizing properties. Cutan. Ocul. Toxicol. 2011, 30, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Madsen, J.T.; Vogel, S.; Johansen, J.D.; Sorensen, J.A.; Andersen, K.E.; Nielsen, J.B. Percutaneous penetration characteristics and release kinetics of contact allergens encapsulated in ethosomes. Cutan. Ocul. Toxicol. 2011, 30, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Madsen, J.T.; Vogel, S.; Karlberg, A.T.; Simonsson, C.; Johansen, J.D.; Andersen, K.E. Ethosome formulation of contact allergens may enhance patch test reactions in patients. Contact Dermat. 2010, 63, 209–214. [Google Scholar] [CrossRef] [PubMed]
- Simonsson, C.; Madsen, J.T.; Graneli, A.; Andersen, K.E.; Karlberg, A.T.; Jonsson, C.A.; Ericson, M.B. A study of the enhanced sensitizing capacity of a contact allergen in lipid vesicle formulations. Toxicol. Appl. Pharmacol. 2011, 252, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Bonefeld, C.M.; Nielsen, M.M.; Rubin, I.M.; Vennegaard, M.T.; Dabelsteen, S.; Gimenez-Arnau, E.; Lepoittevin, J.P.; Geisler, C.; Johansen, J.D. Enhanced sensitization and elicitation responses caused by mixtures of common fragrance allergens. Contact Dermat. 2011, 65, 336–342. [Google Scholar] [CrossRef] [PubMed]
- Sakeena, M.H.; Elrashid, S.M.; Muthanna, F.A.; Ghassan, Z.A.; Kanakal, M.M.; Laila, L.; Munavvar, A.S.; Azmin, M.N. Effect of limonene on permeation enhancement of ketoprofen in palm oil esters nanoemulsion. J. Oleo Sci. 2010, 59, 395–400. [Google Scholar] [PubMed]
- Song, Y.H.; Gwak, H.S.; Chun, I.K. The effects of terpenes on the permeation of lidocaine and ofloxacin from moisture-activated patches. Drug Deliv. 2009, 16, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Heydorn, S.; Andersen, K.E.; Johansen, J.D.; Menne, T. A stronger patch test elicitation reaction to the allergen hydroxycitronellal plus the irritant sodium lauryl sulfate. Contact Dermat. 2003, 49, 133–139. [Google Scholar] [CrossRef]
- Thyssen, J.P.; Carlsen, B.C.; Menne, T.; Johansen, J.D. Trends of contact allergy to fragrance mix I and myroxylon pereirae among danish eczema patients tested between 1985 and 2007. Contact Dermat. 2008, 59, 238–244. [Google Scholar] [CrossRef] [PubMed]
- Brared Christensson, J.; Karlberg, A.T.; Andersen, K.E.; Bruze, M.; Johansen, J.D.; Garcia-Bravo, B.; Gimenez Arnau, A.; Goh, C.L.; Nixon, R.; White, I.R. Oxidized limonene and oxidized linalool—Concomitant contact allergy to common fragrance terpenes. Contact Dermat. 2016, 74, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Heisterberg, M.V.; Menne, T.; Johansen, J.D. Fragrance allergy and quality of life—Development and validation of a disease-specific quality of life instrument. Contact Dermat. 2014, 70, 69–80. [Google Scholar] [CrossRef] [PubMed]
- Heisterberg, M.V.; Menne, T.; Johansen, J.D. Fragrance allergy and quality of life—A case-control study. Contact Dermat. 2014, 70, 81–89. [Google Scholar] [CrossRef] [PubMed]
| Substance (INCI Name) | Chemical Structure | CAS No. | EC3 Value (% w/v) a | References | ||
|---|---|---|---|---|---|---|
| Pure b | Oxidized b | Oxidation Time | ||||
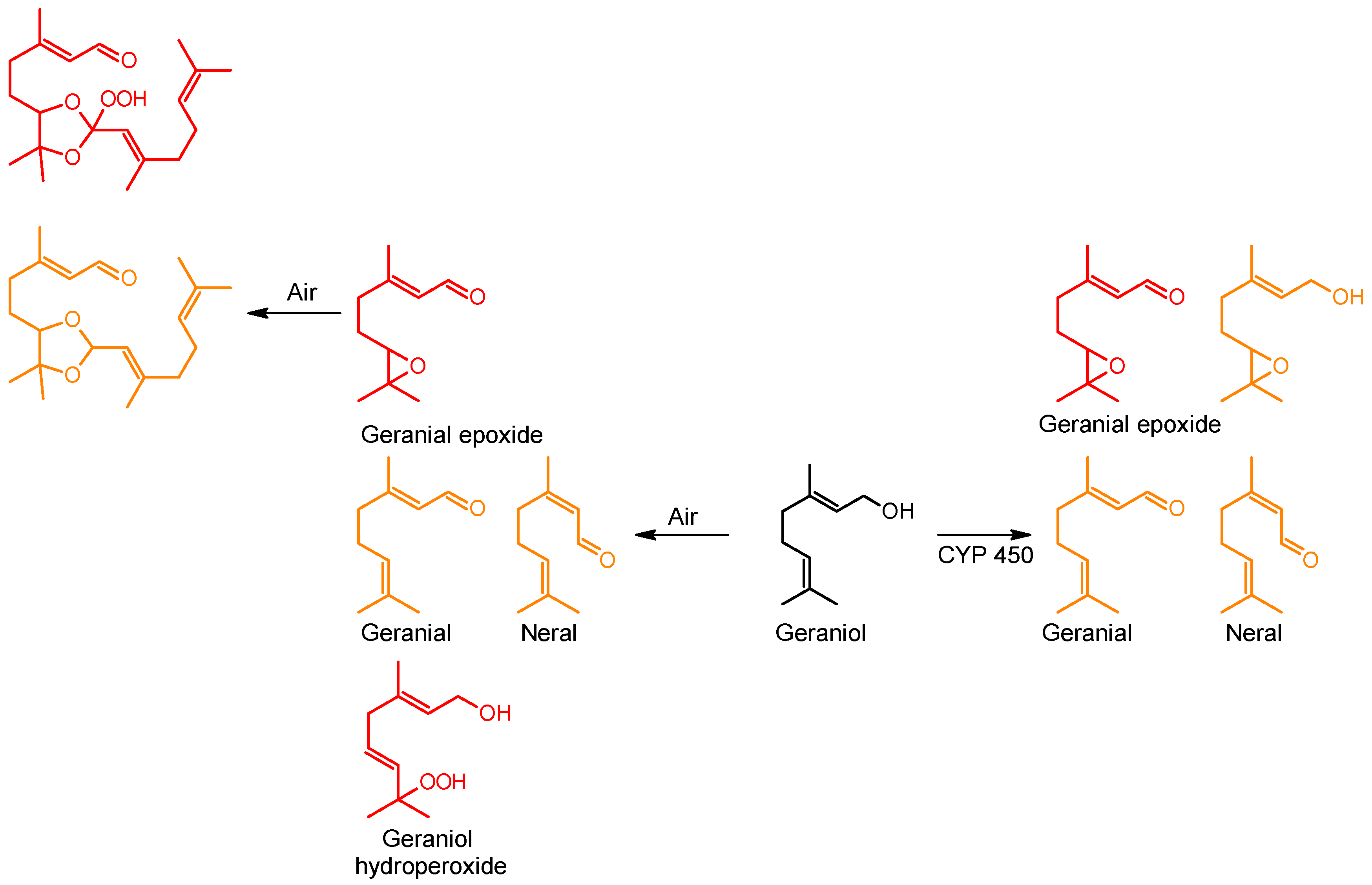
| β-Caryophyllene | 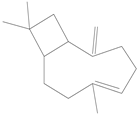 | 87-44-5 | NT c | 26.2 | 10 weeks | [29] |
| Cinnamyl alcohol | 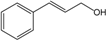 | 104-54-1 | 20.1 d | 4.9 | 2 weeks | [30,31] |
| Geranial | 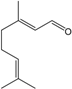 | 141-27-5 | 6.8 | 1.3 | 5 weeks | [32,33] |
| Geraniol | 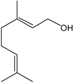 | 106-24-1 | 22.4 | 4.4 | 10 weeks | [33] |
| 5.8 | 45 weeks | |||||
| d-Limonene e | 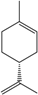 | 5989-27-5 | 30 | 3.0 | 10 weeks | [34] |
| Linalool | 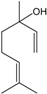 | 78-70-6 | 46.2 | 9.4 | 10 weeks | [35,36] |
| 4.8 | 45 weeks | |||||
| Linalyl acetate | 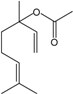 | 115-95-7 | 25 | 3.6 | 10 weeks | [37] |
| α-Terpinene | 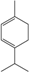 | 99-86-5 | 8.9 | 0.94 | 3 weeks | [38,39] |
| Lavender oil | Main components: Linalool, linalyl acetate, caryophyllene | 8000-28-0 | 36 (Not oxidized) | 11 | 10 weeks | [40] |
| 4.4 | 45 weeks | |||||
| Substance | Pure or Not Intentionally Oxidized | Oxidized, See Details in References Given | ||||||
|---|---|---|---|---|---|---|---|---|
| Test Conc. (% w/w) a | n Positive | n Tested (%) | Reference | Test Conc. (% w/w) a | n Positive | n Tested (%) | Reference | |
| Geraniol | 2 | 3/2227 | 0.13% | [45] | 2 | 12/2179 | 0.55% | [45] |
| 4 | 1/655 | 0.15% | [43] | 4 | 6/655 | 0.92% | [43] | |
| 6 | 3/649 | 0.46% | 6 | 15/655 | 2.3% | |||
| 11 | 7/655 | 1.1% | 11 | 30/653 | 4.6% | |||
| d-Limonene b | 2 | 0/1200 | – | [46] | 3 | 63/2273 | 2.8% | [47] |
| 3 | 49/1812 | 2.3% | [48] | |||||
| l-Limonene c | 2 | 11/1241 | 0.88% | [49] | 3 | 36/1812 | 2.0% | |
| d- and/or l-Limonene | 2 | 0/320 | – | [18] | 3 | 63/2411 | 2.6% | |
| d-Limonene | 2 | 3/2396 | 0.1% | [42] | 3 (0.3% hydroperoxides) | 152/2900 | 5.2% | [50] |
| 10 | 11/4731 | 0.2% | [51] | 3 (0.3% hydroperoxides) | 237/4731 | 5.0% | [51] | |
| Lavender oil | – | – | – | – | 6 | 47/1693 | 2.8% | [52] |
| Linalool | 20 | 3/1825 | 0.2% | [53] | 2 | 14/1693 | 0.83% | [54] |
| 10 | 2/320 | 0.6% | [18] | 4 | 67/2075 | 3.2% | ||
| 10 | 4/792 | 0.5% | [55] | 6 | 91/1725 | 5.3% | ||
| 5 and 1 | 0/100 | – | [56] | 11 | 72/1004 | 7.2% | ||
| 10 | 7/2401 | 0.3% | [42] | 3 | 11/483 | 2.3% | [57] | |
| 10 | 2/985 | 0.2% | [49] | 6 (1% hydroperoxides) | 200/2900 | 6.9% | [58] | |
| 10 | 12/4731 | 0.3% | [51] | 6 (1% hydroperoxides) | 281/4731 | 5.9% | [51] | |
| 30 | 0/179 | – | [59] | 2 | 20/1511 | 1.3% | [60] | |
| Caryophyllene | 5 | 10/1606 | 0.6% | [61] | 3.9 | 2/1511 | 0.1% | |
| Myrcene | – | – | – | – | 3 | 1/1511 | 0.1% | |
| Linalyl acetate | 1, 5 | 0/100 | – | [56] | 6 | 37/1717 | 2.2% | [62] |
| 10 | 4/1855 | 0.2% | [63] | |||||
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bråred Christensson, J.; Hagvall, L.; Karlberg, A.-T. Fragrance Allergens, Overview with a Focus on Recent Developments and Understanding of Abiotic and Biotic Activation. Cosmetics 2016, 3, 19. https://doi.org/10.3390/cosmetics3020019
Bråred Christensson J, Hagvall L, Karlberg A-T. Fragrance Allergens, Overview with a Focus on Recent Developments and Understanding of Abiotic and Biotic Activation. Cosmetics. 2016; 3(2):19. https://doi.org/10.3390/cosmetics3020019
Chicago/Turabian StyleBråred Christensson, Johanna, Lina Hagvall, and Ann-Therese Karlberg. 2016. "Fragrance Allergens, Overview with a Focus on Recent Developments and Understanding of Abiotic and Biotic Activation" Cosmetics 3, no. 2: 19. https://doi.org/10.3390/cosmetics3020019
APA StyleBråred Christensson, J., Hagvall, L., & Karlberg, A.-T. (2016). Fragrance Allergens, Overview with a Focus on Recent Developments and Understanding of Abiotic and Biotic Activation. Cosmetics, 3(2), 19. https://doi.org/10.3390/cosmetics3020019





