Abstract
Whitening cosmetics with anti-melanogenesis activity are very popular worldwide. Many companies have tried to identify novel ingredients that show anti-melanogenesis effects for new product development. Among many plant-derived compounds, polyphenols are thought to be one of the most promising anti-melanogenesis ingredients. In order to prepare effective whitening polyphenols, 3,3,4,5,7-pentahydrosyflavone (quercetin) has been widely researched and applied to commercial products because it is present in high levels in many edible plants. Quercetin is thus a representative polyphenol and has recently gained attention in the cosmetics field. There are many controversies, however, regarding the effect of quercetin, based on in vitro studies, cell line experiments, and human trials. In this review, toxicity and efficacy data for quercetin and its derivatives in various experimental conditions (i.e., various cell lines, concentration ranges, and other parameters) were examined. Based on this analysis, quercetin itself is shown to be ineffective for hypopigmentation of human skin. However, a few types of quercetin derivatives (such as glycosides) show some activity in a concentration-dependent manner. This review provides clarity in the debate regarding the effects of quercetin.
1. Introduction
Melanin plays a significant role in the prevention of skin damage [1]. However, the accumulation of an abnormal amount of melanin in various parts of the skin results in the development of pigmented patches that might be viewed as an aesthetic problem [2]. Excessive production of melanin and abnormal hyperpigmentation from overexposure to ultraviolet (UV) radiation may cause excessive generation of reactive species, which can lead to various skin injuries, including inflammation, age spots, melasma, and freckles [1]. In recent years, skin-whitening ingredients have become the most important components of cosmetic and hygiene products. Therefore skin-whitening ingredients that show hypo-pigmentation efficacy (i.e., anti-melanogenesis activity) are particularly important. Many researchers in academia, research institutes, and companies have attempted to identify effective and safe anti-melanogenesis and/or safe skin-whitening ingredients [3]. Skin-whitening ingredients often function via the inhibition of melanogenesis, and can also be referred to as anti-melanogenesis agents.
This review describes the fundamental synthesis of melanin, melanin signaling pathways, and the factors involved in melanogenesis and pigmentation disorders. It also examines the effects of the well-known whitening compound quercetin on the inhibition of melanogenesis. Quercetin is a representative polyphenol and has recently gained attention in the cosmetics field for its antimelanogenic properties. There are many controversies, however, regarding the effects of quercetin, based on in vitro studies, cell line experiments, and human trials. Specifically, it is unclear whether quercetin leads to an increase or decrease in melanin formation. Toxicity and efficacy data regarding quercetin and its derivatives for various experimental conditions (i.e., various cell lines, concentration ranges, and other parameters) are also examined in this review.
2. Melanogenesis and Its Signal Pathway
2.1. Melanogenesis Mechanism and the Regulation of Melanin Biosynthesis
Alterations to human skin, hair, and eye color are related to the type, amount, stage, and distribution of melanin [4,5]. Human skin color is determined by the outermost layer of the skin, the epidermis, where pigment-producing cells (i.e., melanocytes) are localized for melanin production. Melanin plays an important role in protecting human skin from the harmful effects of UV radiation. Upon exposure of the skin to UV radiation, melanogenesis is enhanced via the activation of the key enzyme tyrosinase, resulting in excessive production of melanin as well as DNA damage, inflammation, or other skin injuries [6,7,8,9,10]. Melanocytes are derived from fibroblasts in the dermis and keratinocytes in the epidermis (basal and suprabasal keratinocytes), where melanocytes transfer melanin pigments into the basal layers of the epidermis [11]. When UV radiation from sunlight exposes the skin to (photo)-oxidative stress, reactive oxygen species and reactive nitrogen species are generated, resulting in cutaneous abnormalities such as DNA-damaged epidermal hyperplasia, collagen breakdown, and inflammation. Humans naturally produce melanin pigments for photo-protection. Stem cell factor (SCF), adrenaline noradrenaline, α-melanocyte-stimulating hormone (α-MSH), and Wnt hormones are involved in physiological responses and interact with c-Kit, adrenergic receptors, melanocortin 1 receptor (MC1R), and Wnt receptors (Figure 1). For example, MC1R signaling regulates 3′,5′-cyclic adenosine monophosphate (cAMP) and protein kinase A (PKA), promotes cAMP-response element binding protein (CREB), and ultimately upregulates microphthalmia-associated transcription factor (MITF) in the nucleus. Upregulated MITF activates tyrosinase-related protein 1 (TRP1) in the Golgi apparatus. Subsequently, biochemical melanin synthesis (blackish-brown colored eumelanin and yellowish-red colored pheomelanin) ensues, moving these proteins to melanosomes. Melanosomes are characterized by four maturation stages and reaction directions. The combined glutathione or cysteine in DOPAquinone is converted to cysteinylDOPA or glutathionylDOPA, and pheomelanin is formed. The eumelanin stages are involved in the conversion from DOPAquinone to l-3,4-dihydroxyphenylalanine (l-DOPA) or leukodopachrome without glutathione or cysteine [12,13,14,15].
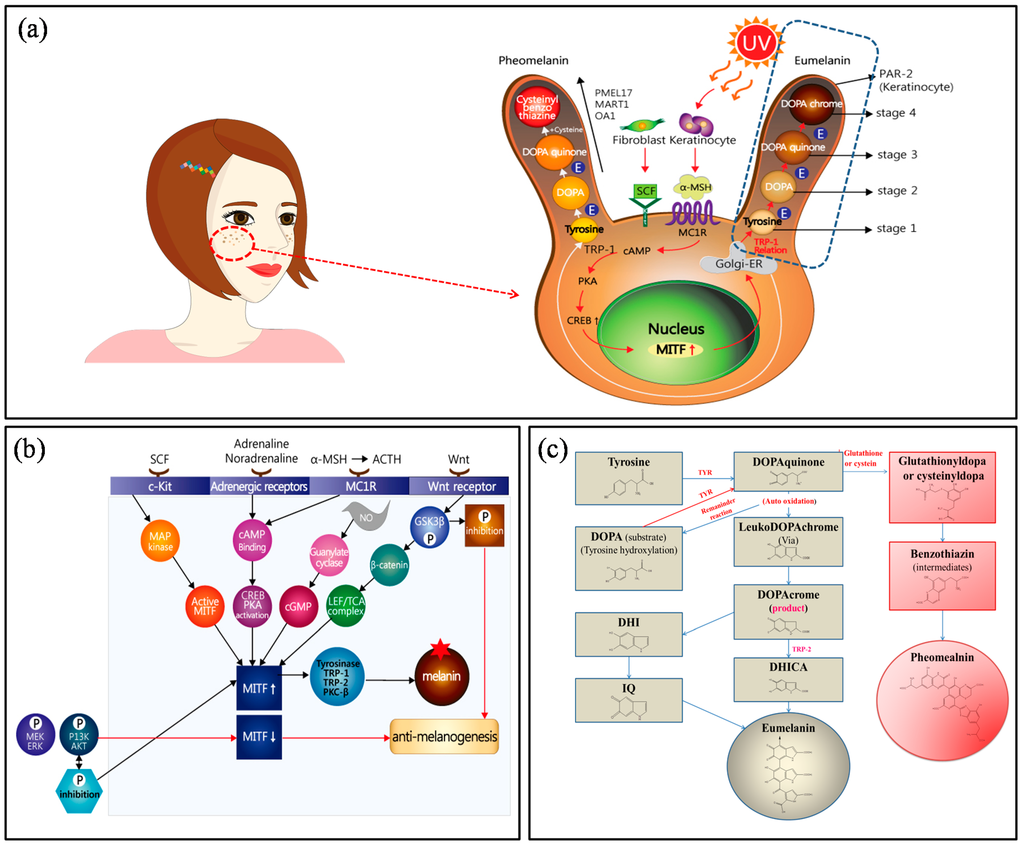
Figure 1.
(a) Schematic representation of melanosome and melanin formation mechanism; (b) signal transduction pathway for anti-melanogenesis activity; and (c) chemical reaction-based melanin formation.
2.2. Signaling Pathways Activating Melanogenesis
To understand the signaling pathway of melanogenesis in physiological responses, four receptors must be considered. First, the c-Kit receptor is activated by SCF, followed by the activation of MAP (mitogen-activated protein) kinase and MITF. Second, adrenergic receptors bind adrenaline and noradrenaline, which activate cAMP binding, followed by CREB and PKA activation. Third, MC1R receptors are activated by ACTH and α-MSH, and interact with cAMP, via the identical adrenergic receptor pathway. In another pathway, nitrogen oxygen (NO) radicals activate guanylate cyclase, which activates cGMP and MITF. In particular, Wnt receptor activates GSK3β, which promotes phosphorylation and accelerates anti-melanogenesis. The inhibition of phosphorylation in GSK3β increases β-catenin and the LEF/TCA complex, and activates MITF [16,17,18,19]. For the above process, activated MITF promotes the expression of tyrosinase, TRP-1 (DCT), TRP-2 (i.e., DOPA chrome tautomerase (DCT)), and PKC-β. As a result, melanin is formed. In contrast, in the extracellular signaling process, the phosphorylation of MEK/ERK and P13K/AKT downregulates MITF, leading to anti-melanogenesis effects, while the dephosphorylation process activates MITF (Figure 1b). The biochemical response for eumelanin and pheomelanin has been explained in Section 2.1.
2.3. Inhibition of Melanogenesis through Tyrosinase Inhibition
Every anti-melanogenesis ingredient acts via inhibitory mechanisms. Five approaches are used to inhibit melanogenesis: (1) inhibition of tyrosinase mRNA transcription; (2) aberrant tyrosinase maturation; (3) inhibition of tyrosinase catalytic activity; (4) acceleration of tyrosinase degradation; and (5) indirect regulation of tyrosinase activity [20]. Until now, tyrosinase has been the most common target for therapeutic agents intended to alleviate hyperpigmentation [6,7,8,9,21,22]. Multiple approaches could potentially be used to control pigmentation via the regulation of tyrosinase activity. The transcription of its mRNA, its maturation via glycosylation, its trafficking to melanosomes, as well as the modulation of its catalytic activity and/or stability are all targets for tyrosinase regulation. Well-known tyrosinase inhibitors and their sources include kojic acid, arbutin, glutathione, vitamin A (retinol), vitamin B3 (niacinamide), vitamin C, mulberry, papaya, and licorice root. Among them, the utilization of kojic acid and arbutin is still common because these agents have repeatedly been demonstrated to be effective whitening agents. The need for new kinds of natural whitening ingredients increases substantially with the growth of the market for whitening products. Some whitening products contain a mixture of many extracts known to contain tyrosinase inhibitors, but other extracts may instead act as antioxidant or anti-inflammatory agents. A number of tyrosinase inhibitors from both natural and synthetic sources have been identified. Currently, natural sources, such as polyphenols, have been gaining attention, reflecting public desire for safe and effective ingredients.
3. Polyphenols and Melanogenesis
3.1. Antioxidant Activity and Melanogenesis
The antioxidant defense system is resistant to free radicals generated in the metabolic processes [23]. Free radicals damage lipids, proteins, and DNA, and cause many diseases. Therefore, antioxidants have beneficial effects on the overall health condition of the skin and protect against UV radiation, ozone, and smoke, among other harmful substances responsible for epithelial cell and basal cell cancer and immune suppression. Representative antioxidant chemicals are plant-derived polyphenols. Polyphenols are typically divided into four primary classes: phenolic acids, flavonoids, lignans, and stilbenes (Figure 2a). Of these, phenolic acids and flavonoids are the most prevalent in nature and the most widely studied. In general, these molecules share common structural features, i.e., multiple aromatic rings and attached hydroxyl groups [24,25].
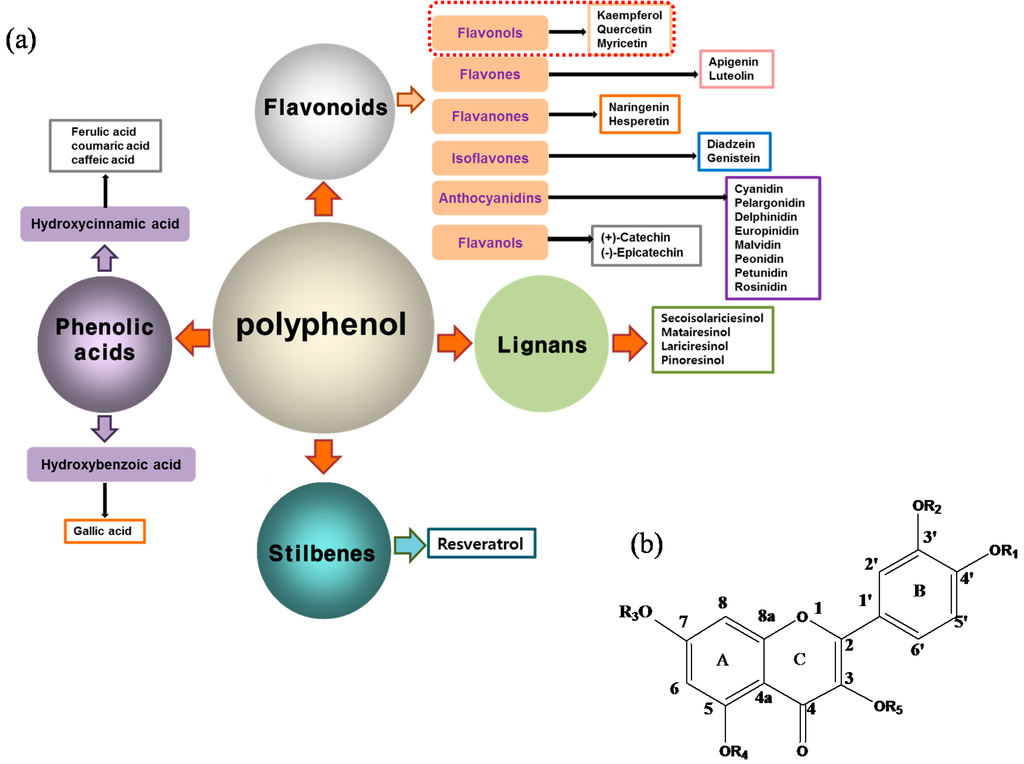
Figure 2.
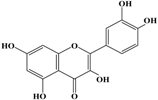
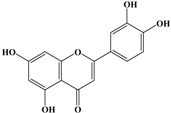
(a) Classification of polyphenols and (b) chemical structure of quercetin.
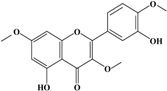
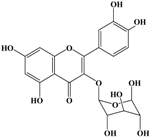
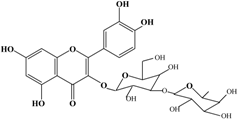
With respect to antioxidant activity, quercetin is a popular flavonoid aglycone that is found in a variety of fruits and vegetables, such as onions, curly kale, leeks, broccoli, and blueberries [24,25]. Quercetin is a potent tyrosinase inhibitor, melanogenesis inhibitor in mouse B16 melanoma cells, and an antioxidant and anticancer agent [26,27,28,29,30]. However, it has the opposite effect as a melanogenesis accelerator in human melanoma cells [26,31,32,33,34,35,36]. It has been shown to decrease intracellular tyrosinase activity and inhibit mushroom tyrosinase activity in a cell-free system [28,29]. In addition, quercetin inhibits melanin production in B16 melanoma cells in a dose-dependent manner [37]. However, some studies report the stimulatory effects of quercetin on cellular melanogenesis. Nagata et al. (2004) reported that quercetin enhances melanogenesis by increasing the activity and synthesis of tyrosinase in human melanoma cells and normal human melanocytes [31]. Quercetin and its derivatives also stimulate melanogenesis in the absence of α-MSH in B16 murine melanoma cells [38]. These opposing effects of quercetin are widely debated, considering the potential application of quercetin in inhibiting tyrosinase enzyme activity in the cosmetic field. Like other flavones, quercetin contains a heterocyclic pyrone ring in its structure, which is connected on both sides to phenolic moieties. It exists in the form of rutin (quercetin-3-rutinoside), a glycoside containing a disaccharide covalently attached to the quercetin unit (Figure 2b) [34,35,36,38,39]. As a therapeutic agent, quercetin plays an important role in biological activities, e.g., as an anti-allergic, anti-inflammatory, anti-melanogenesis, and anti-carcinogenic agent [40,41,42], suggesting that it acts as a free radical scavenging agent for superoxide anions and lipid peroxyl species.
Thus, among its various biological activities, the antioxidant activity of quercetin is particularly important for anti-melanogenesis. Quercetin is considered an antioxidative agent that inactivates the tyrosinase enzyme, mediating the relationship between its antioxidant properties and anti-melanogenesis. It is closely associated with the free radical scavenging effect of antioxidant functions in quercetin for defense against oxidative stress. As a primary condition for anti-melanogenesis effects, the antioxidant property of polyphenols (herein, quercetin) has been extensively investigated, and these studies have focused on whether or not quercetin shows anti-melanogenesis effects.
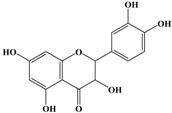
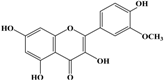
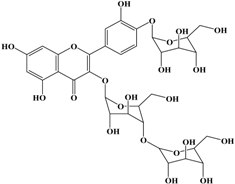
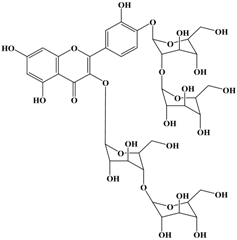
Anti-melanogenesis depends on the position of OH functional groups in the quercetin structure (Figure 2b) [34,35,36,37]. Structurally, the IC50 value for melanin inhibition by quercetin is 26.5 μM, while that for inhibition by quercetin-4′-O-glucoside is 130.6 μM. However, the cell viabilities for quercetin and quercetin-4′-O-glucoside are 88% and 82%, respectively. Quercetin-3,4′-O-diglucoside and quercetin-3,4′-O-rhamnoside (rutin) are safe because they have high antioxidant activity with a related B ring, but have little effect on hypo-pigmentation. Thus, although quercetin and quercetin-4′-O-glucoside have the ability to reduce melanin content, they also demonstrate cell toxicity [43]. Conclusively, C4′ glycosylation of the quercetin structure doubles its antioxidant activity but decreases the inhibition of melanogenesis five-fold. Quercetin presents a dilemma with respect to skin-whitening agents; its safety should be considered for practical human applications. In addition, 4′-O-β-d-glucopyranosyl-quercetin-3-O-β-d-glucopyranosyl-(1 → 4)-β-d-glucopyranoside shows an increase in melanin content by MITF expression with no cytotoxic effects at 10 μM, suggesting that the C4′ site plays a critical role in melanogenesis [35]. Yamauchi et al. (2013) determined that R5 substituted with –OCH3 groups stimulates melanogenesis activity, and both R3 and R5 substituted with –OCH3 groups result in a cell viability of ~60% at 12.5 μM [35]. These observations support the role of C4 in melanogenesis. In an expanded analysis, Yamauchi et al. (2014) reported that synthesized quercetin glycosides have an anti-melanogenesis effect and demonstrate less cell toxicity for R1 = cellobiose, R3 = OH, R5 = cellobiose, or R1 = OH, R3 = cellobiose, R5 = cellobiose, or R1 = glucose, R3 = OH, R5 = glucose [34]. Other quercetin glycosides show either no anti-melanogenesis effect (or the acceleration of melanogenesis), cell toxicity, or their combination. Therefore, quercetin glycosides are not suitable for use as whitening agents [36].
3.2. In Vitro and in Vivo Anti-Melanogenesis Effects of Quercetin
Most anti-melanogenesis experiments using quercetin have been in vitro, rather than in vivo, assays. The results for cellular tests of melanin content, tyrosinase activity, mRNA expression, and protein level expression based on in vitro investigations of quercetin and its derivatives, including compounds derived from natural extracts, are summarized in Table 1. Generally, B16F10/B16F1 melanoma cells and mouse melanoma cells have been utilized, but human melanoma of vagina (HMVII) cells and normal human epidermal melanocytes (NHEM) have also been used, though infrequently. Depending on the concentration of quercetin or its compounds in natural extracts, at low concentrations, in the range of 10–20 μM, melanin content in cells increases, while at concentrations of 20–50 μM, it decreases. At a concentration in the range of 50–100 μM, melanin content decreases and cytotoxicity increases (Figure 3). By contrast, at concentrations of 10–500 μM, the melanin content increases; however, these concentrations are safe with respect to cell viability. This means that natural quercetin compounds show less cell toxicity. However, importantly, those compounds are not effective in reducing melanin content. Furthermore, as shown in Table 1, Nagata et al. found that tyrosinase activity in cell systems is enhanced, but there is no effect on mRNA expression, resulting in the overexpression of the tyrosinase protein owing to quercetin treatment at concentrations of 1–20 μM [31]. In a cell-free system, tyrosinase activity is inhibited at 10–100 μM. Interestingly, in a cell system, tyrosinase activity increased at 5–10 μM, but was inhibited at 20–50 μM. Tyrosinase, TRP-1, and TRP-2 show different protein expression patterns for the tested quercetin concentrations. Specifically, at quercetin concentrations of 10 and 20 μM, tyrosinase is overexpressed, but at 50 μM, it is expressed at slightly lower levels. At quercetin concentrations of 10, 20, and 50 μM, TRP-1 shows gradually lower expression, while at 5 and 10 μM, TRP-2 is expressed, and at 20 and 50 μM, its expression gradually decreases [41]. In particular, Takekoshi et al. (2013) reported an increase in melanin content at quercetin concentrations >50 μM [26]. Tyrosinase and TRP-2 are overexpressed at quercetin concentrations of 5–160 μM, but there is no effect on TRP-1 at quercetin concentrations of 50–160 μM. Likewise, the same group showed that at 10 μM quercetin, melanin content increases and tyrosinase is overexpressed after three days [21].

Table 1.
Literature summary of in vitro anti-melanogenesis effect by quercetin and its derivatives.
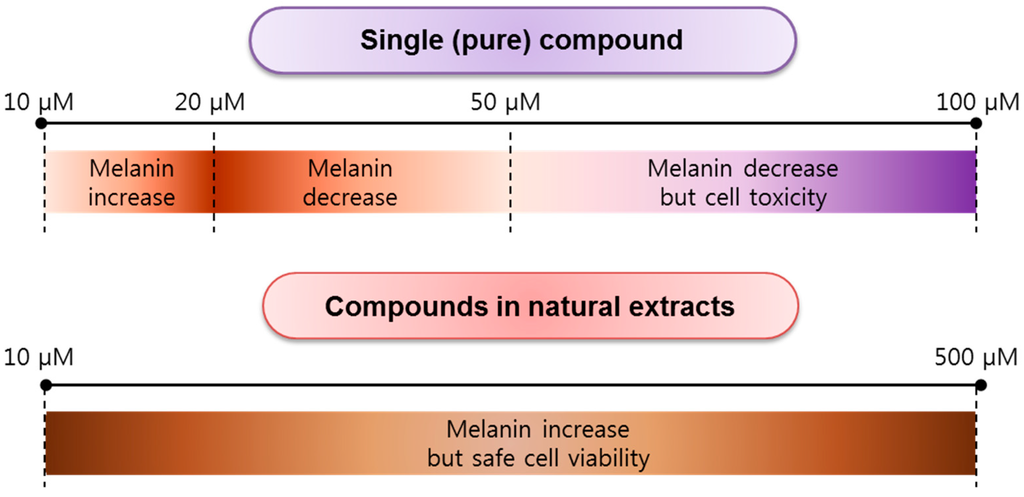
Figure 3.
Concentration dependency of pure quercetin and natural quercetin in cell viability and anti-melanogenesis.
For quercetin in the Capparis spinosa L. extract, the melanin content increases at 50–500 μM and tyrosinase is expressed at 300 μM after 24 h [33]. Masuda et al. (2012) showed that at 12.5–50 μM, the melanin content increases, while at 100 and 200 μM, the melanin content decreases [44]. In a cell system, tyrosinase activity was enhanced at quercetin concentrations of 200 μM. As P-38 MAPK is overexpressed, MITF is reduced, and ERK1/2 is overexpressed, but the activation of MITF results in low expression of tyrosinase at the protein level quercetin concentrations of 5 and 200 μM [44]. Quercetin extracted from rosehip (Rosa canina L.) results in a decrease in melanin content at 20 μM and tyrosinase activity in both cell and cell-free systems is inhibited at 10–40 μM, resulting in low tyrosinase expression at the protein level at these concentrations [37]. An et al. (2008) tested quercetin derivatives with taxifolin and luteolin as additives, and the melanin content decreased at 200 μM [39]. In the presence of taxifolin and luteolin in a cell system, tyrosinase activity is inhibited and tyrosinase is overexpressed at quercetin concentration of 200 μM [38]. Synthesized quercetin is related to decreased melanin content at concentrations of 6.25–100 μM. It is associated with low expression of tyrosinase, TRP-1, TRP-2, and p38 MAPK and a lack of stimulation of MITF and phosphorylated-p38 (p-p38) MAPK [34]. Most quercetin derivatives lead to increased melanin content. However, quercetin-galactose-rhamnose-xylose and quercetin-glucose-rhamnose result in decreases at 72 μM after 72 h and the mRNA expression levels of tyrosinase, TRP-1, TRP, MITF, and MC1R are downregulated. As a result, the tyrosinase protein expression level is low. Arung et al. (2011) found that quercetin glucoside (Allium cepa) decreases the melanin content at 1–100 μM [43].
In summary, melanin content is not closely associated to tyrosinase activity or mRNA and protein expression levels [45]. However, Chao et al. (2013) reported that the melanin content is related to a decrease in tyrosinase activity, a downregulation of mRNA expression, and a decrease in protein expression levels [46]. Clearly, the anti-melanogenesis effect of quercetin is not clear and it remains to be determined whether it induces an increase or decrease in melanin content. It is possible that the effect depends on the quercetin concentration, and this should be tested in vitro using melanoma cells.
In order to evaluate the anti-melanogenesis effect of quercetin, in vivo analyses were performed using the zebrafish, useful for studies of melanogenic inhibitors or stimulators in terms of deformed morphologies or cell killing [47], because of its cost-effective and rapid analyses, its close physiological relevance to humans [48], and the establishment of a transgenic zebrafish for superficial skin ablation [49]. Chen et al. (2011) found that zebrafish larvae exhibit low toxicity owing to the radical oxygen scavenging properties of the antioxidant quercetin [42]. Mitochondrial ATPase as a target in zebrafish embryos modulates pigmentation in both melanocytes and melanoma cells [50]. Xenopus laevis pigment cell development can be tested using NSC 86153 compound but, unfortunately, not using quercetin [51]. However, an anti-melanogenesis effect of quercetin has not been observed in the zebrafish model. Therefore, our group recently tested zebrafish for an anti-melanogenic effect based on quercetin concentration, and found a negligible effect on hypo-pigmentation and cell mortality at quercetin concentrations >100 μM. These data will be shown elsewhere.
4. Concluding Remarks and Perspectives
Based on a number of in vitro studies and relatively few in vivo studies, quercetin and its derivatives are not effective anti-melanogenesis agents. Pure quercetin, at >50 μM, results in a decreased melanin content, while at 10–20 μM, the melanin content increases in a concentration-dependent manner (Figure 3). Therefore, quercetin is not effective in cosmetic applications as a whitening ingredient. However, quercetin glycosides may be suitable for anti-melanogenesis purposes with no cytotoxicity. Other quercetin derivatives, including cyanidins, merit further tests of their anti-melanogenesis effects in zebrafish, humans, and other animals. To minimize cell toxicity, the use of vitamin C and arbutin with quercetin has been suggested. The growing demand for whitening cosmetics has had a positive impact on the search for anti-melanogenesis ingredients. The consistent development of new and innovative agents drives market growth; hence, new assay techniques and delivery systems are being developed to improve the application of the agents in cosmetic products.
Acknowledgments
This work was supported by the Human Resource Training Program for Regional Innovation and Creativity through the Ministry of Education and National Research Foundation of Korea (2015H1C1A1035883).
Author Contributions
Moon-Hee Choi and Hyun-Jae Shin equally contributed to the writing of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| ACTH | adrenocorticotropin melanocyte stimulating hormone |
| cAMP | cyclic adenosinemonophosphate |
| cGMP | cyclic guanosine monophosphate |
| CREB | cAMP-response element binding protein |
| DCT | DOPA chrome tautomerase |
| DHI | 5,6-dihydroxyindole |
| DHICA | 5,6-dihydroxyindole-2-carboxylic acid |
| DOPA | 3,4-dihydroxyphenylalanine |
| ER | endoplasmic reticulum |
| ERK | extracellular signal-regulated kinase |
| HBTA | 5-hydroxyl-1,4-benzothiazinylalanine |
| HMVII | human melanoma of vagina |
| ICAQ | indole-2-carboxylic acid-5,6-quinone |
| IQ | indole-5,6-quinone |
| L-DOPA | l-3,4-dihydroxyphenylalanine |
| LEF | lymphoid-enhancing factor |
| MEK | methyl ethyl ketone |
| MAP kinase | mitogen-activated protein kinase |
| MART1 | melan-A, MC1R; melanocortin 1 receptor |
| MC1R | melanocortin 1 receptor |
| MITF | microphthalmia-associated transcription factor |
| MTT | 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide |
| NHEM | normal human epidermal melanocytes |
| NO | nitric oxide |
| OA1 | ocular albinism type 1 |
| PAR-2 | protease activated receptor 2 |
| PKA | protein kinase A |
| PKC-β | protein kinase C-β |
| PMEL17 | Premelanosome protein 17 |
| P13K | phosphoinositide 3-kinase |
| SCF | stem cell factor |
| TCA | tricarboxylic acid |
| TRP-1 | tyrosinase-related protein 1 |
| TRP-2 (DCT) | tyrosinase-related protein 2 (DOPA chrome tautomerase) |
| TYR | tyrosinase |
| UV | ultraviolet |
| Wnt | wingless type |
References
- Taieb, A.; Cario-Andre, M.; Briganti, S.; Picardo, M. Inhibitors and enhancers of melanogenesis. In Melanins and Melanosomes; Borovansky, J., Riley, P.A., Eds.; Wiley-Blackwell: Weinheim, Germany, 2011; pp. 117–166. [Google Scholar]
- Chang, T.S. An updated review of tyrosinase inhibitors. Int. J. Mol. Sci. 2009, 10, 2440–2475. [Google Scholar] [PubMed]
- Saxena, S.; Andersen, R.; Maibach, H.I. What do we know about depigmenting agents? Cosmet. Toilet. 2015, 130, 26–29. [Google Scholar]
- Lin, J.Y.; Fisher, D.E. Melanocyte biology and skin pigmentation. Nature 2007, 445, 843–850. [Google Scholar] [CrossRef] [PubMed]
- Chang, T.S. Natural melanogenesis inhibitors acting through the down-regulation of tyrosinase activity. Materials 2012, 5, 1661–1685. [Google Scholar] [CrossRef]
- Solano, F.; Briganti, S.; Picardo, M.; Ghanem, G. Hypopigmenting agents: An updated review on biological, chemical and clinical aspects. Pigment Cell Res. 2006, 19, 550–571. [Google Scholar] [CrossRef] [PubMed]
- Gillbro, J.M.; Olsson, M.J. The melanogenesis and mechanisms of skin-lightening agents—Existing and new approaches. Int. J. Cosmet. Sci. 2011, 33, 210–221. [Google Scholar] [CrossRef] [PubMed]
- Simon, J.D.; Peles, D.; Wakamatsu, K.; Ito, S. Current challenges in understanding melanogenesis: Bridging chemistry, biological control, morphology, and function. Pigment Cell Melanoma Res. 2009, 22, 563–579. [Google Scholar] [CrossRef] [PubMed]
- D’Lschia, M.; Wakamatsu, K.; Napolitano, A.; Briganti, S.; Garcia-Borron, J.C.; Kovacs, D.; Meredith, P.; Pezzella, A.; Picardo, M.; Sarna, T.; et al. Melanins and melanogenesis: Methods, standards, protocols. Pigment Cell Melanoma Res. 2013, 26, 616–633. [Google Scholar]
- Haq, R.; Fisher, D.E. Targeting melanoma by small molecules: Challenges ahead. Pigment Cell Melanoma Res. 2013, 26, 464–469. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.P.; Sun, X.X. Melanin: Biosynthesis, Functions, and Health Effects; Nova Science Publishers, Inc.: New York, NY, USA, 2012; pp. 3–12. [Google Scholar]
- Cao, H.H.; Tse, A.J.W.; Kwan, H.Y.; Yu, H.; Cheng, C.Y.; Su, T.; Fong, W.F.; Yu, Z.L. Quercetin exerts anti-melanoma activities and inhibits STAT3 signaling. Biochem. Pharmacol. 2014, 87, 424–434. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.C.; Chou, Y.C.; Wu, C.Y.; Chang, T.M. [8]-Ginerol inhibits melanogenesis in murine melanoma cells through down-regulation of the MAPK and PKA signal pathways. Biochem. Biophys. Res. Commun. 2013, 438, 375–381. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.N.; Yang, H.M.; Kang, S.M.; Kim, D.; Ahn, G.; Jeon, Y.J. Octaphlorethol A isolated from Ishige foliacea inhibits α-MSH-stimulated induced melanogenesis via ERK pathway in B16F10 melanoma cells. Food Chem. Toxicol. 2013, 59, 521–526. [Google Scholar] [CrossRef] [PubMed]
- Su, T.R.; Lin, J.J.; Tsai, C.C.; Huang, T.K.; Yang, Z.Y.; Wu, M.O.; Zheng, Y.Q.; Su, C.C.; Wu, Y.J. Inhibition of melanogenesis by gallic acid: Possible involvement of the PI3K/Akt, MEK/ERK and Wnt/β-catenin signaling pathways in B16F10 cells. Int. J. Mol. Sci. 2013, 14, 20443–20458. [Google Scholar] [CrossRef] [PubMed]
- Chung, K.W.; Jeong, H.O.; Jang, E.J.; Choi, Y.J.; Kim, D.H.; Kim, S.R.; Lee, K.J.; Lee, H.J.; Chun, P.; Byun, Y.; et al. Characterization of a small molecule inhibitor of melanogensis that inhibits tyrosinase activity and scavenges nitric oxide (NO). Biochim. Biophys. Acta-Gen. Subj. 2013, 1830, 4752–4761. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.S.; Kim, M.J.; Choi, Y.H.; Kim, B.K.; Kim, K.S.; Park, K.J.; Park, S.M.; Lee, N.H.; Hyun, C.G. Down-regulation of tyrosinase, TRP-1, TRP-2 and MITF expressions by citrus press-cakes in murine B16F10 melanoma. Asian Pac. J. Trop. Biomed. 2013, 3, 617–622. [Google Scholar] [CrossRef]
- Kim, A.; Yang, Y.; Lee, M.S.; Yoo, Y.D.; Lee, H.G.; Lim, J.S. NDRG2 gene expression in B16F10 melanoma cells restrains melanogenesis via inhibition of Mitf expression. Pigment Cell Melanoma Res. 2008, 21, 653–664. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.K.; Sarkar, C.; Mallick, S.; Saha, B.; Bera, R.; Bhadra, R. Human placental lipid induces melanogenesis through p38 MAPK in B16F10 mouse melanoma. Pigment Cell Res. 2005, 18, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Ando, H.; Kondoh, H.; Ichihashi, M.; Hearing, V.J. Approaches to identify inhibitors of melanin biosynthesis via the quality control of tyrosinase (review). J. Investig. Dermatol. 2007, 127, 751–761. [Google Scholar] [CrossRef] [PubMed]
- Takekoshi, S.; Nagata, H.; Kitatani, K. Flavonoids enhance melanogenesis in human melanoma cells. Tokai J. Exp. Clin. Med. 2014, 39, 116–121. [Google Scholar] [PubMed]
- Xie, L.P.; Chen, Q.X.; Huang, H.; Wang, H.Z.; Zhang, R.Q. Inhibitory effects of some flavonoids on the activity of mushroom tyrosinase. Biochemistry 2003, 68, 598–602. [Google Scholar]
- Boots, A.W.; Li, H.; Schins, R.P.F.; Duffin, R.; Heemskerk, J.W.M.; Bast, A.; Haenen, G.R.M.M. The quercetin paradox. Toxicol. Appl. Pharmacol. 2007, 222, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Heo, S.J.; Ko, S.C.; Cha, S.H.; Kang, D.H.; Park, H.S.; Choi, Y.U.; Kim, D.; Jung, W.K.; Jeon, Y.J. Effect of phlorotannins isolated from Ecklonia cava on melanogenesis and their protective effect against photo-oxidative stress induced by UV-B radiation. Toxicol. In Vitro 2009, 23, 1123–1130. [Google Scholar] [CrossRef] [PubMed]
- Siwarungson, N.; Ali, I.; Damsud, T. Comparative analysis of antioxidant and antimelanogenesis properties of three local guava (Psidium guajava L.) varieties of Thailand, via different extraction solvents. J. Food Meas. Charact. 2013, 7, 207–214. [Google Scholar] [CrossRef]
- Takekoshi, S.; Matsuzaki, K.; Kitatani, K. Quercetin stimulates melanogenesis in hair follicle melanocyte of the mouse. Tokai J. Exp. Clin. Med. 2013, 38, 129–134. [Google Scholar] [PubMed]
- Kim, Y.J. Hyperin and quercetin modulate oxidative stress-induced melanogenesis. Biol. Pharm. Bull. 2012, 35, 2023–2027. [Google Scholar] [CrossRef] [PubMed]
- Chun, H.J.; Choi, W.H.; Baek, S.H.; Woo, W.H. Effect of quercetin on melanogenesis in melan-a melanocyte cells. Korean J. Pharmacogn. 2002, 33, 245–251. [Google Scholar]
- Chun, H.J.; Hwang, S.G.; Kim, C.K.; Jeon, B.H.; Baek, S.H.; Woo, W.H. In vitro modulation of proliferation and melanization of B16/F10 melanoma cells by quercetin. Yakhak Hoeji 2002, 46, 75–80. [Google Scholar]
- Cho, H.W.; Jung, W.S.; An, B.G.; Cho, J.H.; Jung, S.Y. Isolation of compounds having inhibitory activity toward tyrosinase from Receptaculum nelumbinis. Korean J. Pharmacogn. 2013, 44, 1–5. [Google Scholar]
- Nagata, H.; Takekoshi, S.; Takeyama, R.; Homma, T.; Osamura, R.Y. Quercetin enhances melanogenesis by increasing the activity and synthesis of tyrosinase in human melanoma cells and normal human melanocytes. Pigment Cell Res. 2004, 17, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Takeyama, R.; Takekoshi, S.; Nagata, H.; Osamura, R.Y.; Kawana, S. Quercetin-induced melanogenesis in a reconstituted three-dimensional human epidermal model. J. Mol. Histol. 2004, 35, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Matsuyama, K.; Villareal, M.O.; Omri, A.E.; Han, J.; Kchouk, M.E.; Isoda, H. Effect of Tunisian Capparis spinosa L. extract on melanogenesis in B16 murine melanoma cells. J. Nat. Med. 2009, 63, 468–472. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, K.; Mitsunaga, T.; Inagaki, M.; Suzuki, T. Synthesized quercetin derivatives stimulate melanogenesis in B16 melanoma cells by influencing the expression of melanin biosynthesis proteins MITF and p38 MAPK. Bioorg. Med. Chem. 2014, 22, 3331–3340. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, K.; Mitsunaga, T.; Batubara, I. Novel quercetin glucosides from Helminthostachys zeylanica root and acceleratory activity of melanin biosynthesis. J. Nat. Med. 2013, 67, 369–374. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, K.; Mitysunaga, T.; Batubara, I. Synthesis of quercetin glycosides and their melanogenesis stimulatory activity in B16 melanoma cells. Bioorg. Med. Chem. 2014, 22, 937–944. [Google Scholar] [CrossRef] [PubMed]
- Fujii, T.; Saito, M. Inhibitory effect of quercetin isolated form Rose hip (Rosa canina L.) against melanogenesis by mouse melanoma cells. Biosci. Biotechnol. Biochem. 2009, 73, 1989–1993. [Google Scholar] [CrossRef] [PubMed]
- Taira, J.; Tsuchida, E.; Uehara, M.; Ohhama, N.; Ohmine, W.; Ogi, T. The leaf extract of Mallotus japonicas and its major active constituent, rutin, suppressed on melanin production in murine B16F1 melanoma. Asian Pac. J. Trop. Biomed. 2015, 5, 819–823. [Google Scholar] [CrossRef]
- An, S.M.; Kim, H.J.; Kim, J.E.; Boo, Y.C. Flavonoids, taxifolin and luteolin attenuate cellular melanogenesis despite increasing tyrosinase protein levels. Phytother. Res. 2008, 22, 1200–1207. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Cordovés, C.; Bartolomé, B.; Vieira, W.; Virador, V.M. Effects of wine phenolics and sorghum tannins on tyrosinase activity and growth of melanoma cells. J. Agric. Food Chem. 2001, 49, 1620–1624. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.M.; Son, Y.O.; Lee, S.A.; Jeon, Y.M.; Lee, J.C. Quercetin inhibits α-MSH-stimulated melanogenesis in B16F10 melanoma cells. Phytother. Res. 2011, 25, 1166–1173. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.H.; Yang, Z.S.; Wen, C.C.; Chang, Y.S.; Wang, B.C.; Hsiao, C.A.; Shih, T.L. Evaluation of the structure-activity relationship of flavonoids as antioxidants and toxicants of zebrafish larvae. Food Chem. 2012, 134, 717–724. [Google Scholar] [CrossRef] [PubMed]
- Arung, E.T.; Furuta, S.; Ishikawa, H.; Kusuma, I.W.; Shimizu, K.; Kondo, R. Anti-melanogenesis properties of quercetin- and its derivative-rich extract from Allium cepa. Food Chem. 2011, 124, 1024–1028. [Google Scholar] [CrossRef]
- Masuda, M.; Itoh, K.; Murata, K.; Naruto, S.; Uwaya, A.; Isami, F.; Matsuda, H. Inhibitory effects of Morinda citrifolia extract and its constituents on melanogenesis in murine B16 melanoma cells. Biol. Pharm. Bull. 2012, 35, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, S.; Matsuda, H.; Oda, Y.; Nakamura, S.; Xu, F.; Yoshikawa, M. Melanogenesis inhibitors from the desert plant Anastatica hierochuntica in B16 melanoma cells. Bioorg. Med. Chem. 2010, 18, 2337–2345. [Google Scholar] [CrossRef] [PubMed]
- Chao, H.C.; Najjaa, H.; Villareal, M.O.; Ksouri, R.; Han, J.; Neffati, M.; Isoda, H. Arthrophytum scoparium inhibits melanogenesis through the down-regulation of tyrosinase and melanogenic gene expressions in B16 melanoma cells. Exp. Dermatol. 2013, 22, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Choi, T.Y.; Kim, J.H.; Ko, D.H.; Kim, C.H.; Hwang, J.S.; Ahn, S.; Kim, S.Y.; Kim, C.D.; Lee, J.H.; Yoon, T.J. Zebrafish as a new model for phenotype-based screening of melanogenic regulatory compounds. Pigment Cell Res. 2007, 20, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Shiraki, T.; Kojima, D.; Fukada, Y. Light-induced body color change in developing zebrafish. Photochem. Photobiol. Sci. 2010, 9, 1498–1504. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.F.; Chu, C.Y.; Chen, T.H.; Lee, S.J.; Shen, C.N.; Hsiao, C.D. Establishment of a transgenic zebrafish line for superficial skin ablation and functional validation of apoptosis modulators in vivo. PLoS ONE 2011, 6, e20654. [Google Scholar] [CrossRef] [PubMed]
- Jung, D.W.; Williams, D.; Khersonsky, S.M.; Kang, T.W.; Heidary, N.; Chang, Y.T.; Orlow, S.J. Identification of the F1F0 mitochondrial ATPase as a target for modulating skin pigmentation by screening a tagged triazine library in zebrafish. Mol. Biosyst. 2005, 1, 85–92. [Google Scholar] [PubMed]
- Tomlinson, M.L.; Rejzek, M.; Fidock, M.; Field, R.A.; Wheeler, G.N. Chemical genomics identifies compounds affecting Xenopus laevis pigment cell development. Mol. Biosyst. 2009, 5, 376–384. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).