1. Introduction
Besides providing a natural protective barrier against foreign bodies for the eyes, eyelashes also possess an aesthetic function [
1,
2]. In modern society, longer and fuller eyelashes are considered a desirable physical attribute for women and a sign of femininity and beauty [
3]. To enhance the overall prominence of their eyelashes, women have employed a number of techniques (eye shadow, eyeliner, and mascara), some dating back millennia [
4,
5].
Hypotrichosis is characterized by a less than normal amount of hair, and eyelashes hypotrichosis is the term for an inadequate amount of eyelashes [
6]. Causes of eyelashes hypotrichosis are many, including hereditary, aging, chemotherapy, other medical treatments and unknown causes [
6]. Physical trauma involving the face, eye surgery and trichotillomania may also cause thin or absent lash growth [
7,
8].
To date, few pharmacologic agents have been identified and investigated in specific clinical trials to stimulate eyelash growth. Since the beginning of prostaglandin analogs (PGAs) use as ocular hypotensive agents for the treatment of glaucoma, eyelashes hypertrichosis has been reported as a side effect [
9,
10].
Currently, a synthetic PGA, bimatoprost, besides being used to lower intraocular pressure is also approved only by American Food and Drug Administration, but not by European Union for cosmetic purposes to increase eyelash length, thickness, and darkness in normal patients and in patients with palpebral hypotrichosis [
3,
6,
11]. The effectiveness of bimatoprost for eyelash growth has been demonstrated by clinician ratings, digital image analysis, and patient satisfaction [
12,
13,
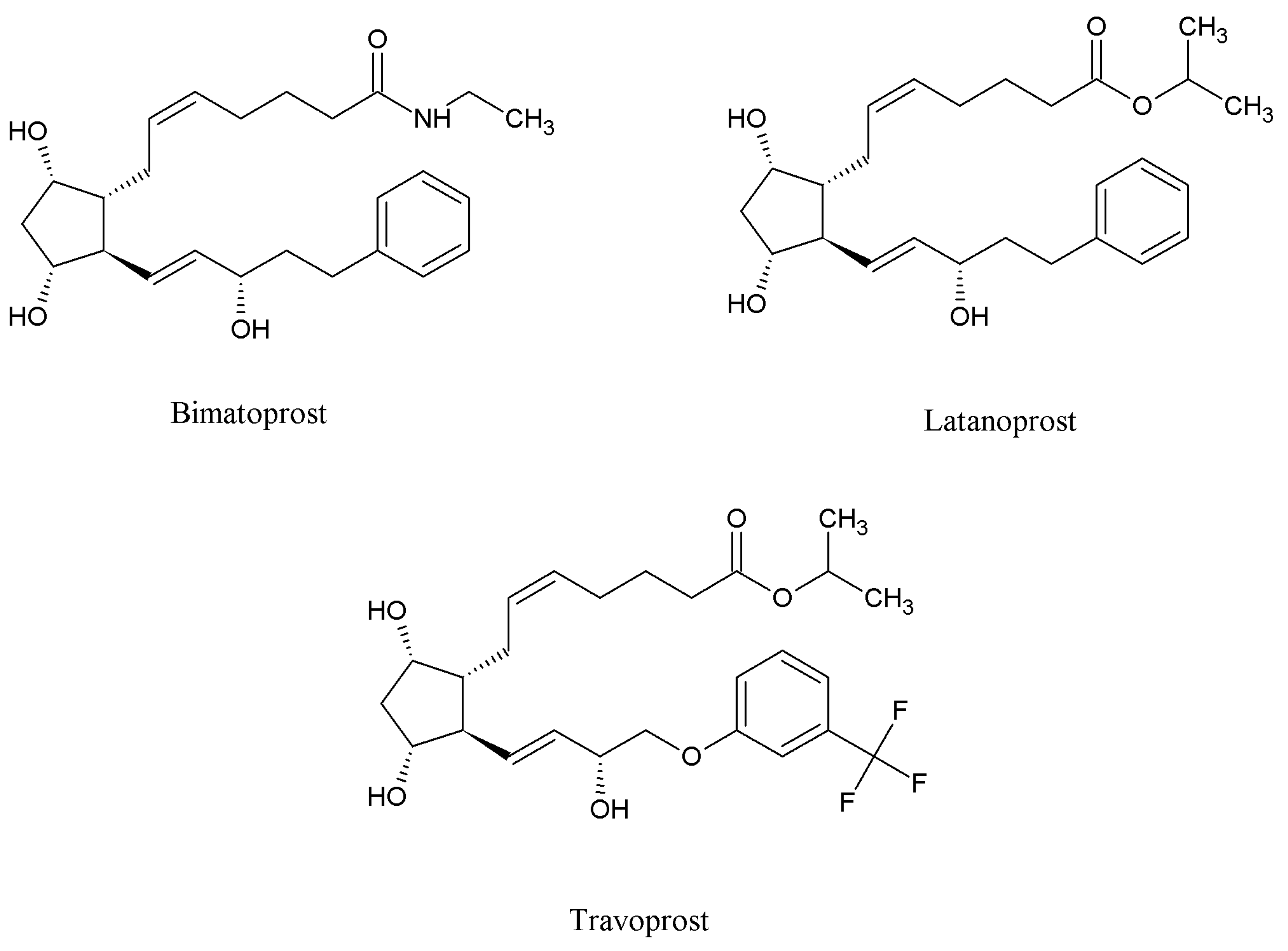
14], but the ability of PGAs to increase eyelash growth does not appear limited to bimatoprost. Two other PGAs, latanoprost and travoprost, both used to treat ocular hypertension, have been associated with eyelash changes, including increases in length and darkness (
Figure 1) [
15,
16].
Figure 1.
Structures of the prostaglandin analogs under investigation.
Figure 1.
Structures of the prostaglandin analogs under investigation.
There are other prostaglandin analogs, such as tafluprost and others (free acid, ethyl amide, isopropyl esther,
etc.), that could be used in cosmetics because they appear capable of influencing eyelash growth, but their efficacy and safety when applied to the upper-eyelid margins has not been fully studied and evaluated [
17].
Article 2 of the Cosmetic Products Regulation (EC) No 1223/2009, aiming at regulating the production of cosmetic products, defined exactly what can be considered a cosmetic: “‘cosmetic product’ means any substance or mixture intended to be placed in contact with the external parts of the human body (epidermis, hair system, nails, lips and external genital organs) or with the teeth and the mucous membranes of the oral cavity with a view exclusively or mainly to cleaning them, perfuming them, changing their appearance, protecting them, keeping them in good condition or correcting body odours” [
18].
Although cosmetic products have no therapeutic purposes and cannot claim any therapeutic action, the use of prostaglandin analogs, pharmacologically active substances present in pharmaceutical preparations, as cosmetic eyelash enhancers is becoming popular. The mechanisms by which prostaglandins trigger eyelash growth are not clear [
6]. It is suggested that hypertrichosis following administration of prostaglandin analogs is probably a result of the induction of the anagen phase (growing) in telogen phase (rest) follicles of eyelashes [
19].
Only two published methods report the determination of these substances in cosmetic products [
17,
20]. The first has been developed for the simultaneous determination of numerous prostaglandin analogs and require The QuEChERS extraction method. This is based on the liquid/liquid extraction, followed by an induced liquid–liquid partition after the addition of salts and a dispersive solid-phase extraction (D-SPE) cleanup step [
17].
The second is a general screening procedure for unknown active pharmaceutical products (e.g., weight loss substances, sexual potency enhancers, Corticosteroids and also prostaglandin analogs) in medicinal products, food supplements and herbal formulations using liquid chromatography coupled to quadrupole time of flight mass spectrometry and nuclear magnetic resonance spectroscopy [
20].
In this study, a high performance liquid chromatography tandem mass spectrometry (HPLC-MS/MS) with a rapid and simple extraction was developed to investigate the presence of bimatoprost, latanoprost and travoprost in eyelash and eyebrow enhancing cosmetic products by matrix-matched calibration process to obtain the most possible accurate data. The developed method has been applied to the analysis of prostaglandin analogs in eyelash growth serums freely sold on Internet websites.
2. Experimental
2.1. Chemicals, Reagents and Samples
All prostaglandin analogs (bimatoprost, latanoprost and travoprost), internal standard (reserpine), ultrapure water and all other reagents of HPLC-MS grade were obtained from Sigma-Aldrich (St. Louis, MO, USA).
Seven different cosmetic eyelash and/or eyebrow enhancing serums of the 27 required (20 products were not authorized for export outside the United States) were purchased from Internet websites, where these products were sold as “powerful peptide-infused night treatment to nourish lashes and to bring out their fullness and length potential”. The products presented a label with indications such as “use one or twice a day” passing.
2.2. Preparation of Standard Solutions, Calibration and Quality Control Samples
Standard stock solutions of all prostaglandin analogs (bimatoprost, latanoprost and travoprost) at 1, 0.1 and 0.01 mg/mL and reserpine (IS) at 0.5 mg/mL were prepared in methyl alcohol and stored at −20 °C.
Calibration standards containing 5 μg IS and different PGAs amounts were prepared for each analytical batch by adding suitable amounts of standard stock solutions to 100 mg pre-checked blank cosmetic serum (1, 5, 10, 50, 100, 500 μg/g). Calibration samples, injected in triplicate, were treated and processed as unknown samples, which were also analyzed in triplicate.
Several aliquots of PGAs quality control (QC) samples (low, medium, and high, respectively) at 2, 80, and 450 μg/g were prepared to be used for calculation of validation parameters.
2.3. Samples Extraction
For PGAs extraction from cosmetic serum, 100 mg product added with 5 μL of IS (1 mg/mL) were transferred into screw-capped glass tube and 995 μL of HPLC mobile phase (5 mM ammonium acetate with 0.02% formic acid (mobile phase A) and 5 mM ammonium acetate in acetonitrile/water (95/5; v/v) with 0.02% formic acid (mobile phase B), 50:50 v/v) were added. After ultrasonication for 10 min, the mixture was centrifuged at 3000 g for 5 min and 10 μL of the clear liquid phase was injected into the high performance liquid chromatography tandem mass spectrometry (HPLC-MS/MS) system.
2.4. HPLC-MS/MS for PGAs Determination
Analyses of bimatoprost, latanoprost and travoprost were performed with a high performance liquid chromatography system (Waters Acquity UPLC, Waters Corporation, Milford, MA, USA) coupled with a triple quadrupole mass spectrometer (Waters Xevo TQ, Waters Corporation). Chromatography was carried out using an kinetex biphenyl 100A (2.1 mm × 100 mm, 2.6 μm) using a linear gradient elution with two solvents 5 mM ammonium acetate with 0.02% formic acid (mobile phase A) and 5 mM ammonium acetate in acetonitrile/water (95/5; v/v) with 0.02% formic acid (mobile phase B) Solvent B was maintained at 5% for the first 4.0 min. It was increased to 95% from 4.0 to 18 min then increased to 100% from 18.0 to 18.1 min and held from 18.1 to 22 min to wash the column and then decreased to 5% from 22.0 to 22.1 min, held to 5% from 22.1 to 24 min to re-equilibration. The flow rate was kept constant at 0.25 mL/min during the analysis.
The separated analytes were detected with a triple quadrupole mass spectrometer operated in multiple reaction-monitoring (MRM) mode via positive electrospray ionization (ESI). The applied ESI conditions were the following: capillary voltage 3.0 kV, desolvation temperature 550 °C, source temperature 150 °C, cone gas flow rate 50 L/h, desolvation gas flow rate 600 L/h and collision gas flow rate 0.12 mL/min. Cone energy voltages, collision energy voltages and MRM transitions were established for each analyte and the values are listed in
Table 1.
Table 1.
HPLC-MS/MS parameters for the multiple reaction monitoring (MRM) acquisition mode.
Table 1.
HPLC-MS/MS parameters for the multiple reaction monitoring (MRM) acquisition mode.
| Analytes | Retention Time (min) | MRM Transitions |
|---|
| Quantification | Confirmation |
|---|
| m/z | CV (V) | CE (eV) | m/z | CV (V) | CE (eV) |
|---|
| Bimatoprost | 10.51 | 398.1 > 362.1 | 20 | 19 | 398.1 > 317.0 | 20 | 21 |
| Latanoprost | 12.78 | 433.3 > 337.4 | 20 | 15 | 433.3 > 397.3 | 20 | 10 |
| Travoprost | 12.84 | 501.0 > 321.4 | 20 | 5 | 501.0 > 248.9 | 20 | 10 |
| Reserpine (IS) | 13.22 | 609.2 > 397.3 | 20 | 30 | 609.2 > 195.2 | 20 | 30 |
2.5. Validation Protocol
Prior to application to real samples, HPLC-MS/MS method was tested in a validation protocol scheme following the accepted criteria for bioanalytical method validation [
21,
22].
Validation protocol applied in the present study included linearity, limits of detection (LOD) and quantification (LOQ), precision, accuracy, selectivity, carryover, matrix effect, ion suppression, recovery, and stability, as elsewhere described [
23]. LOD was defined as the lowest analyte concentration that can be detected and identified with a given degree of certainty. Standard deviation (SD) of the mean noise level over the retention time window of each analyte was used to determine LOD. A minimum requirement for signal to noise of 3 is widely accepted.
LOQ was the lowest concentration that met LOD criteria and a signal-to-noise ratio of at least 10. Validation parameters were calculated using five different daily replicates of QC samples (low, medium, and high quality control) along five subsequent working days.
Matrix effect and recovery were determined using the experimental design proposed by Matuszewski
et al. [
24]. Set 1 were five replicates of QC samples prepared in the mobile phase. Sets 2 and 3 were five replicates of blank cosmetic serum fortified with QC material after and before extraction, respectively. Matrix effect was determined by dividing mean peak areas of set 2 by those of set 1, multiplied by 100. Recovery was determined by comparing the mean peak areas of set 3 by those of set 2 multiplied by 100.
3. Results and Discussion
3.1. HPLC-MS/MS and Validation Parameters
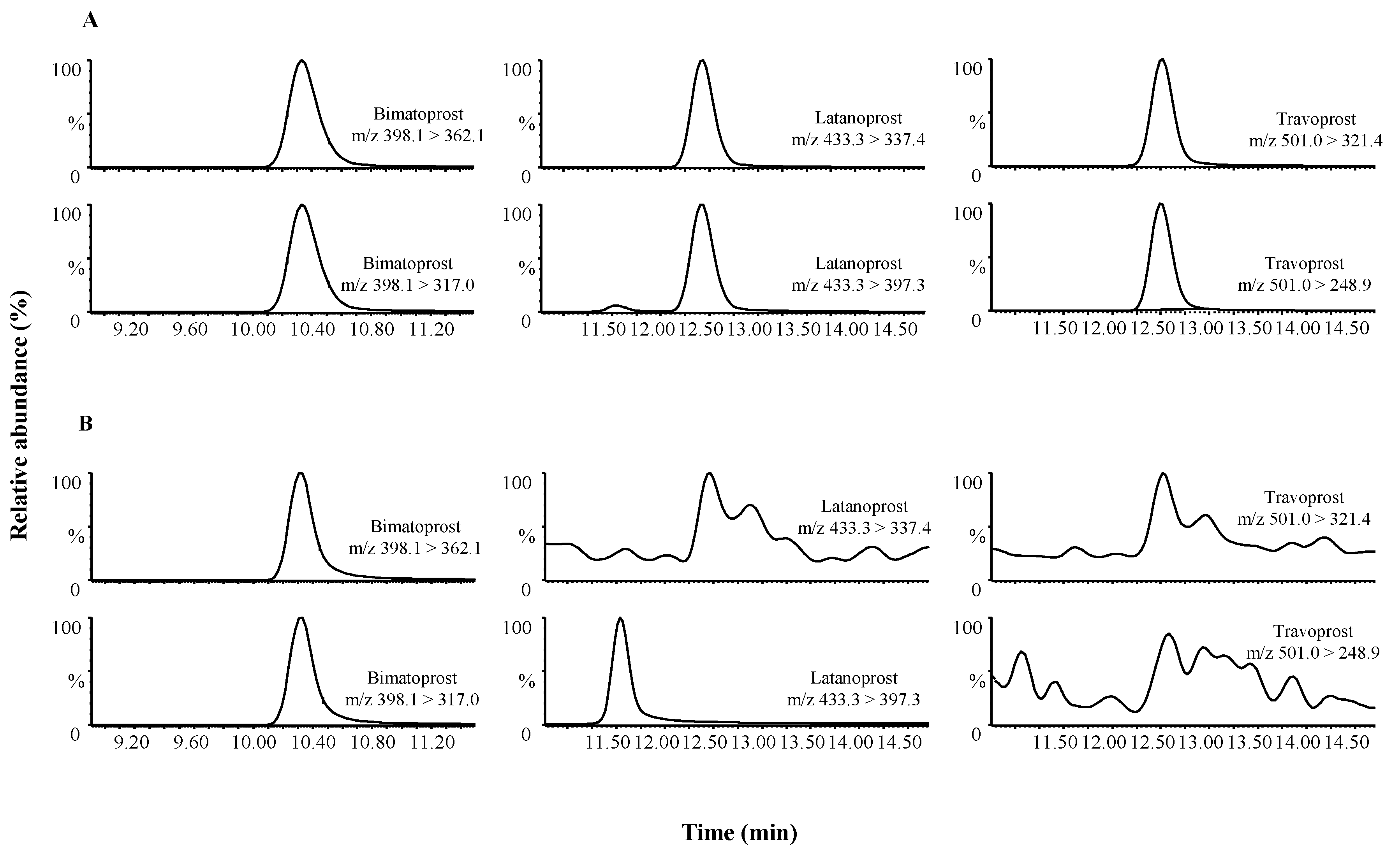
Representative chromatograms obtained following the extraction of a blank serum samples spiked with 450 μg/g PGAs under investigation and of an eyelash enhancing product containing 629.3 μg/g bimatoprost are shown in
Figure 2A,B, respectively.
Figure 2.
HPLC-MS/MS chromatograms of (A) the quantifying and qualifying transitions of all three prostaglandin analogs (450 μg/g) and (B) the quantifying and qualifying transitions obtained following the extraction of eyelash enhancing product containing 629.3 μg/g of bimatoprost.
Figure 2.
HPLC-MS/MS chromatograms of (A) the quantifying and qualifying transitions of all three prostaglandin analogs (450 μg/g) and (B) the quantifying and qualifying transitions obtained following the extraction of eyelash enhancing product containing 629.3 μg/g of bimatoprost.
In the optimization of chromatographic method, different columns and different mobile phase gradients were tested to improve separation and to reduce the time of analyses. The column and the gradient elution chosen for the present method allowed good peaks shape, good separation and absence of interfering peaks and most importantly a low back pressure.
Linear calibration curves for bimatoprost, latanoprost and travoprost in cosmetic serum samples showed correlation coefficients (
r2) equal or higher than 0.990. LOD and LOQ values calculated for PGAs in serum samples were adequate for the purpose of the present study and mean absolute analytical recoveries obtained for the three different QC samples were always above 90% (
Table 2). The intra- and inter-assay precision (measured as coefficient of variation, CV%) and accuracy (measured as % error) values were always lower than 11% (
Table 3).
Table 2.
Calibration curve parameters, LODs, LOQs and recovery of prostaglandin analogs in eyelash enhancing products.
Table 2.
Calibration curve parameters, LODs, LOQs and recovery of prostaglandin analogs in eyelash enhancing products.
| Analytes | Correlation Coefficient (r2) a | LOD | LOQ | Mean Recovery (%) b |
|---|
| Low QC Samples | Medium QC Samples | High QC Samples |
|---|
| Bimatoprost | 0.992 ± 0.001 | 1.5 | 5.0 | 105.0 | 97.2 | 102.4 |
| Latanoprost | 0.991 ± 0.003 | 0.3 | 1.0 | 102.2 | 92.1 | 95.2 |
| Travoprost | 0.990 ± 0.002 | 0.3 | 1.0 | 94.1 | 95.4 | 102.9 |
No additional peaks due to endogenous substances, which could have interfered with the detection of the analytes under investigation, were observed in PGAs-free eyelash enhancing products.
No significant ion suppression/enhancement (less than 10% analytical signal suppression due to matrix effect) occurred during chromatographic runs.
With respect to the two previous published methods for prostaglandin analogs analysis in cosmetic products, this one proposes a simpler and cheaper samples extraction (e.g., solvent addition ultrasonication and centrifugation) than the QuEChERS extraction method presented by Wittenberg
et al. [
17] and does not require an expensive and complex quadrupole time of flight mass spectrometry and nuclear magnetic resonance spectroscopy.
Table 3.
Intra- (n = 5 for each QC sample) and inter-assay (n = 15) precision and accuracy for prostaglandin analogs (PGAs) in eyelash enhancing cosmetic serums.
Table 3.
Intra- (n = 5 for each QC sample) and inter-assay (n = 15) precision and accuracy for prostaglandin analogs (PGAs) in eyelash enhancing cosmetic serums.
| Analytes | Intra-Assay |
|---|
| Precision (CV%) | Accuracy (% Error) |
|---|
| Low QC Sample | Medium QC Sample | High QC Sample | Low QC Sample | Medium QC Sample | High QC Sample |
|---|
| Bimatoprost | 10.1 | 8.8 | 7.2 | 5.2 | 8.5 | 6.9 |
| Latanoprost | 9.7 | 7.8 | 10.1 | 6.4 | 6.2 | 4.3 |
| Travoprost | 9.1 | 10.8 | 7.3 | 7.0 | 5.1 | 7.1 |
| Analytes | Inter-Assay |
| Precision (CV%) | Accuracy (% Error) |
| Low QC Sample | Medium QC Sample | High QC Sample | Low QC Sample | Medium QC Sample | High QC Sample |
| Bimatoprost | 9.9 | 8.3 | 5.6 | 10.6 | 9.2 | 7.8 |
| Latanoprost | 9.6 | 10.8 | 4.4 | 9.8 | 7.2 | 7.6 |
| Travoprost | 9.8 | 9.9 | 6.7 | 9.0 | 7.1 | 6.5 |
3.2. Prostaglandin Analogs Content in Eyelash and/or Eyebrow Enhancing Products
Seven cosmetic products were investigated for the eventual presence of one or more of the three prostaglandins analogs (bimatoprost, latanoprost and travoprost). Only two of the seven products analyzed tested positive: one to dechloro-dihydroxy-difluoro-ethylcloprostenolamide (or tafluprost ethylamide), a bimatropost analogue labelled in the list of the ingredients, one to bimatoprost, not labelled in the list of ingredients.
The tafluprost ethyl amide, another prostaglandins analogue found in this type of products [
19], has been identified by retention time and its specific spectrum pattern (fragments
m/
z 438 > 306; 438 > 232) in comparison with the spectrum spectra library available in the instrument and in agreement with previous study [
17]. Anyway, the quantification was not possible because at the time of analysis, the pure standard was not available.
The bimatoprost content in the product resulted to be 629.3 μg/g, corresponding to 0.06%
w/
w, a double percentage with respect to the one present in the ophthalmic solution Latisse
®, the first and only Food and Drug Administration (FDA)-approved treatment for inadequate or not enough lashes [
11]. As already observed in previous investigations [
23,
25], this is the case of a non-allowed substance in a cosmetic obtained through websites of unknown origin.
Since autumn 2012 the Swedish Medical Products Agency has carried out an investigation to disclose the extent to which eyelash serums were added with prostaglandin analogs. Nine out of 26 investigated products contained prostaglandin analogs and the Agency considered this result as a very serious issue [
26]. For this reason, during the autumn of 2012 the Swedish Medical Products Agency decided to ban some eyelash serums that contained prostaglandin analogs [
26].
Certain cosmetic products require special attention from the regulators due to their scientific complexity or higher potential risk for consumers’ health. Even though not mentioned in the reported Annex II (list containing non allowed substances) of Cosmetic Products Regulation (EC) No 1223/2009, prostaglandin analogs should not be added to a cosmetic product, since they are also pharmacologically active compounds requiring medical prescription even when present in topical preparations.
4. Conclusions
Special test should be conducted on eyelash enhancing products, specially if sold on websites, to determine the presence of prostaglandin analogs that can cause side effects such as eye irritation, itching, eye pain, change of eye color and darker pigmentation around the eye.
An assay including a simple sample treatment and a high performance liquid chromatography tandem mass spectrometry method was developed for the measurement of the principal prostaglandin analogs (bimatoprost, latanoprost and travoprost) in eyelash enhancing products.
The analytical results show that prostaglandin substances can be identified in eyelash growth serums and that mass spectrometric techniques are and will be indispensable in cosmetic control. This type of detection can identify with a high grade of certainty, based on their structure and molecular weight, unknown substances that can be illegally added in cosmetics.