Abstract
During recent years, microwave irradiation has been extensively used for performing green organic synthesis. The aim of this study was to synthesize, through a microwave-assisted irradiation process, a natural surfactant with O/W emulsifying properties. Our attention was focused on polyglycerol esters of fatty acids that are biocompatible and biodegradable non-ionic surfactants widely used in food and cosmetic products. The emulsifier was obtained using vegetable raw material from renewable sources: polyglycerol derived from vegetable glycerol and rice bran oil fatty acids. The natural emulsifier obtained was then characterized and evaluated for its emulsifying properties using different doses, oil phases, rheological additives, waxes, etc. The potential application in solar products, in comparison with other natural emulsifiers, was also evaluated.
1. Introduction
Recently, both the cosmetics market and research are moving towards natural cosmetics and organic products, in order to obtain more effective and dermo-safe products according to the demands of an increasing amount of legislation [1].
The tendency of consumers to prefer natural products has oriented the scientific research in the cosmetic sector towards the study and realization of organic or natural cosmetics.
A cosmetic product can be considered natural only when it is made from natural raw materials. Natural raw materials mean those natural substances extracted from plants, animals, or minerals by physical means only, without any chemical changes having taken place.
It has been known that the surface-active agents have a negative environmental impact, therefore the biodegradability and biocompatibility have become very important and fundamental requirements, almost as much as the functionality [2,3,4,5]. The increased concern towards the environment has determined, for the surfactants, a greater interest to those of natural origin [6,7,8]. A natural surfactant, strictly speaking, is a compound derived directly from a natural source, of animal or vegetable origin, obtained by extraction, precipitation, and distillation [3,8].
There are not many surfactants in use that meet these criteria; the main reason why they are so few is not for a lack of availability (on the contrary, amphiphilic compounds are abundant in the animal domain and in vegetables), but for the expensive processes required for their production [8].
Biotechnological processes could solve this problem. Yeast and bacteria can be efficient producers of surfactants. Thus with the processes of fermentation we can get the “biosurfactants”, also known as “green surfactants”, obtained by microorganisms or extracted from biomass, or obtained from these after biotransformation [9]. Biosurfactants are structurally diverse, depending on the microorganism from which they derived, the substrates employed in the bioprocess, and the fermentation conditions. They are generally classified into acylpolyols [10], glycolipids [11], and lipopeptides [12].
However, surfactants synthesized from natural sources are considered “natural”, such as alkyl polyglycosides, sugar fatty acid esters, amino acid-based surfactants, and polyglycerol esters of fatty acids [3,13,14,15,16]. Chemical processes such as amidation, etherification, and esterification are allowed in the Cosmos-standard certification of natural ingredients [17].
Polyglycerol esters of fatty acids are biocompatible and biodegradable non-ionic surfactants widely used in foods, cosmetics, and other industrial products. Depending on their hydrophilic/lipophilic balance (HLB), polyglycerol esters can act as W/O or O/W emulsifiers. The possibility of obtaining emulsifiers with the desired HLB depends on the appropriate selection of the fatty acid and polyglycerol, their ratio, and producing conditions [16,18].
The aim of this work was to synthesize, through a process of green chemistry, a polyglycerol ester with O/W emulsifying properties. The synthesis was carried out by microwave irradiation without using either chemical reagents and/or even organic solvents.
During recent years, microwave irradiation has been extensively used for carrying out chemical reactions and has been a useful non-conventional energy source for performing organic synthesis [19,20,21]. The effect of microwave irradiation in chemical reactions is a combination of the thermal effect (i.e., overheating, hot spots, selective heating) and non-thermal effects of the highly polarizing field, in addition to effects on the mobility and diffusion that may increase the probabilities of effective contacts [20].
The microwave technology shows many advantages: rapid reactions, high purity of products, improved yields, simplified and improved synthetic procedure, wider usable range of temperature, higher energy efficiency, low environmental impact, possibility of not using “classic catalyst”, and opportunity to use water as a solvent [20,21].
The emulsifier was obtained using raw materials of vegetable origins from renewable sources: polygycerol produced from glycerol of vegetable origins and rice bran oil fatty acids. Rice bran oil has been chosen for its emollient, moisturizing, and smoothing properties on the skin [22,23]. It contains mainly oleic acid (38.4%), linoleic acid (34.4%), and α-linolenic acid (2.2%) as unsaturated fatty acids, and palmitic (21.5%) and stearic (2.9%) acids as saturated fatty acids. In contrast to most common refined vegetable oils, crude rice bran oil contains a rich unsaponifiable fraction (up to 5%) mainly composed of sterols (43%), triterpene alcohols (28%), 4-methyl sterols (10%), and less polar components (19%) [24,25]. Phytosterols include β-sitosterol, campesterol, stigmasterol, squalene, and γ-oryzanol [25,26,27]. Rice bran oil contains a little variable quantity of tocotrienols (especially β and γ), but it is naturally very rich in tocopherol [26]. Cosmetic industries use rice bran oil in sunscreen formulations, anti-aging and skin-lightening products, and in treatments for skin diseases [28,29].
The natural emulsifier obtained was then evaluated for its emulsifying properties by varying the percentage of use, the nature of the oil phase, the type of the rheological additive, and wax and consistency factors. The potential application in solar products was also evaluated.
2. Experimental Section
2.1. Materials
Materials were obtained from commercial suppliers and used as received. The following were used to synthesize the surfactant: Polyglycerol-3 (Spiga Nord S.p.A., Genova, Italy); Arginine (Sigma-Aldrich, Milan, Italy); Rice Bran Fatty Acids (Tsuno Rice Fine Chemical, Wakayama, Japan).
To prepare emulsions we used: Emulsifiers: Cetearyl alcohol, Cetearyl Glucoside (Montanov 68, Seppic, Milan, Italy); Cetearyl Olivate, Sorbitan Olivate (Olivem 1000, HallStar, Arcore, Italy); Glyceryl Stearate Citrate (Imvitor 372P, Sasol, GmbH, Witten, Germany). Oils: Cetearyl Isononanoate (Cetiol SN, Cognis, Monheim, Germany); Simmondsia Chinensis Oil (Jojoba Oil, Biochim S.R.L., Milan, Italy); Persea Gratissima Oil (Avocado Oil, Balestrini G. S.R.L., Milan, Italy); Olea Europaea (Olive) Fruit Oil (Olive Oil, Res Pharma S.R.L., Milan, Italy); Oryza Sativa Bran Oil (Rice Bran Oil, Pharmacosm Polli, Milan Italy); C12–15 Alkyl Benzoate (Cosmacol EBI, Sasol, GmbH), C12–13 Alkyl Tartrate (Cosmacol ETI, Sasol, GmbH); Caprylic/Capric Triglyceride (Myritol 318, Cognis); Dicaprylyl ether (Cetiol OE, Cognis); Octyldodecanol (Eutanol G, Cognis); Paraffinum liquidum (Mineral oil, Galeno, Prato, Italy); Olea Europaea Oil Unsaponifiables (Pantrofina OLV, Res Pharma S.R.L.). Rheological additives: Carbomer (Carbopol Ultrez 10, Noveon Italia, Milan, Italy); Acrylates/C10–30 Alkyl Acrylate Crosspolymer (Carbopol ETD 2020, Noveon Italia); Sodium Polyacrylate (Cosmedia SP, Cognis); Xanthan Gum (Keltrol T, CPKelco, GmbH, Witten, Germany); Dehydroxanthan Gum (Amaze XT, AkzoNobel, Sempach Station, Neuenkirch, Switzerland); Hydroxyethylcellulose (Natrosol 250M, Eigenmann & Veronelli, Rho, Milan, Italy); Hydroxypropyl Guar (Jaguar HP105, Rhodia Italia SPA, Bollate, Italy); Magnesium Aluminum Silicate (Veegum Ultra, Vanderbilt R.T., Norwalk, CT, USA); Hydroxypropyl Starch Phosphate (Structure XL, AkzoNobel). Factors of consistency and waxes: Cetearyl Alcohol (Lanette O, Cognis); Glyceryl Stearate (Cutina GMS, Cognis); Olea Europea Extract (Wax-Olea, Sinerga S.P.A., Varese, Italy); Prunus Armeniaca Kernel Extract (Albiwax, Sinerga S.P.A.); Triticum Vulgare Germ Extract (Granowax, Sinerga S.P.A.). UV filters: Ethylhexyl Methoxycinnamate (Parsol MCX, DSM, Basel, Switzerland); Butyl Methoxydibenzoylmethane (Parsol 1789, DSM). Preservatives and other materials: Phenoxyethanol, Methyl Paraben, Propyl Paraben, Ethyl Paraben, Butyl Paraben (Fenossiparaben, Sinerga S.P.A.); Sodium Benzoate (Sodium Benzoate, Pentagon Fine Chemicals Ltd., Widnes, UK); Potassium Sorbate (Potassium Sorbate, Tri-K Industries, Danville, NH, USA); Methylchloro Isothiazolinone, Methyl Isothiazolinone (Kathon CG, Dow Italia s.r.l., Milan, Italy); Imidazolidinyl urea (Gram 1, Sinerga S.P.A.); Disodium EDTA (Edeta BD, Basf Italia, Cesano Maderno, Italy); Glycerin (Glicerina, Akzo Nobel GMBH, Emmerich, Germany), Dimethicone (SF 18-350, GE Bayer Silicones, Erkrath, Germany); Aminomethyl propanol (AMP Ultra PC 1000, Azelis Italia S.r.l., Milan, Italy).
2.2. Methods
2.2.1. Synthesis of Polyglycerol Rice Bran Fatty Acid Esters
The fatty acids (2 g) and polyglyceryl-3 (1:1 molar ratio) were heated in a pressure tube at 160 °C under microwave irradiation for 6 h and magnetic stirring. Microwave irradiation was carried out using a monomode reactor (Discover from CEM). The internal temperature was monitored through an internal IR sensor and the maximum internal pressure monitored and maintained under the value of 1 bar. Then the reaction mixture was cooled to room temperature. The product was recovered and used without further purification. Arginine (about 1%) was added to neutralize the unreacted acids that may be present.
2.2.2. Characterization of Polyglycerol Rice Bran Fatty Acid Esters
1H NMR data were acquired at room temperature on a Bruker AC 200 and a Bruker Avance (Bruker Instruments Inc., Billerica, MA, USA) operating at 200 and 400 MHz, respectively. The spectra were recorded in CDCl3, chemical shifts (δ) are expressed in part per million with reference to tetramethylsilane (TMS) used as internal standard. The assignment of the relative chemical shifts (δ) is shown below:
1H-NMR δ: 0.75 (m, CH3); 1.12-1.17 (bp, (CH2)n–); 1.33 (m, CH2CH2COOH); 1.47 (bp, CH2CH2COOH); 1.88 (bm, CH2–CH=CH–CH2); 2.15 (m, –CH2COOH); 2.63 (m, CH=CHCH2–CH=CH); 2.73 (m, CH=CH–CH2–CH=CH); 3.3–3.65 and 3.9–4.2 (polyglycerol-3); 4.75 (m, CH=CH); 5.08–5.18 (m, CH=CH).
Infrared data were acquired with a Thermo FITR spectrometer Nicolet 5700 (Thermo Electron Corp., Madison, WI, USA) equipped with ATR (Attenuated Total Reflectance) accessory with an Internal Reflection Element of Ge and with MCT (Mercurium Cadmium Telluride) detector. The spectra were recorded accumulating 128 scans at a resolution of 2 cm−1.
Infrared radiation (IR) cm−1: 2967–2945 C–H Aliphatic Stretch; 3400 O–H Stretch; 1737 C=O Stretching frequency of ester; 1463 CH2 Stretch; 1376 CH2 Stretch.
2.2.3. Emulsifier–Xanthan Gum Interaction
This study was performed by 1H-NMR analysis. The solutions of the emulsifier (5 mg/mL), Xanthan gum (5 mg/mL) and mixture emulsifier:xanthan gum (5 mg:0.5 mg in 1 mL) were prepared using DMSO-d6 as solvent. The 1H-NMR spectra were recorded at room temperature on a Bruker Avance operating at 400 MHz.
2.2.4. Emulsions Preparation
The emulsions were prepared using a Silverson SL mixer (Silverson Machines Inc., East Longmeadow, MA, USA) and a Kirk 510 stirring paddle. The most suitable percentage for use and the influence of the amount and the type of lipophilic phase on the stability and viscosity were tested in O/W emulsions for which compositions are given in Table 1, Table 2 and Table 3.

Table 1.
Composition of O/W basic emulsions containing a different percentage of emulsifier.
| Phase | No | Ingredients | INCI Name | %w/w | ||
|---|---|---|---|---|---|---|
| E1 | E2 | E3 | ||||
| A | 1 | Emulsifier | Polyglycerol rice bran fatty acids esters | 2.50 | 5.00 | 7.50 |
| 2 | Cetiol SN | Cetearyl Isononanoate | 14.00 | |||
| 3 | SF 18-350 | Dimethicone | 1.00 | |||
| 4 | Fenossiparaben | Phenoxyethanol, Methylparaben, Ethylparaben, Propylparaben, Butylparaben | 0.50 | |||
| B | 5 | Water | Aqua | qs 100.00 | ||
| 6 | Glyceryn | Glyceryn | 4.00 | |||
| C | 7 | Sodium Benzoate | Sodium Benzoate | 0.30 | ||
| 8 | Potassium Benzoate | Potassium Benzoate | 0.30 | |||
| 9 | Edeta BD | Disodium EDTA | 0.15 | |||

Table 2.
Composition of O/W basic emulsions containing a different percentage of oil phase.
| Phase | No | Ingredients | INCI Name | %w/w | |||
|---|---|---|---|---|---|---|---|
| E4 | E5 | E6 | E7 | ||||
| A | 1 | Emulsifier | Polyglycerol rice bran fatty acids esters | 5.00 | |||
| 2 | Cetiol SN | Cetearyl Isononanoate | 9.00 | 19.00 | 24.00 | 29.00 | |
| 3 | SF 18-350 | Dimethicone | 1.00 | ||||
| 4 | Fenossiparaben | Phenoxyethanol, Methylparaben, Ethylparaben, Propylparaben, Butylparaben | 0.50 | ||||
| B | 5 | Water | Aqua | qs 100.00 | |||
| 6 | Glyceryn | Glyceryn | 4.00 | ||||
| C | 7 | Sodium Benzoate | Sodium Benzoate | 0.30 | |||
| 8 | Potassium Benzoate | Potassium Benzoate | 0.30 | ||||
| 9 | Edeta BD | Disodium EDTA | 0.15 | ||||

Table 3.
Composition of O/W basic emulsions containing oils with different polarity.
| Phase | No | Ingredients | INCI Name | %w/w |
|---|---|---|---|---|
| E8(a–h) | ||||
| A | 1 | Emulsifier | Polyglycerol rice bran fatty acids esters | 5.00 |
| 2 | Mineral Oil Pantrofina OLV Cetiol OE Avocade Oil Olive Oil Rice Bran Oil Myritol 318 Jojoba Oil Eutanol G | Paraffinum Liquidum Olea Europaea Oil Unsaponifiables Dicaprylyl Ether Persea Gratissima Oil Olea Europea Fruit Oil Oryza Sativa Bran Oil Caprylic/Capric Triglyceride Simmondsia Chinensis Oil Octyldodecanol | 14.00 | |
| 3 | SF 18-350 | Dimethicone | 1.00 | |
| 4 | Fenossiparaben | Phenoxyethanol, Methylparaben, Ethylparaben, Propylparaben, Butylparaben | 0.50 | |
| B | 5 | Water | Aqua | qs 100.00 |
| 6 | Glyceryn | Glyceryn | 4.00 | |
| C | 7 | Sodium Benzoate | Sodium Benzoate | 0.30 |
| 8 | Potassium Benzoate | Potassium Benzoate | 0.30 | |
| 9 | Edeta BD | Disodium EDTA | 0.15 |
The influence of the rheological additive on the stability and viscosity was tested on O/W emulsions for which compositions are given in Table 4.

Table 4.
Composition of O/W basic emulsions containing different rheological additives.
| Phase | No | Ingredients | INCI Name | %w/w |
|---|---|---|---|---|
| E9(a–i) | ||||
| A | 1 | Emulsifier | Polyglycerol rice bran fatty acids esters | 5.00 |
| 2 | Cetiol SN | Cetearyl Isononanoate | 5.00 | |
| 3 | Myritol 318 | Caprylic/Capric Triglyceride | 5.00 | |
| 4 | Cetiol OE | Dicaprylyl Ether | 4.00 | |
| 5 | SF 18-350 | Dimethicone | 1.00 | |
| 6 | Fenossiparaben | Phenoxyethanol, Methylparaben, Ethylparaben, Propylparaben, Butylparaben | 0.50 | |
| B | 7 | Water | Aqua | qs 100.00 |
| 8 | Carbopol Ultrez 10 | Carbomer | 0.10–1.00 * | |
| Carbopol ETD 2020 | Acrylates/C10-30 Alkyl Acrylate Crosspolymer | |||
| Cosmedia SP | Sodium Polyacrylate | |||
| Keltrol T | Xanthan Gum | |||
| Amaze XT | Dehydroxanthan Gum | |||
| Natrosol 250M | Hydroxyethylcellulose | |||
| Structure XL | Hydroxypropylstarch Phosphate | |||
| Jaguar HP 105 | Hydroxypropyl Guar | |||
| Veegum Ultra | Magnesium Aluminum Silicate | |||
| 9 | Glycerin | Glycerin | 4.00 | |
| 10 | AMP Ultra PC 1000 | Aminomethyl propanol | 0.06–0.12 | |
| C | 11 | Sodium Benzoate | Sodium Benzoate | 0.3 |
| 12 | Potassium Benzoate | Potassium Benzoate | 0.3 | |
| 13 | Edeta BD | Disodium EDTA | 0.15 |
* E9a1: 0.10%; E9a2: 0.15%; E9a3: 0.20%; E9b1: 0.10%; E9b2: 0.15%; E9c: 0.50%; E9d1: 0.30; E9d2: 0.50; E9e1: 0.30%; E9e2: 0.50%; E9f: 0.50%; E9g1: 0.50%; E9g2: 1.00%; E9h: 0.50%; E9i1: 0.50%; E9i2: 1.00%.
In Table 5, we report the compositions of the emulsions prepared to evaluate the influence of vegetable wax and consistency factors on the stability and viscosity.

Table 5.
Compositions of O/W basic emulsions containing vegetable wax and consistency factors.
| Phase | No | Ingredients | INCI Name | %w/w | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| E9 | E10 | E11 | E12 | E13 | E14 | E15 | ||||
| A | 1 | Emulsifier | Polyglycerol rice bran fatty acids esters | 5.00 | ||||||
| 2 | Cetiol SN | Cetearyl Isononanoate | 5.00 | |||||||
| 3 | Myritol 318 | Caprylic/Capric Triglyceride | 5.00 | |||||||
| 4 | Cetiol OE | Dicaprylyl Ether | 4.00 | |||||||
| 5 | Cutina GMS | Glyceryl Stearate | – | 1.00 | – | 1.00 | – | – | – | |
| Lanette O | Cetearyl Alcohol | – | – | 1.00 | 1.00 | – | – | – | ||
| 6 | Wax-Olea | Olea Europea Extract | – | – | – | – | 1.50 | – | – | |
| Albiwax | Prunus Armeniaca Extract | – | – | – | – | – | 1.50 | – | ||
| Granowax | Triticum Vulgare Germ Extract | – | – | – | – | – | – | 1.50 | ||
| 7 | SF 18-350 | Dimethicone | 1.00 | |||||||
| 8 | Fenossiparaben | Phenoxyethanol, Methylparaben, Ethylparaben, Propylparaben, Butylparaben | 0.50 | |||||||
| B | 9 | Water | Aqua | qs 100.00 | ||||||
| 10 | Glycerin | Glycerin | 4.00 | |||||||
| C | 11 | Sodium Benzoate | Sodium Benzoate | 0.30 | ||||||
| 12 | Potassium Benzoate | Potassium Benzoate | 0.30 | |||||||
| 13 | Edeta BD | Disodium EDTA | 0.15 | |||||||
In Table 6, we report the compositions of the solar emulsions (UVB and UVA) prepared to evaluate the influence of different emulsifiers on Sun Protection Factor (SPF) values.

Table 6.
Compositions of O/W emulsions containing UVB (E16a–d) and UVA (E17a–d) filters prepared with different emulsifiers.
| Phase | No | Ingredients | INCI Name | %w/w | |
|---|---|---|---|---|---|
| E16a–d | E17a–d | ||||
| A | 1 | (a) Emulsifier | Polyglycerol rice bran fatty acids esters | 6.00 | |
| (b) Olivem 1000 | Cetearyl Olivate, Sorbitan Olivate | ||||
| (c) Montanov 68 | Cetearyl alcohol, Cetearyl Glucoside | ||||
| (d) Imvitor 372P | Glyceryl Stearate Citrate | ||||
| 2 | Parsol MCX | Ethylhexyl Methoxycinnamate | 6.50 | – | |
| 3 | Parsol 1789 | Butyl Methoxydibenzoylmethane | – | 3.50 | |
| 4 | Cutina GMS | Glyceryl Stearate | 3.00 | 3.00 | |
| 5 | Lanette O | Cetearyl Alcohol | 1.00 | 1.00 | |
| 6 | Cosmacol EBI | C12–15 Alkyl Benzoate | 6.75 | 8.25 | |
| 7 | Cosmacol ETI | C12–13 Alkyl Tartrate | 6.75 | 8.25 | |
| 8 | SF 18-350 | Dimethicone | 1.00 | 1.00 | |
| B | 9 | Water | Aqua | qs 100.00 | |
| 10 | Glycerin | Glycerin | 4.00 | ||
| C | 11 | Kathon CG | Methylchloro Isothiazolinone, Methyl Isothiazolinone | 0.05 | |
| 12 | Gram 1 | Imidazolidinyl urea | 0.30 | ||
| 13 | Edeta BD | Disodium EDTA | 0.15 | ||
General procedure: mix ingredients of phase A and heat to 65 °C; heat water to 70 °C and disperse rheological additive using a turbo-emulsifier to obtain a homogeneous system; then add glycerin. Add A to B while stirring and homogenize; then neutralize with Aminomethyl propanol when Carbomer and Acrylates/C10–30 Alkyl Acrylate Crosspolymere were used, and homogenize again. Let the emulsion cool under stirring, then at 40 °C add phase C dispersed in water (10 mL).
Evaluation of Emulsions
All preparations were evaluated by measuring the pH at 25 °C (10% in water) using an Orma pHmeter and the viscosity at 0.5 and 1 rpm at 25 °C using a Brookfield DV-II rotational viscosimeter. Emulsion stability was evaluated using the following accelerated aging processes at some time during 3 months: (a) storage at 4, 25, and 40 °C; (b) storage at hot/cold cycle (4–40 °C, two cycles per 24 h); (c) centrifugation at 3000 rpm at room temperature. The physical parameters were measured on fresh and stored emulsions in triple.
The structure of the emulsion was investigated with an optical microscope (Axio Vert.A1 Inverted Microscope, Carl Zeiss Microscopy GmbH, Gottingen, Germany) connected with a camera (AxioCam ERc5s, Carl Zeiss Microlmaging GmbH, Jena, Germany) and picture analysis software. The prepared emulsion was placed on the microscope slide. A cover slip was placed on the sample. No air, or bubbles, were trapped between the sample and cover slip, and the samples were tested with a 40× objective.
2.2.5. Sun Protection Factor Evaluation
The protective efficacy of the solar emulsions (UVB and UVA) was examined by measuring the in vitro Sun Protection Factor (SPF) [30,31] using a Labsphere spectrophotometer (UV-1000S Ultraviolet Transmittance Analyzer, Labsphere Inc., North Sutton, NH, USA), a quality control tool designed specifically for this purpose. According to the COSMETICS EUROPE protocol [32], 2 mg/cm2 of the emulsion were spread on TransporeTM tape and the SPF was measured after 15 min, according to the Diffey and Robson equation reported below, where Tλ is the sunscreen transmittance at wavelength λ, Eλ is the spectral irradiance of “standard sun” corresponding to the COLIPA “SPF method” (sunlight expected for a clear sky at noon in midsummer for a latitude of 40°N and solar altitude 70°), and Bλ is the erythema action spectrum adopted by the International Commission on Illumination (CIE) [33]. The UV-1000S calculates the SPF of the sunscreen sample by measuring the spectral transmittance of UV radiation (290–400 nm) through the TransporeTM substrate before and after application of the sunscreen product. The term transmittance refers to the percentage of the radiant flux transmitted through the sample, relative to the incident flux. Five measurements were made for each sample and the mean standard deviations were calculated.
2.2.6. Photostability Test
The photostability of the solar emulsions were evaluated through the determination of SPF values before and after irradiation. Samples were exposed to the solar simulator equipped with a xenon lamp (Universal Arc Lamp Housing model 66000 and Arc Lamp Power Supply model 68805, LOT ORIEL Italia, Milan, Italy). Before each measurement the xenon arc lamp was calibrated with a radiometer (Goldilux Smart Meter model 70234, LOT ORIEL Italia, Milan, Italy) equipped with a UVB and UVA probes. Samples were placed 40 cm from the lamp, irradiated with 600 mJ/cm2, equivalent to 20 MED (Minimal Erythemal Dose: 1 MED = 25 mJ/cm2 for skin phototype II) [34], and air-cooled during irradiation.
2.2.7. Statistical Analysis
Findings were compared by analysis of variance (ANOVA). p < 0.05 was considered statistically significant.
3. Results and Discussion
A natural surfactant with O/W emulsifying properties was synthesized through a microwave-assisted irradiation process. The reaction was carried out using natural raw materials such as polyglycerol-3 and rice bran oil fatty acids. The synthesis does not involve ethylene oxide and performs without using either a chemical reagent or even an organic solvent. The reaction scheme and the structure of the polyglycerol esters are represented in Figure 1.
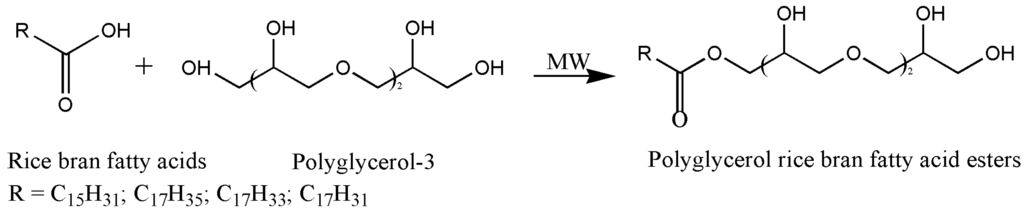
Figure 1.
Reaction scheme and structure of the polyglycerol esters synthesized by microwave-assisted irradiation process.
The product does not require purification but only the neutralization of unreacted fatty acids. For this purpose the amino acid arginine was chosen. The product was characterized by 1H-NMR and IR analyses. The NMR data show the presence of the signals of the protons of fatty acids and polyglycerol. In particular, the signals at δ = 0.75–2.73 for the protons bonded to saturated carbons and the signals at δ = 4.75–5.18 for the protons bonded to unsaturated carbons. The signals at δ = 3.3–3.65 (corresponding to CH and CH2 protons) and at δ = 3.9–4.2 (corresponding to OH protons) are due to the polyglycerol moiety.
The emulsifier is hydrodispersible: it can form liquid crystal lamellar structures without the help of other co-emulsifiers, regardless of the chemical structure and polarity of the substances present in the internal phase of the emulsion. Under a microscope with polarized light their liquid crystal structure is quiet clear, as the following photograph, taken at ×250, demonstrates (Figure 2).
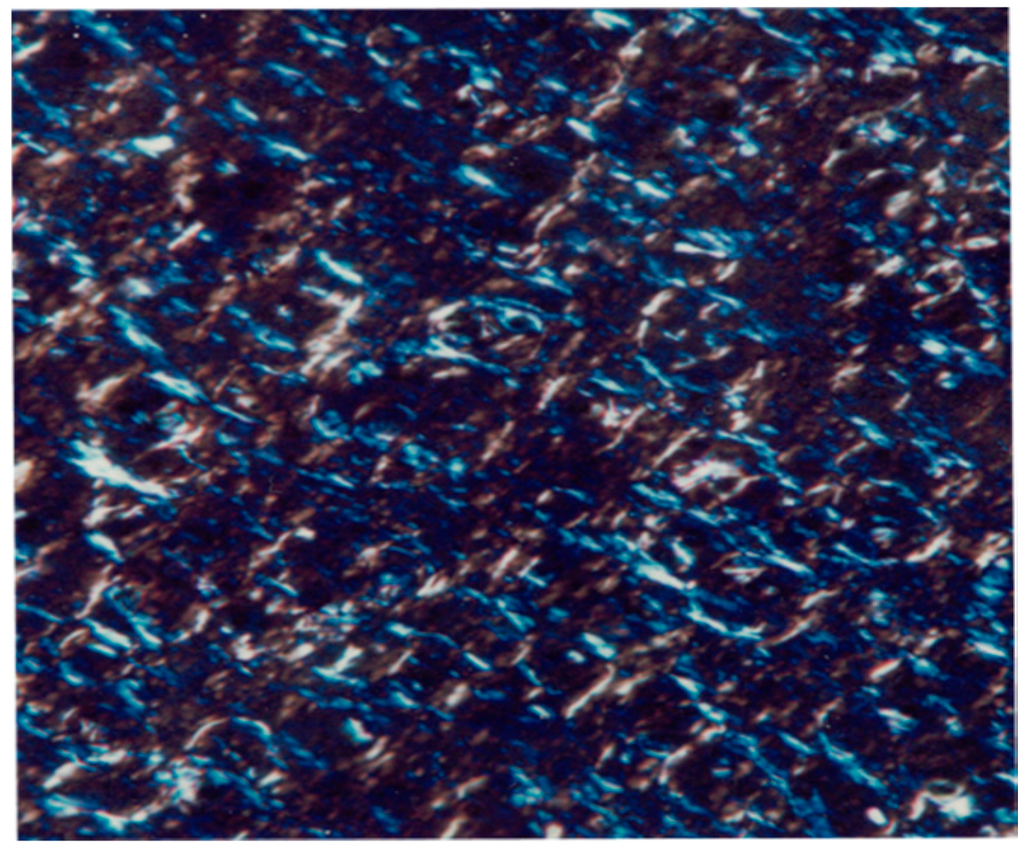
Figure 2.
Liquid crystal structure observed under a polarizing microscope.
This feature is interesting because the liquid crystal system could enhance the stability and moisturizing ability of the emulsion [35,36,37,38].
The emulsifying properties were evaluated through the preparation of a series of O/W emulsions.
For this purpose, we designed experimental emulsions containing a few essential ingredients. The compatibility between the emulsifier with various ingredients (oils, rheological additives, consistency factors) was investigated. The emulsions were added with a preservative system that, according to our experience, was suitable for the storage of the preparation during the time necessary for the stability assessment.
The study began with the preparation of emulsions containing three different concentrations of emulsifier (Table 1): 2.5%, 5%, and 7.5%, with the same kind and amount of oil phase (14%), choosing a medium polar oil such as Cetearyl isononanoate, and without the use of any rheological additive, to detect the minimum concentration of emulsifier necessary for the stability of the system.
The best stable emulsion was obtained with 5% minimum of emulsifier (E2), while those with 2.5% (E1) have not passed the test of stability. Emulsion E1 did not pass the test in the centrifuge and was separated after two weeks of storage at 40 °C and after three weeks of storage at hot/cold cycle. Emulsion viscosity increased with the emulsifier concentration (Figure 3).
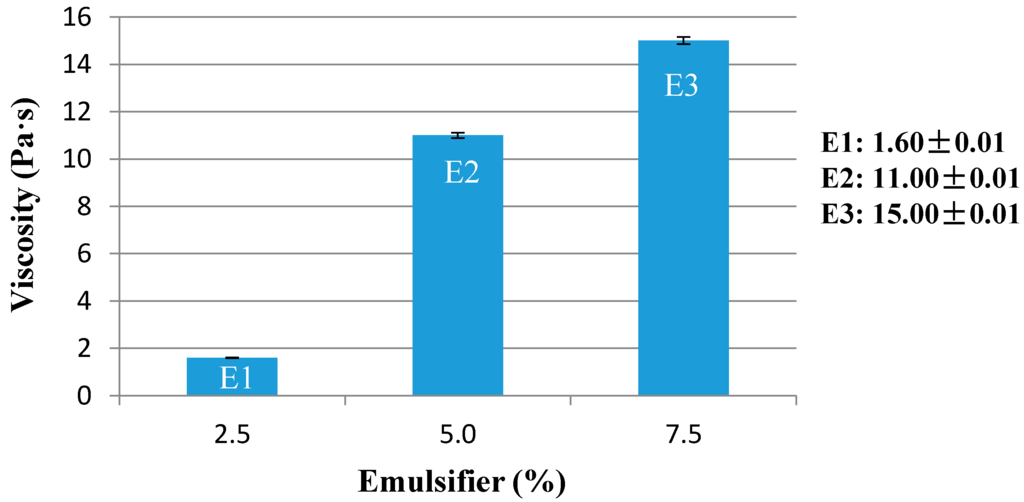
Figure 3.
Viscosity of O/W basic emulsions (E1–E3) containing a different percentage of emulsifier.
Therefore, the results show that the emulsifier can be used in a range of 2.5%–7.5%. The more suitable dose is 5%. The emulsion prepared with the lowest dose requires, for the stabilization, a rheological additive, as will be shown later.
In a second step, the percentage of internal phase volume was varied and stability was investigated. Keeping constant the concentration of emulsifier (5%) and the nature of the oil phase (Cetearyl isononanoate), the concentration of oil phase has been changed from 9% to 29% (Table 2). All these emulsions have passed the test of stability showing that variable amounts of oils could be emulsified from 9% to 29%. The formulations containing 14% (E2) and 19% (E5) of the oil phase showed the best organoleptic characteristics (white color, polished appearance, and good texture). The amount of oil phase affects the viscosity of the emulsion, as shown in Figure 4.
The viscosity increase up to 25% (E6) of internal phase volume then returns to decrease (E7). The decrease of viscosity could be due to the increase of the size of the internal Phase [39,40,41]. The emulsion E7, with lower viscosity, showed bigger droplets (15–40 μm) compared to the emulsion E6 (5–6 μm), as shown in Figure 5.
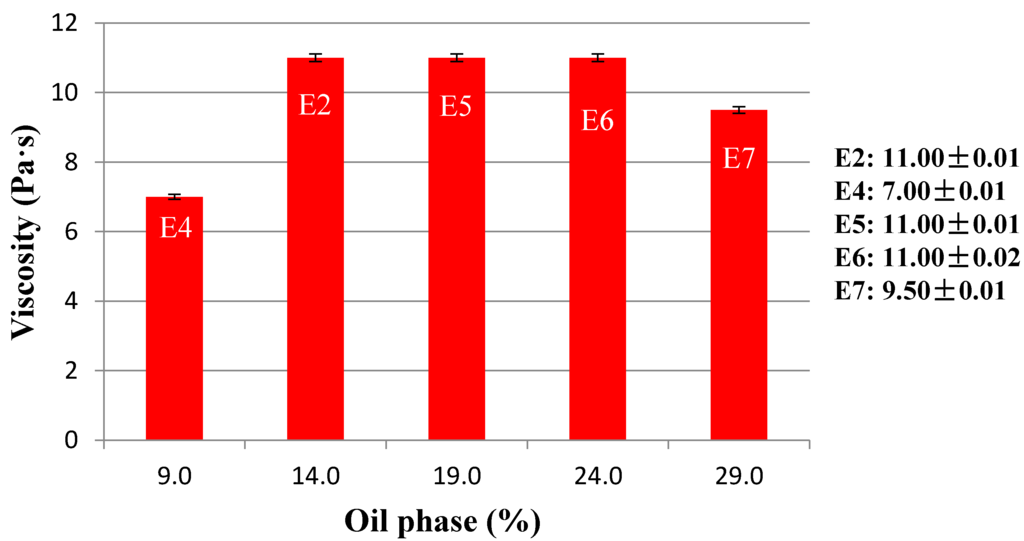
Figure 4.
Viscosity of O/W basic emulsions (E2, E4–E7) containing different amounts of oil phase.
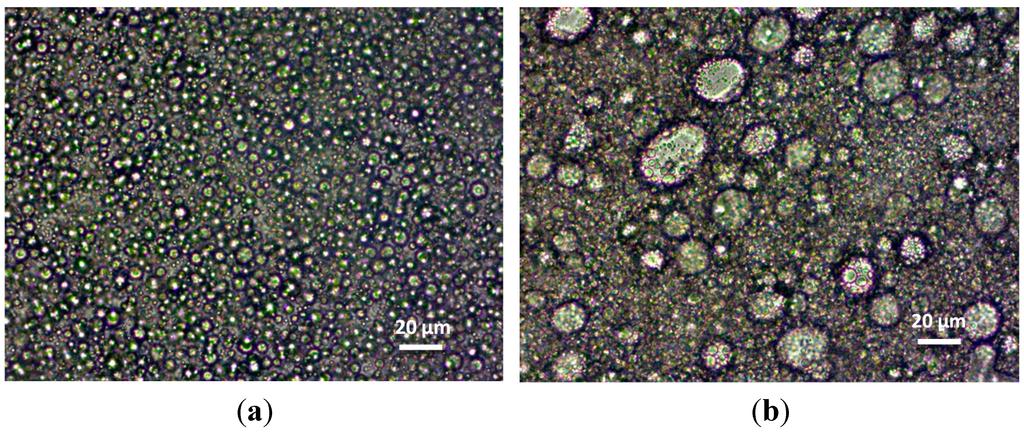
Figure 5.
Morphology of the emulsions E6 (a) and E7 (b) observed under optical microscope.
In the third step, the concentration of emulsifier at 5% and the oil phase at 14% were kept constant and various emulsions were prepared by changing the nature of the internal phase (Table 3). The synthetic ester (E2) was replaced with other oils with different polarity: hydrocarbons (E8a), ether (E8c), natural (E8d–f) and synthetic triglycerides (E8g), vegetable ester (E8h), and alcohol (E8i).
All these emulsions have passed the test of stability except those prepared with Octyldodecanol (E8i) and mineral oil (E8a). The results obtained highlighted the higher affinity of the emulsifier to the moderately polar oils (ethers, triglycerides, esters). Also in these case, the type of oil phase affects the viscosity of the system (Figure 6). The hydrocarbon (apolar) and the alcohol (the more polar ingredient used) showed a fluidifying effect. The unsaponifiable of olive oil, used as oil phase (emulsion E8b), containing a mixture of hydrocarbons, alcohols, sterols, tocopherols, etc., showed the same behavior. This result confirms the low compatibility of the emulsifier with ingredients apolar and polar.
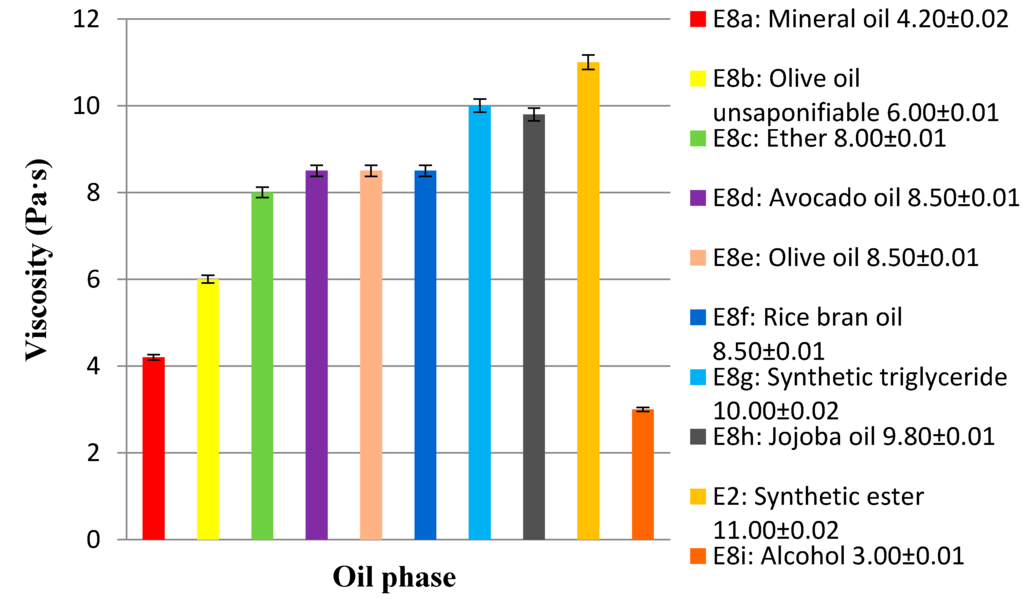
Figure 6.
Viscosity of O/W basic emulsions (E2, E8a–i) prepared with oils of different polarity. The emulsions are reported according to the increasing polarity of the oil phase.
In the next step, the influence of a rheological additive on the stability and viscosity was evaluated. For this purpose a reference basic emulsion was added with different types of rheological additives at various concentrations (Table 4). The basic emulsion (E9) was prepared maintaining the concentration of emulsifier at 5% and the oil phase at 14%, choosing a blend of three oils that gave the best results: Cetearyl Isononanoate, Caprylic/Capric Triglyceride, and Dicaprylyl Ether. All the emulsions have passed the test of stability except those prepared with 0.5% of Xanthan gum (E9d2) and 1% of Magnesium Aluminum Silicate (E9i2). In Figure 7 are reported the results obtained. As we can see the rheological additives used have a different effect on the viscosity. Substances which have shown a fluidizing effect, and therefore not compatible, are the inorganic derivatives (E9i1 and E9i2), xanthan gum (E9d1 and E9d2), hydroxyethylcellulose (E9f) and hydroxypropyl guar (E9h).
The results obtained showed that rheological additives of polysaccharide nature caused a decrease of viscosity which in turn, in the case of xanthan gum, determined a destabilizing effect of the emulsion. Surfactants–polymers interactions are known [42,43,44,45,46,47]. Thus we searched for the presence of an interaction between the emulsifier and xanthan gum. This study was performed by NMR analysis.
1H-NMR spectra of the free emulsifier and of the emulsifier in the presence of the polymer in the same ratio used in the emulsion (5:0.5 emulsifier:polymer) were acquired. The experiments were carried out in DMSO-d6, a solvent in which both substances are soluble. The comparison of the spectra showed a change in the signals of the protons of polyglycerol when it was mixed with the xanthan gum. The peaks are more broad and this effect influences the signals of both OH and CH and CH2. This fact highlights the influence of an interaction between the polymer and the hydrophilic head of the emulsifier.
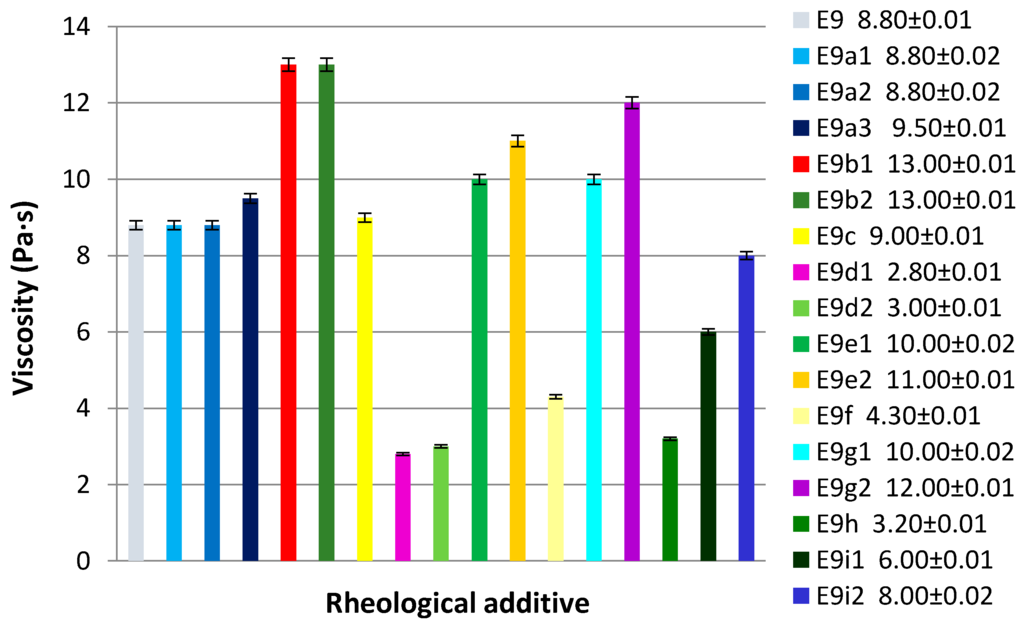
Figure 7.
Effect of rheological additive on the viscosity of basic emulsion (E9): E9a1: Carbomer 0.1%; E9a2: Carbomer 0.15%; E9a3: Carbomer 0.20%; E9b1: Acrylates/C10–30 Alkyl Acrylate Crosspolymer 0.10%; E9b2: Acrylates/C10–30 Alkyl Acrylate Crosspolymer 0.15%; E9c: Sodium Polyacrylate; E9d1: Xanthan gum 0.30%; E9d2: Xanthan gum 0.50%; E9e1: Dehydroxanthan gum 0.30%; E9e2: Dehydroxanthan gum 0.50%; E9f: Hydroxyethylcellulose; E9g1: Hydroxypropylstarch phosphate 0.50%; E9g2: Hydroxypropylstarch phosphate 1.00%; E9h: Hydroxypropyl guar; E9i1: Magnesium Aluminum Silicate 0.50%; E9i2: Magnesium Aluminum Silicate 1.00%.
The high molecular weight of xanthan gum and the formation of aggregates via hydrogen bonding are the reasons why its solutions exhibit high viscosity [48]. We can hypothesize that the interaction between xanthan gum and emulsifier may decrease the formation of aggregates with a consequent decrease of the viscosity. Furthermore, this interaction could promote the migration of the emulsifier from the interface oil/water followed by the separation of the emulsion.
The effect on the viscosity of two consistency factors and three different vegetable waxes was also studied and compared to a basic emulsion (E9) not containing any of them (always keeping constant the concentration of emulsifier at 5% and the oil phase at 14%, and using the same mixture of oils previously used (Table 5). In Table 7, the results obtained are reported.
All these emulsions have passed the test of stability, except for the emulsion prepared with Prunus Armeniaca Kernel extract (E14).
Moreover, it was evaluated the stabilizing effect of the rheological additives and consistency factors in formulations that in earlier tests, were not stable (i.e., emulsion containing 2.5% emulsifier, E1). Only the emulsion prepared with Acrylates/C10–30 Alkyl Acrylate Crosspolymer 0.20% has passed the test of stability.

Table 7.
Viscosity values of emulsions containing consistency factors and vegetable waxes.
| No | Emulsion | Viscosity (Pa·s) |
|---|---|---|
| E9 | Base emulsion | 8.80 ± 0.01 |
| E10 | Base emulsion + Glyceryl stearate 1.0% | 12.00 ± 0.01 |
| E11 | Base emulsion + Cetearyl alcohol 1.0% | 12.00 ± 0.02 |
| E12 | Base emulsion + Cetearyl alcohol 1.0% + Glyceryl stearate 1.0% | 18.00 ± 0.01 |
| E13 | Base emulsion + Olea Europe extract 1.5% | 11.00 ± 0.01 |
| E14 | Base emulsion + Prunus Armeniaca Kernel extract 1.5% | 7.00 ± 0.01 |
| E15 | Base emulsion + Triticum vulgare germ extract 1.5% | 11.00 ± 0.01 |
Finally, the study was completed with the preparation of solar formulations. Specifically, the effect of the polyglycerol derivative in comparison to other different natural emulsifiers on emulsions’ SPF and their photostability has been studied. Sunscreen can interact with components of the vehicle, and these interaction can affect sunscreen efficacy. The effectiveness of a sunscreen agent applied in an emulsion is influenced mainly by the emulsifier and fatty components. Emulsifiers are able to affect surface tension during the film formation phase, the rheological behavior, and the distribution of the emulsion on the skin [49]. Rheological behavior has a fundamental importance in the formulation of sunscreens, because the formation of an evenly-distributed film is critically influenced by the flowing properties of the formulation [50]. Emulsions containing UVB (Ethylhexyl Methoxycinnamate) and UVA (Butyl Methoxydibenzoylmethane) filters and basic emulsions without filters as reference were prepared (Table 6). The results obtained are reported in Figure 8 and Figure 9.
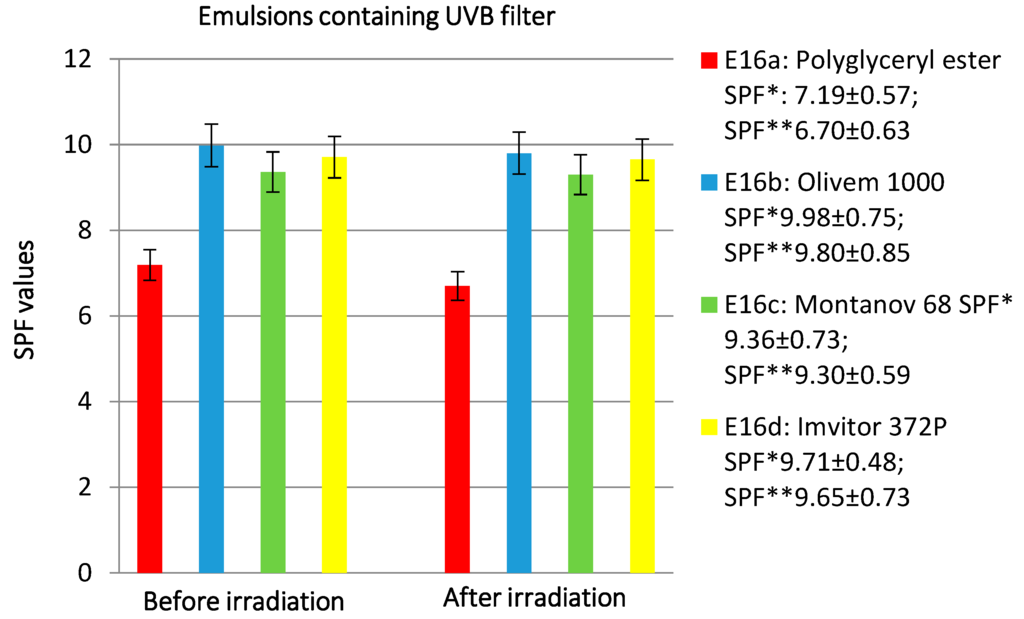
Figure 8.
The effect of different emulsifiers on SPF values and photostability after irradiation at 20 MED of emulsions containing UVB filter. SPF *: SPF value before irradiation; SPF **: SPF value after irradiation.
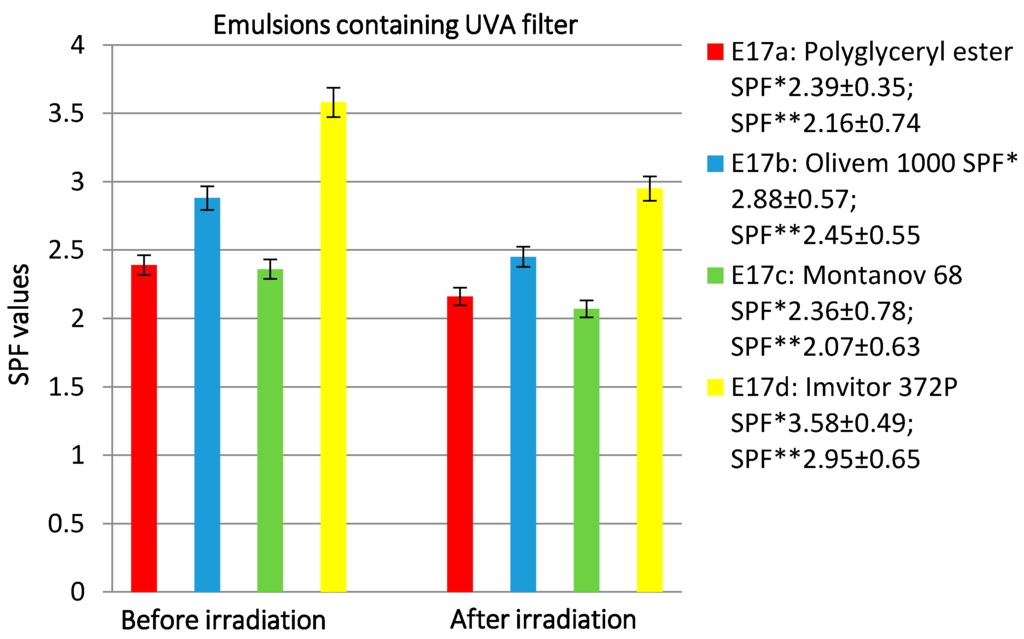
Figure 9.
The effect of different emulsifiers on SPF values and photostability after irradiation at 20 MED of emulsions containing UVA filter. SPF *: SPF value before irradiation; SPF **: SPF value after irradiation.
Regarding the emulsions containing the UVB filter (E16a–d), the Polyglycerol rice bran fatty acid esters did not give good results (Figure 8). In fact, it showed the lowest SPF value and less photostability, while it improved UVA filter photostability. As we can see from Figure 9, the emulsion prepared with the polyglycerol esters has an SPF value comparable to that of the emulsion obtained with the glucoside derivative (Montanov 68) but provides better photostability. The decrease of SPF value is higher for the emulsion prepared with Imvitor 372P (17.60%), followed by Olivem 1000 (14.93%), Montanov 68 (12.28%), and Polyglycerol esters (9.62%).
The different SPF values obtained could be due to the different rheological properties of the emulsions prepared with the various emulsifiers. The emulsion E16a showed the lowest viscosity value (Table 8), consequently a thinner film of the product on the substrate could be obtained. The film thickness of the product applied is an important parameter that influences the effectiveness of sunscreen [49,50,51].

Table 8.
Viscosity values of emulsions containing UV filters.
| Emulsion | Emulsifier | Viscosity (Pa·s) |
|---|---|---|
| E16a | Polyglycerol rice bran fatty acids esters | 15.00 ± 0.02 |
| E16b | Cetearyl Olivate, Sorbitan Olivate | 28.00 ± 0.01 |
| E16c | Cetearyl alcohol, Cetearyl Glucoside | 25.00 ± 0.01 |
| E16d | Glyceryl Stearate Citrate | 26.00 ± 0.01 |
| E17a | Polyglycerol rice bran fatty acids esters | 10.00 ± 0.01 |
| E17b | Cetearyl Olivate, Sorbitan Olivate | 15.00 ± 0.01 |
| E17c | Cetearyl alcohol, Cetearyl Glucoside | 11.00 ± 0.02 |
| E17d | Glyceryl Stearate Citrate | 18.00 ± 0.01 |
4. Conclusions
The study has allowed us to obtain an O/W natural emulsifier through a green chemistry process that responds to the current market trends. The synthesis is simple, rapid, and easily transferable at the industrial level. Moreover, the product can be used as such without further purification. The studied emulsifier is an ester obtained by an innovative combination of a special polyglyceryl derivative of fatty acids from rice bran oil neutralized with arginine, specially balanced to offer outstanding emulsifying properties.
The emulsifier can be used in formulations whose phases consist of fatty substances of varied chemical nature and different polarity, including vegetable triglycerides. Emulsion of varying fluidity and consistency can be made, regardless of the ratio of the two phases and the composition of the internal phase and depending on the chemical nature of the rheological additive. The great versatility of this product is of considerable interest and it opens the way for a new generation of ecoproducts.
Acknowledgments
The support of Progressus Srl is gratefully acknowledged.
Author Contributions
The study design was undertaken by Cecilia Anselmi, Marisanna Centini and Ibrahim Hanno, while experimental work was performed by Ibrahim Hanno with the support of Claudia Bibiani. The analysis and interpretation was mainly conducted by Cecilia Anselmi together with Marisanna Centini and Ibrahim Hanno. The manuscript was written by Cecilia Anselmi, Marisanna Centini and Ibrahim Hanno.
Conflicts of Interest
The authors declare no conflict of interest.
References
- European Commission Regulation No 1223/2009 of the European Parliament and of the Council of 30 November 2009 on Cosmetic Products. Available online: http://ec.europa.eu/health/endocrine_disruptors/docs/cosmetic_1223_2009_regulation_en.pdf (accessed on 8 October 2015).
- Swisher, R.D. Surfactant biodegradation. In Surfactant Science Series, 2nd ed.; Swisher, R.D., Ed.; Marcel Dekker Inc.: New York, NY, USA, 1987; Volume 18. [Google Scholar]
- Holmberg, K. Novel Surfactants: Preparation, Application, and Biodegradability, Second Edition, Revised and Expanded; Holmberg, K., Ed.; Marcel Dekker Inc.: New York, NY, USA, 2003. [Google Scholar]
- Myers, D. Surfactant in the Environment. In Surfactant Science and Technology, 3rd ed.; Myers, D., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2006; pp. 21–23. [Google Scholar]
- Rhein, L.D. Surfactants in cosmetics. In Surfactant Series Science, 2nd ed.; Rieger, M.M., Rhein, L.D., Eds.; Marcel Dekker Inc.: New York, NY, USA, 1997. [Google Scholar]
- Deleue, M.; Paquot, M. From renewable vegetables resources to microorganisms: New trends in surfactants. Comp. Rend. Chim. 2004, 7, 641–646. [Google Scholar] [CrossRef]
- Kosaric, N. Biosurfactants: Production, Properties and Applications. In Surfactant Science Series; Marcel Dekker Inc.: New York, NY, USA, 1983; Volume 48. [Google Scholar]
- Holmberg, K. Natural surfactants. Curr. Opin. Colloid Interface Sci. 2001, 6, 148–159. [Google Scholar] [CrossRef]
- Berger, H. Environmentally compatible surfactants for the cosmetic industry. Int. J. Cosmet. Sci. 1997, 19, 227–237. [Google Scholar] [CrossRef] [PubMed]
- Haferburg, D.; Hommel, R.; Claus, R.; Kleber, H.-P. Extracellular microbial lipids as biosurfactants. In Bioproducts; Springer: Berlin, Germany, 1986; pp. 53–93. [Google Scholar]
- Lourith, N.; Kanlayavattanakul, M. Natural surfactants used in cosmetics: Glycolipids. Int. J. Cosmet. Sci. 2009, 31, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Sen, R.; Swaminathan, T. Application of response-surface methodology to evaluate the optimal environmental conditions for the enhanced production of surfactin. Appl. Microbiol. Biotechnol. 1997, 47, 358–363. [Google Scholar] [CrossRef]
- Baker, I.; Matthews, B.; Suares, H. Sugar fatty acid ester surfactants: Structure and ultimate aerobic biodegradation. J. Surfactants Deterg. 2000, 3, 1–12. [Google Scholar] [CrossRef]
- Infante, M.R.; Perez, L.; Pinazo, A.; Clapes, P.; Moran, M.C.; Angelet, M.; Garcia, M.T.; Vinardell, M.P. Amino acid-based surfactants. Comp. Rend. Chim. 2004, 7, 583–593. [Google Scholar] [CrossRef]
- Kato, T.; Nakamura, T.; Yamashita, M.; Kawaguchi, M.; Kato, T.; Itoh, T. Surfactants properties of purified polyglycerol monolaurates. J. Surfactants Deterg. 2003, 6, 331–337. [Google Scholar] [CrossRef]
- Myers, D. The Organic Chemistry of Surfactants: Nonionic Surfactant: Derivatives of Polyglycerols and Other Polyols. In Surfactant Science and Technology, 3rd ed.; Myers, D., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2006; pp. 70–72. [Google Scholar]
- Lower, E. The cosmetic advantages of polyglycerols. Manufact. Chem. 1997, 68, 30–32. [Google Scholar]
- Cosmos-Standard: Cosmetics Organic and Natural Standard; Version 2.0—21st October 2013. Available online: http://www.ecocert.de/system/files/COSMOS-standard-v2.pdf (accessed on 20 April 2015).
- Lidstrom, P.; Tierney, J.; Wathey, B.; Westman, J. Microwave assisted organic synthesis—A review. Tetrahedron 2001, 57, 9225–9283. [Google Scholar] [CrossRef]
- De la Hoz, A.; Diaz-Ortiz, A.; Moreno, A. Microwaves in organic synthesis. Thermal and non-thermal microwave effects. Chem. Soc. Rev. 2005, 34, 164–178. [Google Scholar] [CrossRef] [PubMed]
- Surati, M.A.; Jauhari, S.; Desai, K.R. A brief review: Microwave assisted organic reaction. Arch. Appl. Sci. Res. 2012, 4, 645–661. [Google Scholar]
- Choi, S.P.; Kim, S.P.; Kang, M.Y.; Nam, S.H.; Friedman, M. Protective effects of Black Rice Bran against chemically-induced inflammation of mouse skin. J. Agric. Food Chem. 2010, 58, 10007–10015. [Google Scholar] [CrossRef] [PubMed]
- Manosroi, A.; Chutoprapat, R.; Abe, M.; Manosroi, W.; Manosroi, J. Anti-aging effect of topical formulations containing niosomes entrapped with rice bran bioactive compounds. Pharm. Biol. 2012, 50, 208–224. [Google Scholar] [CrossRef] [PubMed]
- Sayre, B.; Sanders, R. Rice bran and rice bran oil. Lipid Technol. 1990, 2, 72–76. [Google Scholar]
- Lilitchan, S.; Tangprawat, C.; Aryusuk, K.; Krisnangkura, S.; Chokmoh, S.; Krisnangkura, K. Partial extraction method for rapid analysis of total lipids and gamma-oryzanol contents in rice bran. Food Chem. 2008, 106, 752–759. [Google Scholar] [CrossRef]
- Cicero, A.F.G.; Derosa, G. Rice bran and its main components: Potential role in the management of coronary risk factors. Curr. Topics Nutr. Res. 2005, 3, 29–46. [Google Scholar]
- Patel, M.; Naik, S.N. Gamma-oryzanol from rice bran oil—A review. J. Sci. Ind. Res. 2004, 63, 569–578. [Google Scholar]
- Bernardi, D.S.; Pereira, T.A.; Maciel, N.R.; Bortoloto, J.; Viera, G.S.; Oliveira, G.C.; Rocha-Filho, P.A. Formation and stability of oil-in water nanoemulsions containing rice bran oil: in vitro and in vivo assessments. J. Nanobiotechnol. 2011, 9, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Miyazawa, M.; Oshima, T.; Koshio, K.; Itsuzaki, Y.; Anzai, J. Tyrosinase inhibitor from Black Rice Bran. J. Agric. Food Chem. 2003, 51, 6953–6956. [Google Scholar] [CrossRef] [PubMed]
- Diffey, B.L.; Robson, J. A new substrate to measure sunscreen protection factors throughout the ultraviolet spectrum. J. Soc. Cosmet. Chem. 1989, 40, 127–133. [Google Scholar]
- Labsphere Technical Note. SPF Analysis of Sunscreens Using the Labsphere UV-1000S Ultraviolet Transmittance Analyzer. Available online: http://webx.ubi.pt/~hgil/FotoMetria/PDF's-DOC's/Labsphere/SPF-20of-20Sunscreens.pdf (accessed on 15 April 2015).
- Standardisation Mandate Assigned to CEN Concerning Methods For Testing Efficacy of Sunscreen Products. Available online: http://ec.europa.eu/growth/sectors/cosmetics/products/sunscreen/docs/standardisation_mandate_en.pdf (accessed on 15 April 2015).
- Technical Collection, 90 (Publication CIE); International Commission on Illumination (CIE): Viena, Austria, 1991.
- Pathak, M.A. Photoprotection against harmful effects of solar UVB and UVA radiation: An update. In Sunscreens Development, Evaluation, and Regulatory Aspects; Lowe, N.J., Shaath, N.A., Pathak, M.A., Eds.; Marcel Dekker Inc.: New York, NY, USA, 1997; pp. 59–79. [Google Scholar]
- Iwai, H.; Fukasawa, J.; Suzuki, T. A liquid crystal application in skin care cosmetics. Int. J. Cosmet. Sci. 1998, 20, 87–102. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.; Lin, L.-H.; Kwan, C.-C. Surface properties and morphologies of pheohydrane/liquid crystal moisturizer product. Int. J. Cosmet. Sci. 2010, 32, 258–265. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, S.; Morita, T.; Fukuoka, T.; Imura, T.; Yanagidani, S.; Sogabe, A.; Kitamoto, D.; Kitagawa, M. The Moisturizing Effects of Glycolipid Biosurfactants, Mannosylerythritol Lipids, on Human Skin. J. Oleo Sci. 2012, 61, 407–412. [Google Scholar] [CrossRef] [PubMed]
- Duerr-Auster, N.; Kohlbrecher, J.; Zuercher, I.; Gunde, R.; Fischer, R.; Windhab, E. Microstructure and stability of lamellar liquid crystalline and gel phase formed by a polyglyceryl ester mixture in dilute aqueous solution. Langmuir 2007, 23, 12827–12834. [Google Scholar] [CrossRef] [PubMed]
- Sepulveda, E.; Kildsig, D.O.; Ghaly, E.S. Relationship between Internal Phase Volume and Emulsion Stability: The Cetyl Alcohol/Stearyl Alcohol System. Pharm. Dev. Technol. 2003, 8, 263–275. [Google Scholar] [CrossRef] [PubMed]
- Baudonnet, L.; Grossiord, J.-L.; Rodriguez, F. Physicochemical Characterization, and in Vitro Release of Salicylic Acid from O/W Emulsions Prepared with Montanov 68®: Effect of Formulation Parameters. Drug Dev. Ind. Pharm. 2004, 30, 975–984. [Google Scholar] [CrossRef] [PubMed]
- Kowalska, M.; Ziomek, M.; Zbikowska, A. Stability of cosmetic emulsion containing different amount of hemp oil. Int. J. Cosmet. Sci. 2015, 37, 408–416. [Google Scholar] [CrossRef] [PubMed]
- Panmai, S.; Prud’homme, R.K.; Peiffer, D.G.; Jockusch, S.; Turro, N.J. Interactions between hydrophobically modified polymers and surfactants: A fluorescence study. Langmuir 2002, 18, 3860–3864. [Google Scholar] [CrossRef]
- Thuresson, K.; Lindman, B.; Nyström, B. Effect of hydrophobic modification of a nonionic cellulose derivative on the interaction with surfactants. Rheology. J. Phys. Chem. B 1997, 101, 6450–6459. [Google Scholar] [CrossRef]
- Rebiha, M.; Moulai-Mostefa, N.; HadjSadok, A. Investigations of the effects of xanthan and sodium caseinate on the formation and stability of an oil-in-water emulsion stabilized by a nonionic surfactant using a response surface method. J. Dispers. Sci. Technol. 2012, 33, 429–436. [Google Scholar] [CrossRef]
- Veggeland, K.; Nilsson, S. Polymer–surfactant interactions studies by phase behavior, GPC, and NMR. Langmuir 1995, 11, 1885–1892. [Google Scholar] [CrossRef]
- Mukherjee, I.; Sarkar, D.; Moulik, S.P. Interaction of gums (Guar, Carboxymethylhydroxypropyl Guar, Diutan and Xanthan) with surfactants (DTAB, CTAB, and TX-100) in aqueous medium. Langmuir 2010, 26, 17906–17912. [Google Scholar] [CrossRef] [PubMed]
- Krstonosic, V.; Dokić, L.; Milanović, J. Micellar properties of OSA starch and interaction with xanthan gum in aqueous solution. Food Hydrocoll. 2011, 25, 361–367. [Google Scholar] [CrossRef]
- Katzbaner, B. Properties and applications of xanthan gum. Polym. Degrad. Stab. 1998, 59, 81–84. [Google Scholar] [CrossRef]
- Dahms, G.H. Choosing Emollients and Emulsifier for Sunscreen Products. Cosmet. Toilet. 1994, 109, 45–52. [Google Scholar]
- Gaspar, L.R.; Maia Campos, P.M.B.G. Rheology behavior and the SPF of sunscreens. Int. J. Pharm. 2003, 250, 35–44. [Google Scholar] [CrossRef]
- Liu, W.; Wang, X.; Lai, W.; Yan, T.; Wu, Y.; Wan, M.; Yi, J.; Matsui, M.S. Sunburn protection as a function of sunscreen application thickness differs between high and low SPFs. Photodermatol. Photoimmunol. Photomed. 2012, 28, 120–126. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).