Influence of the Systemic Application of Blue–Green Spirulina platensis Algae on the Cutaneous Carotenoids and Elastic Fibers in Vivo
Abstract
:1. Introduction
2. Experimental Section
2.1. Applied Substance
2.2. Volunteers
2.3. Study Design
2.4. Determination of the SAAID
2.5. Determination of the Carotenoid Concentration
2.6. Statistical Analysis
3. Results
3.1. Carotenoid Concentration in the Skin
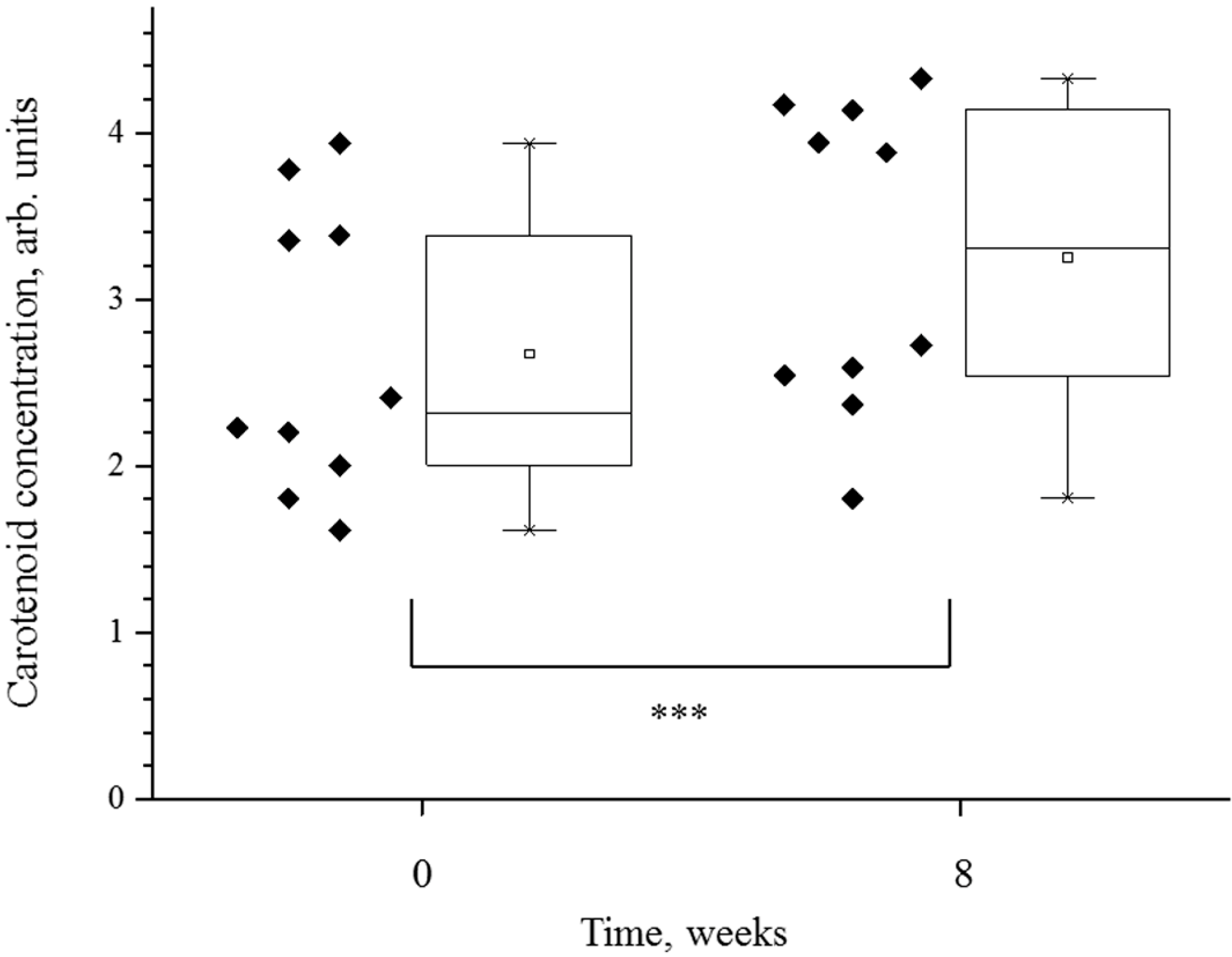
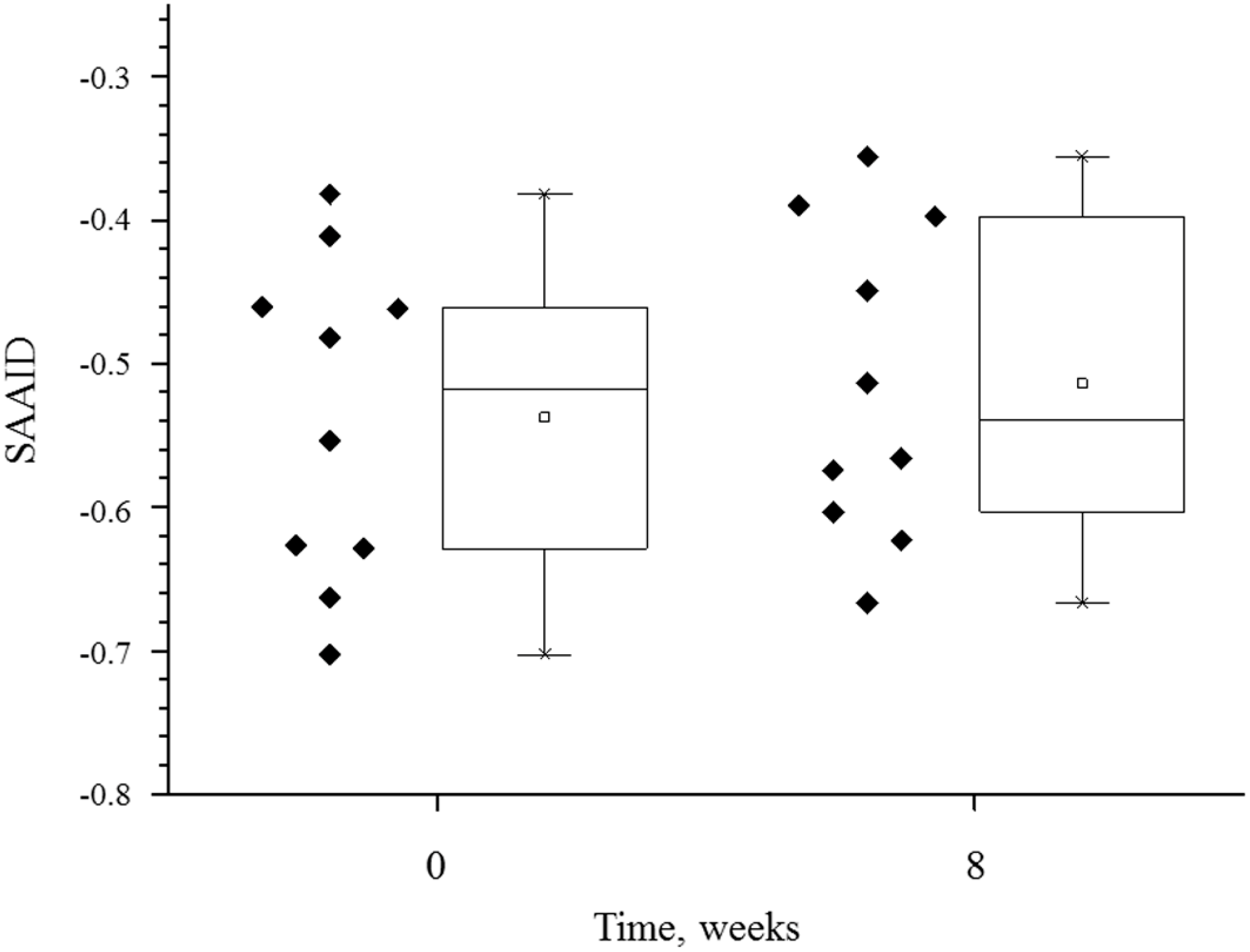
| Volunteer’s Number | Carotenoids (arb. units) | SAAID = (SHG − AF)/(SHG + AF) | ||
|---|---|---|---|---|
| Before Application | After Application | Before Application | After Application | |
| 1 | 3.3850 | 4.1358 | −0.48228 | −0.35563 |
| 2 | 3.9346 | 3.8812 | −0.41115 | −0.44907 |
| 3 | 2.0042 | 1.8042 | −0.38178 | −0.38967 |
| 4 | 3.3532 | 3.9430 | −0.46049 | −0.39780 |
| 5 | 2.2032 | 4.1676 | −0.62912 | −0.62319 |
| 6 | 1.6136 | 2.5906 | −0.46187 | −0.51366 |
| 7 | 2.4084 | 2.5426 | −0.66280 | −0.56581 |
| 8 | 3.7788 | 4.3250 | −0.70285 | −0.66643 |
| 9 | 2.2284 | 2.7234 | −0.62638 | −0.60336 |
| 10 | 1.8060 | 2.3704 | −0.55371 | −0.57417 |
| Mean ± SD | 2.67154 ± 0.85594 | 3.24838 ± 0.92721 | −0.53724 ± 0.1128 | −0.51388 ± 0.10939 |
| p-value | 0.00000011911 | 0.33 | ||
3.2. Collagen/Elastin Index (SAAID)
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lademann, J.; Kocher, W.; Yu, R.; Meinke, M.C.; Na, L.B.; Jung, S.; Sterry, W.; Darvin, M.E. Cutaneous carotenoids: The mirror of lifestyle? Skin Pharmacol. Physiol. 2014, 27, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Naval, J.; Alonso, V.; Herranz, M.A. Genetic polymorphisms and skin aging: The identification of population genotypic groups holds potential for personalized treatments. Clin. Cosmet. Invest. Dermatol. 2014, 7, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Yu, R.-X.; Köcher, W.; Darvin, M.E.; Büttner, M.; Jung, S.; Lee, B.-N.; Klotter, C.; Hurrelmann, K.; Meinke, M.C.; Lademann, J. Spectroscopic biofeedback on cutaneous carotenoids as part of a prevention program could be effective to raise health awareness in adolescents. J. Biophotonics 2014, 7, 926–937. [Google Scholar] [CrossRef] [PubMed]
- Zastrow, L.; Groth, N.; Klein, F.; Kockott, D.; Lademann, J.; Renneberg, R.; Ferrero, L. The missing link-light-induced (280–1600 nm) free radical formation in human skin. Skin Pharmacol. Physiol. 2009, 22, 31–44. [Google Scholar] [PubMed]
- Akhalaya, M.Y.; Maksimov, G.V.; Rubin, A.B.; Lademann, J.; Darvin, M.E. Molecular action mechanisms of solar infrared radiation and heat on human skin. Ageing Res. Rev. 2014, 16, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Vandersee, S.; Beyer, M.; Lademann, J.; Darvin, M.E. Blue-violet light irradiation dose dependently decreases carotenoids in human skin, which indicates the generation of free radicals. Oxid. Med. Cell. Longev. 2015, 2015. [Google Scholar] [CrossRef] [PubMed]
- Krutmann, J.; Morita, A.; Chung, J.H. Sun exposure: What molecular photodermatology tells us about its good and bad sides. J. Invest. Dermatol. 2012, 132, 976–984. [Google Scholar] [CrossRef] [PubMed]
- Huls, A.; Schikowski, T.; Kramer, U.; Sugiri, D.; Stolz, S.; Vierkoetter, A.; Krutmann, J. 286. Ozone exposure and extrinsic skin aging: Results from the salia cohort. J. Invest. Dermatol. 2015, 135, S49–S57. [Google Scholar]
- Huls, A.; Yang, Y.; Gao, W.; Vierkoetter, A.; Schikowski, T.; Ding, A.; Zhang, J.; Matsui, M.S.; Kan, H.; Jin, L.; et al. 288. Evidence that outdoor air pollutants including particulate matter (pm) as well as gases influence skin aging in a Chinese population. J. Invest. Dermatol. 2015, 135, S49–S57. [Google Scholar]
- Saladi, R.N.; Nektalova, T.; Fox, J.L. Induction of skin carcinogenicity by alcohol and ultraviolet light. Clin. Exp. Dermatol. 2010, 35, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Darvin, M.E.; Sterry, W.; Lademann, J.; Patzelt, A. Alcohol consumption decreases the protection efficiency of the antioxidant network and increases the risk of sunburn in human skin. Skin Pharmacol. Physiol. 2013, 26, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.; Darvin, M.E.; Chung, H.S.; Jung, B.; Lee, S.H.; Lenz, K.; Chung, W.S.; Yu, R.X.; Patzelt, A.; Lee, B.N.; et al. Antioxidants in asian-korean and caucasian skin: The influence of nutrition and stress. Skin Pharmacol. Physiol. 2014, 27, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Pavlou, P.; Rallis, M.; Deliconstantinos, G.; Papaioannou, G.; Grando, S.A. In-vivo data on the influence of tobacco smoke and UV light on murine skin. Toxicol. Ind. Health 2009, 25, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Wang, B. Photoaging: A review of current concepts of pathogenesis. J. Cutan. Med. Surg. 2011, 15, 374–377. [Google Scholar]
- Wolfle, U.; Seelinger, G.; Bauer, G.; Meinke, M.C.; Lademann, J.; Schempp, C.M. Reactive molecule species and antioxidative mechanisms in normal skin and skin aging. Skin Pharmacol. Physiol. 2014, 27, 316–332. [Google Scholar] [CrossRef] [PubMed]
- Valko, M.; Izakovic, M.; Mazur, M.; Rhodes, C.J.; Telser, J. Role of oxygen radicals in DNA damage and cancer incidence. Mol. Cell. Biochem. 2004, 266, 37–56. [Google Scholar] [CrossRef] [PubMed]
- Chamcheu, J.C.; Pal, H.C.; Siddiqui, I.A.; Adhami, V.M.; Ayehunie, S.; Boylan, B.T.; Noubissi, F.K.; Khan, N.; Syed, D.N.; Elmets, C.A.; et al. Prodifferentiation, anti-inflammatory and antiproliferative effects of delphinidin, a dietary anthocyanidin, in a full-thickness three-dimensional reconstituted human skin model of psoriasis. Skin Pharmacol. Physiol. 2015, 28, 177–188. [Google Scholar] [CrossRef] [PubMed]
- Thiele, J.J.; Schroeter, C.; Hsieh, S.N.; Podda, M.; Packer, L. The antioxidant network of the stratum corneum. Curr. Probl. Dermatol. 2001, 29, 26–42. [Google Scholar] [PubMed]
- Masaki, H. Role of antioxidants in the skin: Anti-aging effects. J. Dermatol. Sci. 2010, 58, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Darvin, M.; Zastrow, L.; Sterry, W.; Lademann, J. Effect of supplemented and topically applied antioxidant substances on human tissue. Skin Pharmacol. Physiol. 2006, 19, 238–247. [Google Scholar] [CrossRef] [PubMed]
- Darvin, M.E.; Sterry, W.; Lademann, J.; Vergou, T. The role of carotenoids in human skin. Molecules 2011, 16, 10491–10506. [Google Scholar] [CrossRef]
- Nguyen, L.M.; Scherr, R.E.; Linnell, J.D.; Ermakov, I.V.; Gellermann, W.; Jahns, L.; Keen, C.L.; Miyamoto, S.; Steinberg, F.M.; Young, H.M.; et al. Evaluating the relationship between plasma and skin carotenoids and reported dietary intake in elementary school children to assess fruit and vegetable intake. Arch. Biochem. Biophys. 2015, 572, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.H. Health-promoting components of fruits and vegetables in the diet. Adv. Nutr. 2013, 4, 384–392. [Google Scholar] [CrossRef] [PubMed]
- Wrona, M.; Korytowski, W.; Rozanowska, M.; Sarna, T.; Truscott, T.G. Cooperation of antioxidants in protection against photosensitized oxidation. Free Radic. Biol. Med. 2003, 35, 1319–1329. [Google Scholar] [CrossRef] [PubMed]
- Pisoschi, A.M.; Pop, A. The role of antioxidants in the chemistry of oxidative stress: A review. Eur. J. Med. Chem. 2015, 97, 55–74. [Google Scholar] [CrossRef] [PubMed]
- Darvin, M.E.; Patzelt, A.; Knorr, F.; Blume-Peytavi, U.; Sterry, W.; Lademann, J. One-year study on the variation of carotenoid antioxidant substances in living human skin: Influence of dietary supplementation and stress factors. J. Biomed. Opt. 2008, 13, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Krinsky, N.I. Carotenoids as antioxidants. Nutrition 2001, 17, 815–817. [Google Scholar] [CrossRef]
- Jomova, K.; Valko, M. Health protective effects of carotenoids and their interactions with other biological antioxidants. Eur. J. Med. Chem. 2013, 70, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Haag, S.F.; Taskoparan, B.; Darvin, M.E.; Groth, N.; Lademann, J.; Sterry, W.; Meinke, M.C. Determination of the antioxidative capacity of the skin in vivo using resonance Raman and electron paramagnetic resonance spectroscopy. Exp. Dermatol. 2011, 20, 483–487. [Google Scholar] [CrossRef] [PubMed]
- Meinke, M.C.; Friedrich, A.; Tscherch, K.; Haag, S.F.; Darvin, M.E.; Vollert, H.; Groth, N.; Lademann, J.; Rohn, S. Influence of dietary carotenoids on radical scavenging capacity of the skin and skin lipids. Eur. J. Pharm. Biopharm. 2013, 84, 365–373. [Google Scholar] [CrossRef] [PubMed]
- Darvin, M.E.; Fluhr, J.W.; Schanzer, S.; Richter, H.; Patzelt, A.; Meinke, M.C.; Zastrow, L.; Golz, K.; Doucet, O.; Sterry, W.; et al. Dermal carotenoid level and kinetics after topical and systemic administration of antioxidants: Enrichment strategies in a controlled in vivo study. J. Dermatol. Sci. 2011, 64, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Mayne, S.T.; Cartmel, B.; Scarmo, S.; Jahns, L.; Ermakov, I.V.; Gellermann, W. Resonance raman spectroscopic evaluation of skin carotenoids as a biomarker of carotenoid status for human studies. Arch. Biochem. Biophys. 2013, 539, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Blume-Peytavi, U.; Rolland, A.; Darvin, M.E.; Constable, A.; Pineau, I.; Voit, C.; Zappel, K.; Schafer-Hesterberg, G.; Meinke, M.; Clavez, R.L.; et al. Cutaneous lycopene and beta-carotene levels measured by resonance raman spectroscopy: High reliability and sensitivity to oral lactolycopene deprivation and supplementation. Eur. J. Pharm. Biopharm. 2009, 73, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B. The antioxidant paradox: Less paradoxical now? Br. J. Clin. Pharmacol. 2013, 75, 637–644. [Google Scholar] [CrossRef] [PubMed]
- Darvin, M.E.; Gersonde, I.; Albrecht, H.; Sterry, W.; Lademann, J. Resonance raman spectroscopy for the detection of carotenolds in foodstuffs. Influence of the nutrition on the antioxidative potential of the skin. Laser Phys. Lett. 2007, 4, 452–456. [Google Scholar] [CrossRef]
- Meinke, M.C.; Darvin, M.E.; Vollert, H.; Lademann, J. Bioavailability of natural carotenoids in human skin compared to blood. Eur. J. Pharm. Biopharm. 2010, 76, 269–274. [Google Scholar] [CrossRef] [PubMed]
- Darvin, M.E.; Fluhr, J.W.; Meinke, M.C.; Zastrow, L.; Sterry, W.; Lademann, J. Topical beta-carotene protects against infra-red-light-induced free radicals. Exp. Dermatol. 2011, 20, 125–129. [Google Scholar] [CrossRef] [PubMed]
- Darvin, M.E.; Meinke, M.C.; Sterry, W.; Lademann, J. Optical methods for noninvasive determination of carotenoids in human and animal skin. J. Biomed. Opt. 2013, 18. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, V.E.; Neves, M.A.; Soares, M.C.; Edwards, H.G.; de Oliveira, L.F. Study of carotenoids in cyanobacteria by Raman spectroscopy. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2015, 150, 373–380. [Google Scholar] [CrossRef] [PubMed]
- Kulshreshtha, A.; Zacharia, A.J.; Jarouliya, U.; Bhadauriya, P.; Prasad, G.B.; Bisen, P.S. Spirulina in health care management. Curr. Pharm. Biotechnol. 2008, 9, 400–405. [Google Scholar] [CrossRef] [PubMed]
- Tobon-Velasco, J.C.; Palafox-Sanchez, V.; Mendieta, L.; Garcia, E.; Santamaria, A.; Chamorro-Cevallos, G.; Limon, I.D. Antioxidant effect of spirulina (arthrospira) maxima in a neurotoxic model caused by 6-OHDA in the rat striatum. J. Neural Transm. 2013, 120, 1179–1189. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, S.M.; Khosravi-Darani, K.; Mozafari, M.R. Nutritional and medical applications of spirulina microalgae. Mini Rev. Med. Chem. 2013, 13, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, K.; Hayashi, T.; Kojima, I. A natural sulfated polysaccharide, calcium spirulan, isolated from Spirulina platensis: In vitro and ex vivo evaluation of anti-herpes simplex virus and anti-human immunodeficiency virus activities. AIDS Res. Hum. Retrovir. 1996, 12, 1463–1471. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, T.; Hayashi, K.; Maeda, M.; Kojima, I. Calcium spirulan, an inhibitor of enveloped virus replication, from a blue-green alga spirulina platensis. J. Nat. Prod. 1996, 59, 83–87. [Google Scholar] [CrossRef] [PubMed]
- Deng, R.; Chow, T.J. Hypolipidemic, antioxidant, and antiinflammatory activities of microalgae spirulina. Cardiovasc. Ther. 2010, 28, 33–45. [Google Scholar] [CrossRef] [PubMed]
- Joventino, I.P.; Alves, H.G.; Neves, L.C.; Pinheiro-Joventino, F.; Leal, L.K.; Neves, S.A.; Ferreira, F.V.; Brito, G.A.; Viana, G.B. The microalga spirulina platensis presents anti-inflammatory action as well as hypoglycemic and hypolipidemic properties in diabetic rats. J. Complement. Integr. Med. 2012, 9. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.M.; Lee, E.H.; Cho, H.H.; Moon, Y.H. Inhibitory effect of mast cell-mediated immediate-type allergic reactions in rats by spirulina. Biochem. Pharmacol. 1998, 55, 1071–1076. [Google Scholar] [CrossRef]
- Cheong, S.H.; Kim, M.Y.; Sok, D.E.; Hwang, S.Y.; Kim, J.H.; Kim, H.R.; Lee, J.H.; Kim, Y.B.; Kim, M.R. Spirulina prevents atherosclerosis by reducing hypercholesterolemia in rabbits fed a high-cholesterol diet. J. Nutr. Sci. Vitaminol. 2010, 56, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Konickova, R.; Vankova, K.; Vanikova, J.; Vanova, K.; Muchova, L.; Subhanova, I.; Zadinova, M.; Zelenka, J.; Dvorak, A.; Kolar, M.; et al. Anti-cancer effects of blue-green alga spirulina platensis, a natural source of bilirubin-like tetrapyrrolic compounds. Ann. Hepatol. 2014, 13, 273–283. [Google Scholar] [PubMed]
- Grawish, M.E.; Zaher, A.R.; Gaafar, A.I.; Nasif, W.A. Long-term effect of spirulina platensis extract on DMBA-induced hamster buccal pouch carcinogenesis (immunohistochemical study). Med. Oncol. 2010, 27, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Ismail, M.F.; Ali, D.A.; Fernando, A.; Abdraboh, M.E.; Gaur, R.L.; Ibrahim, W.M.; Raj, M.H.; Ouhtit, A. Chemoprevention of rat liver toxicity and carcinogenesis by spirulina. Int. J. Biol. Sci. 2009, 5, 377–387. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.; Shobha, J.C.; Mohan, I.K.; Naidu, M.U.; Sundaram, C.; Singh, S.; Kuppusamy, P.; Kutala, V.K. Protective effect of spirulina against doxorubicin-induced cardiotoxicity. Phytother. Res. 2005, 19, 1030–1037. [Google Scholar] [CrossRef] [PubMed]
- Bermejo-Bescos, P.; Pinero-Estrada, E.; del Fresno, A.M. Neuroprotection by spirulina platensis protean extract and phycocyanin against iron-induced toxicity in SH-SY5Y neuroblastoma cells. Toxicol. In Vitro 2008, 22, 1496–1502. [Google Scholar] [CrossRef] [PubMed]
- Yogianti, F.; Kunisada, M.; Nakano, E.; Ono, R.; Sakumi, K.; Oka, S.; Nakabeppu, Y.; Nishigori, C. Inhibitory effects of dietary spirulina platensis on UVB-induced skin inflammatory responses and carcinogenesis. J. Invest. Dermatol. 2014, 134, 2610–2619. [Google Scholar] [CrossRef] [PubMed]
- Fitzpatrick, T.B. The validity and practicality of sun-reactive skin types I through VI. Arch. Dermatol. 1988, 124, 869–871. [Google Scholar] [CrossRef] [PubMed]
- Darvin, M.E.; Konig, K.; Kellner-Hoefer, M.; Breunig, H.G.; Werncke, W.; Meinke, M.C.; Patzelt, A.; Sterry, W.; Lademann, J. Safety assessment by multiphoton fluorescence/second harmonic generation/hyper-Rayleigh scattering tomography of ZnO nanoparticles used in cosmetic products. Skin Pharmacol. Physiol. 2012, 25, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Choe, C.S.; Ahlberg, S.; Meinke, M.C.; Alexiev, U.; Lademann, J.; Darvin, M.E. Penetration of silver nanoparticles into porcine skin ex vivo using fluorescence lifetime imaging microscopy, Raman microscopy, and surface-enhanced raman scattering microscopy. J. Biomed. Opt. 2015, 20. [Google Scholar] [CrossRef]
- Koehler, M.J.; Konig, K.; Elsner, P.; Buckle, R.; Kaatz, M. In vivo assessment of human skin aging by multiphoton laser scanning tomography. Opt. Lett. 2006, 31, 2879–2881. [Google Scholar] [CrossRef] [PubMed]
- Darvin, M.E.; Richter, H.; Ahlberg, S.; Haag, S.F.; Meinke, M.C.; le Quintrec, D.; Doucet, O.; Lademann, J. Influence of sun exposure on the cutaneous collagen/elastin fibers and carotenoids: Negative effects can be reduced by application of sunscreen. J. Biophotonics 2014, 7, 735–743. [Google Scholar] [CrossRef] [PubMed]
- Darvin, M.E.; Gersonde, I.; Ey, S.; Brandt, N.N.; Albrecht, H.; Gonchukov, S.A.; Sterry, W.; Lademann, J. Noninvasive detection of beta-carotene and lycopene in human skin using Raman spectroscopy. Laser Phys. 2004, 14, 231–233. [Google Scholar]
- Krinsky, N.I.; Johnson, E.J. Carotenoid actions and their relation to health and disease. Mol. Aspects Med. 2005, 26, 459–516. [Google Scholar] [CrossRef] [PubMed]
- Darvin, M.E.; Gersonde, I.; Albrecht, H.; Meinke, M.; Sterry, W.; Lademann, J. Non-invasive in vivo detection of the carotenoid antioxidant substance lycopene in the human skin using the resonance Raman spectroscopy. Laser Phys. Lett. 2006, 3, 460–463. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Darvin, M.E.; Jung, S.; Schanzer, S.; Richter, H.; Kurth, E.; Thiede, G.; Meinke, M.C.; Lademann, J. Influence of the Systemic Application of Blue–Green Spirulina platensis Algae on the Cutaneous Carotenoids and Elastic Fibers in Vivo. Cosmetics 2015, 2, 302-312. https://doi.org/10.3390/cosmetics2030302
Darvin ME, Jung S, Schanzer S, Richter H, Kurth E, Thiede G, Meinke MC, Lademann J. Influence of the Systemic Application of Blue–Green Spirulina platensis Algae on the Cutaneous Carotenoids and Elastic Fibers in Vivo. Cosmetics. 2015; 2(3):302-312. https://doi.org/10.3390/cosmetics2030302
Chicago/Turabian StyleDarvin, Maxim E., Sora Jung, Sabine Schanzer, Heike Richter, Elke Kurth, Gisela Thiede, Martina C. Meinke, and Juergen Lademann. 2015. "Influence of the Systemic Application of Blue–Green Spirulina platensis Algae on the Cutaneous Carotenoids and Elastic Fibers in Vivo" Cosmetics 2, no. 3: 302-312. https://doi.org/10.3390/cosmetics2030302
APA StyleDarvin, M. E., Jung, S., Schanzer, S., Richter, H., Kurth, E., Thiede, G., Meinke, M. C., & Lademann, J. (2015). Influence of the Systemic Application of Blue–Green Spirulina platensis Algae on the Cutaneous Carotenoids and Elastic Fibers in Vivo. Cosmetics, 2(3), 302-312. https://doi.org/10.3390/cosmetics2030302








