Determination of the Antioxidant Status of the Skin by In Vivo-Electron Paramagnetic Resonance (EPR) Spectroscopy
Abstract
:1. Introduction
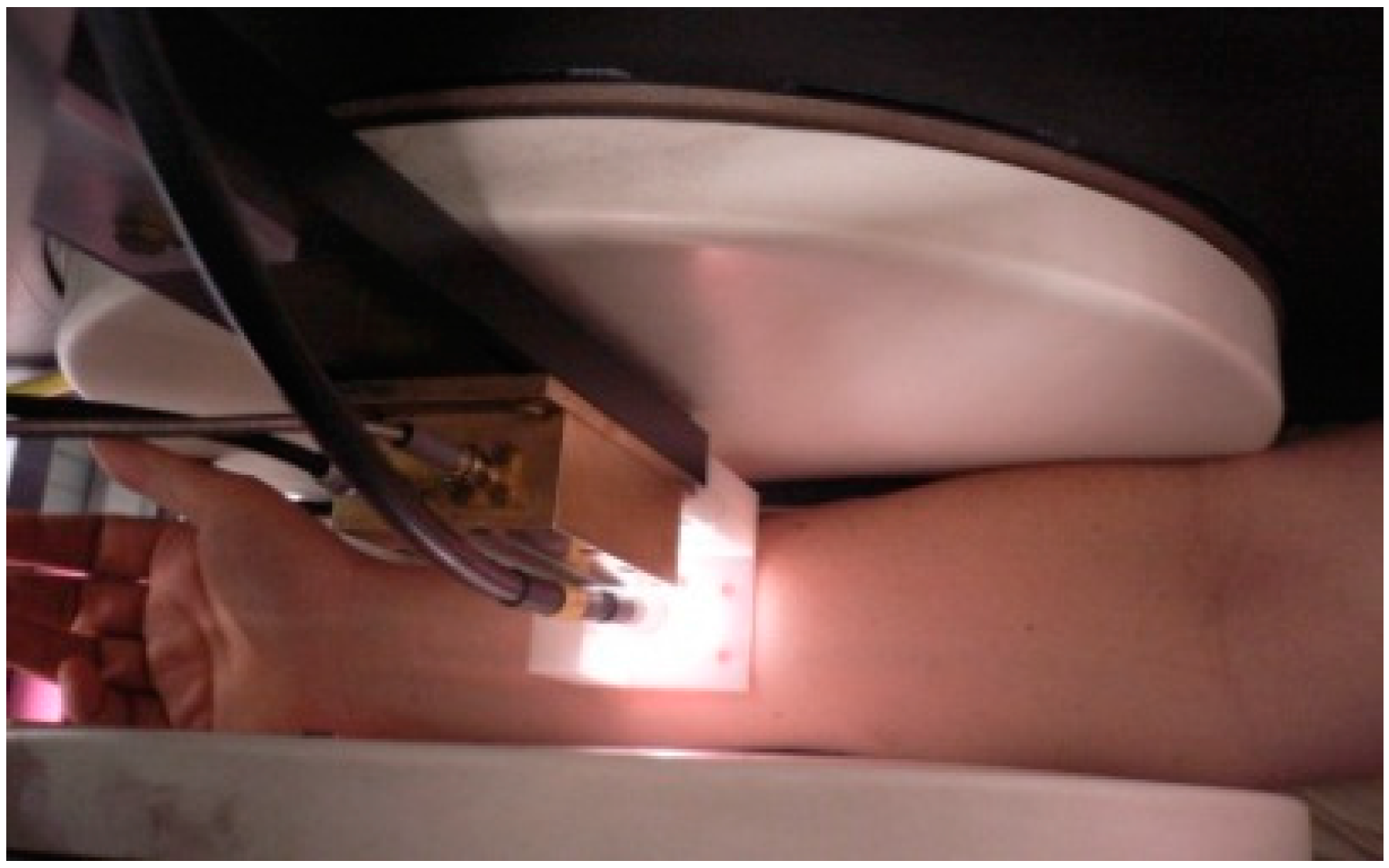
2. Technical Aspects of the Performance of In Vivo EPR-Measurements
3. Discussion
3.1. Effect of Radical Formation in the Visible and Near Infrared Spectral Range
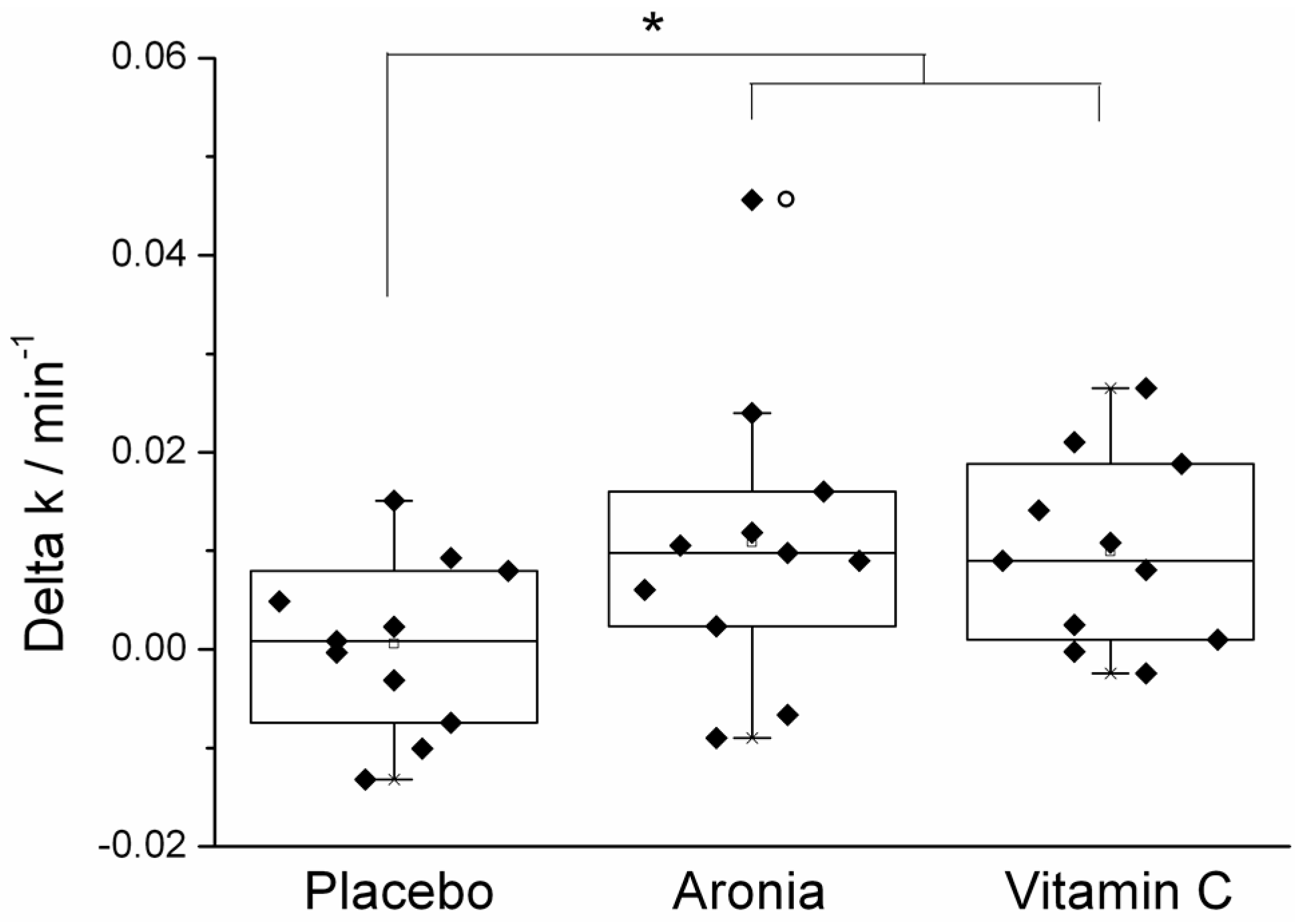
3.2. Effect of Orally Ingested Antioxidants
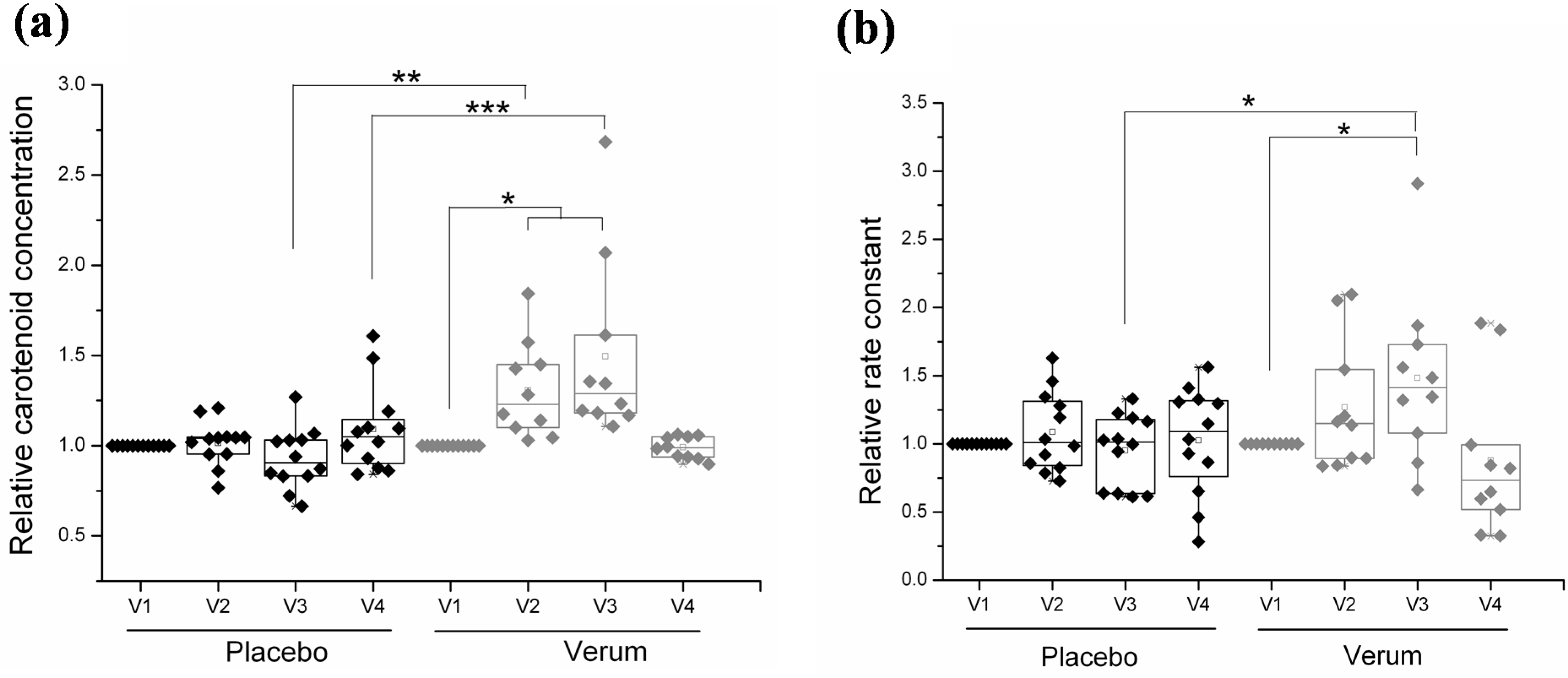
3.3. Effect of Topical Application
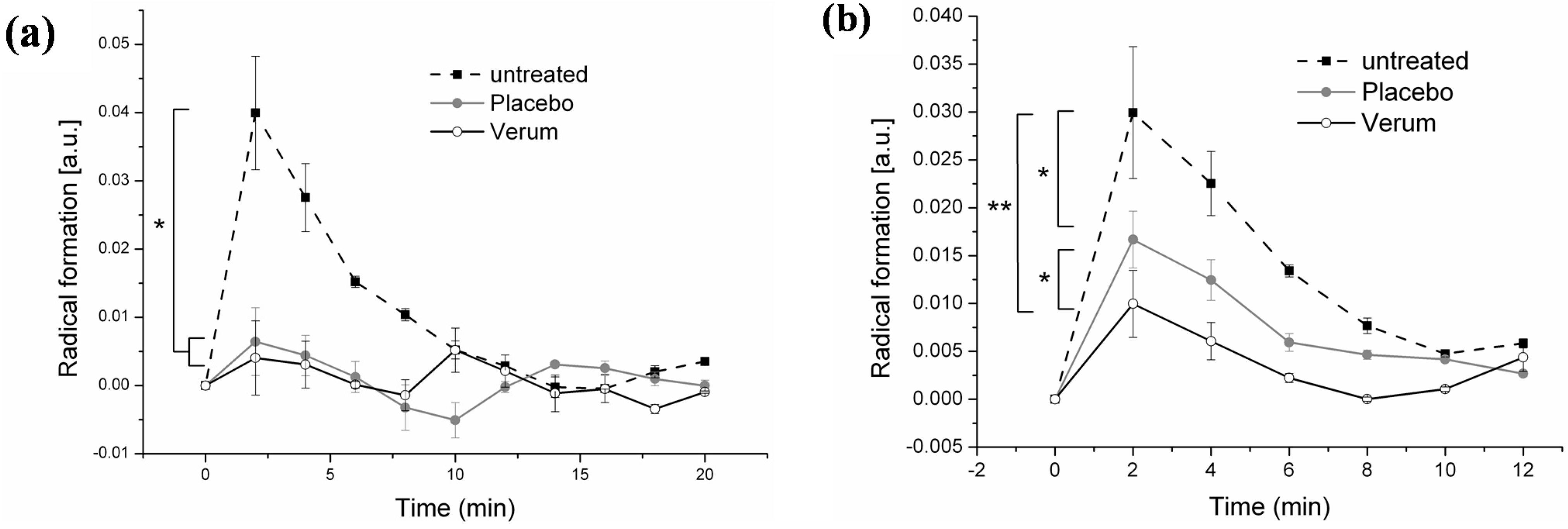
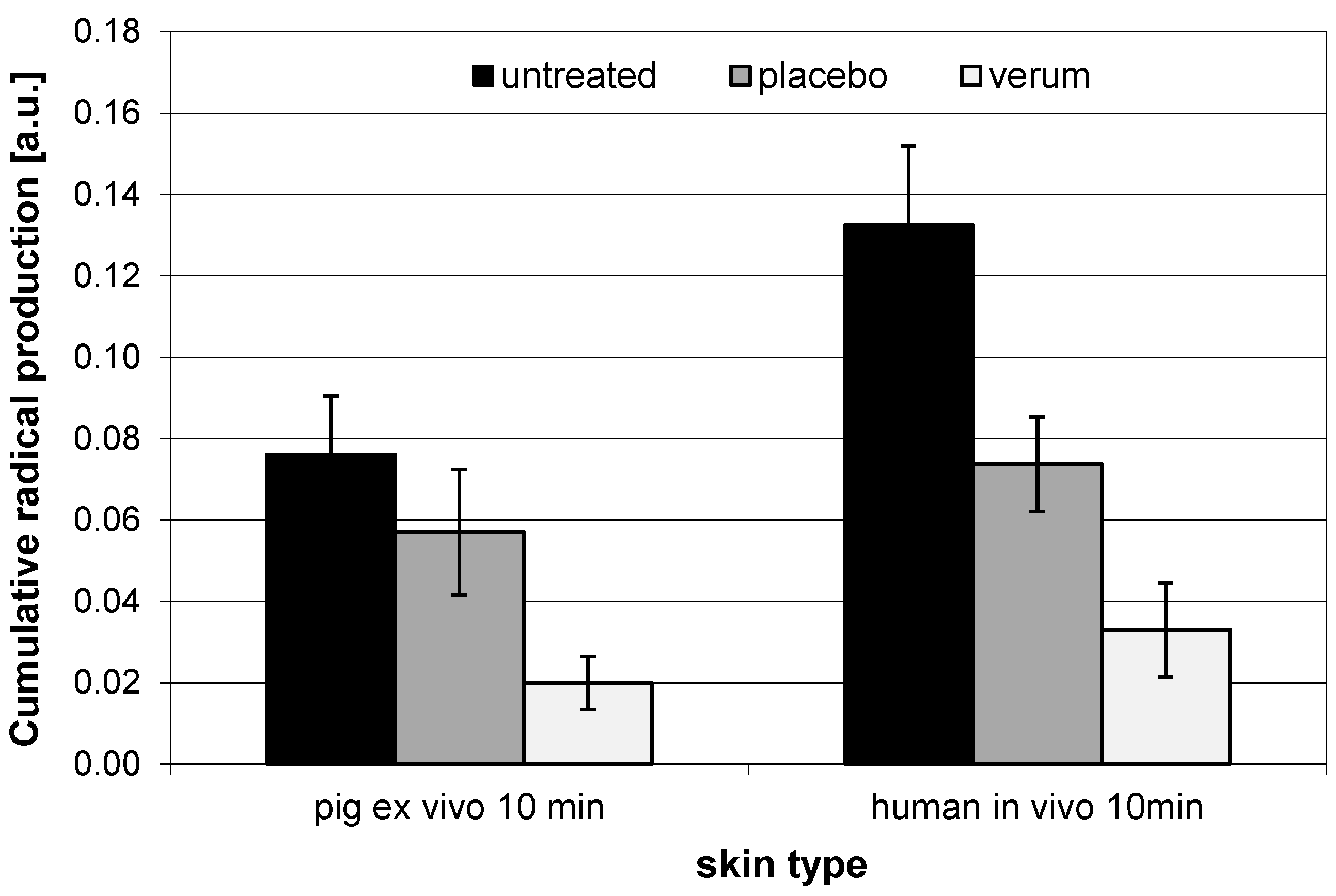
3.4. Comparison of In Vivo and Ex Vivo Investigations
4. Conclusion
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Chen, L.; Hu, J.Y.; Wang, S.Q. The role of antioxidants in photoprotection: A critical review. J. Am. Acad. Dermatol. 2012, 67, 1013–1024. [Google Scholar] [CrossRef] [PubMed]
- Zastrow, L.; Groth, N.; Klein, F.; Kockott, D.; Lademann, J.; Renneberg, R.; Ferrero, L. The missing link-light-induced (280–1600 nm) free radical formation in human skin. Skin Pharmacol. Physiol. 2009, 22, 31–44. [Google Scholar] [PubMed]
- Gutteridge, J.M.; Halliwell, B. Free radicals and antioxidants in the year 2000: A historical look to the future. Ann. N.Y. Acad. Sci. 2000, 899, 136–147. [Google Scholar] [CrossRef] [PubMed]
- Lademann, B.; Gerber, D.M.; Olbertz, M.E.; Darvin, L.; Stauf, K.; Ueberholz, V.; Heinrich, J.; Lademann, V. Non-invasive spectroscopic determination of the antioxidative status of gravidae and neonates. Skin Pharmacol. Physiol. 2015, 28, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Wolfle, U.; Seelinger, G.; Bauer, G.; Meinke, M.C.; Lademann, J.; Schempp, C.M. Reactive molecule species and antioxidative mechanisms in normal skin and skin aging. Skin Pharmacol. Physiol. 2014, 27, 316–332. [Google Scholar] [CrossRef] [PubMed]
- Sies, H. Oxidative stress: Oxidants and antioxidants. Exp. Physiol. 1997, 82, 291–295. [Google Scholar] [CrossRef] [PubMed]
- Briganti, S.; Picardo, M. Antioxidant activity, lipid peroxidation and skin diseases. What’s new. J. Eur. Acad. Dermatol. Venereol. 2003, 17, 663–669. [Google Scholar] [CrossRef] [PubMed]
- Harman, D. Aging: A theory based on free radical and radiation chemistry. J. Gerontol. 1956, 11, 298–300. [Google Scholar] [CrossRef] [PubMed]
- Sander, C.S.; Chang, H.; Hamm, F.; Elsner, P.; Thiele, J.J. Role of oxidative stress and the antioxidant network in cutaneous carcinogenesis. Int. J. Dermatol. 2004, 43, 326–335. [Google Scholar] [CrossRef] [PubMed]
- Thiele, J.J.; Schroeter, C.; Hsieh, S.N.; Podda, M.; Packer, L. The antioxidant network of the stratum corneum. Curr. Probl. Dermatol. 2001, 29, 26–42. [Google Scholar] [PubMed]
- Lademann, J.; Kocher, W.; Yu, R.; Meinke, M.C.; Na Lee, B.; Jung, S.; Sterry, W.; Darvin, M.E. Cutaneous carotenoids: The mirror of lifestyle? Skin Pharmacol. Physiol. 2014, 27, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Zamzami, N.; Kroemer, G. The mitochondrion in apoptosis: How Pandora’s box opens. Nat. Rev. Mol. Cell Biol. 2001, 2, 67–71. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Jones, D.P. Mitochondrial redox signaling during apoptosis. J. Bioenerg. Biomembr. 1999, 31, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Lademann, J.; Patzelt, A.; Schanzer, S.; Richter, H.; Meinke, M.C.; Sterry, W.; Zastrow, L.; Doucet, O.; Vergou, T.; Darvin, M.E. Uptake of antioxidants by natural nutrition and supplementation: Pros and cons from the dermatological point of view. Skin Pharmacol. Physiol. 2011, 24, 269–273. [Google Scholar] [CrossRef] [PubMed]
- Shindo, Y.; Witt, E.; Han, D.; Epstein, W.; Packer, L. Enzymic and non-enzymic antioxidants in epidermis and dermis of human skin. J. Invest. Dermatol. 1994, 102, 122–124. [Google Scholar] [CrossRef] [PubMed]
- Uddin, S.; Ahmad, S. Antioxidants protection against cancer and other human diseases. Compr. Ther. 1995, 21, 41–45. [Google Scholar] [PubMed]
- Robert, C.; Bonnet, M.; Marques, S.; Numa, M.; Doucet, O. Low to moderate doses of infrared A irradiation impair extracellular matrix homeostasis of the skin and contribute to skin photodamage. Skin Pharmacol. Physiol. 2015, 28, 196–204. [Google Scholar] [CrossRef] [PubMed]
- Park, G.; Kim, H.G.; Hong, S.P.; Kim, S.Y.; Oh, M.S. Walnuts (seeds of Juglandis sinensis L.) protect human epidermal keratinocytes against UVB-induced mitochondria-mediated apoptosis through upregulation of ROS elimination pathways. Skin Pharmacol. Physiol. 2014, 27, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Meinke, M.C.; Haag, S.F.; Schanzer, S.; Groth, N.; Gersonde, I.; Lademann, J. Radical protection by sunscreens in the infrared spectral range. Photochem. Photobiol. 2011, 87, 452–456. [Google Scholar] [CrossRef] [PubMed]
- He, G.; Samouilov, A.; Kuppusamy, P.; Zweier, J.L. In vivo imaging of free radicals: Applications from mouse to man. Mol. Cell. Biochem. 2002, 234, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Gallez, B.; Swartz, H.M. In vivo EPR: When, how and why? NMR Biomed. 2004, 17, 223–225. [Google Scholar] [CrossRef] [PubMed]
- Fujii, H.; Berliner, L.J. Detection of bioradicals by in vivo L-band electron spin resonance spectrometry. NMR Biomed. 2004, 17, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Zastrow, T.H.L.; Berliner, L.J.; Ferrero, L.; Groth, N. In-vivo measurements of free radicals in human skin. IFSCC Mag. 2003, 6, 295–301. [Google Scholar]
- He, G.; Samouilov, A.; Kuppusamy, P.; Zweier, J.L. In vivo EPR imaging of the distribution and metabolism of nitroxide radicals in human skin. J. Magn. Reson. 2001, 148, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Herrling, T.; Jung, K.; Fuchs, J. Measurements of UV-generated free radicals/reactive oxygen species (ROS) in skin. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2006, 63, 840–845. [Google Scholar] [CrossRef] [PubMed]
- Haag, S.F.; Taskoparan, B.; Bittl, R.; Teutloff, C.; Wenzel, R.; Fahr, A.; Chen, M.; Lademann, J.; Schafer-Korting, M.; Meinke, M.C. Stabilization of reactive nitroxides using invasomes to allow prolonged electron paramagnetic resonance measurements. Skin Pharmacol. Physiol. 2011, 24, 312–321. [Google Scholar] [CrossRef] [PubMed]
- Haag, S.F.; Taskoparan, B.; Darvin, M.E.; Groth, N.; Lademann, J.; Sterry, W.; Meinke, M.C. Determination of the antioxidative capacity of the skin in vivo using resonance Raman and electron paramagnetic resonance spectroscopy. Exp. Dermatol. 2011, 20, 483–487. [Google Scholar] [CrossRef] [PubMed]
- Vogt, O.; Lademann, J.; Rancan, F.; Meinke, M.C.; Schanzer, S.; Stockfleth, E.; Sterry, W.; Lange-Asschenfeldt, B. Photoprotective properties of the fluorescent europium complex in UV-irradiated skin. Skin Pharmacol. Physiol. 2013, 26, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Weil, J.A.; Bolton, J.R.; Wertz, J.E. (Eds.) Electron Paramagnetic Resonance; Wiley-Interscience: Hoboken, NY, USA, 1994.
- Swartz, H.M.; Khan, N.; Buckey, J.; Comi, R.; Gould, L.; Grinberg, O.; Hartford, A.; Hopf, H.; Hou, H.; Hug, E.; et al. Clinical applications of EPR: Overview and perspectives. NMR Biomed. 2004, 17, 335–351. [Google Scholar] [CrossRef] [PubMed]
- Berliner, L.J.; Fujii, H. In vivo spin trapping of nitric oxide. Antioxid. Redox Signal. 2004, 6, 649–656. [Google Scholar] [CrossRef] [PubMed]
- Gallez, B.; Bacic, G.; Goda, F.; Jiang, J.; O’Hara, J.A.; Dunn, J.F.; Swartz, H.M. Use of nitroxides for assessing perfusion, oxygenation, and viability of tissues: In vivo EPR and MRI studies. Magn. Reson. Med. 1996, 36, 97–106. [Google Scholar] [CrossRef]
- Gallez, B.; Mader, K.; Swartz, H.M. Noninvasive measurement of the pH inside the gut by using pH-sensitive nitroxides. An in vivo EPR study. Magn. Reson. Med. 1996, 36, 694–697. [Google Scholar] [CrossRef] [PubMed]
- Ibragimova, M.I.; Petukhov, V.Y.; Zheglov, E.P.; Khan, N.; Hou, H.; Swartz, H.M.; Konjukhov, G.V.; Nizamov, R.N. Quinoid radio-toxin (QRT) induced metabolic changes in mice: An ex vivo and in vivo EPR investigation. Nitric Oxide 2008, 18, 216–222. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, J.; Groth, N.; Herrling, T. In vivo measurement of oxidative stress status in human skin. Methods Enzymol. 2002, 352, 333–339. [Google Scholar] [PubMed]
- Fuchs, J.; Freisleben, H.J.; Podda, M.; Zimmer, G.; Milbradt, R.; Packer, L. Nitroxide radical biostability in skin. Free Radic. Biol. Med. 1993, 15, 415–423. [Google Scholar] [CrossRef]
- Herrling, T.; Fuchs, J.; Rehberg, J. UV-induced free radicals in the skin detected by ESR spectroscopy and imaging using nitroxides. Free Radic. Biol. Med. 2003, 35, 59–67. [Google Scholar] [CrossRef]
- Meinke, M.C.; Friedrich, A.; Tscherch, K.; Haag, S.F.; Darvin, M.E.; Vollert, H.; Groth, N.; Lademann, J.; Rohn, S. Influence of dietary carotenoids on radical scavenging capacity of the skin and skin lipids. Eur. J. Pharm. Biopharm. 2013, 84, 365–373. [Google Scholar] [CrossRef] [PubMed]
- Herrling, T.; Zastrow, L.; Groth, N. Detection and Influencing of the Antioxidative Potential (AOP) of Human Skin. SÖFW 1996, 122, 472–476. [Google Scholar]
- Haag, S.F.; Tscherch, K.; Arndt, S.; Kleemann, A.; Gersonde, I.; Lademann, J.; Rohn, S.; Meinke, M.C. Enhancement of skin radical scavenging activity and stratum corneum lipids after the application of a hyperforin-rich cream. Eur. J. Pharm. Biopharm. 2014, 86, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Meinke, M.C.; Muller, R.; Bechtel, A.; Haag, S.F.; Darvin, M.E.; Lohan, S.B.; Ismaeel, F.; Lademann, J. Evaluation of carotenoids and reactive oxygen species in human skin after UV irradiation: A critical comparison between in vivo and ex vivo investigations. Exp. Dermatol. 2015, 24, 194–197. [Google Scholar] [CrossRef] [PubMed]
- Meinke, M.C.; Schanzer, S.; Haag, S.F.; Casetti, F.; Muller, M.L.; Wolfle, U.; Kleemann, A.; Lademann, J.; Schempp, C.M. In vivo photoprotective and anti-inflammatory effect of hyperforinis associated with high antioxidant activity in vitro and ex vivo. Eur. J. Pharm. Biopharm. 2012, 81, 346–350. [Google Scholar] [CrossRef] [PubMed]
- Terman, M.; Terman, J.S. Light therapy for seasonal and nonseasonal depression: Efficacy, protocol, safety, and side effects. CNS Spectr. 2005, 10, 647–663. [Google Scholar] [PubMed]
- Kochevar, I.E.; Pathak, M.A.; Parrish, J.A. Photophysics, photochemistry, and photobiology. In Fitzpatrick’s Dermatology in General Medicine; Freedberg, I.M., Eisen, A.Z., Wolff, K., Eds.; McGraw-Hill: New York, NY, USA, 1999. [Google Scholar]
- Schieke, S.M.; Schroeder, P.; Krutmann, J. Cutaneous effects of infrared radiation: From clinical observations to molecular response mechanisms. Photodermatol. Photoimmunol. Photomed. 2003, 19, 228–234. [Google Scholar] [CrossRef] [PubMed]
- Kligman, L.H. Intensification of ultraviolet-induced dermal damage by infrared radiation. Arch. Dermatol. Res. 1982, 272, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Akhalaya, M.Y.; Maksimov, G.V.; Rubin, A.B.; Lademann, J.; Darvin, M.E. Molecular action mechanisms of solar infrared radiation and heat on human skin. Ageing Res. Rev. 2014, 16, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Darvin, M.E.; Patzelt, A.; Meinke, M.C.; Sterry, W.; Lademann, J. Influence of two different IR radiators on the antioxidative potential of the human skin. Laser Phys. Lett. 2009, 6, 229–234. [Google Scholar] [CrossRef]
- Schroeder, P.; Lademann, J.; Darvin, M.E.; Stege, H.; Marks, C.; Bruhnke, S.; Krutmann, J. Infrared radiation-induced matrix metalloproteinase in human skin: Implications for protection. J. Invest. Dermatol. 2008, 128, 2491–2497. [Google Scholar] [CrossRef] [PubMed]
- Buettner, G.R.; Jurkiewicz, B.A. Catalytic metals, ascorbate and free radicals: Combinations to avoid. Radiat. Res. 1996, 145, 532–541. [Google Scholar] [CrossRef] [PubMed]
- Furst, A. Can nutrition affect chemical toxicity? Int. J. Toxicol. 2002, 21, 419–424. [Google Scholar] [CrossRef] [PubMed]
- Dayan, S.H.; Arkins, J.P.; Sharma, V.; Paterson, E.; Barnes, D. A phase 2, double-blind, randomized, placebo-controlled trial of a novel nutritional supplement product to promote healthy skin. J. Drugs Dermatol. 2011, 10, 1106–1114. [Google Scholar] [PubMed]
- Lauer, A.C.; Groth, N.; Haag, S.F.; Darvin, M.E.; Lademann, J.; Meinke, M.C. Dose-dependent vitamin C uptake and radical scavenging activity in human skin measured with in vivo electron paramagnetic resonance spectroscopy. Skin Pharmacol. Physiol. 2013, 26, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Lauer, A.C.; Groth, N.; Haag, S.F.; Darvin, M.E.; Lademann, J.; Meinke, M.C. Radical scavenging capacity in human skin before and after vitamin C uptake: An in vivo feasibility study using electron paramagnetic resonance spectroscopy. J. Invest. Dermatol. 2013, 133, 1102–1104. [Google Scholar] [CrossRef] [PubMed]
- Meinke, M.C.; Lauer, A.-C.; Haag, S.F.; Darvin, M.E.; Groth, N.; Lademann, J. Cutaneous radical scavenging effects of orally administered antioxidants measured by electron paramagnetic resonance spectroscopy. E-SPEN J. 2012, 7, 160–166. [Google Scholar] [CrossRef]
- Shindo, Y.; Witt, E.; Packer, L. Antioxidant defense mechanisms in murine epidermis and dermis and their responses to ultraviolet light. J. Invest. Dermatol. 1993, 100, 260–265. [Google Scholar] [CrossRef] [PubMed]
- Gaziano, J.M.; Glynn, R.J.; Christen, W.G.; Kurth, T.; Belanger, C.; MacFadyen, J.; Bubes, V.; Manson, J.E.; Sesso, H.D.; Buring, J.E. Vitamins E and C in the prevention of prostate and total cancer in men: The Physicians’ Health Study II randomized controlled trial. JAMA 2009, 301, 52–62. [Google Scholar] [CrossRef] [PubMed]
- Meinke, M.C.; Darvin, M.E.; Vollert, H.; Lademann, J. Bioavailability of natural carotenoids in human skin compared to blood. Eur. J. Pharm. Biopharm. 2010, 76, 269–274. [Google Scholar] [CrossRef] [PubMed]
- Darvin, M.E.; Fluhr, J.W.; Schanzer, S.; Richter, H.; Patzelt, A.; Meinke, M.C.; Zastrow, L.; Golz, K.; Doucet, O.; Sterry, W.; et al. Dermal carotenoid level and kinetics after topical and systemic administration of antioxidants: Enrichment strategies in a controlled in vivo study. J. Dermatol. Sci. 2011, 64, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Lademann, J.; Schanzer, S.; Meinke, M.; Sterry, W.; Darvin, M.E. Interaction between carotenoids and free radicals in human skin. Skin Pharmacol. Physiol. 2011, 24, 238–244. [Google Scholar] [CrossRef] [PubMed]
- De Spirt, S.; Sies, H.; Tronnier, H.; Heinrich, U. An encapsulated fruit and vegetable juice concentrate increases skin microcirculation in healthy women. Skin Pharmacol. Physiol. 2012, 25, 2–8. [Google Scholar] [CrossRef] [PubMed]
- Sapuntsova, S.G.; Lebed’ko, O.A.; Shchetkina, M.V.; Fleyshman, M.Y.; Kozulin, E.A.; Timoshin, S.S. Status of free-radical oxidation and proliferation processes in patients with atopic dermatitis and lichen planus. Bull. Exp. Biol. Med. 2011, 150, 690–692. [Google Scholar] [CrossRef] [PubMed]
- Aly, D.G.; Shahin, R.S. Oxidative stress in lichen planus. Acta Dermatoven APA 2010, 19, 3–11. [Google Scholar]
- Arndt, S.; Haag, S.F.; Kleemann, A.; Lademann, J.; Meinke, M.C. Radical protection in the visible and infrared by a hyperforin-rich cream—In vivo vs. ex vivo methods. Exp. Dermatol. 2013, 22, 354–357. [Google Scholar] [CrossRef] [PubMed]
- Schempp, C.M.; Windeck, T.; Hezel, S.; Simon, J.C. Topical treatment of atopic dermatitis with St. John’s wort cream—A randomized, placebo controlled, double blind half-side comparison. Phytomedicine 2003, 10, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Schempp, C.M.; Pelz, K.; Wittmer, A.; Schopf, E.; Simon, J.C. Antibacterial activity of hyperforin from St John’s wort, against multiresistant Staphylococcus aureus and gram-positive bacteria. Lancet 1999, 353, 2129–2130. [Google Scholar] [CrossRef]
- Schempp, C.M.; Meinke, M.C.; Lademann, J.; Ferrari, Y.; Brecht, T.; Gehring, W. Topical antioxidants protect the skin from chemical-induced irritation in the repetitive washing test: A placebo-controlled, double-blind study. Contact Dermat. 2012, 67, 234–237. [Google Scholar] [CrossRef] [PubMed]
- Esser, P.R.; Wolfle, U.; Durr, C.; von Loewenich, F.D.; Schempp, C.M.; Freudenberg, M.A.; Jakob, T.; Martin, S.F. Contact sensitizers induce skin inflammation via ROS production and hyaluronic acid degradation. PLoS One 2012, 7. [Google Scholar] [CrossRef] [PubMed]
- Senaldi, G.; Pointaire, P.; Piguet, P.F.; Grau, G.E. Protective effect of N-acetylcysteine in hapten-induced irritant and contact hypersensitivity reactions. J. Invest. Dermatol. 1994, 102, 934–937. [Google Scholar] [CrossRef] [PubMed]
- Kuriyama, K.; Shimizu, T.; Horiguchi, T.; Watabe, M.; Abe, Y. Vitamin E ointment at high dose levels suppresses contact dermatitis in rats by stabilizing keratinocytes. Inflamm. Res. 2002, 51, 483–489. [Google Scholar] [CrossRef] [PubMed]
- Schäfer, U.F.; Loth, H. An ex vivo model for the study of drug penetration into human skin. Pharm. Res. 1996, 13, 336–341. [Google Scholar]
- Haag, S.F.; Bechtel, A.; Darvin, M.E.; Klein, F.; Groth, N.; Schafer-Korting, M.; Bittl, R.; Lademann, J.; Sterry, W.; Meinke, M.C. Comparative study of carotenoids, catalase and radical formation in human and animal skin. Skin Pharmacol. Physiol. 2010, 23, 306–312. [Google Scholar] [CrossRef] [PubMed]
- Jung, K.; Seifert, M.; Herrling, T.; Fuchs, J. UV-generated free radicals (FR) in skin: Their prevention by sunscreens and their induction by self-tanning agents. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2008, 69, 1423–1428. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, T.P.; Eaglstein, W.H.; Davis, S.C.; Mertz, P. The pig as a model for human wound healing. Wound Repair Regen 2001, 9, 66–76. [Google Scholar] [CrossRef] [PubMed]
- Regensburger, J.; Knak, A.; Maisch, T.; Landthaler, M.; Baumler, W. Fatty acids and vitamins generate singlet oxygen under UVB irradiation. Exp. Dermatol. 2012, 21, 135–139. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lohan, S.B.; Lauer, A.-C.; Arndt, S.; Friedrich, A.; Tscherch, K.; Haag, S.F.; Darvin, M.E.; Vollert, H.; Kleemann, A.; Gersonde, I.; et al. Determination of the Antioxidant Status of the Skin by In Vivo-Electron Paramagnetic Resonance (EPR) Spectroscopy. Cosmetics 2015, 2, 286-301. https://doi.org/10.3390/cosmetics2030286
Lohan SB, Lauer A-C, Arndt S, Friedrich A, Tscherch K, Haag SF, Darvin ME, Vollert H, Kleemann A, Gersonde I, et al. Determination of the Antioxidant Status of the Skin by In Vivo-Electron Paramagnetic Resonance (EPR) Spectroscopy. Cosmetics. 2015; 2(3):286-301. https://doi.org/10.3390/cosmetics2030286
Chicago/Turabian StyleLohan, Silke Barbara, Anna-Christina Lauer, Sophia Arndt, Annette Friedrich, Kathrin Tscherch, Stefan F. Haag, Maxim E. Darvin, Henning Vollert, Anke Kleemann, Ingo Gersonde, and et al. 2015. "Determination of the Antioxidant Status of the Skin by In Vivo-Electron Paramagnetic Resonance (EPR) Spectroscopy" Cosmetics 2, no. 3: 286-301. https://doi.org/10.3390/cosmetics2030286
APA StyleLohan, S. B., Lauer, A.-C., Arndt, S., Friedrich, A., Tscherch, K., Haag, S. F., Darvin, M. E., Vollert, H., Kleemann, A., Gersonde, I., Groth, N., Lademann, J., Rohn, S., & Meinke, M. C. (2015). Determination of the Antioxidant Status of the Skin by In Vivo-Electron Paramagnetic Resonance (EPR) Spectroscopy. Cosmetics, 2(3), 286-301. https://doi.org/10.3390/cosmetics2030286








