Main Benefits and Applicability of Plant Extracts in Skin Care Products
Abstract
:1. Natural Ingredients in Cosmetics
2. Benefits of Plant Extracts
2.1. Antioxidant Activity
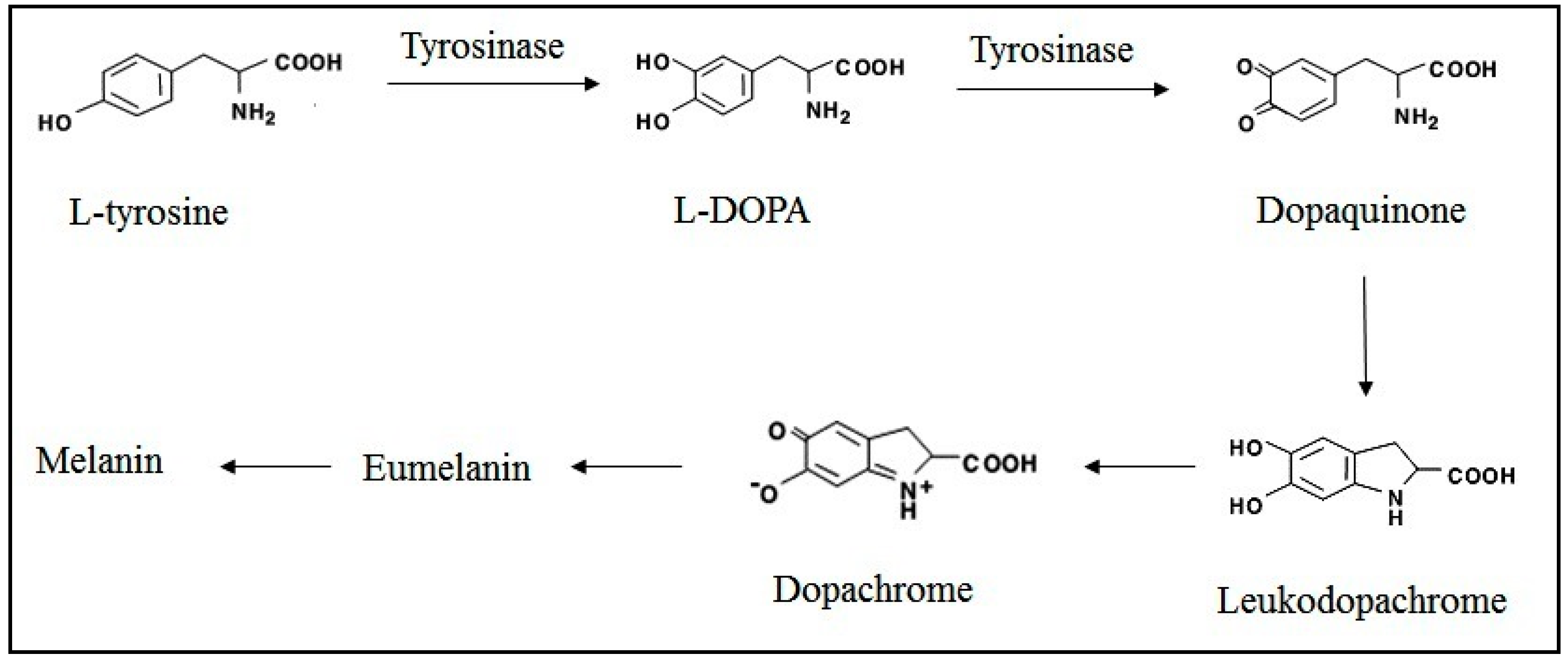
2.2. Tyrosinase Inhibition Effect
2.3. Antimicrobial Activity
3. Plant Extracts and Skin Care Products
3.1. Castanea Sativa
3.2. Prunus Dulcis
3.3. Juglans Regia L.
3.4. Olea Europaea
3.5. Helichrysum Stroechas (L.) Moench
3.6. Quercus Robur
3.7. Glycyrrhiza Glabra
3.8. Vitis Vinifera
3.9. Crataegus Monogyna Jacq
3.10. Pinus Pinaster
4. Conclusions
Author Contributions
Conflicts of Interest
References
- Rawlings, A.V.; Scott, I.R.; Harding, C.R.; Bowser, P.A. Stratum corneum moisturization at the molecular level. J. Investig. Dermatol. 1994, 103, 731–740. [Google Scholar] [CrossRef] [PubMed]
- Hardin, C.R.; Watkinson, A.; Rawlings, A.V. Dry skin, moisturization and corneodesmolysis. Int. J. Cosmet. Sci. 2000, 22, 21–52. [Google Scholar] [CrossRef] [PubMed]
- Draelos, Z.D. The cosmeceutical realm. Clin. Dermatol. 2008, 26, 627–632. [Google Scholar] [CrossRef] [PubMed]
- Mukul, S.; Surabhi, K.; Atul, N. Cosmeceuticals for the skin: An overview. Asian J. Pharm. Clin. Res. 2011, 4, 1–6. [Google Scholar]
- Fowler, J.F., Jr.; Woolery-Loyd, H.; Waldorf, H.; Saini, R. Innovations in natural ingredients and their use in skin care. J. Drugs Dermatol. 2010, 9, s72–s81. [Google Scholar] [PubMed]
- Dorland’s Illustrated Medical Dictionary, 29th ed.; W.B. Saunders Company: Philadelphia, PA, USA, 2000.
- Dweck, A.C. Botanicals—Research of actives. Cosmet. Toilet. 1996, 111, 45–57. [Google Scholar]
- Aburjai, T.; Natsheh, F.M. Plants used in cosmetics. Phytother. Res. 2003, 17, 987–1000. [Google Scholar] [CrossRef] [PubMed]
- Laroche, M.; Bergeron, J.; Barbaro-Forleo, G. Targeting consumers who are willing to pay more for environmentally friendly products. J. Consum. Mark. 2001, 18, 503–520. [Google Scholar] [CrossRef]
- Dureja, H.; Kaushik, D.; Gupta, M.; Kumar, V.; Lather, V. Cosmeceuticals: An emerging concept. Indian J. Pharm. 2005, 37, 155–159. [Google Scholar] [CrossRef]
- Dubey, N.K.; Kumar, R.; Tripathi, P. Global promotion of herbal medicine: India’s opportunity. Curr. Sci. 2004, 86, 37–41. [Google Scholar]
- Chaudhari, P.M.; Kawade, P.V.; Funne, S.M. Cosmeceuticals—A review. Int. J. Pharm. Technol. 2011, 3, 774–798. [Google Scholar]
- Kaur, G.; Jabbar, Z.; Athar, M.; Alam, M.S. Punica granatum (pomegranate) flower extract possesses potent antioxidant activity and abrogates Fe-NTA induced hepatotoxicity in mice. Food Chem. Toxicol. 2006, 44, 984–993. [Google Scholar] [CrossRef] [PubMed]
- Yamakoshi, J.; Otsuka, F.; Sano, A.; Tokutake, S.; Saito, M.; Kikuchi, M.; Kubota, Y. Lightening effect on ultraviolet-induced pigmentation of guinea pig skin by oral administration of a proanthocyanidin-rich extract from grape seeds. Pigment Cell Res. 2003, 16, 629–638. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.P.; Agarwal, R. Cosmeceuticals and silibinin. Clin. Dermatol. 2009, 27, 479–484. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.-Y.; Selim, M.A.; Shea, C.R.; Grichnik, J.M.; Omar, M.M.; Monteiro-Riviere, N.A.; Pinnell, S.R. UV photoprotection by combination topical antioxidants vitamin C and vitamin E. J. Am. Acad. Dermatol. 2003, 48, 866–874. [Google Scholar] [CrossRef] [PubMed]
- Marquele-Oliveira, F.; Fonseca, Y.M.; de Freitas, O.; Fonseca, M.J.V. Development of topical functionalized formulations added with propolis extract: Stability, cutaneous absorption and in vivo studies. Int. J. Pharm. 2007, 342, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Burke, K.E. Photodamage of the skin: Protection and reversal with topical antioxidants. J. Cosmet. Dermatol. 2004, 3, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Kornsteiner, M.; Wagner, K.-H.; Elmadfa, I. Tocopherols and total phenolics in 10 different nut types. Food Chem. 2006, 98, 381–387. [Google Scholar] [CrossRef]
- Velioglu, Y.S.; Mazza, G.; Gao, L.; Oomah, B.D. Antioxidant activity and total phenolics in selected fruits, vegetables, and grain products. J. Agric. Food Chem. 1998, 46, 4113–4117. [Google Scholar] [CrossRef]
- Figueiredo, A.C.; Barroso, J.G.; Pedro, L.G.; Scheffer, J.J.C. Factors affecting secondary metabolite production in plants: Volatile components and essential oils. Flavour. Fragr. J. 2008, 23, 213–226. [Google Scholar] [CrossRef]
- Maqsood, S.; Benjakul, S. Comparative studies of four different phenolic compounds on in vitro antioxidative activity and the preventive effect on lipid oxidation of fish oil emulsion and fish mince. Food Chem. 2010, 119, 123–132. [Google Scholar] [CrossRef]
- Zhang, Z.; Liao, L.; Moore, J.; Wu, T.; Wang, Z. Antioxidant phenolic compounds from walnut kernels (juglans regia L.). Food Chem. 2009, 113, 160–165. [Google Scholar] [CrossRef]
- Silva, E.M.; Souza, J.N.S.; Rogez, H.; Rees, J.F.; Larondelle, Y. Antioxidant activities and polyphenolic contents of fifteen selected plant species from the amazonian region. Food Chem. 2007, 101, 1012–1018. [Google Scholar] [CrossRef]
- Anitha, T. Medicinal plants used in skin protection. Asian J. Pharm. Clin. Res. 2012, 5, 35–38. [Google Scholar]
- Jakopič, J.; Veberič, R.; Štampar, F. Extraction of phenolic compounds from green walnut fruits in different solvents. Acta Agric. Slov. 2009, 93, 11–15. [Google Scholar]
- Mapunya, M.B.; Nikolova, R.V.; Lall, N. Melanogenesis and antityrosinase activity of selected south african plants. Evid.-Based Complement. Alternat. Med. 2012, 2012. [Google Scholar] [CrossRef] [PubMed]
- Gillbro, J.M.; Olsson, M.J. The melanogenesis and mechanisms of skin-lightening agents—Existing and new approaches. Int. J. Cosmet. Sci. 2011, 33, 210–221. [Google Scholar] [CrossRef] [PubMed]
- Satooka, H.; Kubo, I. Effects of thymol on mushroom tyrosinase-catalyzed melanin formation. J. Agric. Food Chem. 2011, 59, 8908–8914. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Liu, X.-M.; Zhang, J.; Zhang, Y.-Q. An efficient preparation of mulberroside a from the branch bark of mulberry and its effect on the inhibition of tyrosinase activity. PLoS ONE 2014, 9. [Google Scholar] [CrossRef] [PubMed]
- Lall, N.; Kishore, N. Are plants used for skin care in south africa fully explored? J. Ethnopharmacol. 2014, 153, 61–84. [Google Scholar] [CrossRef] [PubMed]
- Grimes, P.; Nordlund, J.J.; Pandya, A.G.; Taylor, S.; Rendon, M.; Ortonne, J.P. Increasing our understanding of pigmentary disorders. J. Am. Acad. Dermatol. 2006, 54, S255–S261. [Google Scholar] [CrossRef] [PubMed]
- Solano, F.; Briganti, S.; Picardo, M.; Ghanem, G. Hypopigmenting agents: An updated review on biological, chemical and clinical aspects. Pigment Cell Res. 2006, 19, 550–571. [Google Scholar] [CrossRef] [PubMed]
- Augustin, M.; Hoch, Y. Phytotherapie bei Hauterkrankungen; Urban & Fischer Verlag/Elsevier GmbH: Munich, Germany, 2004. (In German) [Google Scholar]
- Cowan, M.M. Plant products as antimicrobial agents. Clin. Microbiol. Rev. 1999, 12, 564–582. [Google Scholar] [PubMed]
- Taguri, T.; Tanaka, T.; Kouno, I. Antibacterial spectrum of plant polyphenols and extracts depending upon hydroxyphenyl structure. Biol. Pharm. Bull. 2006, 29, 2226–2235. [Google Scholar] [CrossRef] [PubMed]
- Tian, F.; Li, B.; Ji, B.; Zhang, G.; Luo, Y. Identification and structure-activity relationship of gallotannins separated from galla chinensis. LWT-Food Sci. Technol. 2009, 42, 1289–1295. [Google Scholar] [CrossRef]
- Rains, J.L.; Jain, S.K. Oxidative stress, insulin signaling, and diabetes. Free Radic. Biol. Med. 2011, 50, 567–575. [Google Scholar] [CrossRef] [PubMed]
- Amaral, L.F.; Moriel, P.; Foglio, M.A.; Mazzola, P.G. Caryocar brasiliense supercritical CO2 extract possesses antimicrobial and antioxidant properties useful for personal care products. BMC Complement Altern. Med. 2014, 14. [Google Scholar] [CrossRef] [PubMed]
- Calliste, C.A.; Trouillas, P.; Allais, D.P.; Duroux, J.L. Castanea sativa Mill. leaves as new sources of natural antioxidant: An electronic spin resonance study. J. Agric. Food Chem. 2005, 53, 282–288. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, B.; Rangel, J.; Valentão, P.C.; Andrade, P.B.; Pereira, J.A.; Bölke, H.; Seabra, R.M. Organic acids in two portuguese chestnut (Castanea sativa Miller) varieties. Food Chem. 2007, 100, 504–508. [Google Scholar] [CrossRef]
- Barreira, J.C.M.; Ferreira, I.C.F.R.; Oliveira, M.B.P.P.; Pereira, J.A. Antioxidant activities of the extracts from chestnut flower, leaf, skins and fruit. Food Chem. 2008, 107, 1106–1113. [Google Scholar] [CrossRef]
- Barreira, J.C.; Casal, S.; Ferreira, I.C.; Peres, A.M.; Pereira, J.A.; Oliveira, M.B. Chemical characterization of chestnut cultivars from three consecutive years: Chemometrics and contribution for authentication. Food Chem. Toxicol. 2012, 50, 2311–2317. [Google Scholar] [CrossRef] [PubMed]
- Basile, A.; Sorbo, S.; Giordano, S.; Ricciardi, L.; Ferrara, S.; Montesano, D.; Vuotto, M.L.; Castaldo Cobianchi, R.; Ferrara, L. Antibacterial and allelopathic activity of extract from Castanea sativa leaves. Fitoterapia 2000, 71, S110–S116. [Google Scholar] [CrossRef] [PubMed]
- Almeida, I.F.; Maleckova, J.; Saffi, R.; Monteiro, H.; Goios, F.; Amaral, M.H.; Costa, P.C.; Garrido, J.; Silva, P.; Pestana, N.; et al. Characterization of an antioxidant surfactant-free topical formulation containing Castanea sativa leaf extract. Drug Dev. Ind. Pharm. 2015, 41, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Cordeiro, V.; Monteiro, A. Almond growing in Trás-os-Montes region (Portugal). Acta Hortic. 2002, 591, 161–165. [Google Scholar]
- Martins, M.; Tenreiro, R.; Oliveira, M.M. Genetic relatedness of Portuguese almond cultivars assessed by rapd and issr markers. Plant Cell Rep. 2003, 22, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Rao, H.J. Therapeutic applications of almonds (Prunus amygdalus L.): A review. J. Clin. Diagn. Res. 2012, 6, 130–135. [Google Scholar]
- Milbury, P.E.; Chen, C.Y.; Dolnikowski, G.G.; Blumberg, J.B. Determination of flavonoids and phenolics and their distribution in almonds. J. Agric. Food Chem. 2006, 54, 5027–5033. [Google Scholar] [CrossRef] [PubMed]
- Pinelo, M.; Rubilar, M.; Sineiro, J.; Núñez, M.J. Extraction of antioxidant phenolics from almond hulls (Prunus amygdalus) and pine sawdust (Pinus pinaster). Food Chem. 2004, 85, 267–273. [Google Scholar] [CrossRef]
- Sang, S.; Lapsley, K.; Jeong, W.-S.; Lachance, P.A.; Ho, C.-T.; Rosen, R.T. Antioxidative phenolic compounds isolated from almond skins (Prunus amygdalus Batsch). J. Agric. Food Chem. 2002, 50, 2459–2463. [Google Scholar] [CrossRef] [PubMed]
- Takeoka, G.R.; Dao, L.T. Antioxidant constituents of almond [Prunus dulcis (Mill.) D.A. Webb] hulls. J. Agric. Food Chem. 2003, 51, 496–501. [Google Scholar] [CrossRef] [PubMed]
- Wijeratne, S.S.; Abou-Zaid, M.M.; Shahidi, F. Antioxidant polyphenols in almond and its coproducts. J. Agric. Food Chem. 2006, 54, 312–318. [Google Scholar] [CrossRef] [PubMed]
- Wijeratne, S.K.; Amarowicz, R.; Shahidi, F. Antioxidant activity of almonds and their by-products in food model systems. J. Am. Oil Chem. Soc. 2006, 83, 223–230. [Google Scholar] [CrossRef]
- Siriwardhana, S.K.W.; Shahidi, F. Antiradical activity of extracts of almond and its by-products. J. Am. Oil Chem. Soc. 2002, 79, 903–908. [Google Scholar] [CrossRef]
- Monagas, M.; Garrido, I.; Lebron-Aguilar, R.; Bartolome, B.; Gomez-Cordoves, C. Almond (Prunus dulcis (Mill.) D.A. Webb) skins as a potential source of bioactive polyphenols. J. Agric. Food Chem. 2007, 55, 8498–8507. [Google Scholar] [CrossRef] [PubMed]
- Keser, S.; Demir, E.; Yilmaz, O. Phytochemicals and antioxidant activity of the almond kernel (Prunus dulcis mill.) from Turkey. J. Chem. Soc. Pak. 2014, 36, 534–541. [Google Scholar]
- Barreira, J.C.M.; Ferreira, I.C.F.R.; Oliveira, M.B.P.P.; Pereira, J.A. Antioxidant potential of chestnut (Castanea sativa L.) and almond (Prunus dulcis L.) by-products. Food Sci. Technol. Int. 2010, 16, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Stampar, F.; Solar, A.; Hudina, M.; Veberic, R.; Colaric, M. Traditional walnut liqueur—Cocktail of phenolics. Food Chem. 2006, 95, 627–631. [Google Scholar] [CrossRef]
- Wojciechowska, K.; Zun, M.; Dwornicka, D.; Serefko, A.; Świąder, K.; Poleszak, E. Physical and chemical properties of cosmetic cream made of ingredients obtained from Juglans regia L. Curr. Issues Pharm. Med. Sci. 2012, 25, 190–193. [Google Scholar] [CrossRef]
- Pereira, J.A.; Oliveira, I.; Sousa, A.; Ferreira, I.C.F.R.; Bento, A.; Estevinho, L. Bioactive properties and chemical composition of six walnut (Juglans regia L.) cultivars. Food Chem. Toxicol. 2008, 46, 2103–2111. [Google Scholar] [CrossRef] [PubMed]
- Samaranayaka, A.G.P.; John, J.A.; Shahidi, F. Antioxidant activity of English walnut (Juglands regla L.). J. Food Lipids 2008, 15, 384–397. [Google Scholar] [CrossRef]
- Bruneton, J. Pharmacognosie, Phytochimie, Plantes Medicinales; Tec & Doc Lavoisier: Paris, France, 1999. (In French) [Google Scholar]
- Almeida, I.F.; Fernandes, E.; Lima, J.L.F.C.; Costa, P.C.; Bahia, M.F. Walnut (Juglans regia) leaf extracts are strong scavengers of pro-oxidant reactive species. Food Chem. 2008, 106, 1014–1020. [Google Scholar] [CrossRef]
- Pereira, J.A.; Oliveira, I.; Sousa, A.; Valentão, P.; Andrade, P.B.; Ferreira, I.C.F.R.; Ferreres, F.; Bento, A.; Seabra, R.M.; Estevinho, L.M. Walnut (Juglans regia L.) leaves: Phenolic compounds, antimicrobial activity ans antioxidant potential of different cultivars. Food Chem. Toxicol. 2007, 45, 2287–2295. [Google Scholar] [CrossRef] [PubMed]
- El, S.N.; Karakaya, S. Olive tree (Olea europaea) leaves: Potential beneficial effects on human health. Nutr. Rev. 2009, 67, 632–638. [Google Scholar] [CrossRef] [PubMed]
- Bisignano, G.; Tomaino, A.; Cascio, R.L.; Crisafi, G.; Uccella, N.; Saija, A. On the in vitro antimicrobial activity of oleuropein and hydroxytyrosol. J. Pharm. Pharmacol. 1999, 51, 971–974. [Google Scholar] [CrossRef] [PubMed]
- Capasso, R.; Evidente, A.; Schivo, L.; Orru, G.; Marcialis, M.A.; Cristinzio, G. Antibacterial polyphenols from olive oil mill waste waters. J. Appl. Bacteriol. 1995, 79, 393–398. [Google Scholar] [CrossRef] [PubMed]
- Kris-Etherton, P.M.; Hecker, K.D.; Bonanome, A.; Coval, S.M.; Binkoski, A.E.; Hilpert, K.F.; Griel, A.E.; Etherton, T.D. Bioactive compounds in foods: Their role in the prevention of cardiovascular disease and cancer. Am. J. Med. 2002, 113, 71–88. [Google Scholar] [CrossRef]
- Owen, R.W.; Giacosa, A.; Hull, W.E.; Haubner, R.; Spiegelhalder, B.; Bartsch, H. The antioxidant/anticancer potential of phenolic compounds isolated from olive oil. Eur. J. Cancer 2000, 36, 1235–1247. [Google Scholar] [CrossRef] [PubMed]
- Haloui, E.; Marzouk, B.; Marzouk, Z.; Bouraoui, A.; Fenina, N. Hydroxytyrosol and oleuropein from olive leaves: Potent anti-inflammatory and analgesic activities. J. Food Agric. Environ. 2011, 9, 128–133. [Google Scholar]
- Aeschbach, R.; Löliger, J.; Scott, B.C.; Murcia, A.; Butler, J.; Halliwell, B.; Aruoma, O.I. Antioxidant actions of thymol, carvacrol, 6-gingerol, zingerone and hydroxytyrosol. Food Chem. Toxicol. 1994, 32, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Visioli, F.; Poli, A.; Gall, C. Antioxidant and other biological activities of phenols from olives and olive oil. Med. Res. Rev. 2002, 22, 65–75. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulos, G.; Boskou, D. Antioxidant effect of natural phenols on olive oil. J. Am. Oil Chem. Soc. 1991, 68, 669–671. [Google Scholar] [CrossRef]
- Pérez-Bonilla, M.; Salido, S.; van Beek, T.A.; Altarejos, J. Radical-scavenging compounds from olive tree (Olea europaea L.) wood. J. Agric. Food Chem. 2013, 62, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Miralles, P.; Chisvert, A.; Salvador, A. Determination of hydroxytyrosol and tyrosol by liquid chromatography for the quality control of cosmetic products based on olive extracts. J. Pharm. Biomed. Anal. 2015, 102, 157–161. [Google Scholar] [CrossRef] [PubMed]
- Alu’datt, M.H.; Alli, I.; Ereifej, K.; Alhamad, M.; Al-Tawaha, A.R.; Rababah, T. Optimisation, characterisation and quantification of phenolic compounds in olive cake. Food Chem. 2010, 123, 117–122. [Google Scholar] [CrossRef]
- Goulas, V.; Papoti, V.T.; Exarchou, V.; Tsimidou, M.Z.; Gerothanassis, I.P. Contribution of flavonoids to the overall radical scavenging activity of olive (Olea europaea L.) leaf polar extracts. J. Agric. Food Chem. 2010, 58, 3303–3308. [Google Scholar] [CrossRef] [PubMed]
- Papoti, V.T.; Tsimidou, M.Z. Impact of sampling parameters on the radical scavenging potential of olive (Olea europaea L.) leaves. J. Agric. Food Chem. 2009, 57, 3470–3477. [Google Scholar] [CrossRef] [PubMed]
- Haddouchi, F.; Chaouche, T.M.; Ksouri, R.; Medini, F.; Sekkal, F.Z.; Benmansour, A. Antioxidant activity profiling by spectrophotometric methods of aqueous methanolic extracts of Helichrysum stoechas subsp. rupestre and Phagnalon saxatile subsp. saxatile. Chin. J. Nat. Med. 2014, 12, 415–422. [Google Scholar]
- Albayrak, S.; Aksoy, A.; Sagdic, O.; Hamzaoglu, E. Compositions, antioxidant and antimicrobial activities of helichrysum (asteraceae) species collected from Turkey. Food Chem. 2010, 119, 114–122. [Google Scholar] [CrossRef]
- Lourens, A.C.; Viljoen, A.M.; van Heerden, F.R. South African helichrysum species: A review of the traditional uses, biological activity and phytochemistry. J. Ethnopharmacol. 2008, 119, 630–652. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, A.M. Plantas y Sabiduría Popular del Parque Natural de Montesinho: Un Estudío Etnobotánico en Portugal; CSIC, Biblioteca de Ciencias: Madrid, Spain, 2010. (In Spanish) [Google Scholar]
- Carini, M.; Aldini, G.; Furlanetto, S.; Stefani, R.; Facino, R.M. LC coupled to ion-trap MS for the rapid screening and detection of polyphenol antioxidants from Helichrysum stoechas. J. Pharm. Biomed. Anal. 2001, 24, 517–526. [Google Scholar] [CrossRef] [PubMed]
- Barros, L.; Oliveira, S.; Carvalho, A.M.; Ferreira, I.C.F.R. In vitro antioxidant properties and characterization in nutrients and phytochemicals of six medicinal plants from the Portuguese folk medicine. Ind. Crops Prod. 2010, 32, 572–579. [Google Scholar] [CrossRef]
- Barroso, M.R.; Barros, L.; Dueñas, M.; Carvalho, A.M.; Santos-Buelga, C.; Fernandes, I.P.; Barreiro, M.F.; Ferreira, I.C.F.R. Exploring the antioxidant potential of Helichrysum stoechas (L.) moench phenolic compounds for cosmetic applications: Chemical characterization, microencapsulation and incorporation into a moisturizer. Ind. Crops Prod. 2014, 53, 330–336. [Google Scholar] [CrossRef]
- Vaya, J.; Belinky, P.A.; Aviram, M. Antioxidant constituents from licorice roots: Isolation, structure elucidation, and antioxidative capacity toward LDL oxidatio. Free Radic. Biol. Med. 1997, 23, 302–313. [Google Scholar] [CrossRef] [PubMed]
- Leung, A.T.; Foste, S. Encyclopedia of Common Natural Ingredients Used in Food, Drugs and Cosmetics; Wiley: New York, USA, 1996. [Google Scholar]
- Morteza-Semnani, K.; Saeedi, M.; Shahnavaz, B. Comparison of antioxidant activity of extract from roots of licorice (Glycyrrhiza glabra L.) to commercial antioxidants in 2% hydroquinone cream. J. Cosmet. Sci. 2003, 54, 551–558. [Google Scholar] [PubMed]
- Upadhyay, S.; Ghosh, A.K.; Singh, V. Hair growth promotant activity of petroleum ether root extract of Glycyrrhiza glabra L. (Fabaceae) in female rats. Trop. J. Pharm. Res. 2012, 11, 753–758. [Google Scholar]
- Geetha, R.V.; Roy, A. In vitro evaluation of anti bacterial activity of ethanolic root extract of Glycyrrhiza glabra on oral microbes. Int. J. Drug Dev. Res. 2012, 4, 161–165. [Google Scholar]
- Fu, B.; Li, H.; Wang, X.; Lee, F.S.; Cui, S. Isolation and identification of flavonoids in licorice and a study of their inhibitory effects on tyrosinase. J. Agric. Food Chem. 2005, 53, 7408–7414. [Google Scholar] [CrossRef] [PubMed]
- Nerya, O.; Vaya, J.; Musa, R.; Izrael, S.; Ben-Arie, R.; Tamir, S. Glabrene and isoliquiritigenin as tyrosinase inhibitors from licorice roots. J. Agric. Food Chem. 2003, 51, 1201–1207. [Google Scholar] [CrossRef] [PubMed]
- Hara, T.; Matsui, H.; Shimizu, H. Suppression of microbial metabolic pathways inhibits the generation of the human body odor component diacetyl bystaphylococcusspp. PLoS ONE 2014, 9. [Google Scholar] [CrossRef] [PubMed]
- Fiume, M.M.; Bergfeld, W.F.; Belsito, D.V.; Hill, R.A.; Klaassen, C.D.; Liebler, D.C.; Shank, R.C.; Marks, J.G., Jr.; Slaga, T.J.; Slaga, T.J.; et al. Safety assessment of Vitis vinifera (Grape)-derived ingredients as used in cosmetics. Int. J. Toxicol. 2014, 33, 48S–83S. [Google Scholar] [CrossRef] [PubMed]
- Personal Care Products Council. Available online: http://online.personalcarecouncil.org/jsp/Home.jsp (accessed on 17 February 2015).
- Food and Drug Administration (FDA). Frequency of Use of Cosmetic Ingredients; FDA: Washington, DC, USA, 2012.
- Yamakoshi, J.; Sano, A.; Tokutake, S.; Saito, M.; Kikuchi, M.; Kubota, Y.; Kawachi, Y.; Otsuka, F. Oral intake of proanthocyanidin-rich extract from grape seeds improves chloasma. Phytother. Res. 2004, 18, 895–899. [Google Scholar] [CrossRef] [PubMed]
- Proença da Cunha, A.; da Silva, A.P.; Roque, O.R.; Cunha, E. Plantas e Produtos Vegetais em Cosmética e Dermatologia; Fundação Calouste Gulbenkian: Lisbon, Portugal, 2004. (In Portuguese) [Google Scholar]
- Barros, L.; Carvalho, A.M.; Ferreira, I.C. Comparing the composition and bioactivity of crataegus monogyna flowers and fruits used in folk medicine. Phytochem. Anal. 2011, 22, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.P. The development of Chinese herbal medicine and the Pen-ts’ao. Am. J. Chin. Med. 1977, 5, 117–122. [Google Scholar] [CrossRef]
- European Pharmacopoeia 8.0, 8th ed.; EDQM—European Directorate for the Quality of Medicines & Healthcare (Council of Europe): Strasburg, France, 2014.
- Zhang, Z.; Chang, Q.; Zhu, M.; Huang, Y.; Ho, W.K.; Chen, Z. Characterization of antioxidants present in hawthorn fruits. J. Nutr. Biochem. 2001, 12, 144–152. [Google Scholar] [CrossRef] [PubMed]
- Yao, M.; Ritchie, H.E.; Brown-Woodman, P.D. A reproductive screening test of hawthorn. J. Ethnopharmacol. 2008, 118, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Tadic, V.M.; Dobric, S.; Markovic, G.M.; Dordevic, S.M.; Arsic, I.A.; Menkovic, N.R.; Stevic, T. Anti-inflammatory, gastroprotective, free-radical-scavenging, and antimicrobial activities of hawthorn berries ethanol extract. J. Agric. Food Chem. 2008, 56, 7700–7709. [Google Scholar] [CrossRef] [PubMed]
- Shalizar Jalali, A.; Hasanzadeh, S. Crataegus monogyna fruit aqueous extract as a protective agent against doxorubicin-induced reproductive toxicity in male rats. Avicenna J. Phytomed. 2013, 3, 159–170. [Google Scholar] [PubMed]
- Neves, J.M.; Matos, C.; Moutinho, C.; Queiroz, G.; Gomes, L.R. Ethnopharmacological notes about ancient uses of medicinal plants in Tras-os-Montes (northern of Portugal). J. Ethnopharmacol. 2009, 124, 270–283. [Google Scholar] [CrossRef] [PubMed]
- Bahorun, T.; Gressier, B.; Trotin, F.; Brunet, C.; Dine, T.; Luyckx, M.; Vasseur, J.; Cazin, M.; Cazin, J.C.; Pinkas, M. Oxygen species scavenging activity of phenolic extracts from hawthorn fresh plant organs and pharmaceutical preparations. Arzneimittel-Forschung 1996, 46, 1086–1089. [Google Scholar] [PubMed]
- Ljubuncic, P.; Azaizeh, H.; Portnaya, I.; Cogan, U.; Said, O.; Saleh, K.A.; Bomzon, A. Antioxidant activity and cytotoxicity of eight plants used in traditional arab medicine in Israel. J. Ethnopharmacol. 2005, 99, 43–47. [Google Scholar] [CrossRef] [PubMed]
- Ponomarenko, J.; Trouillas, P.; Martin, N.; Dizhbite, T.; Krasilnikova, J.; Telysheva, G. Elucidation of antioxidant properties of wood bark derived saturated diarylheptanoids: A comprehensive (DFT-supported) understanding. Phytochemistry 2014, 103, 178–187. [Google Scholar] [CrossRef] [PubMed]
- Marini, A.; Grether-Beck, S.; Jaenicke, T.; Weber, M.; Burki, C.; Formann, P.; Brenden, H.; Schonlau, F.; Krutmann, J. Pycnogenol® effects on skin elasticity and hydration coincide with increased gene expressions of collagen type I and hyaluronic acid synthase in women. Skin Pharmacol. Physiol. 2012, 25, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Nishigori, C.; Hattori, Y.; Toyokuni, S. Role of reactive oxygen species in skin carcinogenesis. Antioxid. Redox Signal. 2004, 6, 561–570. [Google Scholar] [CrossRef] [PubMed]
- Belcaro, G.; Cesarone, M.R.; Errichi, B.M.; Ledda, A.; di Renzo, A.; Stuard, S.; Dugall, M.; Pellegrini, L.; Gizzi, G.; Rohdewald, P.; et al. Diabetic ulcers: Microcirculatory improvement and faster healing with pycnogenol. Clin. Appl. Thromb. Hemost. 2006, 12, 318–323. [Google Scholar] [CrossRef] [PubMed]
- Iravani, S.; Zolfaghari, B. Pharmaceutical and nutraceutical effects of Pinus pinaster bark extract. Res. Pharm. Sci. 2011, 6, 1–11. [Google Scholar] [PubMed]
- Vertuani, S.; Buzzoni, V.; Manfredini, S.B.B. Evaluation of the stability of oligomeric proanthocyanidins from Pinus pinaster ait. in cosmetics formulations. SOFW J. 2001, 127, 20–23. [Google Scholar]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ribeiro, A.S.; Estanqueiro, M.; Oliveira, M.B.; Sousa Lobo, J.M. Main Benefits and Applicability of Plant Extracts in Skin Care Products. Cosmetics 2015, 2, 48-65. https://doi.org/10.3390/cosmetics2020048
Ribeiro AS, Estanqueiro M, Oliveira MB, Sousa Lobo JM. Main Benefits and Applicability of Plant Extracts in Skin Care Products. Cosmetics. 2015; 2(2):48-65. https://doi.org/10.3390/cosmetics2020048
Chicago/Turabian StyleRibeiro, Ana Sofia, Marilene Estanqueiro, M. Beatriz Oliveira, and José Manuel Sousa Lobo. 2015. "Main Benefits and Applicability of Plant Extracts in Skin Care Products" Cosmetics 2, no. 2: 48-65. https://doi.org/10.3390/cosmetics2020048
APA StyleRibeiro, A. S., Estanqueiro, M., Oliveira, M. B., & Sousa Lobo, J. M. (2015). Main Benefits and Applicability of Plant Extracts in Skin Care Products. Cosmetics, 2(2), 48-65. https://doi.org/10.3390/cosmetics2020048







