Abstract
(1) Background: Low-molecular-weight hyaluronic acid displays moisturizing and anti-aging properties and reduces UV-induced inflammation when applied topically. A 3 kDa sodium hyaluronate oligosaccharide (Extra-Low HA) was designed, and studies were performed to evaluate its safety for cosmetic applications. (2) Methods: The ability of the Extra-Low HA (ExLMW-HA) to penetrate skin was evaluated. Then, pro-inflammatory cytokines were quantified in the culture medium of skin explants following ExLMW-HA application with or without inflammation inducer (PMA). Finally, four predictive in vitro tests (Keratinosens™, kDPRA, Ames’ test, micronucleus test) were conducted to assess the safety of ExLMW-HA. (3) Results: The molecule permeates skin down to the living epidermis and possibly interacts with the dermal compartment. The oligosaccharide did not induce TNF-α, IL-1β, IL-1α, CXCL2, CCL3, or IL-15, neither in basal nor in stressed conditions. ExLMW-HA is not predicted to be a skin sensitizer or a mutagenic or genotoxic substance. (4) Conclusions: This 3 kDA HA is considered safe for use in topical application at the tested dosage.
Keywords:
hyaluronan; skin; permeation; cytokines; inflammation; sensitization; genotoxicity; mutagenicity 1. Introduction
Hyaluronic acid or hyaluronan (HA) is a linear, non-sulfated polysaccharide made of repeating units composed of D-glucuronic acid and N-acetyl glucosamine bonded via β-1,3 and β-1,4 linkages, respectively (Figure 1) [1,2]. In human skin, HA is the most abundant glycosaminoglycan (GAG) and has an important structural and stabilizing role in the extracellular matrix, together with sulfated GAGs and proteoglycans [3]. HA presents a coil structure that is able to bind water and helps to maintain skin plumping and hydration [4]. More than a structural component, HA regulates skin homeostasis, modulating cell proliferation and migration, angiogenesis, and inflammation, especially during the wound healing process [5,6]. The biological effects of HA are mediated by its receptors (CD44, RHAMM, LYVE-1, TLR) and strongly depend on its molecular weight [6,7,8].
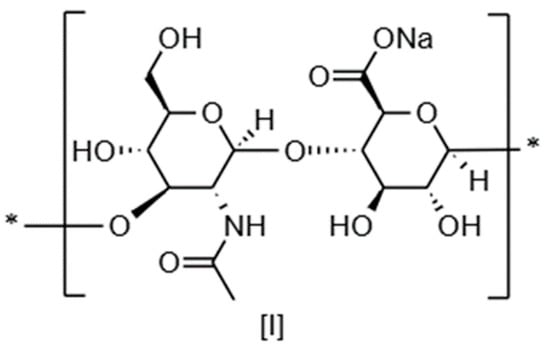
Figure 1.
Chemical structure of sodium hyaluronate dimeric basic unit.
A major difference between low-molecular-weight HA (LMW-HA) and high-molecular-weight HA (HMW-HA, >1000 kDa) resides in their ability to penetrate skin. Raman spectroscopy demonstrated that, following topical application of HMW-HA on skin explants, the molecules do not cross the stratum corneum (SC), while 100–300 kDa HA can penetrate the SC and 20–50 kDa molecules were observed to even reach the deeper epidermis [9]. Another work indicated that 80 kDa HA remained in the SC, while 10 and 30 kDa HA accumulated in the epidermis [10]. However, other methodologies, such as quantitative ELISA and radiolabeling, resulted in different conclusions, observing HA from 10 to 50 kDa remaining in the SC [11,12].
Independent studies on the skin permeability of LMW-HA below 10 kDa—here named HA oligomers (o-HAs)—present matching findings, concluding that these smaller molecules are able to penetrate down to the dermis. Fluorescein-labeled o-HAs of size 5–8 kDa pass through the SC barrier to enter the epidermis and dermis layers, interacting with keratin and lipids in the SC and increasing hydration as previously described [13,14]. A 2 kDA fluorescein-labeled o-HA was also observed to pass through the SC and penetrate to the dermis in excised human skin, with the main signal detected in the epidermis [15]. A hexasaccharide (1137.9 Da) and a tetrasaccharide (776.3 Da) were respectively observed to fully penetrate skin in human explants and a hairless murine model [16,17].
LMW-HAs have several applications in dermatology, as described in a recent review: the treatment of seborrheic dermatitis and rosacea, wound healing, oral care, anti-aging, and drug delivery [18]. There is a growing interest in o-HAs that display moisturizing, anti-aging, and anti-inflammatory activities. Compared to an HMW-HA (1800 kDa), a 7 kDa o-HA better improved xerosis in a clinical investigation on 36 elderly people (60–80 yo.) [19]. An o-HA below 10 kDa induced filaggrin protein and caspase 14 activity in a 3D epidermis model, suggesting a possible increase in natural moisturizing factor and promotion of SC hydration [20]. A 2 kDA o-HA at 0.1% exhibited anti-aging properties in vivo: it improved skin elasticity and hydration, decreased wrinkle depth, and increased dermal collagen scores [15]. The smallest o-HAs were also demonstrated to reduce the deleterious effects of UV exposure. A tetrasaccharide decreased UV-induced inflammation by suppressing IL-6, IL-8, and IL-1β in normal human keratinocytes and UV-induced symptoms in hairless mice [17,21]. A similar molecule also reduced inflammation in a murine model of rosacea by decreasing IL-8 and TNF-α levels [22].
In the literature, HA is reported to be used in 663 formulations, and hydrolyzed HA is reported in 476 formulations [23,24]. Although these ingredients are widely utilized, there is a lack of case reports following topical application.
Our study presents in vitro and ex vivo evaluations of a new 3 kDa sodium hyaluronate designed for topical application, hereinafter named extra-low-molecular-weight HA (ExLMW-HA). This work involved a set of tests aiming at predicting the tolerance and safety of the ExLMW-HA: analyses of skin permeation, the release of inflammatory cytokines, and histology following application on skin explants; sensitization predictive assays; and mutagenicity and genotoxicity tests.
2. Materials and Methods
2.1. ExLMW-HA Raw Material
HA of about 3 kDa molecular weight (about 8 repeating 3-O-(β-D-glucuronide)-N-acetyl-D-glucosamine units in the form of sodium salt) was obtained using an engineered Saccharomyces cerevisiae strain allowing carbon flux optimization and high-precision control of the size of the synthesized polymers. The accumulated polymer was purified using membrane separation technology, and the final size of the purified HA was measured by means of size exclusion chromatography. The result was a white powder containing 95–105% dry matter; the pH of its aqueous solution (concentration close to 10 g/L) was 6.0.
2.2. Skin Penetration Assessment Using Raman Spectroscopy
2.2.1. Skin Explant Culture and Preparation for Raman Spectroscopy
All skin explants examined in this study were obtained from surgical waste with free and informed consent given by the patients who had undergone the surgeries. For this experiment, human skin explants were obtained from a 26-year-old female donor who had undergone abdominoplasty and were kept alive via culturing on biocompatible plastic grids in standard 24-well plates in an air–liquid interface with skin culture medium (Givaudan) at 37 °C with 5% CO2. The next day, a 30 mg/mL (643 µg/cm2) solution of ExLMW-HA diluted in distilled water was topically applied to one skin explant. The baseline control explant received no treatment. The explants were incubated for 8 h at 37 °C under 5% CO2. Following incubation, the skin surface was cleaned to eliminate any excess product. The skin explants were then frozen at −80 °C and sectioned longitudinally using a cryotome to produce 20 µm sections. Three tissue sections were selected and deposited on a CaF2 support for Raman imaging analysis. A total of 9 Raman images were recorded per condition.
2.2.2. Raman Micro-Imaging
Raman images measuring Y: 10 µm/X: 100 µm were acquired with a 5 µm step in both X and Y. Each Raman image was derived from 3 Y spectra and 21 X spectra (63 spectra per image). The parameters were as follows: laser wavelength, 600 nm; magnification, 100 X; long focal length with 0.75 numerical aperture; acquisition time, 25 s; accumulation, 1X; spectral range, 400 to 4000 cm−1; grating, 950T; confocal aperture, 300 µm; slit width, 150 µm (spectral resolution 6.5 cm−1); X step, 5 µm; Y step, 5 µm. The Raman spectrometer was calibrated with silicon before each experiment. The expected Raman peak of 520.7 cm−1 was obtained, thus ensuring reproducible measurements. The laser power was continuously controlled at the sample level. The Raman images were pre-processed to eliminate aberrant spectra (fluorescence, burning, saturation). The spectra were then baseline-corrected, smoothed, despiked, and normalized. Corrected data maps were processed using in-house software based on a least-squares fitting method operating in MATLAB software (Online). Reference spectra—average spectra for hyaluronic acid and clay—were mathematically mapped to the overall spectral image to determine the contribution of each spectrum to the image and the distribution of each compound within the skin explant.
2.3. Ex Vivo Protein Expression of Inflammatory Markers
2.3.1. Skin Explant Culture and Treatment
All skin explants examined in this study were obtained from surgical waste with free and informed consent given by the patients who had undergone the surgeries. For this experiment, skin explants were obtained from a 59-year-old female donor who had undergone breast surgeries. The explants were kept alive via culturing on biocompatible plastic grids in standard 24-well plates in an air–liquid interface with skin culture medium (Givaudan) at 37 °C with 5% CO2. The culture medium was renewed every 24 h for 3 days. Then, the explants were topically treated with either water (untreated condition) or ExLMW-HA at 5 mg/mL (107 µg/cm2) diluted in water. After 24 h of pre-incubation, a group of explants was stressed with PMA (phorbol 12-myristate 13-acetate, Merck France), a pro-inflammatory molecule, pre-diluted at 10 mg/mL in DMSO (Dimethyl sulfoxide, Merck France) then diluted in water to a final concentration of 100 µg/mL. A group of explants exposed to ExLMW-HA was not treated with PMA to retrieve a basal response. After 1 day of incubation, the supernatants were collected and centrifuged at 2000 g for 10 min at 4 °C to eliminate dead cells. The media were stored at −20 °C. The experiment was conducted in n = 4 replicates for each of the four conditions tested (untreated, ExLMW-HA 5 mg/mL, PMA, PMA + ExLMW-HA 5 mg/mL).
2.3.2. Cytokine Quantification
The following pro-inflammatory cytokines were quantified via a multiplex assay (R&D Systems, Minneapolis, USA): CCL/MIP alpha, IL-1 alpha/IL-1F1, IL-1 beta/IL-1F2, IL-15, CXCL2/GRO beta/MIP-2/CINC-3, and TNF-α. Briefly, the samples and the standard range were incubated for 2 h at room temperature in a 96-well plate with specific beads with antibodies against the previously listed cytokines. After three washes with wash buffer using a magnetic device provided by the supplier, a Biotin–antibody cocktail was added to the wells and incubated for 1 h at room temperature under orbital agitation. Following 3 washes, a solution of Streptavidin–PE was added to the wells for 30 min at room temperature. Finally, three more washes were performed, and the microparticles were resuspended in wash buffer. The read was then carried out using the Magpix® system (Luminex, Austin, TX, USA).
2.4. Histological Analysis
Human skin explants were obtained with informed consent from a 44-year-old female donor who had undergone breast surgery and were kept alive via culturing on biocompatible plastic grids in standard 24-well plates in an air–liquid interface with skin culture medium (Givaudan) at 37 °C with 5% CO2. The next day, 5 mg/mL (107 µg/cm2) and 10 mg/mL (214 µg/cm2) solutions of ExLMW-HA diluted in distilled water were topically applied to the skin explants. The explants were incubated for 5 days at 37 °C under 5% CO2. The culture medium was renewed every 24 h. After 5 days of incubation, the skin explant surfaces were rinsed with PBS then fixed in formalin. The skin explants were dehydrated and embedded in paraffin. Slices of 4 µm thickness were cut, then dewaxed, to be stained via the standard hematoxylin and eosin staining method (Abcam, Cambridge, UK) for morphological analysis.
2.5. Skin Sensitization Assessment
2.5.1. Keratinosens™ Assay
Keratinosens™ is a cell-based assay for skin sensitization that is endorsed by the European Union Reference Laboratory for Alternatives to Animal Testing (EURL ECVAM) and OECD Test Guideline 442D [25]. The assay involves a reporter cell line reflecting activation of the Nrf2 pathway. ExLMW-HA was dissolved in water at 12 concentrations, in three repetitions, and was evaluated for its cytotoxicity (MTT assay). Then, ExLMW-HA was tested at up to 400 µg/mL according to the standard operating procedure of Keratinosens™, accessible from the ECVAM DB-ALM database [26].
2.5.2. Kinetic Direct Peptide Reactivity Assay (kDPRA)
This test, endorsed by OECD Test Guideline 442C, determines the speed of the reaction of a test substance toward a peptide, and the reaction constant correlates with the potency of reactive skin sensitizers. ExLMW-HA was dissolved in acetonitrile (0.32–5 mM; as ExLMW-HA is a complex molecule, a default molecular weight of 200 kDa was used to calculate the dosage for the kDPRA gravimetric method according to OECD Test Guideline 442C) and mixed with a cysteine-containing peptide (Cys-peptide Ac-RFAACAA 0.5 mM in phosphate buffer, pH 7.5) to a final volume of 120 µL at five different ratios, according to the standard procedure for the kDPRA. The protocol is accessible from the ECVAM DB-ALM database [26]. The experiment was conducted in three replicates. Peptide depletion was monitored at six different time points via fluorescent derivatization of the parent peptide. At 10, 30, 90, 150, 210, and 1440 min after the start of the incubation, the reaction was stopped via the addition of a 3 mM solution of monobromobimane (40 µL added to 120 µL of incubation solution). The remaining peptide was thus fluorescently labeled after an incubation time of 5 min and could then be quantified by measuring the fluorescence using an excitation filter of 390 nm and an emission filter of 480 nm. The maximal reaction rate with the test peptide, expressed as Log kmax, was determined from a plot of the depletion percentage as a function of reaction time. The log kmax value can be further used in quantitative models to assess sensitizer potency. Log kmax is used in combination with KeratinoSens™ (KS) assay data to calculate a point of departure (PoD) in the form of a predicted Local Lymph Node Assay (LLNA) EC3 value for use in risk assessments [27,28].
2.6. Bacterial Reverse Mutation Assay (Ames Test)
This assay determines the mutagenic activity of a tested item on four Salmonella typhimurium strains (TA98, TA100, TA1535, TA1537) and one Escherichia coli strain (WP2P uvrA), according to OECD Test Guideline No. 471 (26 June 2020). These strains carry a defective gene (mutant) and are unable to synthesize essential amino acids, so they cannot grow on minimal medium. The test target is to quantify the ability of the analyzed compound to induce a mutation allowing these bacteria to regain their ability to grow. ExLMW-HA was prepared in water and added to culture medium in amounts from 50 µg to 5 mg per plate. A cytotoxicity assay was first conducted. Then, a genotoxicity test (Test 1) with and without metabolic activation was conducted with direct incorporation of ExLMW-HA (or controls) on minimal agar in the same concentration range used for the cytotoxicity assay. A second test (Test 2) involving pre-incubation of ExLMW-HA (or controls) with the test system with and without metabolic activation was also realized. The plates were incubated at 37°C for 48 to 72 h. Metabolic activation was induced by a cofactor-enhanced post-mitochondrial fraction isolated from the livers of Sprague Dawley rats treated with an enzymatic inducer, prepared according to Maron and Ames and provided by MOLTOX™ (S9 mix) [29]. It was stored at temperatures below −70 °C and prepared at 10% (v/v). Negative controls were conducted with water (ExLMW-HA vehicle) and no treatment. The positive controls without metabolic activation were strain TA100 with sodium azide, 10 µg per plate; strain TA98 with 2-nitrofluorene, 5 µg per plate; strain TA 1535 with sodium azide, 10 µg per plate; strain TA1537 with 9-aminoacridine, 30 µg per plate; and strain WP2P with N-oxide nitro-4-quinoleine, 1 µg per plate. Positive controls with metabolic activation were performed with amino-2-anthracene at 5 µg per plate, for all strains. Revertant colonies were counted on each plate at the end of incubation and compared to the spontaneous mutation rates of strains in water.
2.7. Micronucleus Assay in Human Lymphocytes
The in vitro micronucleus method investigates the ability of substances to induce chromosomal damage or damage to the mitotic apparatus. This assay tested ExLMW-HA for its ability to induce micronuclei in human lymphocytes cultured in vitro, after treatment in the absence or presence of an S9 metabolizing system, according to OECD Test Guideline No. 487 for the testing of chemicals (4 July 2023). The cytotoxicity of ExLMW-HA (62.5 to 2000 µg/mL, in water) was first evaluated using the cytokinesis-block proliferation index (CBPI). Human lymphocytes were prepared from fresh venous heparinized blood. The culture medium was 500 mL of RPMI 1640 1× (Dutch modification) with 100 mL of fetal calf serum, 6.25 mL of L-glutamine 200 mM, and 1.25 mL of antibiotic solution. The cultures were incubated at 37 °C and were treated approximately 48 h after they were initiated. The cells were centrifuged at 1000 rpm for 10 min and placed in treatment medium for a short treatment or a continuous treatment. The treatment medium consisted of 0.05 mL of ExLMW-HA (500 to 2000 µg/mL) or control solution and culture medium with or without 1 mL of S9 mix. The rat S9 liver tissue fraction was obtained from MOLTOX (Molecular Toxicology, Inc., Boone, NC, USA). The S9 mix was prepared with 1 mL of S9 fraction, 0.4 mL of NADP 0.1 M, 0.5 mL of G6P 0.1 M, 0.2 mL of MgCl2 0.1 M, 5 mL of phosphate buffer 0.2 M, and distilled water up to 10 mL. For the short treatment, cells were incubated for 3 h at 37 °C, and the cell cultures were then centrifuged, washed with PBS, and added to fresh medium. Cytochalasin B was added at a final concentration of 6 µg/mL, and the cells were incubated for a further 32 h. For the continuous treatment, cells were incubated for 3 h at 37 °C; then, cytochalasin B was added at a final concentration of 6 µg/mL, and the cells were incubated for a further 32 h before harvesting. Treating the cultures with cytochalasin B, an inhibitor of actin polymerization, blocks cytokinesis. Cells that have completed one cell cycle after the treatment can be distinguished from non-dividing cells by their binucleate appearance. The positive controls underwent a 3 h treatment with mitomycin-C in the absence of S9 mix or cyclophosphamide in the presence of S9 mix. The negative control used 0.05 mL of water in place of ExLMW-HA solution. Then, micronuclei were scored in the binucleate interphase stage of cell division. At least 1000 binucleated cells per cell culture were scored to assess the frequency of micronucleated cells. The micronucleus diameter must be less than 1/3 but greater than 1/16 of the diameter of the nucleus; no overlapping with the nucleus must be observed; and the micronucleus must be non-refractile and should have the same staining intensity as the main nucleus.
2.8. Data Analysis/Statistical Analysis
For protein expression experiments, a Shapiro–Wilk normality test was performed to evaluate whether the data followed the Gaussian Law. The results did not follow the Gaussian Law. As a consequence, a non-parametric statistical analysis was performed via Kruskal–Wallis ANOVA followed by a Mann–Whitney U test. It was considered a significant result when p < 0.05, indicated with *; p < 0.01, indicated with **; or p < 0.001, indicated with ***. For the sensitization and micronucleus assays, we used Student’s t-test and the modified χ2 test, respectively.
3. Results
3.1. ExLMW-HA Crossed Stratum Corneum and Reached Living Skin Cells
The ability of ExLMW-HA to penetrate skin following topical application was evaluated on skin explants using Raman spectroscopy imagery (Figure 2). ExLMW-HA crossed the SC and penetrated 60 µm of skin tissue; a spot was also observed around 100 µm. The epidermal thickness of women’s abdominal skin is described to reach an average of 79.2 to 127.2 ± 38 µm, depending on the study [30,31]. The highest product concentration was found in the epidermis in our tested conditions, and the main biological effects of ExLMW-HA are expected to occur in this compartment, although interaction with dermal cells is not excluded.
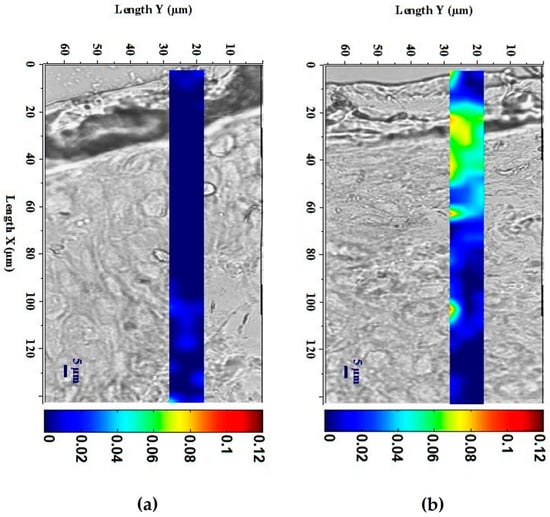
Figure 2.
Confocal micro-imagery from Raman spectroscopy of ExLMW-HA permeation and localization in (a) untreated skin explants and (b) skin explants topically treated with ExLMW-HA at 30 mg/mL for 8 h. Color scale indicates relative amount of ExLMW-HA, from dark blue (low concentration) to dark red (high concentration).
3.2. ExLMW-HA Did Not Induce Cytokine Release in the Culture Medium of Skin Explants
This assay aimed at quantifying the release of different cytokines in the culture medium of skin explants. TNF-α, IL-1β, IL-1α, CXCL2, IL-15, and CCL3/MIPα levels were determined after pre-treatment with ExLMW-HA for 48 h in the basal condition and in the inflammatory context induced by PMA (Figure 3). ExLMW-HA did significantly modify the cytokine concentration in the culture medium. In PMA-treated conditions, pre-treatment with ExLMW-HA did not induce a synergistic effect with the stressor.
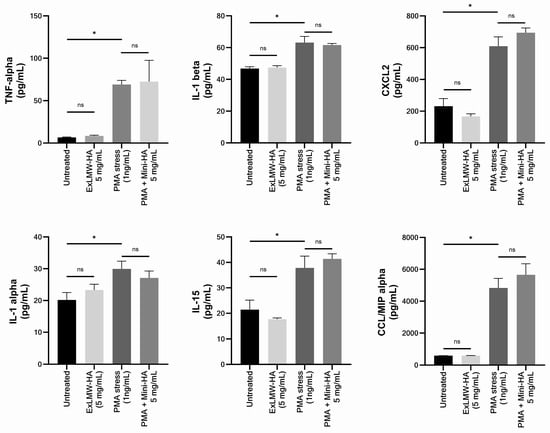
Figure 3.
The impact of ExLMW-HA (5 mg/mL) on the release of TNF-alpha, IL-1 alpha, IL-1 beta, CXCL2, IL-15, and CCL3/MIP alpha proteins in the culture medium of skin explants treated for 48 h and exposed or not to inflammatory inducer PMA (phorbol 12-myristate 13-acetate). Values are the mean ± standard error of mean (SEM) of four repetitions per condition. Mann–Whitney statistical test; ns: not significant; * p < 0.05.
3.3. Histological Study Revealed No Visible Impact of ExLMW-HA on Skin Explants
A histological study was conducted on skin explants treated topically with ExLMW-HA at 5 or 10 mg/mL for 5 days (Figure 4). In the morphological analysis, we were specifically interested in edema, necrosis, modifications of epidermal thickness, changes in cell morphology, and signs of dermal fiber degradation. No modification of the morphology of skin explants was observed at either ExLMW-HA concentration: the SC did not thicken, no edema was observed around the nuclei of epidermal cells that remained organized, the epidermal–dermal junction was cohesive with regular invaginations, and no visible modifications of the dermis appeared.
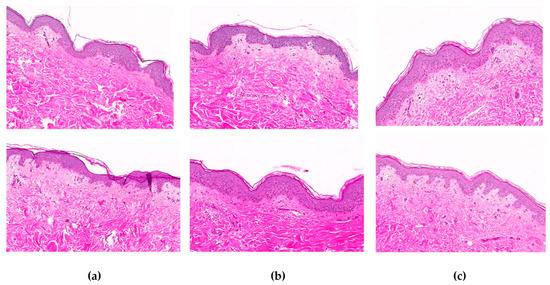
Figure 4.
Histological analysis of skin explants that were (a) untreated, (b) cultured with ExLMX-HA at 5 mg/mL, or (c) cultured with ExLMW-HA at 10 mg/mL, for 5 days. Slices were colored with hematoxylin–eosin stain (magnification ×10). Two representative images are presented for each condition.3.4. ExLMW-HA is not predicted to be a skin sensitizer.
ExLMW-HA permeates skin down to the living epidermis and possibly interacts with the dermal compartment. Due to its extra-low molecular weight, it may penetrate into skin cells. Therefore, it was crucial to demonstrate that keratinocytes, which are the first cells involved in the sensitization process, were not affected. Two predictive assays were conducted to assess the potential sensitization capacity of ExLMW-HA. Keratinosens™ is a reporter cell-line-based assay targeting the induction of the Nrf2 pathway [25]. The kinetic direct peptide reactivity assay (DPRA) is an in chemico test to determine the reaction speed of a test substance towards peptides and produce a quantitative estimate of the Molecular Initiating Event in skin sensitization as an indication of sensitizer potency.
ExLMW-HA’s cytotoxicity (MTT assay) was evaluated on the Keratinosens™ reporter cell line. The 3 kDa HA was not cytotoxic for the Keratinosens™ cells; the IC50 value, reducing the viability by 50%, was not reached and was higher than 400 µg/mL (Figure 5). In three independent repetitions, ExLMW-HA did not induce the luciferase gene above the threshold of 1.5, indicating that it is a non-sensitizer in the standard prediction model of KeratinoSens™.
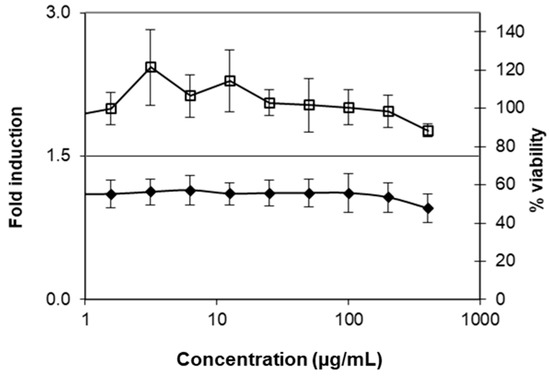
Figure 5.
Dose–response curves for ExLMW-HA. Given is the fold induction of the luciferase gene over the solvent control (filled diamonds) and the percentage of viability as determined via the MTT assay (open squares). Values are the mean ± standard deviation of three repetitions.
The kDPRA assay was conducted to classify ExLMW-HA in the globally harmonized system of chemical classification and labeling established by United Nations experts, also called GHS. ExLMW-HA was minimally reactive in the kDPRA. The default Log Kmax value of −3.5 for a chemical that is rated negative was applied. The substance is therefore not classified and falls into the GHS 1B/NC category, i.e., is not a strong 1A sensitizer, according to the prediction model of the kDPRA. The negative kDPRA result and the negative KeratinoSens™ result were integrated into a predictive model to calculate a point of departure (POD) in the form of a predicted Local Lymph Node Assay EC3 value for use in risk assessment [28]. The POD can be used as a starting point to set health-based exposure limits. The POD was predicted as an EC3 of 75% (>18,750 µg/cm2), rating it as a non-sensitizer. Based on a model integrating the available human evidence, the POD was predicted at 75% (>18,750 µg/cm2).
3.4. ExLMW-HA Is Not Predicted to Be Mutagenic or Pro-Mutagenic
ExLMW-HA permeates skin beyond the stratum corneum and it may penetrate into living cells, triggering activities at the nucleus and DNA level. Therefore, it is crucial to demonstrate that ExLMW-HA fully respects DNA content. A bacterial reverse mutation test determines the mutagenic activity of a tested item. It detects events that revert the mutations present in the test strains and restore the functional capability of the bacteria to synthesize an essential amino acid. Counting revertant colonies in the presence of the evaluated substance and comparing the outcome with the spontaneous rate of reversion allows us to determine the mutagenic potential of this substance. ExLMW-HA displayed no cytotoxic activity against the Salmonella typhimurium and Escherichia coli strains, and it was soluble in all conditions. In both tests without (Test 1) and with (Test 2) pre-incubation with ExLMW-HA, and for all bacterial strains tested, the ratio of the ExLMW-HA-induced reversion rate to the spontaneous rate of reversion did not exceed 1.4, with or without metabolic activation (Table 1). No dose–response relationship was observed, regardless of the test system or conditions of the test. ExLMW-HA was found to be non-mutagenic and non-pro-mutagenic.

Table 1.
Revertant analysis table. Salmonella typhimurium (TA100, TA98, and TA1535 strains) and Escherichia coli (WP2P strain) revertant colonies counted in presence of Ex-LMW-HA solubilized in water (50–5000 µg per plate) or in presence of water alone or without treatment. Positive control: see Materials and Methods Section. R: ratio of number of revertant colonies with ExLMW-HA to number of revertant colonies with water.
3.5. ExLMW-HA Is Not Predicted to Be Genotoxic
The in vitro micronucleus method investigates the ability of substances to induce chromosomal damage or damage to the mitotic apparatus. Cytotoxicity to lymphocytes was evaluated at 62.5 to 2000 µg/mL of ExLMW-HA, after 3 h in the presence or absence of metabolic activation (S9), or after 31 h of treatment without metabolic activation. ExLMW-HA did not induce pH or osmolality changes during the tests and was soluble in water. Cytotoxicity was established through a CBPI calculation that evaluated cytotoxic effects due to exposure to different concentrations of ExLMW-HA. No remarkable cytotoxicity was observed at any dose level, and the CBPI did not differ from that for the negative control (water) (Supplementary Data, Table S1). Based on these results, quantification of micronuclei was conducted on 2000 cells exposed to 500, 1000, or 2000 µg/mL of ExLMW-HA (Table 2). Following the treatment, no statistically significant increase in the incidence of micronucleated cells over the concurrent control value was observed at any dose level in any treatment condition, and no dose–effect relationship was observed.

Table 2.
Evaluation of ExLMW-HA regarding micronucleated lymphocyte incidence. Statistical significance was determined via χ2 test. NA: not applicable; NS: not significant; *** p < 0.001. Cyclophosphamide and colchicine were positive controls.
4. Discussion
Hyaluronic acid is a ubiquitous polymer that can be found in the human body in the vitreous fluid, joints, and derma, where it can be hydrolyzed into lower-molecular-weight molecules [6].
Topically applied HA has proven efficacy in cosmetic applications, with clinical demonstrations having shown its hydrating and anti-aging properties [32,33,34,35,36,37,38]. According to the current knowledge, the use of HMW-HA and LMW-HA at the recommended concentrations in cosmetics is recognized as safe by independent experts [23,24,39]. Mastery of the microbial fermentation process and hyaluronidase-controlled HA depolymerization now allows us to obtain HMW-HA of size over 2 MDa and diverse o-HAs starting from disaccharides [40,41,42]. Recent studies have demonstrated that o-HAs have moisturizing, anti-aging, and anti-inflammatory properties that can surpass those of higher-molecular-weight HAs [15,19,20,21,22].
In this work, a 3 kDa o-HA, called ExLMW-HA, was designed for cosmetic applications, and its safety and dermal tolerability were evaluated. This research was conducted on in vitro and ex vivo models. The absence of in vivo investigations and the use of skin explants from single donors represent limitations to the extrapolation of these results. We first determined the skin penetration of ExLMW-HA using Raman spectroscopy. As previously reported for 2, 5, 8, and 10 kDa o-HAs, ExLMW-HA permeated the SC, accumulated in the living epidermis, and was detected as a spot at 100 µm, suggesting a possible interaction with the dermal compartment [13,15,16]. The published skin penetration depths for various molecular weights of HA have revealed that skin penetration is inversely correlated with size [9]. It would be interesting to compare the skin penetration of ExLMW-HA to that of other sizes of HA under similar experimental conditions in order to fully understand the results obtained.
In vivo, HMW-HA and LMW-HA interact with the innate and adaptive immune systems during skin injuries and contribute to the tissue repair process [6,43,44,45]. TNF-α and IL-1β are key targets in the context of o-HAs and inflammation [46,47,48]. In this work, the impact of ExLMW-HA on inflammation was evaluated on skin explants to obtain an environment as close as possible to the whole skin structure, including immune cells. TNF-α and IL-1β release in the culture medium was quantified. The levels of four other inflammatory cytokines were determined: IL-1α, a member of the IL-1 family along with IL-1β, both binding the same receptor and constitutively expressed in keratinocytes; CXCL2, the level of which was found to increase in fibroblasts under 4.3 kDa o-HA treatment; and CCL3/MIPalpha and IL-15, involved in dermal wound healing [49,50,51,52]. Measurements were also conducted following treatment with PMA, an inducer of inflammatory response, in order to evaluate ExLMW-HA as a potentiator of inflammation. Under basal or inflammatory conditions, ExLMW-HA (5 mg/mL) did not modify protein expression, suggesting that this o-HA did not trigger an inflammatory response. To support our results, a morphological analysis of explants treated with 5 and 10 mg/mL of ExLMW-HA was conducted, and no visible modifications of the epidermis or dermis were found.
ExLMW-HA, as an endogenous hyaluronic acid, can trigger an immune response; therefore, the skin sensitization potential of ExLMW-HA was of interest and was evaluated through Keratinosens™ and kDPRA [53]. Ames’ test was also conducted to assess mutagenicity, and the micronucleus test was used to evaluate genotoxicity. The 3 kDa o-HA was not predicted to be a skin sensitizer, and the safe dosage extrapolated from these results was 90- to 178-fold greater than the concentrations used in the skin explants. The Ames’ test and micronucleus test gave negative results, predicting ExLMW-HA to be non-mutagenic, non-pro-mutagenic, and non-genotoxic.
5. Conclusions
This work provided an in vitro and ex vivo assessment of the safety of a 3 kDa sodium hyaluronate designed for topical cosmetic applications. We confirmed the ability of ExLMW-HA to penetrate skin to the living epidermis, at least. Predictive in vitro tests and a search for pro-inflammatory activity indicated that ExLMW-HA is safe for topical application. Research work on ExLMW-HA will continue at the clinical level to confirm its safety and determine its cosmetic benefits.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/cosmetics12060266/s1, Table S1: Evaluation of ExLMW-HA cytotoxicity on lymphocytes.
Author Contributions
Conceptualization, M.D.T. and A.S.; methodology, validation, and formal analysis, M.D.T., A.C. and A.N.; investigation, A.D., M.B.-D. and T.H.; design and supply of raw material, K.J., M.M. and D.A.; supply of surgical waste, J.T.; writing—review and editing, M.D.T. and A.C.; funding acquisition and resources, R.R. and D.L.; project administration and supervision, A.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article; further inquiries can be directed to the corresponding author.
Acknowledgments
We would like to thank the companies M Conseil, SGS, and ERBC for their support in conducting part of the experiments.
Conflicts of Interest
At the time of this study, the authors were employed by the companies Givaudan France SA and Givaudan International AG. The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The funders had no role in the design of the study; in the collection, analyses or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Abbreviations
The following abbreviations are used in this manuscript:
| ExLMW-HA | Extra-low-molecular-weight hyaluronic acid |
| HA | Hyaluronic acid |
| HMW-HA | High-molecular-weight hyaluronic acid |
| LMW-HA | Low-molecular-weight hyaluronic acid |
| SC | Stratum corneum |
| o-HA | Hyaluronic acid oligosaccharide |
References
- Weissmann, B.; Meyer, K. The Structure of Hyalobiuronic Acid and of Hyaluronic Acid from Umbilical Cord1,2. J. Am. Chem. Soc. 1954, 76, 1753–1757. [Google Scholar] [CrossRef]
- Atkins, E.D.T.; Sheehan, J.K. Structure for Hyaluronic Acid. Nat. New Biol. 1972, 235, 253–254. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.T.; Neo, B.H.; Betts, R.J. Glycosaminoglycans: Sweet as Sugar Targets for Topical Skin Anti-Aging. Clin. Cosmet. Investig. Dermatol. 2021, 14, 1227–1246. [Google Scholar] [CrossRef] [PubMed]
- Laurent, T.C.; Fraser, J.R.E. Hyaluronan. FASEB J. 1992, 6, 2397–2404. [Google Scholar] [CrossRef]
- Anderegg, U.; Simon, J.C.; Averbeck, M. More than Just a Filler—The Role of Hyaluronan for Skin Homeostasis. Exp. Dermatol. 2014, 23, 295–303. [Google Scholar] [CrossRef]
- Litwiniuk, M.; Krejner, A.; Speyrer, M.S.; Gauto, A.R.; Grzela, T. Hyaluronic Acid in Inflammation and Tissue Regeneration. Wounds 2016, 28, 78–88. [Google Scholar]
- Ferguson, E.L.; Roberts, J.L.; Moseley, R.; Griffiths, P.C.; Thomas, D.W. Evaluation of the Physical and Biological Properties of Hyaluronan and Hyaluronan Fragments. Int. J. Pharm. 2011, 420, 84–92. [Google Scholar] [CrossRef]
- Cyphert, J.M.; Trempus, C.S.; Garantziotis, S. Size Matters: Molecular Weight Specificity of Hyaluronan Effects in Cell Biology. Int. J. Cell Biol. 2015, 2015, e563818. [Google Scholar] [CrossRef]
- Essendoubi, M.; Gobinet, C.; Reynaud, R.; Angiboust, J.F.; Manfait, M.; Piot, O. Human Skin Penetration of Hyaluronic Acid of Different Molecular Weights as Probed by Raman Spectroscopy. Skin. Res. Technol. 2016, 22, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.; Zhang, F. Image of the Distribution Profile of Targets in Skin by Raman Spectroscopy-Based Multivariate Analysis. Skin. Res. Technol. 2022, 28, 402–409. [Google Scholar] [CrossRef]
- Henry, L.; Delsuc, N.; Laugel, C.; Lambert, F.; Sandt, C.; Hostachy, S.; Bernard, A.-S.; Bertrand, H.C.; Grimaud, L.; Baillet-Guffroy, A.; et al. Labeling of Hyaluronic Acids with a Rhenium-Tricarbonyl Tag and Percutaneous Penetration Studied by Multimodal Imaging. Bioconjug Chem. 2018, 29, 987–991. [Google Scholar] [CrossRef]
- Grégoire, S.; Man, P.D.; Maudet, A.; Le Tertre, M.; Hicham, N.; Changey, F.; Gaëlle, B.-S.; Tran, C.; Laurence, V. Hyaluronic Acid Skin Penetration Evaluated by Tape Stripping Using ELISA Kit Assay. J. Pharm. Biomed. Anal. 2023, 224, 115205. [Google Scholar] [CrossRef]
- Ni, C.; Zhang, Z.; Wang, Y.; Zhang, Z.; Guo, X.; Lv, H. Hyaluronic Acid and HA-Modified Cationic Liposomes for Promoting Skin Penetration and Retention. J. Control Release 2023, 357, 432–443. [Google Scholar] [CrossRef]
- Witting, M.; Boreham, A.; Brodwolf, R.; Vávrová, K.; Alexiev, U.; Friess, W.; Hedtrich, S. Interactions of Hyaluronic Acid with the Skin and Implications for the Dermal Delivery of Biomacromolecules. Mol. Pharm. 2015, 12, 1391–1401. [Google Scholar] [CrossRef]
- Abe, Y.; Seino, S.; Kurihara, H.; Kage, M.; Tokudome, Y. 2-kDa Hyaluronan Ameliorates Human Facial Wrinkles through Increased Dermal Collagen Density Related to Promotion of Collagen Remodeling. J. Cosmet. Dermatol. 2023, 22, 320–327. [Google Scholar] [CrossRef]
- Legouffe, R.; Jeanneton, O.; Gaudin, M.; Tomezyk, A.; Gerstenberg, A.; Dumas, M.; Heusèle, C.; Bonnel, D.; Stauber, J.; Schnebert, S. Hyaluronic Acid Detection and Relative Quantification by Mass Spectrometry Imaging in Human Skin Tissues. Anal. Bioanal. Chem. 2022, 414, 5781–5791. [Google Scholar] [CrossRef]
- Kage, M.; Tokudome, Y.; Hashimoto, F. Permeation of Hyaluronan Tetrasaccharides through Hairless Mouse Skin: An in Vitro and in Vivo Study. Arch. Dermatol. Res. 2013, 305, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Waggett, S.; Lyles, E.; Schlesinger, T. Update on Low-Molecular Weight Hyaluronic Acid in Dermatology: A Scoping Review. EMJ Dermatol. 2024, 12, 134–146. [Google Scholar] [CrossRef]
- Muhammad, P.; Novianto, E.; Setyorini, M.; Legiawati, L.; Yusharyahya, S.N.; Menaldi, S.L.; Budianti, W.K. Effectiveness of Topical Hyaluronic Acid of Different Molecular Weights in Xerosis Cutis Treatment in Elderly: A Double-Blind, Randomized Controlled Trial. Arch. Dermatol. Res. 2024, 316, 329. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, M.; Maeda, K. New Functions of Low-Molecular-Weight Hyaluronic Acid on Epidermis Filaggrin Production and Degradation. Cosmetics 2021, 8, 118. [Google Scholar] [CrossRef]
- Hu, L.; Nomura, S.; Sato, Y.; Takagi, K.; Ishii, T.; Honma, Y.; Watanabe, K.; Mizukami, Y.; Muto, J. Anti-Inflammatory Effects of Differential Molecular Weight Hyaluronic Acids on UVB-Induced Calprotectin-Mediated Keratinocyte Inflammation. J. Dermatol. Sci. 2022, 107, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.G.; Yoon, M.S.; Kim, D.H.; Shin, J.U.; Lee, H.J. Hyaluronan Oligosaccharides Improve Rosacea-Like Phenotype through Anti-Inflammatory and Epidermal Barrier-Improving Effects. Ann. Dermatol. 2020, 32, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Becker, L.C.; Bergfeld, W.F.; Belsito, D.V.; Klaassen, C.D.; Marks, J.G.; Shank, R.C.; Slaga, T.J.; Snyder, P.W.; Cosmetic Ingredient Review Expert Panel; Andersen, F.A. Final Report of the Safety Assessment of Hyaluronic Acid, Potassium Hyaluronate, and Sodium Hyaluronate. Int. J. Toxicol. 2009, 28, 5–67. [Google Scholar] [CrossRef]
- CIR Portal Ingredient Status Report Starter. Available online: https://cir-reports.cir-safety.org/cir-ingredient-status-report/?id=57efb38d-7d49-ed11-bba2-00224824e39e (accessed on 15 March 2025).
- Natsch, A.; Emter, R. Nrf2 Activation as a Key Event Triggered by Skin Sensitisers: The Development of the Stable KeratinoSens Reporter Gene Assay. Altern. Lab. Anim. 2016, 44, 443–451. [Google Scholar] [CrossRef]
- KeratinosensTM, Protocol 155:P; kDPRA, Protocol 217:P. Available online: https://jeodpp.jrc.ec.europa.eu/ftp/jrc-opendata/EURL-ECVAM/datasets/DBALM/LATEST/online/DBALM_docs/ (accessed on 17 March 2025).
- Natsch, A.; Emter, R.; Gfeller, H.; Haupt, T.; Ellis, G. Predicting Skin Sensitizer Potency Based on in Vitro Data from KeratinoSens and Kinetic Peptide Binding: Global versus Domain-Based Assessment. Toxicol. Sci. 2015, 143, 319–332. [Google Scholar] [CrossRef]
- Natsch, A.; Gerberick, G.F. Integrated Skin Sensitization Assessment Based on OECD Methods (I): Deriving a Point of Departure for Risk Assessment. ALTEX 2022, 39, 636–646. [Google Scholar] [CrossRef]
- Maron, D.M.; Ames, B.N. Revised Methods for the Salmonella Mutagenicity Test. Mutat. Res. 1983, 113, 173–215. [Google Scholar] [CrossRef]
- Lintzeri, D.A.; Karimian, N.; Blume-Peytavi, U.; Kottner, J. Epidermal Thickness in Healthy Humans: A Systematic Review and Meta--analysis. Acad. Dermatol. Venereol. 2022, 36, 1191–1200. [Google Scholar] [CrossRef]
- Oltulu, P.; Tekecik, M.; Taflioglu Tekecik, Z.; Kilinc, F.; Ince, B. Measurement of Epidermis, Dermis, and Total Skin Thicknesses from Six Different Face Regions. Selcuk Tip Derg. 2022, 38, 210–215. [Google Scholar] [CrossRef]
- Blanc Catala, J.; Zanchetta, C.; François, C.; Chapuis, E.; Joset, N.; Meunier, M.; Loeser, F.; Godbille, S.; Scandolera, A.; Reynaud, R.; et al. Evaluation of the Hydrating Benefits of a Cationic Hyaluronic Acid: From Biological Evaluation to Consumer Home Use Trial. Int. J. Cosmet. Sci. 2024, 46, 795–805. [Google Scholar] [CrossRef]
- De Tollenaere, M.; Meunier, M.; Lapierre, L.; Chapuis, E.; Guilleret, A.; Harrison, I.; Jean, T.; Rannou, A.; Scandolera, A.; Reynaud, R. High Molecular Weight Hyaluronic Acid Vectorised with Clay Provides Long-Term Hydration and Reduces Skin Brightness. Skin. Res. Technol. 2024, 30, e13672. [Google Scholar] [CrossRef]
- Jegasothy, S.M.; Zabolotniaia, V.; Bielfeldt, S. Efficacy of a New Topical Nano-Hyaluronic Acid in Humans. J. Clin. Aesthet. Dermatol. 2014, 7, 27–29. [Google Scholar]
- Meunier, M.; Scandolera, A.; Chapuis, E.; Lapierre, L.; Sandré, J.; Brunner, G.; Lovchik, M.; Reynaud, R. The Anti-Wrinkles Properties of Sodium Acetylated Hyaluronate. J. Cosmet. Dermatol. 2022, 21, 2749–2762. [Google Scholar] [CrossRef]
- Nobile, V.; Buonocore, D.; Michelotti, A.; Marzatico, F. Anti-Aging and Filling Efficacy of Six Types Hyaluronic Acid Based Dermo-Cosmetic Treatment: Double Blind, Randomized Clinical Trial of Efficacy and Safety. J. Cosmet. Dermatol. 2014, 13, 277–287. [Google Scholar] [CrossRef]
- Pavicic, T.; Gauglitz, G.G.; Lersch, P.; Schwach-Abdellaoui, K.; Malle, B.; Korting, H.C.; Farwick, M. Efficacy of Cream-Based Novel Formulations of Hyaluronic Acid of Different Molecular Weights in Anti-Wrinkle Treatment. J. Drugs Dermatol. 2011, 10, 990–1000. [Google Scholar]
- Poetschke, J.; Schwaiger, H.; Steckmeier, S.; Ruzicka, T.; Gauglitz, G.G. Anti-wrinkle creams with hyaluronic acid: How effective are they? MMW Fortschr. Med. 2016, 158 (Suppl. 4), 1–6. [Google Scholar] [CrossRef] [PubMed]
- Schlesinger, T.; Rowland Powell, C. Efficacy and Safety of a Low Molecular Weight Hyaluronic Acid Topical Gel in the Treatment of Facial Seborrheic Dermatitis Final Report. J. Clin. Aesthet. Dermatol. 2014, 7, 15–18. [Google Scholar] [PubMed]
- Marcellin, E.; Steen, J.A.; Nielsen, L.K. Insight into Hyaluronic Acid Molecular Weight Control. Appl. Microbiol. Biotechnol. 2014, 98, 6947–6956. [Google Scholar] [CrossRef]
- Pang, B.; Wang, H.; Huang, H.; Liao, L.; Wang, Y.; Wang, M.; Du, G.; Kang, Z. Enzymatic Production of Low-Molecular-Weight Hyaluronan and Its Oligosaccharides: A Review and Prospects. J. Agric. Food Chem. 2022, 70, 14129–14139. [Google Scholar] [CrossRef]
- Qiu, Y.; Ma, Y.; Huang, Y.; Li, S.; Xu, H.; Su, E. Current Advances in the Biosynthesis of Hyaluronic Acid with Variable Molecular Weights. Carbohydr. Polym. 2021, 269, 118320. [Google Scholar] [CrossRef] [PubMed]
- Kaul, A.; Short, W.D.; Keswani, S.G.; Wang, X. Immunologic Roles of Hyaluronan in Dermal Wound Healing. Biomolecules 2021, 11, 1234. [Google Scholar] [CrossRef]
- Avenoso, A.; Bruschetta, G.; D Ascola, A.; Scuruchi, M.; Mandraffino, G.; Saitta, A.; Campo, S.; Campo, G.M. Hyaluronan Fragmentation During Inflammatory Pathologies: A Signal That Empowers Tissue Damage. Mini Rev. Med. Chem. 2020, 20, 54–65. [Google Scholar] [CrossRef]
- Tolg, C.; Messam, B.J.-A.; McCarthy, J.B.; Nelson, A.C.; Turley, E.A. Hyaluronan Functions in Wound Repair That Are Captured to Fuel Breast Cancer Progression. Biomolecules 2021, 11, 1551. [Google Scholar] [CrossRef]
- Dong, Y.; Arif, A.; Olsson, M.; Cali, V.; Hardman, B.; Dosanjh, M.; Lauer, M.; Midura, R.J.; Hascall, V.C.; Brown, K.L.; et al. Endotoxin Free Hyaluronan and Hyaluronan Fragments Do Not Stimulate TNF-α, Interleukin-12 or Upregulate Co-Stimulatory Molecules in Dendritic Cells or Macrophages. Sci. Rep. 2016, 6, 36928. [Google Scholar] [CrossRef]
- Campo, G.M.; Avenoso, A.; Campo, S.; D’Ascola, A.; Nastasi, G.; Calatroni, A. Small Hyaluronan Oligosaccharides Induce Inflammation by Engaging Both Toll-like-4 and CD44 Receptors in Human Chondrocytes. Biochem. Pharmacol. 2010, 80, 480–490. [Google Scholar] [CrossRef]
- Sanchez, B.; Ferraro, S.; Josset-Lamaugarny, A.; Pagnon, A.; Hee, C.K.; Nakab, L.; Sigaudo-Roussel, D.; Fromy, B. Skin Cell and Tissue Responses to Cross-Linked Hyaluronic Acid in Low-Grade Inflammatory Conditions. Int. J. Inflamm. 2023, 2023, 3001080. [Google Scholar] [CrossRef]
- Vistejnova, L.; Safrankova, B.; Nesporova, K.; Slavkovsky, R.; Hermannova, M.; Hosek, P.; Velebny, V.; Kubala, L. Low Molecular Weight Hyaluronan Mediated CD44 Dependent Induction of IL-6 and Chemokines in Human Dermal Fibroblasts Potentiates Innate Immune Response. Cytokine 2014, 70, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Li, X.; Wang, Z.; Li, C.; Wang, D.; Li, C. CCL3 Promotes Cutaneous Wound Healing Through Recruiting Macrophages in Mice. Cell Transplant. 2024, 33, 09636897241264912. [Google Scholar] [CrossRef] [PubMed]
- Cavalli, G.; Colafrancesco, S.; Emmi, G.; Imazio, M.; Lopalco, G.; Maggio, M.C.; Sota, J.; Dinarello, C.A. Interleukin 1α: A Comprehensive Review on the Role of IL-1α in the Pathogenesis and Treatment of Autoimmune and Inflammatory Diseases. Autoimmun. Rev. 2021, 20, 102763. [Google Scholar] [CrossRef]
- Jones, A.M.; Griffiths, J.L.; Sanders, A.J.; Owen, S.; Ruge, F.; Harding, K.G.; Jiang, W.G. The Clinical Significance and Impact of Interleukin 15 on Keratinocyte Cell Growth and Migration. Int. J. Mol. Med. 2016, 38, 679–686. [Google Scholar] [CrossRef] [PubMed]
- Martin, S.F. New Concepts in Cutaneous Allergy. Contact Dermat. 2015, 72, 2–10. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).